Abstract
Objective
To investigate the efficacy and safety of abatacept in combination with etanercept in patients with active rheumatoid arthritis during a 1‐year, randomised, placebo‐controlled, double‐blind phase, followed by an open‐label, long‐term extension (LTE).
Methods
Patients continued etanercept (25 mg twice weekly) and were randomised to receive abatacept 2 mg/kg (n = 85) or placebo (n = 36). As the effective dose of abatacept was established as 10 mg/kg in a separate trial, all patients received abatacept 10 mg/kg and etanercept during the LTE.
Results
A total of 121 patients were randomised; 80 completed double‐blind treatment and entered the LTE. During double‐blind treatment, the difference in the percentage of patients achieving the primary end point (modified American College of Rheumatology (ACR) 20 response at 6 months) was not significant between groups (48.2% v 30.6%; p = 0.072). At 1 year, no notable changes in modified ACR responses were observed. Subsequent to the dosing change, similar modified ACR responses were seen during the LTE. Significant improvements in quality of life were observed with abatacept and etanercept versus placebo and etanercept in five of the eight short‐form 36 subscales at 1 year. More abatacept and etanercept‐treated patients experienced serious adverse events (SAEs) at 1 year than patients receiving placebo and etanercept (16.5% v 2.8%), with 3.5% v 0% experiencing serious infections.
Conclusion
The combination of abatacept (at a dose of 2 mg/kg during the double‐blind phase and 10 mg/kg during the LTE) and etanercept was associated with an increase in SAEs, including serious infections, with limited clinical effect. On the basis of the limited efficacy findings and safety concerns, abatacept in combination with etanercept should not be used for rheumatoid arthritis treatment.
Abatacept—a fully human soluble fusion protein that consists of the extracellular domain of human cytotoxic T lymphocyte‐associated antigen‐4 linked to the modified Fc portion of human IgG1—is the first in a class of agents for the treatment of rheumatoid arthritis that selectively modulates the CD80/CD86:CD28 costimulatory signal required for full T cell activation.
The efficacy of abatacept for the treatment of rheumatoid arthritis was first shown in clinical trials as monotherapy in a phase II pilot study in patients with active early rheumatoid arthritis refractory to disease‐modifying antirheumatic drugs (DMARDs).1 A subsequent phase IIb, dose‐finding, placebo‐controlled study compared the efficacy of abatacept (2 and 10 mg/kg) and methotrexate (MTX) in patients with active rheumatoid arthritis despite MTX treatment.2,3 Owing to the interest in combining biological treatments that have different mechanisms of action, the effects of abatacept in combination with etanercept were assessed.
This pilot phase IIb trial was designed to evaluate the safety and clinical efficacy of abatacept in combination with etanercept in patients with active rheumatoid arthritis despite continued etanercept treatment.
Methods
Patients and study design
This was a multicentre, randomised, double‐blind, placebo‐controlled trial with an open‐label long‐term extension (LTE) phase, conducted at 40 centres in the US between 26 February 2001 and 13 October 2004.
Eligible patients were ⩾18 years of age and met the criteria of the American College of Rheumatology (ACR) for rheumatoid arthritis,4 and were in functional class I, II or III.5 Patients must have received etanercept 25 mg twice weekly for ⩾3 months and have ⩾8 swollen joints (66‐joint count) and ⩾10 tender joints (68‐joint count). The original protocol definition of required C reactive protein (CRP) concentration at entry was ⩾2 mg/dl; however, owing to the effect of etanercept on normalising CRP levels, there was a high initial rate of screen failures. Therefore, the protocol was modified so that CRP elevation was not required for entry and the CRP threshold of ⩾2 mg/dl was never executed. The primary end point (ACR 20) was also modified early in the study to accommodate this finding. Important exclusion criteria included active or latent infection, recent opportunist infection, tuberculosis requiring treatment within the previous 3 years, history of cancer within the previous 5 years or history of drug or alcohol misuse. Pregnant and nursing women were excluded. Patients and clinical assessors were blinded to the treatment assigned for the duration of the study.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by institutional review boards (Schulman Associates Institutional Review Board, Cincinnati, Ohio, USA). All patients gave informed consent before undergoing any screening procedure.
Trial objectives
The primary end point of the double‐blind phase was a modified ACR 20 (defined under “efficacy assessments”) response rate at 6 months. The secondary end point of the double‐blind phase was the proportion of patients achieving a modified ACR 50 response at 6 months. The primary objective of the LTE was to assess the safety and tolerability of abatacept in combination with etanercept during long‐term administration in patients with active rheumatoid arthritis.
Treatment group assignment
At enrolment, each patient was assigned a unique sequential patient number via the Central (Interactive Voice) Randomisation System. Randomisation schedules were generated and kept sealed by the Randomisation Group until the study unblinding. Patients who qualified for treatment were assigned a unique randomisation number in the order of qualification.
Treatment
During the double‐blind phase, patients were randomised in a 2:1 ratio to receive abatacept 2 mg/kg and etanercept, or placebo and etanercept. Etanercept (25 mg, twice weekly) was continued in all patients for the duration of the study. Abatacept was administered intravenously on days 1, 15 and 30, and every 4 weeks thereafter. MTX and other DMARDs were stopped at least 28 days before randomisation, with the exception of leflunomide, which was stopped ⩾60 days before randomisation. Low‐dose corticosteroids (⩽10 mg/day) or non‐steroidal anti‐inflammatory drugs were allowed, provided the dose remained stable during the study. Analgesics were also permitted at all times except ⩽12 h before a joint evaluation; addition of hydroxychloroquine, sulfasalazine, leflunomide or MTX was allowed after 6 months of double‐blind treatment, as considered appropriate by the investigator according to the patient's condition. Patients completing double‐blind treatment were eligible to enter the LTE. All patients entering the LTE were switched to receive abatacept at a fixed dose approximating 10 mg/kg (according to weight range). During the LTE, patients were permitted to increase, decrease or discontinue corticosteroids (to a maximum maintenance dose of 10 mg prednisone equivalent daily), etanercept (to a maximum of 25 mg twice weekly) and non‐steroidal anti‐inflammatory drugs according to their condition.
Efficacy assessments
Owing to the effect of etanercept on normalising CRP levels in this population, the primary and secondary end points were based on modified ACR 20 criteria, defined as ⩾20% improvement in tender and swollen joints and ⩾20% improvement in two of the remaining four core measures (pain, physical function, modified Health Assessment Questionnaire, and patient and physician global assessments). CRP values were excluded from the definition. Secondary efficacy measures included the modified ACR 50 and ACR 70 criteria at 6 months, standard ACR 20, ACR 50 and ACR 70 responses, and improvements in individual ACR criteria components.
Health outcomes were determined using the Short‐Form 36 (SF‐36) Questionnaire, which measures eight subscales. Clinically meaningful changes were defined as a ⩾3‐point change from baseline.6
Safety assessments
All patients were monitored for adverse events and serious adverse events (SAEs). The Medical Dictionary for Regulatory Activities classification of adverse events was used. Adverse events were assessed by the investigators for severity and relationship to study drug. An SAE was defined as an adverse event that met any of the following criteria: was fatal; was life threatening; resulted in or prolonged hospitalisation; resulted in persistent or marked disability or incapacity; was cancer; resulted in an overdose; resulted in the development of drug dependence or drug misuse; or was an important medical event. Serious infections were defined as infections meeting the SAE criteria.
Blood samples were obtained on days 1, 30, 90, 180 and 360, and, in the case of premature discontinuation, for up to 60 days after the last infusion, for analysis of abatacept‐specific antibody levels using an enzyme immunoassay.1 A ⩾9‐fold increase in antibody titre compared with day 1 was considered evidence for seroconversion. This cut‐off point represented a shift of two serial dilutions (1:3) in relation to background activity. Clinical laboratory tests, including complete blood count and chemistry panel, were also performed.
Data analyses and statistics
By using a 2:1 randomisation schedule and assuming a 35% ACR 20 response at 6 months for the placebo group7 and a 10% dropout rate, it was estimated that a sample size of 126 patients (or 141 patients, accounting for expected dropouts) would allow 90% power to detect a treatment difference of 30% at the 5% level of significance (two tailed).
All randomised patients who received ⩾1 infusion of study drug were included in the efficacy and safety analyses. Patients demographics and baseline clinical characteristics were summarised by group. Modified and standard ACR 20, ACR 50 and ACR 70 response rates at 6 months were summarised by group using point estimates and 95% confidence intervals with between‐group differences assessed using χ2 tests at the 5% level of significance. For the primary analysis of modified ACR 20 responses during the double‐blind phase, patients who discontinued the study owing to a lack of efficacy were considered to be non‐responders subsequent to withdrawal, and patients who discontinued for other reasons had their ACR response at the time of discontinuation carried forward. The analysis of ACR responses was also performed as a sensitivity analysis, in which patients who discontinued for any reason were considered to be non‐responders subsequent to withdrawal. During the LTE, ACR response analysis was based on as‐observed data. In addition, ACR response rates were imputed for patients who had missing data at a given visit. This imputation was performed by analysing data from the visits that occurred immediately before and immediately after the visit for which data were missing. If positive responses were observed at both visits, a positive response was imputed. If a positive response was not observed at both the prior and subsequent visits, the response was set to missing. If data were missing for the last scheduled efficacy visit, imputation was dependent on responses observed at both the previous consecutively scheduled efficacy visits. If positive responses were observed at both prior visits, a positive response was imputed for the final visit; otherwise, the response was set to missing. For the individual components of the ACR criteria, the mean change from baseline to year 1, and from year 1 to year 2 was determined, based on the last observation carried forward dataset. Student's t test was used to compare the difference between each time point, Fisher's exact test was used to compare the rate of adverse events, and the log rank test was used to compare the pattern of discontinuation from study among treatment groups. All other safety outcomes were summarised by group.
Results
Study population
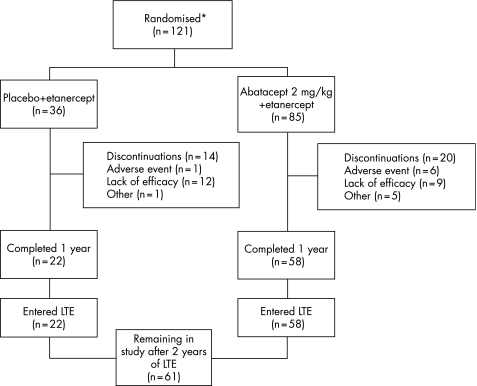
A total of 121 patients were randomised to receive abatacept 2 mg/kg and etanercept (n = 85) or placebo and etanercept (n = 36). Enrolment was prematurely discontinued because of a shortage of etanercept supply, and this led to a lack of available patients. One additional patient was randomised but did not receive the study drug and was not included in any analyses. Patients' demographics and baseline clinical characteristics were similar between groups (table 1). Most patients were women (76%), Caucasian (96%), with a mean disease duration of 13 years. On average, patients had 29 tender and 20 swollen joints at baseline.
Table 1 Demographics and baseline clinical characteristics of study population.
| Characteristic* | Abatacept 2 mg/kg+etanercept (n = 85) | Placebo+etanercept (n = 36) |
|---|---|---|
| Mean (years) age (range) | 49.8 (23–73) | 54.3 (28–71) |
| Women (%) | 78 | 72 |
| Caucasian (%) | 94 | 100 |
| Disease duration (years) | 13 (10.1) | 12.8 (8.6) |
| Tender joint count† (n) | 28.7 (14) | 29.2 (13.2) |
| Swollen joint count‡ (n) | 19.6 (9.4) | 20.1 (10.5) |
| Physical function, mHAQ score 0–3 | 1 (0.5) | 0.9 (0.5) |
| CRP level (mg/dl)§ | 2 (2.7) | 2.4 (4.3) |
| Normal CRP level (%) (<2 mg/dl) | 67 | 66.7 |
CRP, C reactive protein; mHAQ, modified Health Assessment Questionnaire.
All patients received etanercept 25 mg twice weekly.
*Values are mean (SD), unless otherwise mentioned.
†68 joints were assessed for tenderness.
‡66 joints were assessed for swelling.
§Reference range 0–0.4 mg/dl.
Overall, 80 patients (66%) completed the double‐blind phase of the study (abatacept 2 mg/kg and etanercept, n = 58; placebo and etanercept, n = 22), with all entering the LTE. Of these, 68 (85%) patients were exposed to etanercept after 1 year of LTE, and 36 (45%) after 2 years of LTE. Approximately 80% received etanercept for ⩾18 months during the LTE. At the end of 2 years, 61 patients remained in the study (fig 1).
Figure 1 *Patient disposition. Excludes one patient that was randomised but did not receive the study drug. LTE, long‐term extension.
Efficacy
ACR response rates
Double‐blind phase
No significant differences were seen between the abatacept 2 mg/kg and etanercept, and placebo and etanercept groups in modified ACR 20 (48.2% v 30.6%; p = 0.072) or modified ACR 50 response rates (25.9% v 19.4%; p = 0.448) at 6 months (table 2). A significant increase in modified ACR 70 response rates was observed with abatacept 2 mg/kg and etanercept versus placebo and etanercept at the 5% level (10.6% v 0%; p = 0.042).
Table 2 Modified American College of Rheumatology response rates at 6 months and 1 year.
| Modified ACR response rate | 6 months | 1 year | ||
|---|---|---|---|---|
| Abatacept (2 mg/kg) +etanercept (n = 85) | Placebo+etanercept (n = 36) | Abatacept (2 mg/kg)+etanercept (n = 85) | Placebo+etanercept (n = 36) | |
| ACR 20 | 41 (48.2) | 11 (30.6) | 41 (48.2) | 11 (30.6) |
| ACR 50 | 22 (25.9) | 7 (19.4) | 24 (28.2) | 6 (16.7) |
| ACR 70 | 9 (10.6) | 0 (0) | 8 (9.4) | 2 (5.6) |
ACR, American College of Rheumatology.
Values are number (%).
All patients received etanercept 25 mg twice weekly.
At 1 year, modified ACR 20 and ACR 50 response rates remained similar between groups, although modified ACR 70 response rates were no longer significantly different between groups (p = 0.481; table 2).
The modified ACR 20, ACR 50 and ACR 70 response rates were also calculated based on a sensitivity analysis, which considered all patients who received abatacept and discontinued as ACR non‐responders. The findings of this analysis were comparable to the primary analysis at both 6 months (ACR 20: 30.6% v 44.7%; ACR 50: 19.4% v 25.9%; ACR 70: 0% v 10.6%) and 1 year (ACR 20: 30.6% v 43.5%; ACR 50: 16.7% v 9.4%; ACR 70: 5.6% v 9.4%) (placebo plus etanercept v abatacept plus etanercept, respectively).
An additional analysis, performed using standard ACR 20, ACR 50 and ACR 70 response rates, which included CRP values in its criteria, was consistent with the modified ACR response rates: greater increases in ACR 20 (42.4% v 27.8%), ACR 50 (23.5% v 13.9%) and ACR 70 (9.4% v 0%) response rates were observed with abatacept 2 mg/kg and etanercept versus placebo and etanercept; however, these differences were not statistically significant.
Long‐term extension
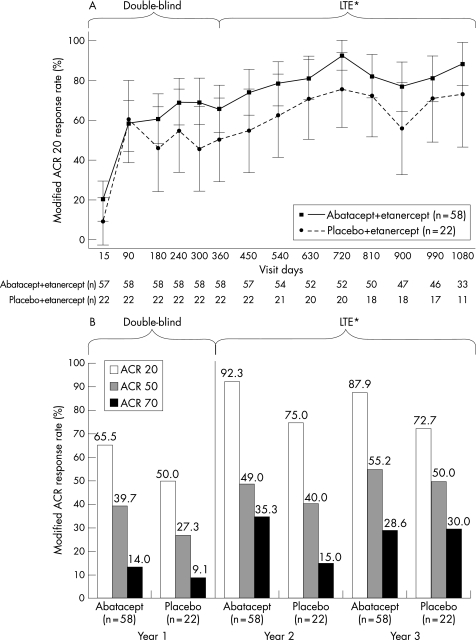
During the LTE, all patients were switched to receive abatacept at a fixed dose approximating 10 mg/kg (according to weight range). In general, ACR response rates were maintained from the start of the LTE (day 360) to day 1080 in the group treated with abatacept 2 mg/kg during the double‐blind phase (fig 2A,B).
Figure 2 (A) American College of Rheumatology (ACR) 20 response rates during 1 year of double‐blind and 2 years of open‐label treatment; (B) ACR 20, ACR 50 and ACR 70 response rates at year 1 (double‐blind phase) and years 2 and 3 for patients who entered the long‐term extension (LTE). Data shown are for all patients entering the LTE, based on the as‐observed analysis. *All patients treated with abatacept 10 mg/kg during LTE; groups described according to double‐blind treatment assignment.
ACR core components
Double‐blind phase
At 1 year, significant improvements from baseline values were seen in all ACR components in the abatacept 2 mg/kg and etanercept group (p<0.001 for all components except for CRP, where p = 0.039). Significant improvements were also seen in four of the seven components in the placebo and etanercept group (table 3).
Table 3 Core American College of Rheumatology component responses at baseline and 1 year.
| ACR component | Abatacept 2 mg/kg+etanercept (n = 85) | Placebo+etanercept (n = 36) | ||||
|---|---|---|---|---|---|---|
| BL | 1 year | Change | BL | 1 year | Change | |
| Tender joints* | 28.7 (14.0) | 17.1 (17.3) | −11.6 (13.9)† | 29.2 (13.2) | 20.4 (15.1) | −8.8 (13.7)† |
| Swollen joints‡ | 19.6 (9.4) | 11.8 (9.5) | −7.8 (9.5)† | 20.1 (10.5) | 15.7 (10.6) | −4.4 (9.2)§ |
| Patient assessment of pain | 65.5 (16.6) | 43.6 (28.2) | −22.0 (29.0)† | 53.2 (23.2) | 47.0 (29.9) | −6.1 (23.2) |
| Patient assessment of function (mHAQ) | 1.0 (0.5) | 0.7 (0.6) | −0.3 (0.5)† | 0.9 (0.5) | 0.8 (0.5) | −0.2 (0.4)¶ |
| Patient assessment of disease activity | 61.6 (18.9) | 43.4 (28.0) | −18.2 (29.1)† | 54.4 (22.5) | 47.4 (29.3) | −7.1 (21.3) |
| Physician assessment of disease activity | 61.8 (17.2) | 36.2 (23.7) | −25.7 (27.0)† | 62.1 (13.5) | 43.8 (28.3) | −18.2 (20.4)† |
| CRP** | 2.0 (2.6) | 1.4 (1.5) | −0.6 (2.4)†† | 2.4 (4.3) | 3.3 (4.9) | −0.9 (3.5) |
ACR, American College of Rheumatology; BL, baseline; CRP, C reactive protein; mHAQ, modified Health Assessment Questionnaire.
All patients received etanercept 25 mg twice weekly.
All values are shown as mean (SD).
*68 joints were assessed for tenderness; †p<0.001; ‡66 joints were assessed for swelling; §p = 0.007; ¶p = 0.040; **reference range 0–0.4 mg/dl; ††p = 0.039.
Long‐term extension
After 1 year of treatment with abatacept 10 mg/kg and etanercept, improvements in ACR components were maintained through year 2 in the group previously receiving abatacept 2 mg/kg and etanercept. Further, significant improvements compared with year 1 were seen in three of these individual components (tender joints, swollen joints and physician assessment of disease activity). The group originally randomised to placebo and etanercept and switched to abatacept 10 mg/kg and etanercept only showed a significant improvement in one of the seven ACR components (swollen joints; table 4). Mean changes in the ACR components were also calculated from years 2 to 3. The general trend was for no further improvement, but low patient numbers (n⩽30 in each case) made these data difficult to interpret.
Table 4 Core American College of Rheumatology component responses at 1 and 2 years.
| ACR component | Abatacept 2 mg/kg switched to 10 mg/kg+etanercept* | Placebo switched to abatacept 10 mg/kg+etanercept† | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | Change | 1 year | 2 years | Change | |
| Tender joints‡ | 10.6 (12.9) | 6.4 (8.8) | −4.2 (9.4)§ | 14.1 (10.7) | 11.1 (13.4) | −3.0 (8.2) |
| Swollen joints¶ | 10.4 (9.5) | 6.0 (5.7) | −4.4 (8.3)** | 12.9 (9.4) | 8.7 (9.3) | −4.2 (7.3)†† |
| Patient assessment of pain | 34.5 (23.5) | 29.2 (19.8) | −5.3 (26.3) | 30.3 (22.6) | 33.1 (19.8) | 2.8 (15.7) |
| Patient assessment of function (mHAQ) | 0.5 (0.4) | 0.4 (0.4) | −0.1 (0.3) | 0.5 (0.4) | 0.5 (0.4) | 0 (0.2) |
| Patient assessment of disease activity | 32.1 (22.3) | 28.0 (19.4) | −4.1 (23.1) | 29.4 (17.7) | 31.0 (19.8) | 1.6 (19.4) |
| Physician assessment of disease activity | 27.4 (19.0) | 19.8 (17.0) | −7.6 (20.6)‡‡ | 25.4 (17.0) | 24.8 (19.2) | −0.6 (23.1) |
| CRP level§§ | 1.0 (1.1) | 0.8 (0.7) | −0.2 (0.9) | 1.0 (0.8) | 0.6 (0.5) | −0.4 (0.8) |
ACR, American College of Rheumatology; CRP, C reactive protein; mHAQ, modified Health Assessment Questionnaire.
All patients received etanercept 25 mg twice weekly; values for year 3 were recorded but excluded owing to the small sample number.
All values are shown as mean (SD).
*n = 46–51; †n = 19–20; ‡68 joints were assessed for tenderness; §p = 0.002; ¶66 joints were assessed for swelling; **p<0.001; ††p = 0.018; ‡‡p = 0.0135; §§reference range, 0–0.4 mg/dl.
Quality of life
Double‐blind phase
At 1 year, the abatacept 2 mg/kg and etanercept group had significantly greater improvements (p<0.05) from baseline compared with placebo plus etanercept in five of the eight mental and physical subscales of the SF‐36 (physical function, role–physical, bodily pain, vitality and social functioning) and in the physical component summary (PCS); the absolute changes in all subscales were considered clinically meaningful (an increase of ⩾3 points). The improvement in the mental component summary (MCS) in the abatacept group was clinically meaningful (3.9 points), although not significantly greater than the placebo group (1.06 points).
Long‐term extension
After 1 year of LTE, improvement from baseline for the MCS and PCS increased to 7.08 and 9.11, respectively, in the group that switched from abatacept 2 mg/kg and etanercept to abatacept 10 mg/kg and etanercept, and to 2.14 and 3.19, respectively, in the group that switched from placebo to abatacept 10 mg/kg and etanercept. After 2 years of LTE, improvements from baseline in the MCS and PCS for the group that switched from placebo to abatacept 10 mg/kg and etanercept were 7.54 and 3.44, respectively, and for the group that switched from abatacept 2 mg/kg and etanercept to abatacept 10 mg/kg and etanercept, improvements were 4.68 and 6.65, respectively.
Safety
Double‐blind phase
At 6 months, overall frequencies of adverse events and SAEs were comparable between groups. However, at 1 year, patients receiving abatacept 2 mg/kg and etanercept had higher frequencies of related adverse events, SAEs, related SAEs and discontinuations due to adverse events than the placebo and etanercept group (table 5). No deaths or opportunistic infections were reported during the double‐blind phase. The most commonly reported adverse event was worsening arthritis, reported in 43.5% of patients receiving abatacept 2 mg/kg and etanercept, and in 36.1% of patients receiving placebo and etanercept (table 5). Other commonly reported adverse events included headache, upper respiratory tract infection, musculoskeletal pain, nausea and vomiting, sinus abnormality and fatigue. Related adverse events, discontinuations due to adverse events, total SAEs, related SAEs and infections reported as SAEs were all higher with abatacept 2 mg/kg and etanercept compared with placebo and etanercept (table 5).
Table 5 Overall adverse events and discontinuations due to adverse events at 1 year and after 2 years of long‐term extension.
| AE | DB phase | LTE | |
|---|---|---|---|
| Abatacept 2 mg/kg+etanercept (n = 85) | Placebo+etanercept (n = 36) | Abatacept 10 mg/kg+etanercept (n = 80) | |
| Deaths | 0 | 0 | 1 (1.3) |
| Total AEs | 79 (92.9) | 32 (88.9) | 78 (97.5) |
| Most common AEs* | |||
| Rheumatoid arthritis | 37 (43.5) | 13 (36.1) | 24 (30) |
| URTI | 20 (23.5) | 5 (13.9) | 23 (28.8) |
| Headache | 20 (23.5) | 5 (13.9) | 10 (12.5) |
| Fatigue | 14 (16.5) | 6 (16.7) | 14 (17.5) |
| Sinusitis | 14 (16.5) | 3 (8.3) | 18 (22.5) |
| Nausea | 13 (15.3) | 1 (2.8) | 12 (15) |
| Arthralgia | 13 (15.3) | 3 (8.3) | 5 (6.3) |
| Dizziness | 13 (15.3) | 2 (5.6) | 8 (10) |
| Diarrhoea | 12 (14.1) | 2 (5.6) | 13 (16.3) |
| Cough | 11 (12.9) | 3 (8.3) | 11 (13.8) |
| Rash | 11 (12.9) | 3 (8.3) | 10 (12.5) |
| Discontinuations due to AEs | 10 (11.8) | 1 (2.8) | 8 (10) |
| Related AEs† | 53 (62.4) | 17 (47.2) | 52 (65) |
| SAEs | 14 (16.5) | 1 (2.8) | 26 (32.5) |
| Musculoskeletal and connective tissue disorders | 1 (1.2) | 0 | 9 (11.3) |
| Vascular disorders | 0 | 1 (1.2) | 6 (7.5) |
| Malignancies | — | — | 3 (3.8) |
| Gastrointestinal disorders | — | — | 3 (3.8) |
| Infections and infestations | 3 (3.5) | 0 | 1 (1.3) |
| Nervous system disorders | 2 (2.4) | 0 | 1 (1.3) |
| Respiratory, thoracic and mediastinal disorders | 30 (35.3) | 7 (9.4) | 32 (40) |
| Related SAEs† | 5 (5.9) | 0 | 3 (3.8) |
| Serious infections | 3 (3.5) | 0 | 1 (1.3) |
AE, adverse event; DB, double blind; LTE, long‐term extension; SAE, serious adverse event; URTI, upper respiratory tract infection.
Values are number (%).
*Occurring in ⩾10% of patients in the abatacept treatment group.
†Related events include those considered by the investigator to be certain, probable or possibly related to the study drug.
Adverse events leading to discontinuation within the abatacept 2 mg/kg and etanercept group were chest pain, bronchitis, ear infection, localised infection, upper respiratory tract infection, ankle fracture, limb injury, tendon injury, worsening arthritis, cough, leucocytoclastic vasculitis and vasculitis. Only 2 of the 10 patients reported a single adverse event that resulted in discontinuation; the remaining patients reported ⩾2 of the above adverse events.
Herpes simplex, herpes zoster or candidiasis infections were reported by 7% of patients receiving abatacept 2 mg/kg and etanercept and 6% of patients receiving placebo and etanercept. One patient experienced a moderate infection of the thumb (skin and subcutaneous tissue) which was treated with intravenous levofloxacin (500 mg, reduced to 250 mg on the third day of treatment) and led to discontinuation. No patients were hospitalised because of infection.
Long‐term extension
The overall pattern and intensity of adverse events during the LTE with the higher dose of abatacept were similar to those observed during the double‐blind period. Most adverse events reported during the LTE were mild to moderate in severity. Serious adverse events were reported in 26 (32.5%) patients. Total infections (mainly respiratory) were seen in 62 (77.5%) patients; a serious infection (bacterial arthritis) was reported in 1 (1.3%) patient. Predefined infections of interest (ie, those that may be associated with the use of immunomodulatory agents or infusion of therapeutic proteins) were reported in 12 (15%) patients during the LTE. Malignant neoplasms were reported for three patients and included basal cell carcinoma, cervix carcinoma and diffuse, large B cell lymphoma. All three patients were receiving concomitant etanercept. One patient died because of diffuse, large B cell lymphoma; the investigator considered the death to be possibly related to treatment with both abatacept and etanercept. Autoimmune symptoms and disorders were reported in 3 (3.8%) patients (psoriasis, two patients; keratoconjunctivitis sicca, one patient) during the LTE; all were mild or moderate in intensity.
There were no clinically relevant laboratory findings, and no patient developed antibodies to abatacept.
Discussion
In this phase II study, when abatacept 2 mg/kg was added to etanercept, the primary end point of a significant improvement in modified ACR 20 response rates at 6 months (compared with etanercept and placebo) was not achieved; however, significant benefits were observed in ACR 70 response rates and quality of life measures. The abatacept 2 mg/kg dose was subsequently found to be suboptimal when used in combination with MTX in MTX‐inadequate responders.2,3 In response to this, all patients entering the LTE of this study were switched to a fixed dose of abatacept approximating 10 mg/kg. In general, however, this higher dose of abatacept did not lead to greater reductions in disease activity in either of the study groups. No additional changes in the responses seen after 1 year of double‐blind treatment were seen through 2 years of LTE. In this study, the quality of life was significant in five of the eight subscales of the SF‐36 and in the PCS. This is in contrast with observations in MTX‐inadequate responders,2,3 where improvements in all eight subscales and both the PCS and MCS were significant and well maintained, thus supporting the finding that the 2 mg/kg dose of abatacept is suboptimal. Firm efficacy conclusions from this study are limited owing to the use of the 2 mg/kg dose of abatacept during the double‐blind phase, and the relatively low numbers of patients who received abatacept 10 mg/kg in the LTE. Furthermore, owing to the shortage of etanercept supply and the resulting lack of available patients, enrolment was discontinued before the planned number of patients per protocol was reached. This reduced the statistical power of the study; however, this study suggests that the addition of abatacept to etanercept was not associated with a meaningful clinical effect.
Although the incidence of adverse events in this study was generally low, abatacept in combination with etanercept was shown to result in an increase in adverse events, including related adverse events, SAEs and serious infections. Data from a recent large‐scale phase III study of abatacept in patients with rheumatoid arthritis receiving background biological and/or non‐biological DMARDs show that abatacept in combination with biological DMARDs produces a less favourable safety profile than the combination of abatacept and non‐biological DMARDs.8 A lack of added benefit and increased rate of infection has also been seen when other biologicals are used in combination (etanercept and anakinra).9 Collectively, these findings suggest that the risk‐to‐benefit ratio of using biological treatments in combination is yet to be established.
In conclusion, this phase II study showed increases in adverse events and SAEs with limited clinical benefit when abatacept was used in combination with etanercept for the treatment of rheumatoid arthritis.
Acknowledgements
We thank Claire Smart, PhD, for her editorial assistance with the manuscript.
Abbreviations
ACR - American College of Rheumatology
CRP - C reactive protein
DMARD - disease‐modifying antirheumatic drug
LTE - long‐term extension
MCS - mental component summary
MTX - methotrexate
PCS - physical component summary
SAE - serious adverse event
SF‐36 - short‐form 36
Footnotes
Funding: This study was funded and sponsored by Bristol‐Myers Squibb, which was involved in the design of the study (conducted between 26 February 2001 and 22 June 2004), the collection and analysis of the data, the writing of the report and the decision to submit the report.
Competing interests: MW has received consulting fees (< US $10 000/year) from the sponsor of this study, Bristol‐Myers Squibb, as a consultant. In addition, he has received research funding for support of this study. He has also received consulting fees (< US $10 000/year) from Wyeth, Amgen, Genentech, Centocor and Abbott and research funding from Amgen, Abbott and Genentech. MS has received research funding and consulting fees from Bristol‐Myers Squibb, Abbott, Amgen, Wyeth and Centocor. JK has received research grants from Bristol‐Myers Squibb and has also served as a consultant for research and on the development of educational presentations. AG and ML have no competing financial interests. TL is an employee of Bristol‐Myers Squibb and owns Bristol‐Myers Squibb stocks. DC and J‐CB are both employees of Bristol‐Myers Squibb.
Clinical investigators: The investigators for this study played a key role in its success. The following clinical investigators provided and cared for the study patients while participating in the study:
Drs Neal Birnbaum, San Francisco, California; Steven Carsons, Mineola, New York; Walter Chase, Austin, Texas; Melvin A Churchill, Lincoln, Nebraska; Stanley Cohen, Dallas, Texas; Geoffrey S Dolan, Long Beach, California; John Donahue, Boston, Massachusetts; Michael Ellman, Chicago, Illinois; Gary Fink, Charleston, South Carolina; Mark Genovese, Palo Alto, California; Allan Goldman, Milwaukee, Wisconsin; Richard Honsinger, Los Alamos, New Mexico; Christopher Jackson, Salt Lake City, Utah; Richard Jimenez, Edmonds, Washington; Alan Kivitz, Duncansville, Pennsylvania; Joel Kremer, Albany, New York; Robert Leff, Duluth, New Mexico; Michael Luggen, Cincinnati, Ohio; Raymond Malamet, Hagerstown, Maryland; Joseph Markenson, New York, New York; Daniel Norden, Norristown, Pennsylvania; David Pierangelo, Springfield, Massachusetts; Michael Schiff, Denver, Colorado; William Shergy, Huntsville, Alabama; Yvonne Sherrer, Ft Lauderdale, Florida; Lee Simon, Boston, Massachusetts; Nowarat Songsiridej, Bismarck, North Dakota; Elizabeth A Tindall, Portland, Oregon; Wayne Tsuji, Seattle, Washington; Daniel J Wallace, Los Angeles, California; Michael Weinblatt, Boston, Massachusetts; and David Wofsy, San Francisco, California.
References
- 1.Moreland L W, Alten R, Van den Bosch F.et al Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose‐finding, double‐blind, placebo‐controlled clinical trial evaluating CTLA‐4Ig and LEA29Y eighty‐five days after the first infusion. Arthritis Rheum 2002461470–1479. [DOI] [PubMed] [Google Scholar]
- 2.Kremer J M, Westhovens R, Leon M.et al Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. N Engl J Med 20033491907–1915. [DOI] [PubMed] [Google Scholar]
- 3.Kremer J M, Dougados M, Emery P.et al Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve‐month results of a phase iib, double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005522263–2271. [DOI] [PubMed] [Google Scholar]
- 4.Arnett F C, Edworthy S M, Bloch D A.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg M C, Chang R W, Dwosh I.et al The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 199235498–502. [DOI] [PubMed] [Google Scholar]
- 6.Samsa G, Edelman D, Rothman M L.et al Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics 199915141–155. [DOI] [PubMed] [Google Scholar]
- 7.Moreland L W, Schiff M H, Baumgartner S W.et al Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 1999130478–486. [DOI] [PubMed] [Google Scholar]
- 8.Weinblatt M, Combe B, Covucci A.et al Safety of the selective co‐stimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and non‐biologic DMARDs: a 1 year randomised study. Arthritis Rheum 2006542807–2816. [DOI] [PubMed] [Google Scholar]
- 9.Genovese M C, Cohen S, Moreland L.et al Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004501412–1419. [DOI] [PubMed] [Google Scholar]