Abstract
Background and objective
Glucosamine is suggested to affect glucose transport and insulin resistance. The effects of oral glucosamine on serum glucose and insulin levels at the initiation and throughout the duration of a 3‐h oral glucose tolerance test were examined.
Methods
Sera from 16 patients with osteoarthritis, but with no other diagnosed medical condition who had fasted overnight, were obtained every 15–30 min during the 3 h of continued fasting and during the 3 h after ingestion of 75 g of glucose with or without ingestion of 1500 mg of glucosamine sulphate. Glucose was analysed by high‐performance liquid chromatography using a Metrohm‐Peak 817 Bioscan, and the area under the curve (AUC) for glucose was calculated. Insulin was measured by radioimmunoassay every 30 min for 2 h.
Results
Three participants who were found to have previously undiagnosed abnormalities of glucose tolerance demonstrated significant (p = 0.04) incremental elevations in glucose levels after ingestion of glucosamine sulphate. The other 13 participants also had mean incremental elevations that were not significant (p = 0.20). Glucosamine sulphate ingestion had no effect on insulin levels.
Conclusion
The results suggest that glucosamine ingestion may affect glucose levels and consequent glucose uptake in patients who have untreated diabetes or glucose intolerance.
Glucosamine is widely marketed as a treatment for osteoarthritis. However, it is not ordinarily found in mammalian tissues as free sugar, but is provided in cells by intracellular synthesis by the conversion of glucose‐6‐phosphate to fructose‐6‐phosphate and then by a controlling step by glutamine:fructose‐6‐phosphate amidotransferase to form glucosamine‐6‐phosphate. Exogenous glucosamine, when provided to cultured cells, enters by 6‐phosphorylation similarly but less efficiently than glucose. This provides a potential accumulation of glucosamine‐6‐phosphate, which has been found to affect glucose transport and insulin resistance,1,2,3 and suggests that ingestion may have similar consequences in humans.
It has been reported that glucosamine given intravenously to animals4,5,6,7 or with organ culture8,9 induces insulin resistance. In contrast, other reports indicated that short‐term intravenous glucosamine did not affect insulin sensitivity or secretion in healthy humans,10,11 and that oral ingestion at standard doses for 4 weeks12 and 12 weeks 13 had no apparent effect on insulin, fasting glucose or glucose levels during a subsequent oral glucose tolerance test. However, these glucose tolerance tests and insulin levels were apparently taken in the morning, 8–12 h after glucosamine had been ingested, when serum glucosamine would have fallen to baseline levels that we have previously determined to be <0.5 μmol/l.14 Thus, no immediate direct effect would be seen. Consequently, we aimed to examine the effects of oral glucosamine sulphate (1500 mg) on serum glucose and insulin levels when glucosamine was taken simultaneously at the beginning of a standard glucose tolerance test rather than the day before. We found slight inconsistent effects on glucose levels with 13 normoglycaemic subjects, but significant effects with three people who had unsuspected diabetes or impaired glucose tolerance curves.
Materials and methods
Materials
Glucosamine sulphate was purchased from Rottapharm Ltd (Neptune, New Jersey, USA) and given as one 1500 mg powder in 295 ml of water. Glucose tolerance test solution, in Trutol Cola 75 g/bottle, was purchased from NERL Diagnostics (Fischer Scientific, East Providence, Rhode Island, USA).
Subjects
Subjects fulfilling the American College of Rheumatology criteria for hand, hip or knee osteoarthritis, but otherwise apparently healthy were recruited over a period of several months from Tufts‐New England Medical Arthritis Treatment Center (Boston, Massachusetts, USA). Subjects were excluded if they had a body mass index (BMI) <20 or >40 kg/m2, known diabetes mellitus, fasting blood glucose level >110 mg/dl, kidney or liver disease, acute illness, previous myocardial infarction, uncontrolled inflammatory condition, malignancy, endocrine disorder, corticosteroid use, anaemia, or pregnancy. Informed consent was obtained with approval by the Human Institutional Review Boards, Tufts‐New England Medical Center and Bedford Veteran's Affairs Hospital (Bedford, Massachusetts, USA).
Of the 18 subjects who began the study, two withdrew after the first visit for reasons unrelated to the study protocol. Analyses were performed for the 11 women and 5 men who completed all study visits. Age (41–74 years, median 62 years), weight (42–132 kg, median 91 kg), BMI (22–40 kg/m2,median 31 kg/m2), area affected by osteoarthritis and previous use of glucosamine by subjects were as published previously.14
Study protocol
The study was performed in three visits, 1–2 weeks apart, and a fourth, several months later, with subjects fasted overnight and all morning drugs withheld. The doses at each visit were as follows: 1500 mg of glucosamine sulphate at visit 1; 75 g of glucose at visit 2; 1500 mg of glucosamine sulphate then 75 g of glucose given within 5 min at visit 3; and control without glucose or glucosamine sulphate at visit 4.
Sample collections
An intravenous catheter was inserted and kept clear with normal saline. Blood was obtained at time 0 and then at 15, 30, 45, 60, 90, 120, 150 and 180 min after ingestion. After clotting at room temperature for 30 min, samples were centrifuged at 1500 rpm at 4°C for 15 min and serum stored at −70°C until assay.
Biochemical analysis
To measure glucose, 50 μl of serum was diluted 1:40 by addition of 1.95 ml of water for automated analysis at 35°C by a Metrohm‐Peak 817 Bioscan (Metrohm‐Peak, Inc., Houston, TX, USA) by pulsed amperometric detection after elution from an ion‐exchange Metrosep Carb 1 (250×4.5 mm) column, and measured against standards as described previously.14
Serum insulin was measured by radioimmunoassay (‘Coat‐a‐count' radioimmunoassay kit, Diagnostic Products Corporation, Los Angeles, California, USA) with an interassay coefficient of variation <10%.
Data analysis
Oral glucose tolerance profiles were classified as normal (2‐h glucose, <140 mg/dl); impaired (2‐h glucose, >140–<200 mg/dl); or diabetic (2‐h glucose, >200 mg/dl) by World Health Organization criteria. Areas under the curve (AUCs) for increase in glucose and insulin over baseline were calculated by trapezoidal rule, and means were tested for significance by paired t test. Mean glucose and insulin blood levels of all subjects were calculated for each time point.
Results
Glucose levels with and without ingestion of glucosamine
Fasting glucose levels remained essentially constant for 3 h with or without ingestion of glucosamine, thus providing an accurate baseline. Table 1 shows incremental glucose AUC over the baseline in mg min/ml for 75 g glucose ingestion with and without glucosamine for each participant. Unexpectedly, three subjects had glucose tolerance curves that indicated previously undiagnosed diabetes or impaired glucose tolerance. There were wide variations among the 13 normoglycaemic participants, with the mean glucose AUC increment being somewhat higher when glucosamine was ingested, but not reaching statistical significance (39 v 29 mg min/ml, p = 0.20). In contrast, the three subjects with abnormal glucose tolerance tests showed a mean higher glucose AUC increment that was significant (191 v 145 mg min/ml, p = 0.04) with glucosamine ingestion.
Table 1 3‐h incremental area under the curve (AUC) glucose and 2‐h total AUC insulin for 75 g oral glucose tolerance test performed with and without 1500 mg glucosamine sulphate.
| Patient ID | 3‐h incremental AUC for glucose (mg min/ml) | 2‐h total AUC insulin (mIU min/ml) | ||
|---|---|---|---|---|
| 75‐g glucose tolerance | 75‐g glucose tolerance | |||
| Without GlcN | With GlcN | Without GlcN | With GlcN | |
| 2 | 74 | 47 | 17.8 | 15.2 |
| 3 | 53 | 33 | 10.0 | 10.4 |
| 4 | 75 | 63 | 6.0 | 5.3 |
| 6 | −19 | 52 | 6.8 | 5.1 |
| 13 | 8 | 38 | 6.0 | 3.2 |
| 14 | 30 | 60 | 6.6 | 6.8 |
| 15 | 28 | 4 | 13.7 | 15.8 |
| 18 | 39 | 40 | 3.3 | 4.1 |
| 19 | 27 | 63 | 5.5 | 6.3 |
| 20 | 6 | 53 | 9.8 | 11.2 |
| 21 | 13 | 4 | 2.8 | 2.4 |
| 22 | 23 | 39 | 3.8 | 5.3 |
| 23 | 16 | 5 | 6.9 | 5.0 |
| Mean (SD) | 29 (27) | 39 (22) | 7.6 (4.1) | 7.4 (4.2) |
| 5 | 66 | 117 | 11.8 | 11.2 |
| 7 | 237 | 297 | 3.5 | 3.8 |
| 17 | 131 | 160 | 11.4 | 10.4 |
| Mean (SD) | 145 (86) | 191 (94) | 8.9 (3.8) | 8.5 (3.3) |
AUC, area under the curve; GIcN, glucosamine
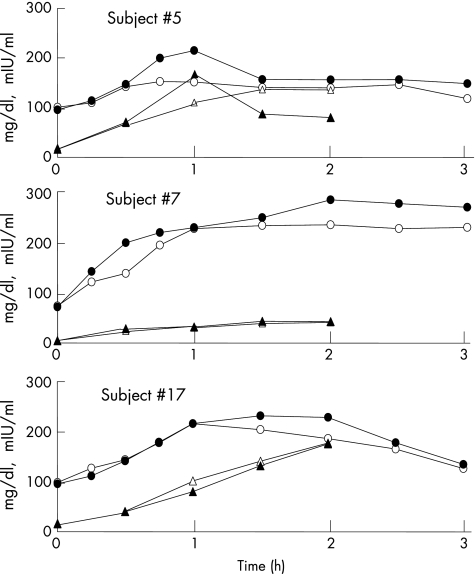
The mean glucose levels at each time interval for the normoglycaemics (fig 1) showed small differences with glucosamine ingestion. The three subjects with abnormal glucose tolerance tests (fig 2) showed much greater differences when glucosamine was ingested, with a tendency for levels to peak slightly later and to be more prolonged. There were no apparent relationships of any glucose levels with parameters of sex, age, weight, BMI or prior glucosamine utilisation.
Figure 1 Mean glucose and insulin levels at timed intervals during glucose tolerance test for 13 normoglycaemic patients. Mean serum glucose levels in mg/dl with (•) and without (○) ingestion of glucosamine sulphate, and mean serum insulin levels in mIU/ml with (▴) and without (▵) ingestion of oral glucosamine sulphate.
Figure 2 Glucose and insulin levels at timed intervals during a glucose tolerance test for three diabetic or glucose intolerant subjects. Serum glucose levels in mg/dl with (•) and without (○) ingestion of glucosamine sulphate, and serum insulin levels in mIU/ml with (▴) and without (▵) ingestion of oral glucosamine sulphate.
Insulin levels with and without ingestion of glucosamine
Table 1 shows incremental insulin in mIU min/ml AUC (time 0–120 min, 75 g glucose tolerance tests with and without glucosamine) for each participant and varied considerably among subjects and in relation to glucose levels. However, mean levels at each time interval (fig 1) and AUC for each individual (table 1) varied little with glucosamine ingestion. Ingestion of glucosamine by the two patients with diabetes did not modify insulin levels at any time interval (fig 2), but there was some modification of the insulin levels of the subject with impaired glucose tolerance. There were no apparent relationships of any insulin levels with parameters of sex, age, weight, BMI or prior glucosamine utilisation.
Discussion
We previously reported14 that ingesting 1500 mg of glucosamine sulphate resulted in a mean serum peak glucosamine level of approximately 5 μmol/l (3.3 μmol/l per g dosage), whereas ingestion of 75 g of glucose resulted in a mean peak glucose level of approximately 5 mmol/l (66 μmol/l per g dosage). Thus, 20 times more glucose than glucosamine reached the peripheral circulation in relation to dosage. Furthermore glucosamine levels returned to <0.5 μmol/l by 8 h, so that little accumulation would occur on a day to day basis. It seems that as much as 1.25 g (7 mmol) of glucosamine travelled directly through the portal system to provide the extracellular 300–400 ml of liver with a concentration as high as 5–20 mmol/l depending on the rapidity of intracellular use. This is as much as 1000 times the concentration that reaches the peripheral circulation.14 Thus, in vitro or intravenous studies using concentrations as high as 1.5 mmol/l11 bypassing the liver may not be pertinent to human consumption and would be less likely to demonstrate any liver‐mediated effects similar to what we have seen. Notably, there was no apparent increase in insulin levels as might be expected to accompany higher glucose levels after glucosamine ingestion by the three diabetic/glucose intolerant subjects, suggesting that the high liver levels may have affected insulin response, perhaps by some unknown mechanism.
Subgroup analysis for the six subjects who were taking glucosamine supplements before this study revealed no consistent differences in glucose or insulin levels from the 10 subjects who had not taken glucosamine previously.
Our study adds to previous reports12,13 that oral doses of glucosamine taken over a period of time have little or no significant effect on glucose metabolism in people who are neither diabetic nor glucose intolerant. However, this is the first study to report glucose and insulin levels when glucosamine is coingested with an oral glucose load and not the morning after, when glucosamine levels would be negligible. This study also provides a better indication of effects on diabetics or glucose intolerant subjects who are taking no drugs for diabetes, in contrast with a reported lack of effect on people with type 2 diabetes taking oral drugs for hyperglycaemia.15
Further investigation is warranted to define the extent and frequency of glucose elevations when glucosamine is provided simultaneously to people with diabetes who have undergone treatment or to untreated people with glucose intolerance or early diabetes, particularly in view of recent reports questioning if glucosamine has any efficacy.
Abbreviations
AUC - area under the curve
BMI - body mass index
Footnotes
This work was supported by the Tufts University General Clinical Research Center, funded by the Division of Research Resources of the NIH under grant no. MO1‐RR00054, US Department of Health and Human Services, National Institutes of Health and Agency for Healthcare Research and Quality, Ruth L Kirschstein National Research Service Award (T‐32), and by funds provided to JES by the Arthritis Foundation and the Medical Research Service of the Department of Veterans Affairs.
Competing interests: None declared.
References
- 1.Marshall S, Bacote V, Traxinger R. Discovery of a metabolic pathway mediating glucose‐induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 19912664706–4712. [PubMed] [Google Scholar]
- 2.Hebert L F, Jr, Daniels M C, Zhou J, Crook E D, Turner R L, Simmons S T.et al Overexpression of glutamine:fructose‐6‐phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest 199698930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClain D, Crook E. Hexosamines and insulin resistance. Diabetes 1996451003–1009. [DOI] [PubMed] [Google Scholar]
- 4.Balkan B, Dunning B E. Glucosamine inhibits glucokinase in vitro and produces a glucose‐specific impairment of in vivo insulin secretion in rats. Diabetes 1994431173–1179. [DOI] [PubMed] [Google Scholar]
- 5.Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest 199596132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankar R R, Zhu J S, Baron A D. Glucosamine infusion in rats mimics the beta‐cell dysfunction of non‐insulin‐dependent diabetes mellitus. Metabolism 199847573–577. [DOI] [PubMed] [Google Scholar]
- 7.Patti M E, Virkamaki A, Landaker E J, Kahn C R, Yki‐Jarvinen H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes 1999481562–1571. [DOI] [PubMed] [Google Scholar]
- 8.Robinson K A, Sens D A, Buse M G. Pre‐exposure to glucosamine induces insulin resistance of glucose transport and glycogen synthesis in isolated rat skeletal muscles. Study of mechanisms in muscle and in rat‐1 fibroblasts overexpressing the human insulin receptor. Diabetes 1993421333–1346. [DOI] [PubMed] [Google Scholar]
- 9.Furnsinn C, Sanderson A L, Radda G K, Leighton B. Effects of glucosamine on insulin‐stimulated glucose metabolism in rat soleus muscle. Int J Biochem Cell Biol 199527805–814. [DOI] [PubMed] [Google Scholar]
- 10.Pouwels M, Jacobs J R, Span P N, Lutterman J A, Smits P, Tack C J. Short‐term glucosamine infusion does not affect insulin sensitivity in humans. J Clin Endocrinol Metab 2001862099–2103. [DOI] [PubMed] [Google Scholar]
- 11.Monauni T, Zenti M G, Cretti A, Daniels M C, Targher G, Caruso B. Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes 200049926–935. [DOI] [PubMed] [Google Scholar]
- 12.Yu J G, Boies S M, Olefsky J M. The effect of oral glucosamine sulfate on insulin sensitivity in human subjects. Diabetes Care 2003261941–1942. [DOI] [PubMed] [Google Scholar]
- 13.Tannis A, Barban J, Conquier M. Effect of glucosamine supplementation on fasting and non‐fasting plasma glucose and serum insulin concentrations in healthy individuals. Osteoarthritis Cartilage 200412506–511. [DOI] [PubMed] [Google Scholar]
- 14.Biggee B A, Blinn C M, McAlindon T E, Nuite M, Silbert J E. Low levels of human serum glucosamine after ingestion of glucosamine sulphate relative to capability for peripheral effectiveness. Ann Rheum Dis 200665222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scroggie D A, Albright A, Harris M D. The effect of glucosamine‐chondroitin supplementation on glycosylated hemoglobin levels in patients with type 2 diabetes mellitus: a placebo‐controlled, double‐blinded, randomized clinical trial. Arch Intern Med 20031631587–1590. [DOI] [PubMed] [Google Scholar]