Abstract
Background
The metabolic syndrome is an independent risk factor for ischaemic heart disease. Patients with systemic lupus erythematosus (SLE) have accelerated atherosclerosis; however, there are no controlled studies of the metabolic syndrome in patients with SLE.
Objective
To compare the prevalence of the metabolic syndrome in patients with SLE and controls and to evaluate its relationship to other cardiovascular risk factors and inflammation.
Methods
102 patients with SLE and 101 controls were studied. The prevalence of the metabolic syndrome was compared in patients and controls using the National Cholesterol Education Program Adult Treatment Panel III (NCEP) and the World Health Organization (WHO) definitions, and associations with cardiovascular risk factors and lupus characteristics were examined.
Results
The metabolic syndrome was present in 32.4% of patients and in 10.9% of controls subjects (p<0.001) using the WHO definition that requires direct determination of insulin resistance, and in 29.4% of patients with SLE and in 19.8% of controls (p = 0.14) using the NCEP definition. Among patients with SLE, both definitions were significantly associated with higher concentrations of C reactive protein (p = 0.001) and the NCEP definition was significantly associated with higher concentrations of homocysteine (p<0.001), lipoprotein (a) (p = 0.02) and cholesterol (p = 0.04). Neither lupus disease activity nor damage scores were associated with the metabolic syndrome.
Conclusions
Patients with SLE have a higher prevalence of insulin resistance and consequently of the WHO‐defined metabolic syndrome than controls. In patients with SLE, the metabolic syndrome was associated with higher levels of inflammation and may provide a link between inflammation and increased cardiovascular risk.
The metabolic syndrome, a cluster of cardiovascular risk factors that includes central obesity, dyslipidaemia, hypertension and disturbed glucose metabolism, is highly prevalent,1 and is an independent predictor of cardiovascular morbidity and mortality2 that identifies substantial additional cardiovascular risk beyond the sum of the individual risk factors.3 In the general population, men with the metabolic syndrome are 1.9–3 times more likely to die of any cause, and 2.9–4.2 times more likely to die from coronary heart disease.4 Women with the metabolic syndrome also have a two‐fold increased risk of major adverse cardiovascular events and death.2 In addition to the cardiovascular risk factors that comprise the metabolic syndrome, there is a strong relationship with inflammation.5 Inflammation is not only associated with the presence of the metabolic syndrome but is also present before individuals develop the syndrome. Thus, in a population‐based study of individuals without diabetes and coronary artery disease, inflammatory markers including C reactive protein (CRP), fibrinogen and white cell count were correlated with several components of the syndrome.6 Furthermore, high levels of CRP obtained 11 years before predicted the presence of the metabolic syndrome as defined by the World Health Organization (WHO), even after adjustment for baseline cardiovascular risk factors and body mass index (BMI; weight (kg)/height2 (m2)).7
There are several mechanisms whereby inflammation could increase the prevalence of components of the metabolic syndrome. Thus, inflammation may increase levels of triglycerides5 and of cytokines such as tumour necrosis factor (TNF)α that facilitate insulin resistance; inflammation also impairs endothelium‐dependent vasodilatation, and thus could facilitate hypertension.8,9
The prevalence of atherosclerosis10,11 is increased in patients with systemic lupus erythematosus (SLE), but the causes are not clear. Identification of mechanisms that are common to both inflammation and cardiovascular disease are of interest, and SLE provides a unique model to consider such questions. We recently reported that obesity was independently associated with cardiovascular risk factors such as CRP in patients with SLE,12 raising the possibility that, as has been suggested,13 the metabolic syndrome may be more frequent in SLE. If true, this would be important because the metabolic syndrome is a stronger predictor of cardiovascular risk than obesity.2 Therefore, we examined the hypotheses that patients with SLE have a higher prevalence of the metabolic syndrome, and that there is an association between the metabolic syndrome and other cardiovascular risk factors and inflammation.
Methods
In all, 102 patients with SLE and 101 control subjects who constitute an ongoing study of cardiovascular risk factors in SLE10,12 were studied. Consecutive eligible patients, aged >18 years, who met the classification criteria of SLE14 and had disease duration >1 year, were enrolled. Controls did not meet the classification criteria for SLE or any other autoimmune disease and were frequency‐matched for age, sex and race, so that the two groups did not differ materially with regard to these variables. Patients were recruited from the practices of local rheumatologists in Nashville, Tennessee, USA, through a Lupus Foundation newsletter and by advertisements. Controls were recruited from the patients' acquaintances, by advertisement and from a database of volunteers maintained by the General Clinical Research Center, Vanderbilt University School of Medicine, Nashville, Tennessee, USA. Exclusion criteria for both patients and controls included a history of myocardial infarction, angina or stroke. The study was approved by the institutional review committee, and all subjects gave written informed consent.
Patients and controls were evaluated using a standardised clinical interview, physical examination, laboratory tests and, in patients, chart review. Family history of coronary disease was defined as a first‐degree relative who had had a myocardial infarction or stroke before the age of 55 years in men, or 65 years in women.15 Height and weight were measured and the BMI was calculated. Waist measurements were obtained. Blood pressure was recorded as the mean of two measurements obtained 5 min apart after participants had rested in a supine position for 10 min. Blood was collected for the measurement of glucose, total cholesterol, high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), triglycerides, lipoprotein (a), homocysteine and insulin after overnight fasting. Insulin concentrations were measured using ELISA (Lincoplex) and reported as pg/ml. In patients with SLE, routine CRP and Westergren erythrocyte sedimentation rate were determined, and disease activity and damage were measured with the use of the Systemic Lupus Erythematosus Disease Activity Index and the Systemic Lupus International Collaborating Clinics Damage Index, respectively.16,17
Metabolic syndrome definitions
Patients with SLE and controls were classified as having the metabolic syndrome based on The National Cholesterol Education Program Adult Treatment Panel III (NCEP)15 and the modified WHO definition.18,19
The NCEP defines the metabolic syndrome as being present if three or more of the following five criteria are fulfilled: (1) central obesity: waist >102 cm in men and >88 cm in women; (2) hypertriglyceridaemia: ⩾150 mg/dl; (3) low HDL <40 mg/dl in men and <50 mg/dl in women; (4) high blood pressure: ⩾130/85 mm Hg or use of drugs for high blood pressure; and (5) high fasting glucose ⩾110 mg/dl.15
The WHO definition requires the presence of insulin resistance defined by any of the following three criteria: a homeostasis model assessment (HOMA) index (fasting glucose (mmol/l)× fasting insulin (μU/ml)/22.5 in the top quartile of a population without diabetes, impaired fasting glucose (⩾110 mg/dl) or diabetes. In addition, two of the following three criteria are also required: (1) central obesity: waist >94 cm in men and >88 cm in women; (2) dyslipidaemia: triglycerides (⩾150 mg/dl) or HDL <40 mg/dl in women or 35 mg/dl in men; and (3) high blood pressure: ⩾140/90 mm Hg or use of drugs for hypertension.19 On the basis of the Study of Inherited Risk of Coronary Atherosclerosis data, we defined a HOMA index >2.114 as representing the top quartile of a population without diabetes.19
The metabolic score was calculated as described by Hunt et al20 as either the sum of the five metabolic syndrome components according to the NCEP or the sum of the four components using the WHO definition.
Statistics
On the basis of preliminary data showing that the prevalence of the WHO‐defined metabolic syndrome was approximately 10% in 40‐year‐old women, the study required 100 patients with SLE and 100 controls to have 80% power and a two‐sided type I error probability of 0.05 to detect a minimum prevalence of 25% of metabolic syndrome among patients with SLE.
Statistical analyses were performed in two phases. Firstly, the prevalence of metabolic syndrome and its components were compared between patients with SLE and controls using Fisher's exact test and Wilcoxon's rank sum test as appropriate. The χ2 test for linear trend was calculated to compare proportions of patients with SLE and controls by metabolic score. Binary logistic regression models were used to estimate unadjusted and adjusted odds ratios (ORs) to evaluate the association between SLE and the prevalence of the metabolic syndrome. Regression covariates were chosen a priori, including age, sex, race and BMI. A secondary analysis included a proportional odds logistic regression model to assess the association between SLE and a higher number of metabolic syndrome criteria21 after controlling for age, sex, race and BMI. The proportional odds assumption was assessed with the score test. Regression models were validated using bootstrapping methods providing an estimate of shrinkage that is obtained as a slope of the calibration plot of observed responses against predicted responses. The shrinkage estimate provides the degree of overfitting to assess the internal validity of the model.22
Secondly, an exploratory analysis including only patients with SLE was performed. Disease characteristics and cardiovascular risk factors in patients with and without the metabolic syndrome were compared with the use of Wilcoxon's rank sum test for continuous variables and Fisher's exact test for categorical variables. κ Statistics were used to determine the agreement between the two definitions of metabolic syndrome among patients with SLE. All analyses used a 5% two‐sided significant level and were performed using STATA V.8.2 and R V.2.1.0 (http://www.r‐project.org).
Results
Table 1 shows the demographic characteristics, lipid profiles, other cardiovascular risk factors, fasting levels of insulin and the HOMA index for the 102 patients with SLE and the 101 controls. Patients with SLE had a median (range) disease duration of 6 (3–11) years; 27 (26.5%) had had renal involvement, 7 (6.9%) had had CNS involvement, 62 (62.6%) were taking corticosteroids and 4 (3.9%) were receiving cyclophosphamide. Patients with SLE were slightly younger than control with an age of 40 (32–47) years v 44 (37–50) years (p = 0.05). Median (interquartile range) concentrations of fasting insulin were significantly higher in patients with SLE (302 (167–526) pg/ml) than in controls (194 (125–409) pg/ml; p = 0.003). Patients with lupus had significantly lower levels of total (p = 0.04) and LDL cholesterol (p = 0.007) than controls; however, concentrations of triglycerides (p = 0.02) and homocysteine (p<0.001) were higher.
Table 1 Clinical characteristics of patients with systemic lupus erythematosus and controls*.
| Patients with SLE (n = 102) | Controls (n = 101) | p Value† | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 40 (32–47) | 44 (37–50) | 0.05 |
| Women (%) | 91.2 | 89.1 | 0.65 |
| Caucasian (%) | 66.7 | 77.2 | 0.11 |
| Education >12 years (%) | 66.7 | 79.2 | 0.06 |
| Insulin (pg/ml) | 302 (167–526) | 194 (125–409) | 0.003 |
| Glucose (mg/dl) | 83 (75–92) | 85 (81–91) | 0.05 |
| Creatinine (mg/dl) | 0.7 (0.8–0.9) | 0.7 (0.8–0.9) | 0.25 |
| Current use of NSAIDs (%) | 50 (49) | 34 (34) | 0.03 |
| Lipid profile | |||
| Total cholesterol (mg/dl) | 166 (143–208) | 181 (163–210) | 0.04 |
| High‐density lipoprotein (mg/dl) | 48 (36–55) | 45 (39–54) | 0.92 |
| Low‐density lipoprotein (mg/dl) | 97 (81–130) | 114 (91–138) | 0.007 |
| Lipoprotein (a) (mg/dl) | 12 (5–39) | 12 (5–34) | 0.89 |
| Triglycerides (mg/dl) | 100 (71–153) | 87 (64–119) | 0.02 |
| Other cardiovascular risk factors | |||
| Family history (%) | 19 | 20 | 0.86 |
| Homocysteine (μmol/l) | 9.2 (7.5–11.1) | 7.6 (6.7–8.7) | <0.001 |
| Total pack‐years of smoking | 0 (0–3) | 0 (0–4) | 0.87 |
| Body mass index (kg/m2) | 28 (24.1–33.2) | 25.9 (23.1–31.0) | 0.11 |
NSAIDs, non‐steroidal anti‐inflammatory drugs; SLE, systemic lupus erythematosus.
*Continuous values are presented as median (interquartile range).
†p Values were calculated using Fisher's exact test for categorical variables and Wilcoxon's rank sum test for continuous variables.
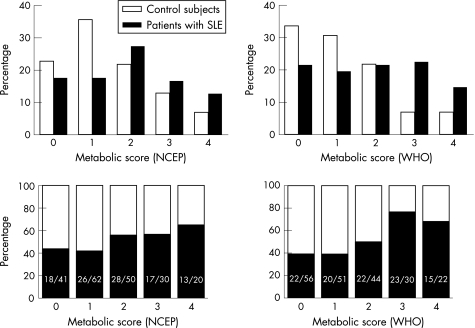
Using the NCEP definition, 30 of 102 (29.4%) patients met the criteria for metabolic syndrome compared with 20 of 101 (19.8%) controls (p = 0.14); unadjusted OR (95% confidence interval (CI)) 1.69 (0.88 to 3.23), p = 0.11; OR adjusted for age, sex, race and BMI was 1.77 (0.91 to 3.45), p = 0.32. The median (interquartile range) NCEP metabolic syndrome score was 2 (1–3) in patients with SLE and 1 (1–2) in controls (p = 0.04). However, the adjusted (for age, sex, race and BMI) association between SLE and the NCEP metabolic score did not remain significant, OR 1.45 (0.68 to 3.07), p = 0.17. Figure 1 shows the frequencies of metabolic syndrome scores in patients and controls.
Figure 1 Prevalence of the metabolic syndrome scores among patients with systemic lupus erythematosus (SLE) and controls. The top two panels represent the proportion of patients with SLE (black) and controls (white) by the number of metabolic syndrome criteria met. The two panels at the bottom represent the proportion of patients with SLE (black) and controls (white) in each individual metabolic syndrome score. p Values using the χ2 test for trend were 0.04 and 0.001 using the National Cholesterol Education Program Adult Treatment Panel III (NCEP) and the World Health Organization (WHO) definitions, respectively.
Using the WHO definition, the metabolic syndrome was present in 33 of 102 (32.4%) patients with SLE and in 11 (10.9%) controls (p<0.001). The unadjusted OR (95% CI) for the prevalence of the metabolic syndrome was 3.91 (1.85 to 8.29); p<0.001, and the association remained significant after adjusting for age, sex, race and BMI (OR 4.37 (1.77 to 10.82); p = 0.001). The median (interquartile range) WHO metabolic syndrome score was 2 (1–3) in patients with SLE and 1 (0–2) in controls (p<0.001). After adjusting for age, sex, race and BMI, the association between SLE and the metabolic score remained significant (OR 4.3 (1.74 to 10.65); p = 0.002)
The model using the WHO definition criteria had a bootstrap shrinkage coefficient of 0.88, and that using the NCEP definition criteria was 0.9, indicating robustness of the regression models.
Table 2 shows the frequency of the individual criteria for the two definitions of the metabolic syndrome. Patients with SLE met the WHO criteria for insulin resistance (44.1% v 24.8%; p = 0.005), hypertension (43.1% v 20.8%; p<0.001) and central obesity (51% v 35.6%; p = 0.03) more often than controls. For the NCEP definition, patients with SLE met the criteria for waist girth (48% v 33.7%; p = 0.05) and hypertension (51% v 34.7%; p = 0.02) more often than controls.
Table 2 Frequency of the metabolic syndrome criteria in patients with SLE and controls*.
| Patients with SLE | Controls | p Value | |
|---|---|---|---|
| NCEP definition | |||
| Waist girth (%) | 48.0 | 33.7 | 0.05 |
| Hypertension† (%) | 51.0 | 34.7 | 0.02 |
| Hypertriglyceridaemia (%) | 27.5 | 16.8 | 0.09 |
| Low HDL (%) | 52.0 | 58.4 | 0.40 |
| Hyperglycaemia (%) | 2.9 | 2.0 | 1.00 |
| Total meeting definition (%) | 29.4 | 19.8 | 0.14 |
| WHO definition | |||
| Insulin resistance‡ (%) | 44.1 | 24.8 | 0.005 |
| Dyslipidaemia (%) | 51.0 | 41.6 | 0.21 |
| Hypertension† (%) | 43.1 | 20.8 | <0.001 |
| Central obesity (%) | 51.0 | 35.6 | 0.03 |
| Total meeting definition (%) | 32.4 | 10.9 | <0.001 |
HDL, high‐density lipoprotein; NCEP, National Cholesterol Education Program Adult Treatment Panel III; SLE, systemic lupus erythematosus; WHO, World Health Organization.
*p Values were calculated using Fisher's exact test.
†Hypertension is defined as ⩾130/85 mm Hg or use of drugs for blood pressure in the NCEP criteria and ⩾140/90 mm Hg or use of drugs for blood pressure in the WHO criteria.
‡Insulin resistance defined by the presence of any of the following three criteria: a homeostasis model assessment index (fasting glucose (mmol/l)×fasting insulin (μU/ml)/22.5 in the top quartile of a population without diabetes (>2.114 in the Study of Inherited Risk of Coronary Atherosclerosis sample), impaired fasting glucose (⩾110 mg/dl) or diabetes.
Among patients with SLE, the metabolic syndrome, as defined either by the NCEP or the WHO criteria, was associated with increased levels of CRP (p = 0.001), but only the NCEP definition of metabolic syndrome was associated with significantly higher concentrations of lipoprotein (a) (p = 0.02), LDL cholesterol (p = 0.02) and homocysteine (p<0.001; table 3) Although insulin resistance is not a criterion for meeting the NCEP classification criteria of the metabolic syndrome, many of the patients identified by the NCEP criteria were also insulin resistant (table 3). There were no significant differences in level of education or cumulative corticosteroid use in patients with and without the metabolic syndrome regardless of the definition used.
Table 3 Characteristics of patients with systemic lupus erythematosus according to the presence or absence of the metabolic syndrome.
| NCEP | WHO | |||||
|---|---|---|---|---|---|---|
| Metabolic syndrome (+) (n = 30) | Metabolic syndrome (−) (n = 72) | p Value | Metabolic syndrome (+) (n = 33) | Metabolic syndrome (−) (n = 69) | p Value | |
| Demographics | ||||||
| Age (years) | 44 (40–50) | 39 (31–46) | 0.06 | 44 (38–48) | 40 (30–46) | 0.18 |
| Female (%) | 86.7 | 93.1 | 0.44 | 93.9 | 89.9 | 0.71 |
| Caucasian (%) | 63.3 | 68.1 | 0.65 | 63.6 | 68.1 | 0.66 |
| Education >12 years (%) | 63.3 | 68.1 | 0.65 | 60.6 | 69.6 | 0.38 |
| Cardiovascular risk factors | ||||||
| Systolic blood pressure (mm Hg) | 128 (120–138) | 113 (105–121) | <0.001 | 120 (113–129) | 115 (105–126) | 0.07 |
| Diastolic blood pressure (mm Hg) | 76 (71–85) | 70 (64–79) | 0.02 | 74 (65–85) | 73 (65–81) | 0.68 |
| BMI (kg/m2) | 33 (29.7–35.9) | 25.8 (22.8–30) | <0.001 | 33.9 (30.8–39) | 25.2 (22.7–28.5) | <0.001 |
| Homocysteine (μmol/l) | 11 (8.8–12.2) | 8.8 (6.6–10.6) | <0.001 | 9.8 (8.5–11.5) | 8.9 (7.3‐11.1) | 0.10 |
| Cumulative smoking (pack‐years) | 0.1 (0–16.8) | 0 (0–1.1) | 0.12 | 0 (0–19) | 0 (0‐1) | 0.10 |
| Cholesterol (mg/dl) | 204 (148–222) | 163 (142–184) | 0.04 | 177 (132–215) | 163 (146–202) | 0.56 |
| Low‐density lipoprotein (mg/dl) | 129 (84–146) | 92 (80–111) | 0.02 | 109 (78–133) | 94 (81–125) | 0.42 |
| High‐density lipoprotein (mg/dl) | 36 (31–46) | 52 (40–58) | <0.001 | 36 (32–51) | 52 (39–58) | 0.003 |
| Lipoprotein (a) (mg/dl) | 25 (6.9–60.8) | 9.6 (4.5–34) | 0.02 | 17 (5–47) | 10.1 (5–34) | 0.29 |
| Triglycerides (mg/dl) | 158 (129–202) | 87 (68–123) | <0.001 | 143 (88–201) | 89 (69–139) | <0.001 |
| Glucose (mg/dl) | 88 (78–98) | 81 (75–88) | 0.006 | 91 (82–94) | 80 (74–86) | <0.001 |
| Insulin (pg/ml) | 485 (377–752) | 222 (134–439) | <0.001 | 529 (461–784) | 208 (128–309) | <0.001 |
| Creatinine (mg/dl) | 0.9 (0.7–1) | 0.8 (0.7–0.9) | 0.04 | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.53 |
| Current use of NSAIDs n (%) | 12 (40) | 38 (52.7) | 0.28 | 14 (42.4) | 36 (52.2) | 0.40 |
| Disease characteristics | ||||||
| Disease duration (years) | 7 (4–11) | 6 (3–12) | 0.41 | 5 (3–11) | 8 (3–11) | 0.35 |
| SLEDAI | 4 (2–6) | 4 (0–6) | 0.30 | 6 (2–6) | 4 (0–6) | 0.10 |
| SLICC | 0 (0–2) | 1 (0–1) | 0.91 | 0 (0–1) | 1 (0–1) | 0.88 |
| Current dose of corticosteroids (mg/day) | 5 (0–10) | 4 (0–6) | 0.24 | 5 (0–9) | 5 (0–5) | 0.36 |
| Cumulative corticosteroid dose (g) | 18.2 (4.6–38.1) | 9.8 (2.7–26.5) | 0.10 | 12.8 (2.7–29.2) | 12.5 (4.1–29.2) | 0.80 |
| Current use of corticosteroids (%) | 70 | 56.9 | 0.27 | 57.6 | 62.3 | 0.67 |
| Current use of hydroxychloroquine (%) | 63.3 | 63.9 | 1 | 66.7 | 62.3 | 0.83 |
| Other markers of inflammation | ||||||
| Erythrocyte sedimentation rate (mm/h) | 24 (13–39) | 16 (7–36) | 0.10 | 24 (13–37) | 16 (7–37) | 0.09 |
| C reactive protein (mg/l) | 6 (3–11) | 3 (3–5) | 0.001 | 7 (4–11) | 3 (3–4) | <0.001 |
NCEP, National Cholesterol Education Program Adult Treatment Panel III; NSAIDs, non‐steroidal anti‐inflammatory drugs; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLICC, Systemic Lupus International Collaborating Clinics Damage Index; WHO, World Health Organization; +, present; −, absent.
Of the 102 patients with SLE, 21 (20.5%) and 60 (58.8%) were classified as having and not having the metabolic syndrome by both sets of criteria, respectively. The κ statistic value was 0.52 (p<0.001), suggesting good agreement (table 4).23
Table 4 Agreement between the WHO and the NCEP definition of the metabolic syndrome in patients with SLE.
| NCEP (+) (%) | NCEP (−) (%) | Total (%) | |
|---|---|---|---|
| WHO (+) | 21 (20.5) | 12 (9.8) | 33 (32.4) |
| WHO (−) | 9 (8.8) | 60 (58.8) | 69 (67.6) |
| Total | 30 (29.4) | 72 (70.6) | 102 (100.0) |
NCEP, National Cholesterol Education Program Adult Treatment Panel III; SLE, systemic lupus erythematosus; WHO, World Health Organization; +, present; −, absent.
κ statistic 0.52, p<0.001.
Discussion
This study shows that patients with SLE have an increased prevalence of the metabolic syndrome, as defined by the WHO criteria, and interestingly this remained significant after adjustment for age, sex, race and BMI, suggesting that these differences are not due to differences in these potential confounders. This novel observation suggests a common mechanism between premature atherosclerosis and inflammation, both features of SLE.
There has been a lack of consensus as to how best define the metabolic syndrome. Thus, first the WHO and then the NCEP published criteria to standardise the definition. Using either definition the prevalence of the metabolic syndrome in the general population is high,24 and the presence of the syndrome is a predictor of cardiovascular outcomes.25 However, the definition of the metabolic syndrome that requires the direct ascertainment of the presence of insulin resistance (the WHO metabolic syndrome) was more strongly associated with SLE than the NCEP syndrome. This is important because inflammation may drive insulin resistance directly, and this observation points to the mechanisms driving the increased prevalence of the WHO metabolic syndrome in patients with SLE. Concordant with this interpretation, insulin resistance was associated with markers of inflammation such as CRP (r = 0.31, p = 0.001) and erythrocyte sedimentation rate (r = 0.26, p = 0.009). The association between inflammation and insulin resistance is of interest because insulin resistance is considered by many to be fundamental to the increased cardiovascular risk attributed to the metabolic syndrome.4,19 In keeping with this notion, data from the general population show that measures of insulin resistance provide additional value to the association between the metabolic syndrome and coronary atherosclerosis.19 By contrast, the NCEP definition, which does not require insulin measurements, is simpler and therefore more commonly used.
Our data suggest that in SLE, the two definitions are reasonably concordant with agreement in 79.4% of subjects and a κ statistic of 0.52. However, approximately 20% of subjects met the classification criteria for only one definition, and 9/30 patients (30%) positive by NCEP criteria were negative by WHO criteria, and 12/33 (36%) patients positive by WHO criteria were negative by NCEP criteria. Studies in the general population reported κ coefficients of 0.45 and 0.51.26
The prevalence of the metabolic syndrome in a population of predominantly Caucasian women aged about 40 years would be expected to be approximately 20% using the NCEP criteria1 and 13% (range 6–24%) using the WHO criteria.27 In our study, the prevalence of the metabolic syndrome in controls was similar to that in the general population. The prevalence of the NCEP‐defined metabolic syndrome was 29.4% in patients with SLE and this was not significantly higher than in controls (19.8%); however, our study was not powered to detect small differences in frequency. The marked differences in prevalence of the WHO‐defined metabolic syndrome between patients with SLE and controls are largely accounted for by higher levels of insulin. We used the Study of Inherited Risk of Coronary Atherosclerosis cut‐off value of 2.114 to define the upper quartile of HOMA. If we used the upper quartile of the HOMA in our control group (2.078), the findings did not change materially, and 32.4% of patients with SLE and 11.8% of controls (p = 0.001) had the WHO‐defined metabolic syndrome.
As recently reported by others,13,28 we found that insulin levels were higher in patients with SLE, and that the metabolic syndrome prevalence was high, but not related to disease activity, use of corticosteroids or use of antimalarial drugs.13 Also, the metabolic syndrome was associated with an increased CRP. These findings thus link inflammatory markers with reduced insulin sensitivity and suggest a potential mechanism for increased cardiovascular risk in SLE. The study also suggests that the WHO criteria, that include a measure of insulin sensitivity, may be more appropriate than the NCEP criteria in the setting of an inflammatory disease.
We found that levels of CRP were higher in patients with the metabolic syndrome regardless of the definition used. In the general population, CRP is also associated with the presence of metabolic syndrome, especially in women.29 Furthermore, CRP adds prognostic information for evaluating cardiovascular risk.30
We found no significant differences in insulin concentrations (308.9 (172.8–528.3) pg/ml v 295.9 (153.4–471.3) pg/ml; p = 0.58), HOMA index (1.7 (0.9–2.8) units v 1.5 (0.7–2.6) units; p = 0.65) or glucose concentrations (82 (76–91) mg/dl v 84 (75–94) mg/dl; p = 0.57) among the 65 patients with SLE currently receiving antimalarial and the 37 patients who were not. These data suggest that, as is the case in subjects without diabetes,31 the use of malaria drugs in this population of patients with SLE was not associated with changes in insulin sensitivity.
Our findings have potential therapeutic implications.32 Recently, a study showed that after 36 weeks of treatment with either simvastatin or atorvastatin, almost 50% of patients with the metabolic syndrome no longer met the classification criteria. Similarly, fluvastatin significantly reduced not only triglyceride, total and LDL cholesterol but also fasting insulin.33 Furthermore, many statins also decrease CRP levels,34 and diet and exercise can reduce markers of inflammation.35 Thus, in addition to immunomodulating drugs to control SLE, interventions to control dyslipidaemia and obesity may be important. Also, several potential mechanisms linking the metabolic syndrome and inflammation may constitute therapeutic targets. These include signalling kinase pathways common to both,36 abnormalities of endothelial function,37 oxidative stress38 and the hypertriglyceridaemia associated with inflammation.5
Some limitations should be considered. Firstly, socioeconomic status is a potential confounder difficult to measure. Education was used as its surrogate, and there was no significant difference in patients with and without the metabolic syndrome. Secondly, controls were slightly older. However, as the prevalence of the metabolic syndrome increases with age, this difference might have biased our results towards the null, and the association remains valid. Thirdly, the patients with SLE included in this study had low disease activity (as determined by the Systemic Lupus Erythematosus Disease Activity Index score) and low damage (as determined by the Systemic Lupus International Collaborating Clinics Damage Index Score). However, the findings regarding insulin resistance are likely to be even more striking in a more severely affected group. Thus, the fact that this finding was present in patients with mild to moderate disease is even more remarkable.
In summary, this study shows that patients with SLE have higher levels of insulin and increased frequency of the metabolic syndrome as determined by the WHO definition, and this is associated with higher levels of inflammation. The metabolic syndrome may constitute a common link between the increased cardiovascular risk and higher levels of inflammation present in SLE.
Acknowledgements
We thank Carol Brannon who assisted in the recruitment of subjects and in entering data.
Abbreviations
BMI - body mass index
CRP - C reactive protein
HDL - high‐density lipoprotein
HOMA - homeostasis model assessment
LDL - low‐density lipoprotein
NCEP - National Cholesterol Education Program Adult Treatment Panel III
SLE - systemic lupus erythematosus
WHO - World Health Organization
Footnotes
Funding: This study was supported by grants (HL04012, HL65082 and GM5M01‐RR00095) from the National Institutes of Health and by grants from the Lupus Foundation of America, Nashville Chapter and the Lupus Clinical Trials Consortium. IA is funded in part by a grant from the American College of Rheumatology
Competing interests: CPC received a CHORD (Centocor Health Outcomes in Rheumatic Diseases) Fellowship in 2004, grants: HL065082, HL067964, 3U18 HS010384‐07S1, HHSA290200500421. IA, ACR REF 2006 Fellowship award. AO, TG, AS, no disclosures. In PR's opinion, this is not relevant to the paper submitted but in the interest of full disclosure is declared: Honoraria (Genzyme). CMS, grants RO1 HL65082, 5K24 HL04012‐05, 5RO1‐HL67964, 2U01 HL65962‐03, 5P01 GM31304‐21, 5U18 HS010384‐07, 1RO1 HL081707‐01, Lupus Foundation, 5 RO1 HL65082‐03. In CMS's opinion, these are not relevant to the paper submitted but in the interest of full disclosure are declared. Consultant fees from Bristol Myers Squibb for preparation of a series of lectures on trial design; royalty fees from the publication of textbooks of rheumatology; fees from medicolegal chart review in two cases of drug toxicity; an honorarium from the American Society of Clinical Pharmacology and Therapeutics for editing their journal; fees from the NIH for participating in peer review. CMS holds a patent on the use of IL2 to monitor ciclosporine activity—the patent has yielded no income.
References
- 1.Ford E S, Giles W H, Dietz W H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002287356–359. [DOI] [PubMed] [Google Scholar]
- 2.Kip K E, Marroquin O C, Kelley D E, Johnson B D, Kelsey S F, Shaw L J.et al Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation 2004109706–713. [DOI] [PubMed] [Google Scholar]
- 3.Reilly M P, Rader D J. The metabolic syndrome: more than the sum of its parts? Circulation 20031081546–1551. [DOI] [PubMed] [Google Scholar]
- 4.Lakka H M, Laaksonen D E, Lakka T A, Niskanen L K, Kumpusalo E, Tuomilehto J.et al The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA 20022882709–2716. [DOI] [PubMed] [Google Scholar]
- 5.Jonkers I J, Mohrschladt M F, Westendorp R G, van der L A, Smelt A H. Severe hypertriglyceridemia with insulin resistance is associated with systemic inflammation: reversal with bezafibrate therapy in a randomized controlled trial. Am J Med 2002112275–280. [DOI] [PubMed] [Google Scholar]
- 6.Festa A, D'Agostino R, Jr, Howard G, Mykkanen L, Tracy R P, Haffner S M. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 200010242–47. [DOI] [PubMed] [Google Scholar]
- 7.Laaksonen D E, Niskanen L, Nyyssonen K, Punnonen K, Tuomainen T P, Valkonen V P.et al C‐reactive protein and the development of the metabolic syndrome and diabetes in middle‐aged men. Diabetologia 2004471403–1410. [DOI] [PubMed] [Google Scholar]
- 8.Rask‐Madsen C, Dominguez H, Ihlemann N, Hermann T, Kober L, Torp‐Pedersen C. Tumor necrosis factor‐alpha inhibits insulin's stimulating effect on glucose uptake and endothelium‐dependent vasodilation in humans. Circulation 20031081815–1821. [DOI] [PubMed] [Google Scholar]
- 9.Hung J, McQuillan B M, Chapman C M, Thompson P L, Beilby J P. Elevated interleukin‐18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol 2005251268–1273. [DOI] [PubMed] [Google Scholar]
- 10.Asanuma Y, Oeser A, Shintani A K, Turner E, Olsen N, Fazio S.et al Premature coronary‐artery atherosclerosis in systemic lupus erythematosus. N Engl J Med 20033492407–2415. [DOI] [PubMed] [Google Scholar]
- 11.Roman M J, Shanker B A, Davis A, Lockshin M D, Sammaritano L, Simantov R.et al Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med 20033492399–2406. [DOI] [PubMed] [Google Scholar]
- 12.Oeser A, Chung C P, Asanuma Y, Avalos I, Stein C M. Obesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosus. Arthritis Rheum 2005523651–3659. [DOI] [PubMed] [Google Scholar]
- 13.El Magadmi M, Ahmad Y, Turkie W, Yates A P, Sheikh N, Bernstein R M.et al Hyperinsulinemia, insulin resistance, and circulating oxidazed low density lipoprotein in women with systemic lupus erythematosus. J Rheumatol 20063350–56. [PubMed] [Google Scholar]
- 14.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 15.National Cholesterol Education Program Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 20012852486–2497. [DOI] [PubMed] [Google Scholar]
- 16.Bombardier C, Gladman D D, Urowitz M B, Caron D, Chang C H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 199235630–640. [DOI] [PubMed] [Google Scholar]
- 17.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M.et al The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 199639363–369. [DOI] [PubMed] [Google Scholar]
- 18.Laaksonen D E, Lakka H M, Niskanen L K, Kaplan G A, Salonen J T, Lakka T A. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol 20021561070–1077. [DOI] [PubMed] [Google Scholar]
- 19.Reilly M P, Wolfe M L, Rhodes T, Girman C, Mehta N, Rader D J. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation 2004110803–809. [DOI] [PubMed] [Google Scholar]
- 20.Hunt M E, O'Malley P G, Feuerstein I, Taylor A J. The relationship between the ‘metabolic score' and sub‐clinical atherosclerosis detected with electron beam computed tomography. Coron Artery Dis 200314317–322. [DOI] [PubMed] [Google Scholar]
- 21.Agresti A.Categorical data analysis. Wesley: Reading, MA, 2001
- 22.Harrel F.Regression modeling strategies. Springer: New York, 2001
- 23.Fleiss J.Statistical methods for rates and proportions. 3rd edn. New York: Wiley, 2005
- 24.Hanley A J, Wagenknecht L E, D'Agostino R B, Jr, Zinman B, Haffner S M. Identification of subjects with insulin resistance and beta‐cell dysfunction using alternative definitions of the metabolic syndrome. Diabetes 2003522740–2747. [DOI] [PubMed] [Google Scholar]
- 25.Hunt K J, Resendez R G, Williams K, Haffner S M, Stern M P. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all‐cause and cardiovascular mortality in the San Antonio Heart Study. Circulation 20041101251–1257. [DOI] [PubMed] [Google Scholar]
- 26.Aguilar‐Salinas C A, Rojas R, Gomez‐Perez F J, Mehta R, Franco A, Olaiz G.et al The metabolic syndrome: a concept hard to define. Arch Med Res 200536223–231. [DOI] [PubMed] [Google Scholar]
- 27.Balkau B, Charles M A, Drivsholm T, Borch‐Johnsen K, Wareham N, Yudkin J S.et al Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab 200228364–376. [PubMed] [Google Scholar]
- 28.Posadas‐Romero C, Torres‐Tamayo M, Zamora‐Gonzalez J, Guilar‐Herrera B E, Posadas‐Sanchez R, Cardoso‐Saldana G.et al High insulin levels and increased low‐density lipoprotein oxidizability in pediatric patients with systemic lupus erythematosus. Arthritis Rheum 200450160–165. [DOI] [PubMed] [Google Scholar]
- 29.Rutter M K, Meigs J B, Sullivan L M, D'Agostino R B, Sr, Wilson P W. C‐reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation 2004110380–385. [DOI] [PubMed] [Google Scholar]
- 30.Ridker P M, Buring J E, Cook N R, Rifai N. C‐reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8‐year follow‐up of 14 719 initially healthy American women. Circulation 2003107391–397. [DOI] [PubMed] [Google Scholar]
- 31.Smith G D, Amos T A, Mahler R, Peters T J. Effect of chloroquine on insulin and glucose homoeostasis in normal subjects and patients with non‐insulin‐dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987294465–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grundy S M, Cleeman J I, Daniels S R, Donato K A, Eckel R H, Franklin B A.et al Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 20051122735–2752. [DOI] [PubMed] [Google Scholar]
- 33.Sonmez A, Baykal Y, Kilic M, Yilmaz M I, Saglam K, Bulucu F.et al Fluvastatin improves insulin resistance in nondiabetic dyslipidemic patients. Endocrine 200322151–154. [DOI] [PubMed] [Google Scholar]
- 34.Costa A, Casamitjana R, Casals E, Alvarez L, Morales J, Masramon X.et al Effects of atorvastatin on glucose homeostasis, postprandial triglyceride response and C‐reactive protein in subjects with impaired fasting glucose. Diabetes Med 200320743–745. [DOI] [PubMed] [Google Scholar]
- 35.Wegge J K, Roberts C K, Ngo T H, Barnard R J. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism 200453377–381. [DOI] [PubMed] [Google Scholar]
- 36.Hotamisligil G S. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 200327(Suppl 3)S53–S55. [DOI] [PubMed] [Google Scholar]
- 37.Dell'Omo G, Penno G, Pucci L, Mariani M, Del Prato S, Pedrinelli R. Abnormal capillary permeability and endothelial dysfunction in hypertension with comorbid metabolic syndrome. Atherosclerosis 2004172383–389. [DOI] [PubMed] [Google Scholar]
- 38.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 200424816–823. [DOI] [PubMed] [Google Scholar]