Abstract
This report describes the preparation of polyacrylate nanoparticles in which an N-thiolated β-lactam antibiotic is covalently conjugated onto the polymer framework. These nanoparticles are formed in water by emulsion polymerization of an acrylated antibiotic pre-dissolved in a liquid acrylate monomer (or mixture of co-monomers) in the presence of sodium dodecyl sulfate as a surfactant and potassium persulfate as a radical initiator. Dynamic light scattering analysis and electron microscopy images of these emulsions show that the nanoparticles are approximately 40 nm in diameter. The emulsions have potent in vitro antibacterial properties against methicillin-resistant Staphylococcus aureus and have improved bioactivity relative to the non-polymerized form of the antibiotic. A unique feature of this methodology is the ability to incorporate water-insoluble drugs directly into the nanoparticle framework without the need for post-synthetic modification. Additionally, the antibiotic properties of the nanoparticles can be modulated by changing the length or location of the acrylate linker on the drug monomer.
The development of antibiotics for control of pathogenic bacteria has been of pressing need in this era of drug-resistant infections.1 N-Methylthiolated β-lactams have recently been identified in our laboratory as a new family of antibacterial agents active against Staphylococcus bacteria, including methicillin-resistant Staphylococcus aureus (MRSA).2 The compounds have also displayed promising anticancer properties.3 Our recent studies have suggested that these lactams exert their growth inhibitory effects on bacteria through a mode of action that is distinctively different to that of other β-lactam antibiotics, and possess structure-activity patterns unlike those already mapped for other β-lactam antibacterials such as the penicillins. One of the major limitations in the potential application of these N-thiolated β-lactam compounds, however, is their exceedingly low water solubility.4 Thus, we were interested in identifying an effective drug delivery platform that would enhance the water solubility of the lactams, without sacrificing inherent bioactivity.
Drug delivery vehicles such as liposomes and gold nanoparticles have been developed to improve bioavailability, efficacy, and specificity of pharmaceutical compounds, particularly for anticancer agents, but nanoparticles have received surprisingly little attention in the antibiotic and infectious disease area.5,6 Some of the few notable examples have included antibiotic-encapsulated polymeric nanoparticles and liposomes,7,8 biodegradable nanospheres,9,10 and surface-coated gold and silver nanoparticles.11-13 This report describes the development of antibacterial polyacrylate nanoparticles based on well-precedented emulsion polymerization procedures.
Scheme I.
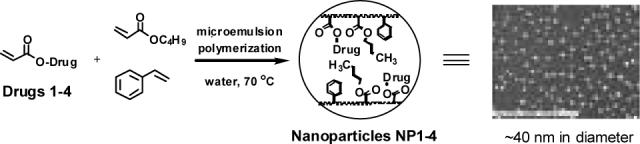
In this process, the antibacterial drug is first converted to an acrylated derivative and then dissolved to homogeneity in a liquid acrylate monomer (or mixture of compatible liquid monomers) at 70°C. This mixture is then pre-emulsified in purified water containing 3% w/w sodium dodecyl sulfate with rapid stirring (Scheme 1). The resulting homogenous solution of micelles is then treated with potassium persulfate (1% w/w), a water-soluble radical initiator, to induce free radical polymerization.14 The resulting emulsion is found to contain uniformly-sized polyacrylate nanoparticles in which the drug monomer is covalently incorporated directly into the polymeric matrix of the nanoparticle. A unique feature of this methodology is that the nanoparticle emulsion containing the antibiotic agent is built from its monomer constituents in one step, without the need for further chemical modification or derivatization. Furthermore, the nanoparticles display potent antibacterial activity against MRSA.
For these studies, four N-thiolated β-lactam derivatives were selected for use as antibiotic drug monomers in order to assess the effect of different lengths and locations of the acrylate linker on the resulting nanoparticle size and anti-MRSA bioactivity (Fig. 1).
Figure 1.
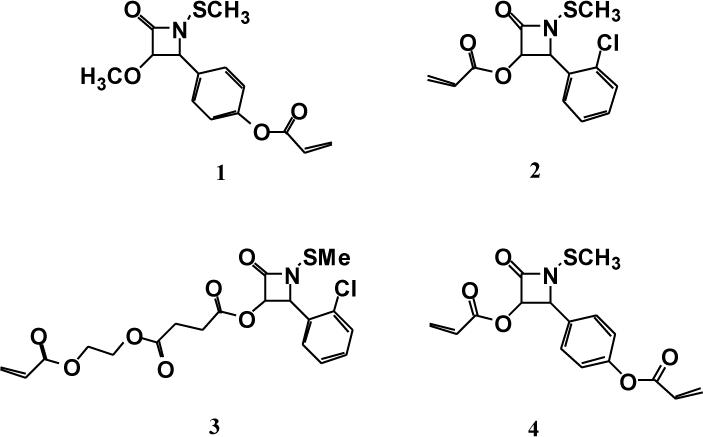
N-Thiolated β-lactam acrylate monomers 1-4.
Lactam 1 was prepared as previously reported. 3 Compound 2 was synthesized in three steps from hydroxy β-lactam 5 by O-acrylation and oxidative deprotection followed by N-methylthiolation of the lactam (Scheme II).
Scheme II.
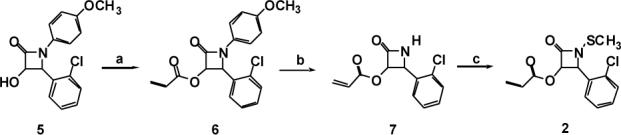
Conditions: a) acryloyl chloride, NaH, CH2Cl2, 85%; b) ceric ammonium nitrate, CH3CN, H2O, 0 °C, 90%; c) N-methylthiophthalimide, CH2Cl2, Hunig's base, reflux, 82%.
Compound 3 was prepared in three steps from 3-hydroxy β-lactam 5 by carbodiimide coupling of the alcohol moiety with mono-2-acryloyloxyethyl succinate, then N-aryl deprotection and N-methylthiolation (Scheme III).
Scheme III.
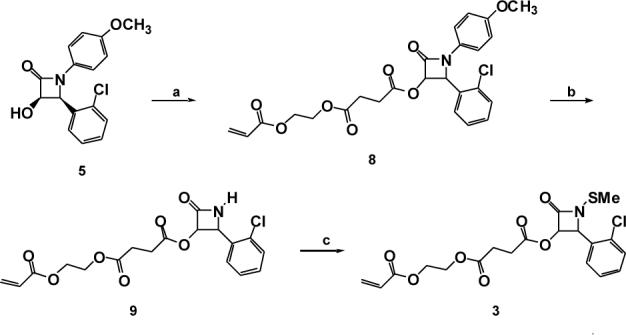
Conditions: a) mono-2-acryloyloxyethyl succinate, EDCI, DMAP, CH2Cl2, r.t., 77%; b) ceric ammonium nitrate, H2O, CH3CN, 0 °C, 86%; c) N-methylthio phthalimide, CH2Cl2, Hunig's base, reflux, 72%.
Bis-acrylated compound 4 was synthesized starting with p-hydroxybenzaldehyde and p-anisidine, as shown in Scheme IV.
Scheme IV.
Conditions: a) CSA, CH2Cl2, rt; b) acetyl chloride, Et3N, CH2Cl2, 92% overall; c) acetoxyacetyl chloride, Et3N, CH2Cl2, 65%; d) KOH, MeOH, acetone, 0°C, 92%; e) acryloyl chloride, Et3N, CH2Cl2, 93%; f) ceric ammonium nitrate, CH3CN, H2O, 0°C, 84%; g) N-methylthiophthalimide, Hunig's base, CH2Cl2 , reflux, 72%.2
Initially, N-methylthio β-lactam monomer 1 was subjected to emulsion polymerization conditions using ethyl acrylate as a liquid acrylate monomer, which gave the desired emulsion. However, upon prolonged storage, this emulsion was found to be sensitive to aggregation and precipitation. Consequently, we examined other combinations of acrylate monomers and found that a mixture of butyl acrylate and styrene (in a 7:3 ratio by weight) afforded the most stable emulsions. In each case, these emulsions contained the polyacrylate (20% by weight) comprised of the bulk monomers and one of the acrylate lactams 1-4 (up to 5% by weight). Purification of the emulsion was performed by continuous extraction using ethyl ether followed by centrifugation on a low-speed benchtop centrifuge. The purification enabled minor impurities such as excess surfactant or residual monomers to be removed without altering the physical characteristics or the antibacterial activity of the nanoparticles in the emulsion. Analysis of the emulsions of nanoparticles NP0-4 by scanning electron microscopy (SEM), transmission electron microscopy (TEM), dynamic light scattering (DLS), and atomic force microscopy (AFM) indicated the formation of spherical nanoparticles having a uniform diameter of 30 to 50 nm (Fig. 2). The zeta potential of the emulsified nanoparticle solutions registered between −50 and −70 mV, reflecting a high anionic charge on the nanoparticle surface and high relative particle stability. Proton nuclear magnetic resonance (NMR) analysis of the thin film produced by evaporation of the nanoparticle emulsions confirmed that essentially all of the butyl acrylate, styrene, and beta-lactam components were incorporated into the skeletal framework of the polymer during the polymerization.15
Figure 2.
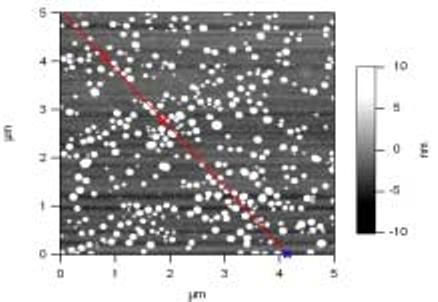
Atomic force microscopy (AFM) image of NP1 indicates that the nanoparticles are uniformly spherical and between 30-50 nm in diameter.
The emulsions of nanoparticles NP0-4 were evaluated for antibacterial activity against S. aureus and MRSA by determining the minimum inhibition concentration (MIC) values in agar. The monomeric acrylates of these compounds (1-4) were also examined for comparison. The MIC values shown in Table 1 represent the lowest concentration of drug (in μg/mL) needed to completely inhibit bacterial proliferation. The data revealed promising MIC's for the drug-conjugated emulsions against both strains of S. aureus, but not for the control (non-drug-conjugated) nanoparticles (NP0). Thus, only the emulsified antibiotic-conjugated nanoparticles (NP1-4) were bioactive while the non-antibiotic-bound nanoparticles, NP0, were completely devoid of antimicrobial activity.
Table 1.
Agar dilution MIC values of nanoparticle emulsions NP0-4 (1% w/w drug in the emulsion) and beta-lactam monomers 1-4 against S. aureus (ATCC 25923) and MRSA (ATCC 43300).
| Sample | S. aureus | MRSA |
|---|---|---|
| Nanoparticles | ||
| NP0 | >256 | >256 |
| NP1 | 32 | 32 |
| NP2 | 16 | 16 |
| NP3 | 8 | 8 |
| NP4 | 16 | 16 |
| Lactam monomers | ||
| 1 | 128 | 128 |
| 2 | 128 | 128 |
| 3 | 64 | 64 |
| 4 | 128 | 128 |
MIC values refer to the lowest concentration of bound drug (μg/mL) needed to completely inhibit bacterial growth for 24 hours. MIC determinations were run in triplicate and the values obtained for each compound were invariant.
The MIC data in Table 1 also indicates that the antibiotic-conjugated polyacrylate nanoparticles NP1-4 had enhanced (four- to eight-fold) anti-MRSA activity compared to their acrylated drug monomers 1-4. This heightened activity may be indicative of an increased bioavailability (membrane permeability or enhanced local concentration) of the antibiotic agent as a consequence of the nanoparticle serving as a delivery vehicle. Furthermore, antibacterial activity of the nanoparticles can be altered by changing the location or the length of the acrylate linker. Thus, positioning the acrylate functionality on the C3 center of the lactam monomer, as shown for monomer 2, rather than on the C4 aryl moiety (lactam 1), produced nanoparticles (NP2) that were two-fold more active against S. aureus. This activity was further enhanced by extending the length of the C3 acrylate tether, as shown for NP3. The increase in biological activity coincided with the relative levels of bioactivity of the two monomers. Also, NP4, prepared from di-acrylated lactam monomer 4, showed the same anti-MRSA capabilities as NP2, which was prepared from the monoacrylated monomer 2.16 This indicates that the presence of two acrylate functionalities on the drug molecule versus only one does not perturb the biological function or the physical properties of the resulting nanoparticle.
In vitro screens determined that these polyacrylate nanoparticles are non-toxic toward human dermal fibroblasts, augmenting the known favorable characteristics and biocompatibilities of polyacrylate biomaterials.14 Control experiments indicated that the nanoparticle emulsions were completely stable for at least 24 hours at elevated temperatures (up to 60°C) and in blood serum, as determined by dynamic light scattering and antibacterial screening (MIC analysis).17
In conclusion, these polyacrylate nanoparticle antibiotics, or nanobiotics, appear to be an effective enhancer of activity for water-insoluble antibiotics such as N-methylthio β-lactams. This study revealed several essential features of polyacrylate nanoparticles that further illustrate their potential advantages as drug delivery platforms: 1) easy, one-step preparation of antibiotic-conjugated polyacrylate frameworks in aqueous media; 2) facile control of nanoparticle size in the 30-50 nm range; 3) ability to incorporate water-insoluble drugs directly onto the polymer framework without the need for post-synthetic modification of the nanoparticle; 4) enhanced anti-MRSA activity of the nanoparticle polymers versus the nonpolymerized form of the drug; and 5) ability to alter bioactivity of the nanoparticle antibiotic by changing the location or length of the acrylate linker. Ongoing studies in our laboratory are focused on the synthesis and evaluation of nanoparticles for other drug classes and polymer compositions, and on full characterization of their in vitro and in vivo properties.
Supplementary Material
Acknowledgements
We thank Dr. Thomas Koob (Shriners Hospital, Tampa) for assistance with cytotoxicity experiments, Gil Brubaker (University of Florida Particle Engineering Research Center) for helping with particle analyses, August Heim for AFM analysis and Betty Loraam for help with TEM imaging. Financial support from the National Institutes of Health (AI05135) is gratefully acknowledged.
Footnotes
Supporting Information Available: Synthetic procedures, physical characterization, antibacterial and cytotoxicity data for lactams 1-4 and nanoparticles NP0-4.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Zafeiris S. Nature. 2004;431:892. [Google Scholar]
- 2.Turos E, Konaklieva MI, Ren RXF, Shi H, Gonzalez J, Dickey S, Lim D. Tetrahedron. 2000;56:5571. [Google Scholar]
- 3.a) Smith DM, Kazi A, Smith L, Long TE, Heldreth B, Turos E, Dou PQ. Molecular Pharmacology. 2002;61:1348. doi: 10.1124/mol.61.6.1348. [DOI] [PubMed] [Google Scholar]; b) Kazi A, Hill R, Long TE, Kuhn DJ, Turos E, Dou PQ. Biochem. Pharmacol. 2004;67:365. doi: 10.1016/j.bcp.2003.09.017. [DOI] [PubMed] [Google Scholar]; c) Kuhn DJ, Yang Y, Minic V, Coates C, Reddy GSK, Shim JY, Chen Di, Piwowar KRL, Miller FR, Turos E, Dou QP. Frontiers in Bioscience. 2005;10:1183. doi: 10.2741/1611. [DOI] [PubMed] [Google Scholar]
- 4.Patel VR, Amiji MM. Pharm. Res. 1996;13:588. doi: 10.1023/a:1016054306763. [DOI] [PubMed] [Google Scholar]
- 5.Alle'mann E, Gurny R, Doelker E. Eur. J. Pharm. Biopharm. 1993;39:173. [Google Scholar]
- 6.Couvreur P, Dubernet C, Puisieux F. Eur. J. Pharm. Biopharm. 1995;41:2. [Google Scholar]
- 7.Couvreur P, Kante B, Roland M, Guiot P, Baudhuin P, Speiser P. J. Pharm. Pharmacol. 1979;31:331. doi: 10.1111/j.2042-7158.1979.tb13510.x. [DOI] [PubMed] [Google Scholar]
- 8.Cavallaro G, Fresta M, Giammona G, Puglisi G, Villari A. Int. J. Pharm. 1994;111:31. [Google Scholar]
- 9.Dillen K, Weyenberg W, Vandervoort J, Ludwig A. Eur. J. Pharm. Biopharm. 2004;58:539. doi: 10.1016/j.ejpb.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Santos-Magalhaes NS, Pontes A, Pereira VMW, Caetano MNP. Int. J. Pharm. 2000;208:71. doi: 10.1016/s0378-5173(00)00546-9. [DOI] [PubMed] [Google Scholar]
- 11.Gu H, Ho PL, Tong E, Wang L, Xu B. Nano. Lett. 2003;3:1261. [Google Scholar]
- 12.Renjis T, Tom V, Suryanarayanan P, Reddy G, Baskaran S, Pradeep T. Langmuir. 2004;20:1909. doi: 10.1021/la0358567. [DOI] [PubMed] [Google Scholar]
- 13.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ. Nanotechnology. 2005;16:2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 14.Odian G. Principles of Polymerization. 3rd Ed. John Wiley and Sons, Inc.; New York: 1991. [Google Scholar]
- 15. Control experiments indicate that less than 2% of residual unreacted acrylate was entrapped in the nanoparticle (see Supporting Information). Moreover, when additional lactam acrylate was added to the emulsion prior to evaporation and thin film formation, none of the monomer was incorporated into the polymer matrix and was able to be recovered quantitatively by extraction of the film with ethyl ether. This demonstrates that all of the acrylated lactam monomer was incorporated covalently into the nanoparticle matrix during emulsion polymerization while creating the nanoparticle, and not the result of further polymerization during the process of evaporation for NMR analysis.
- 16. These results suggest that these polyacrylate nanoparticles are highly crosslinked and that the use of a doubly acrylated co-monomer such as bis-acrylate 4 does not alter size, shape, or bioactivity of the nanoparticle. Polyacrylates made by emulsion polymerization are known to possess extremely high molecular weight as a result of crosslinking via radical backbiting during the polymerization process. See the discussions in reference 14.
- 17. See Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


