Abstract
Serological studies of cottontail rabbits sampled from Nantucket Island, Mass., have suggested exposure to at least two ehrlichiae. The agent of human granulocytic ehrlichiosis (Anaplasma phagocytophilum) is intensely enzootic in rabbits there, but the identity of the other ehrlichial infection remains undescribed. We sampled rabbits over five transmission seasons and tested their blood and tissues for evidence of infection using PCR targeting an Ehrlichia genus-wide 16S rDNA target. Sequence analysis of positive amplicons revealed the presence of Anaplasma bovis, an agent not known to be present in North America. The average annual prevalence of A. bovis within rabbits, as determined by PCR of blood samples, was 18%. Haemaphysalis leporispalustris appears to serve as vector. The public health (human or veterinary) significance of this finding remains speculative.
In 1992, as part of an effort to identify a reservoir for the newly described Ehrlichia chaffeensis, agent of human monocytic ehrlichiosis, cottontail rabbits (Sylvilagus floridanus) from Nantucket Island, Mass., were assayed for seroreactivity to E. chaffeensis antigen. Although a majority of sera were reactive at an immunoglobulin G titer of >1/64 by indirect fluorescent antibody test, no other evidence of infection was detected (J. E. Dawson and S. R. Telford III, unpublished data). Subsequently, E. chaffeensis was demonstrated to be maintained by white-tailed deer (Odocoileus virginianus) and Lone Star ticks (Amblyomma americanum) (5, 8, 13). Whereas white-tailed deer are common on Nantucket, Lone Star ticks are absent. E. chaffeensis has not been detected from deer or any other hosts on Nantucket, rendering the rabbit immunoreactivity to E. chaffeensis antigen an enigma. Nantucket, like many insular sites, is characterized by the intense transmission of zoonoses, particularly those of the guild of deer tick (Ixodes dammini) pathogens. During the course of an analysis of the zoonotic potential of cottontail rabbits there, we examined the possibility that an unidentified Ehrlichia may be present in these hosts, in addition to the prevalent Anaplasma phagocytophilum (the agent of human granulocytic ehrlichiosis). We report herein the discovery of Anaplasma bovis, a bovine-infecting Ehrlichia previously undetected in North America, identify its vector, and describe the dynamics of its transmission.
MATERIALS AND METHODS
Sample collection.
Cottontail rabbits, S. floridanus, were shot or trapped live (Tomahawk Live Traps, Inc., Tomahawk, Wis.) on the grounds of the University of Massachusetts Field Station, Nantucket Island, from 1998 to 2002. Collections occurred during the spring and fall of 1998 and 1999 and then monthly (April to October) from April 2000 to August 2002. Anticoagulated blood was collected from all animals, and spleens were collected from dead animals. All rabbits were visually inspected for the presence of ticks, which were removed by gentle traction with forceps. Most rabbits were caged over water overnight to allow replete ticks to drop off. Animals trapped live were marked with an ear tag and released at the point of capture.
PCR amplification.
DNA was extracted from whole blood and spleens with either the Isoquick blood kit (Orca Research) or the Qiagen tissue kit by following the recommendations of the manufacturers. PCR was performed with an MJ Research thermocycler using Taq polymerase and buffer reagents from Qiagen Corp. as recommended by the manufacturer. General primers ERIK1 (TGCGGRGGAAAGATTTATCGCT) and ERIK2 (GAATTTTACCTCTACACTCGG), designed to amplify a 500-bp region of the 16S rDNA gene for Ehrlichia spp. in addition to that of Rickettsia spp., were used for initial screening. Amplification conditions used were 40 cycles of 94°C for 45 s, 60°C for 45 s, and then 72°C for 45 s. Ten microliters of product was analyzed on a 1.5% agarose gel next to a 100-bp DNA ladder (Gibco BRL). A sample of positive PCR products was purified with Qiagen spin columns and sent to the University of Maine sequencing facility for sequence analysis.
New 16S rDNA primers were designed to specifically amplify A. bovis. A nested reaction was devised to maximize sensitivity. Primers BacA and 1448r were used in the outer reaction as described previously (4). Inner primers Bovis74f (TGTTCTCGTAGCTTGCTATGRG) and Bovis867r (GGAGGTAAAAACCCCCACATC) were designed to amplify a 700-bp piece of the 16S rDNA gene under the same reaction conditions as those described by Chen et al. (4). These primers were tested for specificity on known positive samples containing A. phagocytophilum, the white-tailed deer agent, E. chaffeensis, and Neorickettsia risticii, and produced amplicons only from rabbit samples for which PCR sequencing of the ERIK1 and -2 amplicons indicated the presence of A. bovis. All subsequent PCRs were carried out with these primers. A. bovis had never been received or maintained in the Harvard laboratory. Safe PCR was always practiced: separate rooms were used for reaction setup and analysis, and dedicated pipettors were used for each step in the process; dUTPs and appropriate negative controls were included for all PCRs.
Statistics.
Confidence intervals (CI) around prevalence estimates were calculated by the exact binomial method using the STATA software package.
Phylogenetic analysis.
To identify the rabbit agent, we performed phylogenetic analysis. A large piece of the 16S gene, 1,200 bp, was amplified for use in the phylogenetic analysis with the forward primer ERIK1 and the newly designed reverse primer Ehr1500 (CTTAAATGGCTGCCTCCTTKCG). The PCR was performed as described above. The sequence from the rabbit agent was aligned with those of known Ehrlichia spp. retrieved from GenBank with ClustalX and then adjusted by eye with GeneDoc (K. B. Nicholas and H. B. Nicholas, Jr., GeneDoc: a tool for editing and annotating multiple sequence alignments [distributed by the authors], 1997). Maximum-parsimony analysis using PAUP (17) and neighbor-joining analysis using MEGA (12) were performed. The robustness of the tree topology was assessed by using 500 bootstrap replicates. Rickettsia rickettsii was used as the outgroup, following prior analyses (11, 20).
The following are the GenBank accession numbers for the sequences from the organisms included in the phylogenetic analysis: E. chaffeensis, U60476 and U23503; Ehrlichia ewingii, U96436; Ehrlichia canis, AF162860; Cowdria ruminantium, AF069758; Ehrlichia bovis, U03775, white-tailed deer agent, U27104; E. equi, AF172165; Ehrlichia phagocytophila, M73220; Anaplasma marginale, M60313; Ehrlichia sennetsu, M73225; Rickettsia rickettsii, U11021.
Ticks.
Ticks from rabbits were collected and sorted by species and stage. Partially engorged ticks were crushed individually in 50 μl of phosphate-buffered saline on a 96-well plate and then pooled in groups of six. DNA was extracted by a standard phenol-chloroform procedure and tested by PCR. We were careful to distinguish evidence of infection due to the blood meal from that demonstrating transstadial survival, and therefore ticks collected from PCR-positive animals were removed from subsequent analyses. Replete ticks were held at 24°C and 90% relative humidity and allowed to molt. They were then pooled in groups of five and tested for evidence of infection by PCR.
RESULTS
Identification of the rabbit ehrlichiae.
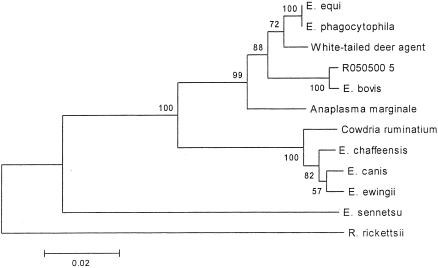
To identify the unknown ehrlichiae infecting cottontail rabbits on Nantucket Island, PCR analysis of blood and spleens was performed with genus-wide primers ERIK1 and ERIK2. PCR analysis for the first year of collections, 1998, yielded 3 (18%) rabbits with a 500-bp band, consistent with the expected 500-bp piece. Sequence analysis of these amplicons showed a 99.5% sequence identity to A. bovis. To confirm the identification of the rabbit agent (GenBank accession no. AY144729), phylogenetic analysis was performed. The topology of our trees was the same regardless of the method of analysis, neighbor-joining or maximum parsimony. The rabbit agent consistently clusters with A. bovis on all trees, with a bootstrap value of 100%. We provide a neighbor-joining bootstrap consensus tree (Fig. 1). From this analysis, we are confident that the organism found in the Nantucket rabbits is A. bovis. Accordingly, all subsequent PCR analyses used the specific A. bovis primers in a nested reaction.
FIG. 1.
Phylogenetic analysis. Shown is a bootstrap consensus neighbor-joining tree of Ehrlichia spp.
Prevalence in rabbits.
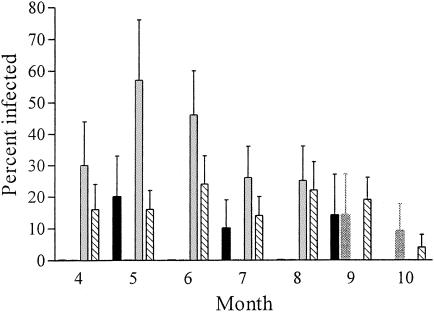
A. bovis is endemic in cottontail rabbits on Nantucket Island; we were able to identify infected rabbits every year during our 5 years of observation. The prevalence of A. bovis in cottontail rabbits from Nantucket Island varied from year to year from 4 to 32% with an overall average of 18% (95% CI, 13 to 24%) (Table 1). Although the differences in prevalence were not significant when assessed on a yearly basis (with overlapping 95% CI), they were significant when assessed on a monthly basis (Fig. 2). For example, no positive rabbits were found throughout the summer in 2001 despite the collection of 29 animals. Infected animals were finally identified in September of that year. In contrast, 2002 demonstrated the greatest prevalence of infection in the early summer months, with infection waning as fall approached.
TABLE 1.
Prevalence of A. bovis in cottontail rabbits by year
| Yr | % infected (95% CI) | Total |
|---|---|---|
| 1998 | 18 (4-48) | 17 |
| 1999 | 4 (0-20) | 25 |
| 2000 | 11 (3-25) | 38 |
| 2001 | 10 (3-21) | 51 |
| 2002 | 32 (21-43) | 73 |
| Overall | 18 (13-24) | 203 |
FIG. 2.
Prevalence of A. bovis in rabbits by month. Black bars, 2000; dark gray bars, 2001; light gray bars, 2002. Data from 1998 and 1999 are not included in this graph because collections occurred only in the spring and fall. Hatched bars, mean prevalence for all years of collection, 1998 to 2002. Note that 2002 collections halted in August, and 2000 collections halted in September.
In 2001 and 2002, we conducted a capture-mark-release study of the rabbits. Nine rabbits that were PCR positive on the initial capture were trapped again in subsequent months. Of seven trapped in the next month, four remained PCR positive. One that was caught 2 months later tested negative, and one that was caught 10 months later also tested negative. We conclude that rabbits may be chronically infected or may be reinfected by A. bovis.
Analysis of ticks.
The majority of ticks collected from these rabbits was identified as H. leporispalustris or Ixodes dentatus. I. dammini was also found. To identify the vector of A. bovis, we tested ticks collected from rabbits by PCR. All species of ticks were determined to be infected (Table 2). None of the I. dammini females and only 1% (minimum infection rate [MIR]; 95% CI, 0 to 7%) of nymphal ticks tested positive. Although no nymphal or female I. dentatus ticks were found to be infected, 2% (MIR; 95% CI, 0.2 to 5%) of I. dentatus males were positive. In contrast, all stages of H. leporispalustris were infected. Although H. leporispalustris appears to be the main vector of A. bovis between rabbits, we cannot exclude a secondary role for Ixodes spp.
TABLE 2.
MIR of A. bovis in ticks collected from rabbits
| Species | MIR (%) (95% CI) for:
|
||
|---|---|---|---|
| Females | Males | Nymphs | |
| H. leporispalustris | 4 (0.5-14) | 3 (0.3-10) | 0.4 (0-2) |
| I. dammini | 0 (0-50) | NDa | 1 (0-7) |
| I. dentatus | 0 (0-1) | 2 (0.2-5) | 0 (0-4) |
ND, not done.
To demonstrate vector competence, we tested the capacity of larvae, fed to repletion on PCR-positive rabbits, to retain the infection through the molt. Both I. dentatus (MIR, 1.8%; 95% CI, 0.2 to 6.4%) and H. leporispalustris (MIR, 5.3%; 95% CI, 1.5 to 13.1%) yielded positive nymphs, but the latter was twice as likely to be infected as the former. We were unable to evaluate the competence of I. dammini because we failed to obtain molted nymphs from A. bovis-infected rabbits. H. leporispalustris transstadially maintains A. bovis, thereby demonstrating an important component of vector competence and further supporting our conclusion that it is the main enzootic vector; but Ixodes spp. may also contribute to perpetuation.
DISCUSSION
We did not expect to identify A. bovis in cottontail rabbits on Nantucket Island. A. bovis is a monocytotropic Ehrlichia sp. that has not previously been reported from North America. It was first described in 1936 during experiments of Theileria sp. transmission, in which Hyalomma sp. ticks from Iran were fed on French cattle (7). It has since been described in cattle and buffalo from Africa, the Middle East, and South America (18). It is morphologically very similar to a pathogen known to infect sheep, “Ehrlichia ovina,” and it is uncertain whether these are distinct entities (14, 19). To our knowledge, our report is the first to describe A. bovis infection of small mammals.
Although infected rabbits were identified every year, the prevalence of A. bovis infecting rabbits seemed highly variable (Fig. 2); this, however, may not reflect actual infection status. Both A. bovis and E. ovina infections are characterized by premunition. It has been demonstrated that splenectomy causes recrudescence 1 year after apparent recovery from the initial infection (14, 16). We did not splenectomize rabbits to determine infection rates and believe that, even using a nested PCR, we probably cannot distinguish between uninfected rabbits and those with small numbers of infected host cells. Monocytes generally comprise less than 1% of all leukocytes in circulating blood, and therefore few infected cells would be present on a blood smear and A. bovis sequences in our extracted DNA would be rare. Preliminary evidence, however, suggests chronic persistence of this infection in rabbits: we identified three rabbits that remained PCR positive when recaptured in a subsequent month. Chronic infection would impart great reservoir capacity, supporting stable transmission.
We have failed, to date, with all attempts to propagate the organism either by inoculation of various laboratory animals or by cell culture methods that have been successful for other Ehrlichia spp. To our knowledge, A. bovis has never been continuously cultivated in vitro, although it has been maintained for a few days in primary leukocyte cultures (15). The starting material for these temporary cultures was 20 ml of buffalo blood. It is possible that we simply did not obtain enough parasitized cells from the limited volume of blood obtained from rabbits to successfully initiate cultures.
Most tick-borne agents have evolved a specific host-parasite interaction with their vector, and it seems axiomatic that a given agent is locally maintained by only one tick species. For example, Lyme disease spirochetes and A. phagocytophilum are maintained only by I. dammini even in sites where Dermacentor variabilis or Amblyomma americanum are sympatric (our unpublished data). The metastriate tick genera Hyalomma, Rhipicephalus, and Amblyomma (16) have previously been reported to transmit A. bovis to cattle. The metastriate H. leporispalustris appears to be the main vector on Nantucket because all stages were found to be infected and were competent for transstadial transmission. However, we were able to identify A. bovis in all three species of ticks tested and can exclude the possibility that this finding reflects contamination by the host blood meal because ticks that were attached to PCR-positive rabbits were not included in our analysis. A. bovis was transstadially maintained in the prostriate I. dentatus, albeit at a lesser efficiency than in H. leporispalustris, suggesting the possibility that it serves as a secondary vector. Thus, perpetuation would be facilitated by the capacity of A. bovis to use either prostriate or metastriate ticks as vectors.
How this infection came to be on Nantucket is a matter of conjecture. However, Nantucket Island was a major port for whaling and shipping during the 1800s and early 1900s. Material goods as well as livestock were imported through Nantucket harbor, and it is possible that either foreign livestock or their ticks introduced the pathogen to the Nantucket fauna. Cottontail rabbits themselves are, in fact, not native to Nantucket and were intensively introduced from the central and southern states (9) during the 1920s and 1930s by sporting clubs. The introduced S. floridanus eventually competitively displaced the New England cottontail, Sylvilagus transitionalis. Interestingly, the agent of tularemia was thereby introduced to New England (2, 3), and it may be that A. bovis and Babesia divergens (10) were introduced similarly. Then too, subadult I. dentatus and H. leporispalustris parasitize birds, which may transport ticks great distances, perhaps even from the tropical Americas, where A. bovis has been reported to infect cattle. We are able to rule out transient introduction by migratory birds, however, by detecting infection in rabbits each year for 5 years and infer that a local cycle is well established.
The human and veterinary public health significance of our findings is uncertain. Reports of disease in cattle are conflicting. A. bovis infection in cattle has been documented as asymptomatic (7) but has also been associated with death within hours to days of the first signs of symptoms (16). We did not determine whether local cattle may be exposed, as there are only a few (a dozen) head of cattle on Nantucket, all of which are carefully maintained. However, the host specificity of H. leporispalustris makes it an unlikely source of infection for cattle. The rabbits collected in this study did not show any obvious signs of infection. There are no known reports of A. bovis infection in humans, although macaques have been experimentally infected and sustain a flu-like illness (6), suggesting that human infection may result in illness. The public health burden would depend on the propensity of the involved ticks to bite humans. I. dentatus and H. leporispalustris feed as adults only on lagomorphs, although subadults may also infest birds. We note that I. dentatus may more frequently attach to humans than previously reported (1). In addition, I. dammini ticks, which feeds on rabbits as larvae, would have the opportunity to transmit A. bovis to humans as nymphs or adults. Accordingly, serologic studies of febrile cases from Nantucket (or other sites where humans commonly see rabbits) should be undertaken if A. bovis is propagated in vitro and a specific antigen becomes available.
REFERENCES
- 1.Armstrong, P. M., L. R. Brunet, A. Spielman, and S. R. Telford III. 2001. Risk of Lyme disease: perceptions of residents of a Lone Star tick-infested community. Bull. W. H. O. 79:916-925. [PMC free article] [PubMed] [Google Scholar]
- 2.Ayes, J. C., and R. Feemster. 1948. Epidemiology of tularemia in Massachusetts with a review of the literature. N. Engl. J. Med. 238:187-194. [DOI] [PubMed] [Google Scholar]
- 3.Belding, D. L., and B. Merrill. 1941. Tularemia in imported rabbits in Massachusetts. N. Engl. J. Med. 224:1085-1087. [Google Scholar]
- 4.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson, J. E., D. E. Stallknecht, E. W. Howerth, C. Warner, K. Biggie, W. R. Davidson, J. M. Lockhart, V. F. Nettles, J. G. Olson, and J. E. Childs. 1994. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J. Clin. Microbiol. 32:2725-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donatien, A., and F. Lestoquard. 1940. Rickettsiose bovine Algerienne a R. bovis. Bull. Soc. Pathol. Exot. 33:245-248. [Google Scholar]
- 7.Donatien, A., and F. Lestoquard. 1936. Rickettsia bovis, novelle espece pathogene pour le boeuf. Bull. Soc. Pathol. Exot. 29:1057-1061. [Google Scholar]
- 8.Ewing, S. A., J. E. Dawson, A. A. Kocan, R. W. Barker, C. K. Warner, R. J. Panciera, J. C. Fox, K. M. Kocan, and E. F. Blouin. 1995. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 32(3):368-374. [DOI] [PubMed] [Google Scholar]
- 9.Godin, A. J. 1977. Wild mammals of New England. Johns Hopkins University Press, Baltimore, Md.
- 10.Goethert, H. K. 2002. Ph.D. thesis. Harvard School of Public Health, Boston, Mass.
- 11.Inokuma, H., P. Brouqui, M. Drancourt, and D. Raoult. 2001. Citrate synthase gene sequence: a new tool for phylogenetic analysis and identification of Ehrlichia. J. Clin. Microbiol. 39:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Arizona State University, Tempe. [DOI] [PubMed]
- 13.Lockhart, J. M., W. R. Davidson, J. E. Dawson, and D. E. Stallknecht. 1995. Temporal association of Amblyomma americanum with the presence of Ehrlichia chaffeensis reactive antibodies in white-tailed deer. J. Wildl. Dis. 31:119-124. [DOI] [PubMed] [Google Scholar]
- 14.Neitz, W. O. 1968. Ehrlichia ovina infection. Bull. Off. Int. Epizoot. 70:337-340. [PubMed] [Google Scholar]
- 15.Sreekumar, C., R. Anandan, S. Balasundaram, and L. John. 2000. Detection of an Ehrlichia bovis-like organism in cultured buffalo monocytes. Trop. Anim. Health Prod. 32:67-72. [DOI] [PubMed] [Google Scholar]
- 16.Stewart, C. G. 1992. Bovine ehrlichiosis, p. 101-107. In B. Fifaz, T. Petney, and I. Horzk (ed.), Tick vector biology: medical and veterinary aspects. Springer-Verlag, Berlin, Germany.
- 17.Swofford, D. 1998. Phylogenetic analysis using parsimony, paup4d61.ppc.ed. Smithsonian Institution, Washington. D.C.
- 18.Uilenberg, G. 1997. General review of tick-borne diseases of sheep and goats world-wide. Parasitologia. 39:161-165. [PubMed] [Google Scholar]
- 19.Uilenberg, G. 1993. Other ehrlichioses of ruminants, p. 269-279. In Z. Woldenhiwet and M. Ristic (ed.), Rickettsial and chlamydial diseases of domestic animals. Pergamon Press, Elmsford, N.Y.
- 20.Yu, X. J., X. F. Zhang, J. W. McBride, Y. Zhang, and D. H. Walker. 2001. Phylogenetic relationships of Anaplasma marginale and ‘Ehrlichia platys’ to other Ehrlichia species determined by GroEL amino acid sequences. Int. J. Syst. E vol. Microbiol. 51:1143-1146. [DOI] [PubMed] [Google Scholar]