Abstract
Background
Many patients with rheumatoid arthritis are currently successfully treated with infliximab (anti‐tumour necrosis factor); however, about 30% of the patients do not respond to infliximab. One of the postulated hypotheses of not responding is the fast clearance of infliximab due to the development of infliximab–anti‐infliximab complexes.
Objective
To investigate the in vivo mechanism of not responding and the role of human anti‐chimeric antibodies (HACAs) by using radiolabelled infliximab.
Methods
Two responding and two non‐responding patients with rheumatoid arthritis, infused with radiolabelled infliximab, were investigated by both imaging and serum analysis.
Results
Images showed predominant presence of infliximab in blood up to 24 h, with a trend of faster blood clearance and of higher liver/spleen uptake in a non‐responding patient. Clinically inflamed joints showed uptake of the drug. The HACA level in the non‐responders was high (1641 and 1008 U/ml), but low or not detectable in responders. Sucrose gradients of serum showed antibody complexes in both non‐responders. Various sizes of antibody complexes, including very large ones, were observed in a non‐responder who developed a serious infusion reaction.
Conclusion
Formation of infliximab–anti‐infliximab complexes were found in non‐responders due to the presence of large amounts of HACA. This finding, supported by both imaging and serum analysis data, may explain failure of infliximab treatment.
Currently, many patients with rheumatoid arthritis are successfully treated with infliximab, a chimeric anti‐tumour necrosis factor α (TNFα) antibody.1,2 Nevertheless, about 30% of the patients do not respond. One of the postulated hypotheses of no response is the fast clearance of infliximab due to the formation of infliximab–anti‐infliximab complexes. Support for this hypothesis was found by others in patients with rheumatoid arthritis and in those with Crohn's disease.1,3 A negative correlation was reported between the presence of human antichimeric antibodies (HACAs) with infliximab levels and clinical response. In addition, we have analysed serum of 51 patients with rheumatoid arthritis treated with infliximab. By means of a radioimmunoassay, we observed that 43% of the patients developed HACAs during a 1‐year course of infliximab treatment.4 The persistence of HACAs relates to a decrease in therapeutic response, probably due to fast clearance. In monkeys treated with infliximab, formation of antibody complexes was seen after injection of iodine‐125‐anti‐infliximab.5 No data on formation of infliximab–anti‐infliximab antibody complexes in patients with rheumatoid arthritis are available yet. The process of antibody complex formation in patients with rheumatoid arthritis treated with infliximab and their meaning in non‐responders was investigated in this study.
Methods
Patients and study protocol
The study was approved by the local ethics committee. Patients fulfilled the criteria of rheumatoid arthritis (according to the American College of Rheumatology Criteria) and were treated with infliximab (3 mg/kg) with 8‐week intervals at inclusion. Two female responders and two female non‐responders to infliximab (age 24–71 years; duration of the disease 6–38 years) were included. Patients received at least five prior infliximab infusions. The European League Against Rheumatism response criteria were used to determine whether a patient was a responder or a non‐responder.6 The presence of HACAs was not known at the start of the study. Any comedication was allowed; all included patients were treated with methotrexate.
Eight weeks after the last infusion of infliximab, patients were infused with 10 mg 740 MBq technetium‐99m infliximab in 50 ml NaCl (0.9%) during 2 h. Simultaneously, they received, via the same infusion needle, 3 mg/kg (−10 mg) unlabelled infliximab in 250 ml NaCl (0.9%) in 2 h. A micropore filter (0.21 μm) was used in the infusion application system. Blood samples were withdrawn for further analysis directly before infusion (t = 0 h), during infusion (t = 5, 10 and 30 min; 1 and 2 h) and post infusion (t = 18 and 24 h). Images were recorded directly after infusion and 24 h later.
Imaging
Infliximab was coupled to 99mTc as described previously.7,899mTc‐infliximab was purified on a PD10 column (PD10 column filled with Sephadex™ G25M, Amershon, Piscataway, New Jersey, USA) with 0.9% NaCl as an eluent and filter sterilised (endotoxin levels <5 units/ml). The radiochemical purity, using high‐performance liquid chromatography and thin‐layer chromatography, was >99.5%. The immunoreactivity of 99mTc‐infliximab, determined using preformed sepharose beads coated with TNF via a non‐competing antibody to TNF,9 was >92%.
Whole‐body images (matrix 1024×256) and spot views of joints (matrix 256×256) were recorded with an ECAM gamma camera (Siemens, Siemens Mediscle Techniek, Zoetermeer, The Netherlands). Regions of interest were drawn on digital images of the whole‐body and on several organs. Whole‐body counts were corrected for machine drift, physical decay and counting time differences. Geometrical means were used for analysis. A region of interest in the left ventricle of the heart, corrected for lung background, was used as measure for blood pool activity. Spleen uptake was also corrected for background activity. Ratios of organ uptake to blood pool activity of 99mTc‐infliximab were calculated.
Serum analysis
Infliximab was quantified as described9 with one modification. Infliximab binding was assessed by incubation with biotinylated rabbit immunoglobulin (Ig) G directed to the infliximab idiotype. Detection limit of this assay is about 0.1 μg infliximab/ml serum. Antibodies to infliximab were measured using a method described previously.10 The size of 99mTc‐infliximab was analysed by sedimentation analysis on sucrose gradients as described previously.11
Results
Administration of 99mTc‐infliximab to patients
Infliximab could be safely administered to the two responders (A and B). One non‐responder (C) developed a light transient pressure on the chest, but the infusion could safely be continued. A serious infusion reaction was observed in the other non‐responder (D), who developed a shock at 45 min of infusion. The infusion was immediately discontinued, followed by proper treatment. The severity of the infusion reaction did not allow this patient to continue the study. Blood samples were collected for up to 30 min; no image data were collected.
Imaging
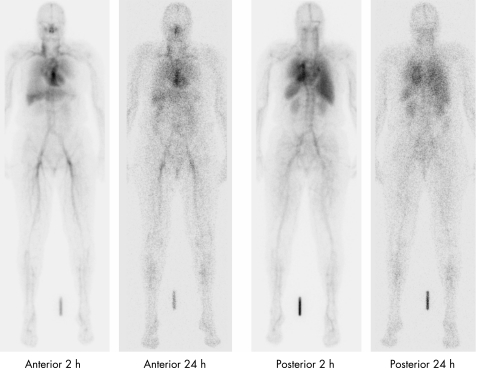
Up to 24 h post infusion, 99mTc‐infliximab was mainly present in the blood pool (fig 1). At 24 h, 100% and 98% of infused 99mTc‐infliximab were still present in the whole‐body of responders A and B, respectively. A relatively faster plasma and whole‐body clearance was found in the non‐responder C; the whole‐body retention at 24 h was 76%. Besides blood pool, liver, spleen and kidneys were also visualised. At 2 h, liver uptake in the non‐responder was higher than that in the responders, with liver to blood pool ratios of 2.6, 1.5 and 2.2, respectively. Spleen to blood pool ratios showed similar differences. At 24 h, no significant differences in liver and spleen uptake were observed. Uptake of 99mTc‐infliximab was seen in clinically inflamed joints (data not shown).
Figure 1 Whole‐body images. Anterior and posterior whole‐body images of a responder directly after (2 h) and 24 h post infusion.
Infliximab and anti‐infliximab levels
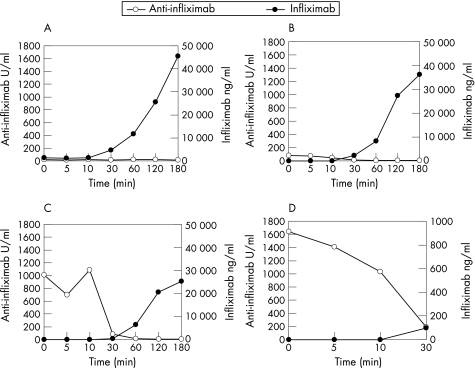
Infliximab levels of responder A, negative for anti‐infliximab, rose to about 45 μg/ml on infusion (fig 2A). Responder B had low levels of anti‐infliximab (82 AU/ml) at t = 0. During infusion, anti‐infliximab disappeared and infliximab levels rose to 35 μg/ml (fig 2B). High anti‐infliximab levels of 1008 and 1641 U/ml were found in both non‐responders, C and D respectively (figs 2C, D). The highest level was detected in patient D, who developed the infusion reaction (fig 2D). In both non‐responders, all infused infliximab was bound to circulating anti‐infliximab within the first 30 min, resulting in low infliximab levels. At 30–60 min of infusion, the concentration of infliximab overruled that of anti‐infliximab, resulting in increasing infliximab concentration (fig 2C).
Figure 2 Infliximab and anti‐infliximab levels in the sera of responders A and B (A,B) and two non‐responders C and D (C,D). (D) The x and y axes were adjusted because of lacking samples after 30 min of patient D and the lower amount of infliximab in the samples up to 30 min.
Infliximab–anti‐infliximab complexes
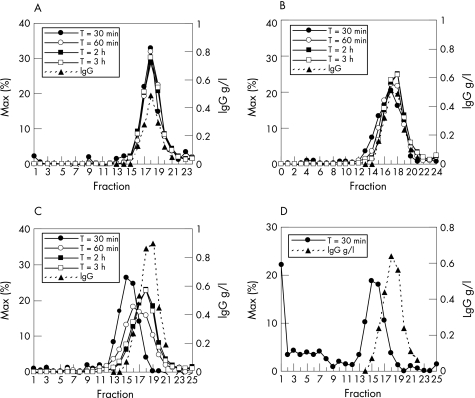
Samples containing sufficient radioactivity (⩾30 min) were analysed. Sucrose gradients of serum of responder A showed a sharp peak of 99mTc‐infliximab at fraction 18, exactly the position at which free IgG was found in the nephelometer (fig 3A). This implies absence of infliximab–anti‐infliximab complexes. In the responder B, a minor radioactivity shift was seen at 30 and 60 min, probably reflecting the formation of small amounts of small complexes that disappeared later (fig 3B). Higher amounts of small complexes were found in the non‐responder C at 30 and 60 min, with a peak at fraction 15 (fig 3C). Their sedimentation properties corresponded to the formation of 1:1 complexes between infliximab and an IgG antibody to infliximab. The serum of non‐responder D showed complexes of various sizes (at fraction 15 and lower fractions), including very large ones (molecular weight >1 000 000; fig 3D). From 1 h onwards, no complexes were found in any of the patients.
Figure 3 Sucrose gradients of sera of responders A and B (A,B) and of non‐responders C and D (C,D). The measured radioactivity in the fractions as a percentage of the maximum radioactivity at each time point (% of maximum) is shown. The values on the y axis in D have been adjusted to the lower amounts of radioactivity in the 30‐min sample to visualise the size of the antibody complexes in this patient.
Discussion
To our knowledge, this is the first report of the formation of infliximab–anti‐infliximab complexes during infliximab infusion in non‐responders with rheumatoid arthritis. Owing to antibody complex formation, low infliximab levels were observed within the first hour of infusion. Images suggested a faster clearance of infliximab in the non‐responding patient, possibly by removal of antibody complexes by the liver and spleen. The quantity of HACAs seemed to be important for responsiveness to treatment. Besides a high HACA level, formation of large antibody complexes was involved in the development of a serious infusion reaction in one non‐responder.
The 2‐h images showed a trend of faster clearance and higher liver and spleen uptake of 99mTc‐infliximab in the non‐responder. Although imaging data of only one non‐responder could be collected, these data correspond to the serum analysis findings. Serum analysis showed antibody complex formation within the first hour of infliximab infusion. These antibody complexes are most likely to accumulate in the macrophage–phagocyte system, which could explain the relatively faster clearance and higher liver and spleen uptake on the 2‐h images. Complex formation was observed in serum up to 60 min; thereafter, the concentration of infliximab overruled that of anti‐infliximab and no antibody complexes and no anti‐infliximab could be measured anymore. This explains why the images at 2 h, and certainly at 24 h, mainly showed blood pool activity, as biodistribution at these time points was dominated by the concentration of unbound infliximab.
Anti‐infliximab levels were high in non‐responders. In responder B, small amounts of antibodies were present. The concentration was probably so low that it had not yet affected the responsiveness to treatment. Therefore, we assume that non‐response occurs when the concentration of anti‐infliximab exceeds a certain critical level. This is confirmed by the results of Beart et al.3 In patients with Crohn's disease, they found a shorter duration of response to infliximab with the presence of anti‐infliximab levels > 8.0 μg/ml. In our study, we calculated that patient D had about 24 μg/ml of anti‐infliximab in the circulation.
Beart et al3 also described a higher risk of infusion reactions in patients with higher anti‐infliximab levels. The finding in our study that development of a serious infusion reaction was observed in the only patient showing formation of large complexes between infliximab and anti‐infliximab suggests that besides the quantity of anti‐infliximab, the quality of the response is related to infusion reactions.
Our study was an effort to investigate the pathophysiological mechanism of non‐response to anti‐TNF treatment. Although the number of investigated patients is low, the observations of our study are new and contribute to further knowledge to anti‐TNF response. Our results showed that the formation of antibody complexes, due to the presence of HACAs, may be responsible for non‐response and are most probably responsible for infusion reactions.
Acknowledgements
We thank GAMS van Dongen for his contribution to the design of the study, the radiolabelling of infliximab and his critical revision of the manuscript. We also thank EFI Comans for his help with the infusions of radiolabelled infliximab, EJF Franssen for his contribution to the design of the study and for pharmacological assistance, and M van der Vlies for his calculations of and advice about the radioactivity burden of 99mTc‐infliximab.
Abbreviations
HACA - human anti‐chimeric antibody
TNF - tumour necrosis factor
Footnotes
Competing interests: None.
References
- 1.Maini R N, Breedveld F C, Kalden J R, Smolen J S, Davis D, MacFarlane J D.et al Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor α monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998411552–1563. [DOI] [PubMed] [Google Scholar]
- 2.Lipsky P E, van der Heijde D M F M, St Clair E W, Furst D E, Breedveld F C, Kalden J R.et al Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 20003431594–1602. [DOI] [PubMed] [Google Scholar]
- 3.Beart F, Noman M, Vermeire S, Van Assche G, D'Haens G, Carbonez A.et al Influence of immunogenicity on the long‐term efficacy of infliximab in Crohn's disease. N Engl J Med 2003348601–608. [DOI] [PubMed] [Google Scholar]
- 4.Wolbink G J, Vis M, Lems W, de Groot E, Nurmohamed M T, Stapel S.et al Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 200654711–715. [DOI] [PubMed] [Google Scholar]
- 5.Rojas J R, Taylor R P, Cunningham M R, Rutkoski T J, Vennarini J, Jang H.et al Formation, distribution, and elimination of infliximab and anti‐infliximab immune complexes in cynomolgus monkeys. J Pharmacol Exp Ther 2005313578–585. [DOI] [PubMed] [Google Scholar]
- 6.Van Gestel A M, Prevoo M L, van't Hof M A, van Rijswijk M H, van de Putte L B, van Riel P L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/Internal League Against Rheumatism criteria. Arthritis Rheum 19963934–40. [DOI] [PubMed] [Google Scholar]
- 7.Visser G, Gerretsen M, Herscheid J, Snow G B, Van Dongen G A M S. Labeling of monoclonal antibodies with 186Re using the MAG3 chelate for radioimmunotherapy of cancer: a technical protocol. J Nucl Med 1993341953–1963. [PubMed] [Google Scholar]
- 8.Van Gog F B, Visser G W M, Stroomer J W G, Snow G B, Van Dongen G A M S. High dose 186Re‐labeling of monoclonal antibodies for clinical application: pitfalls and solutions. Cancer 1997802360–2370. [PubMed] [Google Scholar]
- 9.Wolbink G J, Voskuyl A E, Lems W F, de Groot E, Nurmohamed M T, Tak P P.et al Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 200564704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aalberse R C, van Loghem E, van Munster P J, Nadorp J H. Human antibodies to IgE: a simple method to avoid ambiguous results of the radioimmunosorbent test (RIST). J Lab Clin Med 197483831–839. [PubMed] [Google Scholar]
- 11.Aarden L A, de Groot E R, Feltkamp T E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti‐dsDNA with the immunofluorescence technique. Ann N Y Acad Sci 1975254505–515. [DOI] [PubMed] [Google Scholar]