Abstract
Background
In spite of using heparin-coated extracorporeal circuits, cardiopulmonary bypass (CPB) is still associated with an extensive thrombin generation, which is only partially suppressed by the use of high dosages of heparin. Recent studies have focused on the origins of this thrombotic stimulus and the possible role of retransfused suctioned blood from the thoracic cavities on the activation of the extrinsic coagulation pathway. The present study was designed to find during CPB an association between retransfusion of suctioned blood from the pericardium and pleural space, containing activated factor VIIa and systemic thrombin generation.
Methods
Blood samples taken from 12 consenting patients who had elective cardiac surgery were assayed for plasma factor VIIa, prothrombin fragment 1+2 (F1+2), and thrombin-antithrombin (TAT) concentrations. Blood aspirated from the pericardium and pleural space was collected separately, assayed for F1+2, TAT, and factor VIIa and retransfused to the patient after the aorta occlusion.
Results
After systemic heparinization and during CPB thrombin generation was minimal, as indicated by the lower than base line plasma levels of F1+2, and TAT after correction for hemodilution. In contrast, blood aspirated from the thoracic cavities had significantly higher levels of factor VIIa, F1+2, and TAT compared to the simultaneous samples from the blood circulation (P < 0.05). Furthermore, after retransfusion of the suctioned blood (range, 200–1600 mL) circulating levels of F1+2, and TAT rose significantly from 1.6 to 2.9 nmol/L (P = 0.002) and from 5.1 to 37.5 μg/L (P = 0.01), respectively. The increase in both F1+2, and TAT levels correlated significantly with the amount of retransfused suctioned blood (r = 0.68, P = 0.021 and r = 0.90, P = 0.001, respectively). However, the circulating factor VIIa levels did not correlate with TAT and F1+2 levels.
Conclusions
These data suggest that blood aspirated from the thoracic cavities during CPB is highly thrombogenic. Retransfusion of this blood may, therefore, promote further systemic thrombin generation during CPB.
Background
During CPB thrombin generation is extensive [1,2], therefore heparin is administered at high concentrations. The origins of this thrombotic stimulus have been uncertain and subject to speculation. It was reasoned that the contact of the blood with the extracorporeal circuit, via the intrinsic coagulation pathway, was the main contributor to the increased thrombin generation [3]. Elevated levels of activated coagulation factor XIIa during CPB supported this theory [4]. It is, however, doubtful that the contact activation pathway is the main source of the thrombin generation. First, Lane and coworkers [5-7] found that tissue factor (TF) is most likely the sole trigger of the coagulation process during CPB. Second, Burman and colleagues [8] found a sharp increase in thrombin generation in a patient with factor XII deficiency during closure of an atrioseptal defect without a significant change in factor IX activation. Thus, factor XII activation is not always indispensable for thrombin generation during CPB.
Recent improvements in biocompatibility of the extracorporeal circuit, such as heparin coatings, have resulted in considerably less blood activation [9-13]. Despite these improvements, however, we and also other investigators reported no reduction in thrombin generation in patients undergoing CPB with the use of a heparin-coated extracorporeal circuit [14-17], whereas others have found evidence of some possible benefit of these surfaces on the coagulation cascade [18].
Several investigators [19-22] have suggested that retransfused suctioned blood from the pericardial cavity could be the major source of a thrombin-generating agent. More recently, Philippou et al. [23] demonstrated that pericardium-induced activation of factor VII, due to ineffective local heparinization, resulted in an increased thrombin generation.
Thus, whereas previous studies suggested that blood is activated predominantly by contact activation, more recent studies indicate that the contribution of the material-independent pathway of blood activation -the surgical wound itself- may be of much greater importance than previously thought. The aim of this clinical study was to elucidate the impact of retransfused suctioned blood from the thoracic cavities on systemic TF-driven thrombin generation.
Methods
Patients
Twelve adult patients, subsequently undergoing elective combined heart valve surgery and coronary artery bypass grafting were enrolled in this study. Informed consent was obtained from each patient the day before the operation. The study was approved by the local ethical and research council. No patient had evidence of severe heart failure, renal or hepatic dysfunction, or preoperative coagulopathies. Moreover, no patient was treated with coumarin derivates, platelet-inhibiting drugs, or nonsteroidal anti-inflammatory agents within five days before the operation. The study patients did not receive antifibrinolytic agents during CPB.
Anesthesia and cardiopulmonary bypass
Anesthesia was induced and maintained with weight-related doses of fentanyl, sufentanil, midazolam, and pancuronium. All patients had Swan-Ganz and arterial catheters placed.
The extracorporeal circuit consisted of heparin-coated tubing (Duraflo II, Baxter Bentley Inc., Irvine, CA, USA), a hollow fiber membrane oxygenator (Capiox SX-18, Terumo Corporation, Tokyo, Japan), a heparin-coated venous reservoir (Duraflo II, BMR-1900, Baxter Bentley), an arterial line filter (Pall autovent SV, Pall Biomedical Ltd, Portsmouth, UK), and two biothyl-coated cardiotomy reservoirs (William Harvey H4700, C.R. Bard Inc., Tewsbury, MA, USA). Before connection of the extracorporeal circuit for CPB, each patient received 300 IU/kg heparin (Heparin Leo, Leo Pharmaceutical Products BV, Weesp, The Netherlands) to achieve an activated coagulation time (ACT) > 480 seconds (Hemotec ACT II, Medtronic Inc., Anaheim, CA, USA). An ACT was measured at baseline, after heparinization, and every 30 minutes during CPB. If necessary, additional boluses of heparin were administered to maintain an ACT > 400 seconds. After institution of cardiopulmonary bypass at a flow rate of 2.4 L/min/m2, and after reaching a blood temperature below 28°C (25–28°C), the heart was topically cooled till fibrillation using cold saline 0.9% at 4°C. The aorta was then crossclamped and a single dose of approximately 800 ml (600–1000 ml) of St. Thomas I cardioplegic solution at 4°C was infused into the aortic root to provide myocardial preservation. Topic cooling was maintained during the infusion of the cardioplegic solution. Pulsatile perfusion was used throughout the period of aorta crossclamping. During the conduct of CPB, aortic root venting was separated from the cardiotomy suction and in all cases less than 2% of the calculated flow (< 90 ml/min), using a volume pressure control unit with negative pressures less than 50 mmHg. Cardiotomy suction, i.e. blood aspirated from the pericardium and pleural space, was returned to a separated cardiotomy reservoir that was clamped off. After completion of all the distal anastomoses the cardiotomy reservoir clamp was removed, allowing reinfusion of blood from the pericardium and pleural space into the systemic circulation. After CPB, heparin was reversed by 3 mg/kg protamine chloride (Hoffman/Laroche BV, Mijndrecht, The Netherlands). All pump blood was returned to the patient through the aortic cannula or intravenously via infusion bags without cell washing process or hemoconcentration.
Blood sampling
During and after the operation, blood samples were collected from each patient at seven specific intervals: before heparin administration (time point 1), 5 minutes after heparinization before CPB (time point 2), 5 minutes after the beginning of CPB (time point 3), 5 minutes after release of the aortic crossclamp (time point 4), 5 minutes after reinfusion of cardiotomy blood (time point 5), 15 minutes after protamine administration (time point 6), and 2 hours after surgery (time point 7). Additional samples were taken from the pooled blood recollected by suction from the pericardium and pleural space into a cardiotomy reservoir 5 minutes after the beginning of CPB and 5 minutes after aorta occlusion, before retransfusion.
Laboratory assays
Blood samples were collected in 3.5% sodium citrate (9:1 vol/vol). Platelet-poor plasma for determination of TAT-complexes, F1+2, and factor VIIa was obtained by centrifugation at 4000 g for five minutes, and the supernatant was centrifuged at 12000 g for ten minutes and stored at -70°C until assayed. All samples were corrected for hemodilution.
TAT and F1+2 levels were determined by enzyme-linked immunosorbent assay (Dade-Behring GmbH, Schwalbach, Germany). Factor VIIa levels were determined by a specific immunoassay, which has been described elsewhere [23]. In short, flat-bottomed Maxisorb microtiter plates (Nunc, Roskilde, Denmark) were coated for 2 hours at room temperature or overnight at 4°C with 100 μL coating buffer containing 5 μg/mL of the rabbit polyclonal antibody RB-23, raised to a region of factor VII exposed following its activation by cleavage at Arg152-Ile153. Thereafter, the wells were blocked during 30 minutes at room temperature with 200 μL blocking buffer and then rinsed 5 times with rinse buffer. The wells were incubated for 2 hours at room temperature with 100 μL of a factor VIIa containing sample and then 6 times rinsed with the rinse solution. Finally, the amount of factor VIIa captured by the rabbit anti-human factor VIIa antibody RB-23 was quantified as follows: 100 μL of a solution containing 1:100 diluted thromboplastin (Dade Innovin, Baxter Diagnostics, Deerfield, IL), 10 μmol/L phospholipid (20 mol% phosphatidylserine and 80 mol% phosphatidylcholine), 5 mmol/L Ca2+ and 250 nmol/L bovine factor X [24] in Hepes buffer was added to the well and incubated at 37°C. The activation of factor X was stopped after 30 minutes by the addition of 35 μL Hepes-buffer containing 20 mM EDTA. The amount of formed factor Xa was measured using the chromogenic substrate S2765 as described previously [24]. A calibration curve was constructed with known concentrations of recombinant factor VIIa (Novo Nordisk Pharma, Copenhagen, Denmark).
Statistical analysis
The results were expressed as the mean ± standard error of the mean (SEM), except for the patients' demographics, which was expressed as mean ± standard deviation. Simultaneous samples of the cardiotomy blood and the systemic circulation were compared by the paired Student's t test. This statistic was also used to compare samples during and after CPB with the sample taken before CPB and after heparin administration. Spearman's rank correlation analysis was used to measure the relationship between thrombin generation and the amount of retransfused cardiotomy blood and circulating factor VIIa levels, respectively. A p value less than 0.05 was considered to be significant.
Results
Patients' demographics
The patients (9 men and 3 women) ranged in age from 50 to 76 years, and from 52 to 85 kg in weight. Table 1 gives descriptive data for the patients, operation, and perfusion. No patients developed bleeding complications; no patients died.
Table 1.
Patients' demographics Data are presented as mean ± standard deviation.
| Gender, M / F | 9 / 3 |
| Age, years | 68 ± 8 |
| Height, cm | 170 ± 8 |
| Weight, kg | 75 ± 12 |
| Aortic crossclamp time, min (range) | 66 ± 42 (21–141) |
| Bypass time, min (range) | 98 ± 54 (41–198) |
| Total amount retransfused cardiotomy blood, mL (range) | 617 ± 480 (200 – 1600) |
| Myocardial revascularization | 8 |
| Valve replacement | 2 |
| Valve replacement/repair and myocardial revascularization | 2 |
Factor VIIa
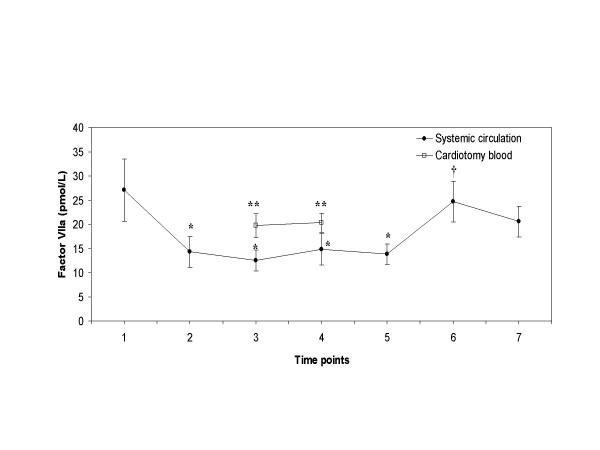
Plasma levels of factor VIIa decreased significantly during bypass from 27.1 to 14.8 ρmol/L (Figure 1); the decreases were significant after heparin infusion (between time points 2 and 4). The mean factor VIIa levels from the cardiotomy suction fluid were significantly higher than the levels measured in the systemic circulation at the same sampling points (time points 3 and 4) during CPB (19.7 and 20.3 ρmol/L versus 12.5 and 14.8 ρmol/L; P = 0.01 for both, respectively). After retransfusion of the cardiotomy blood, surprisingly, no increase in factor VIIa levels was observed in the circulation. Hence, after protamine administration an increase in circulating levels of factor VIIa was noticed.
Figure 1.
Plasma factor VIIa levels Plasma factor VIIa levels with time in patients undergoing CPB (●), and in the cardiotomy blood (□). Blood samples were taken at the various time points as described in the "Methods" section. Data are presented as mean ± SEM. Asterisks indicate a statistically significant difference compared with the sample before heparin administration (P < 0.05 by paired Student's t test); double asterisk indicate a statistically significant difference between the simultaneous samples of the cardiotomy blood and the systemic circulation (P < 0.05 by paired Student's t test); cross signs indicate a statistically significant difference compared with the sample before retransfusion of the cardiotomy blood (P < 0.05 by paired Student's t test).
Prothrombin fragment 1+2
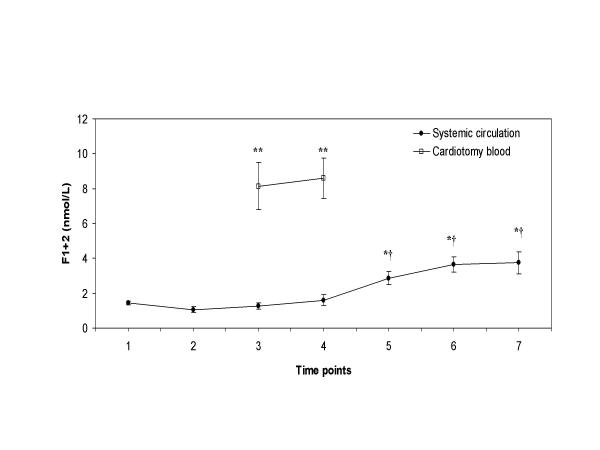
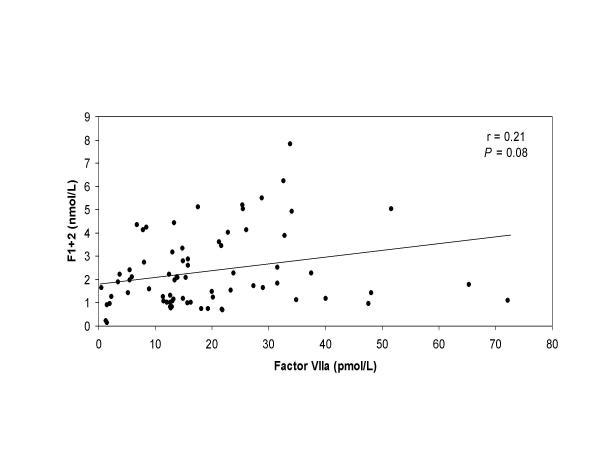
The marker of thrombin generation, F1+2, decreased after heparin infusion and after start of CPB (Figure 2). The mean plasma level of F1+2 measured in the cardiotomy blood was significantly higher (≈ 6-fold increase) than the levels measured at the same time points in the systemic circulation (8.2 and 8.6 nmol/L versus 1.3 and 1.6 nmol/L; P < 0.001 for both, respectively). After retransfusion of the cardiotomy blood, a dramatic and significant increase was observed in the F1+2 levels from the systemic circulation until 2 hours after the operation (from 1.6 nmol/L to 3.8 nmol/L, P = 0.002). In addition, a significant correlation was measured between the amount of retransfused cardiotomy blood and the rise of plasma F1+2 levels in the systemic circulation (r = 0.68, P = 0.021). No correlation was detected between the circulating F1+2 levels and factor VIIa levels, Figure 3 (r = 0.21, P = 0.08).
Figure 2.
Plasma levels of prothrombin fragment 1+2 Plasma levels of F1+2 with time in patients undergoing CPB (●), and in the cardiotomy blood (□). Blood samples were taken at the various time points as described in the "Methods" section. Data are presented as mean ± SEM. Asterisks indicate a statistically significant difference compared with the sample before heparin administration (P < 0.05 by paired Student's t test); double asterisk indicate a statistically significant difference between the simultaneous samples of the cardiotomy blood and the systemic circulation (P < 0.001 by paired Student's t test); cross signs indicate a statistically significant difference compared with the sample before retransfusion of the cardiotomy blood (P < 0.05 by paired Student's t test).
Figure 3.
Correlation between factor VIIa and F1+2 Correlation between circulating factor VIIa levels and F1+2 levels in patients undergoing CPB.
Thrombin-antithrombin
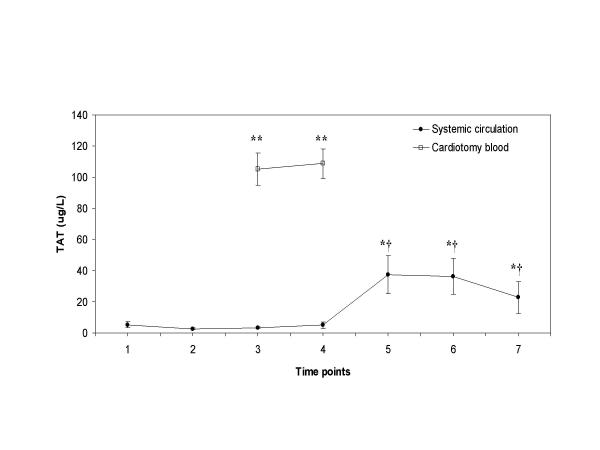
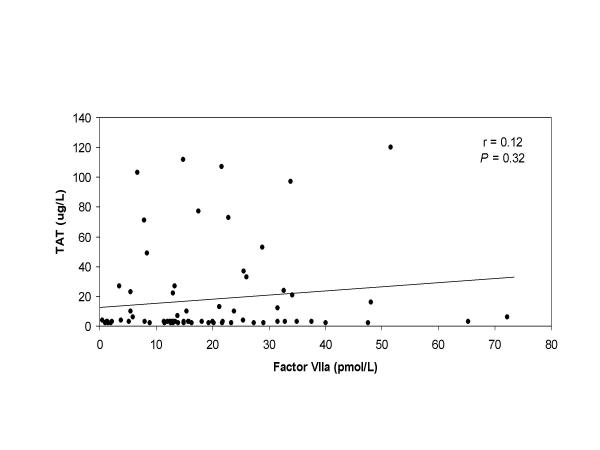
Plasma concentrations of TAT, a marker of the amount of thrombin that is generated, decreased slightly after heparin infusion and up to the phase of retransfusion of the cardiotomy blood (Figure 4). The mean plasma concentration of TAT measured in the cardiotomy blood was significantly higher (>20-fold increase) than the levels measured at the same time points in the systemic circulation (105.2 and 108.8 μg/L versus 3.5 and 5.1 μg/L; P < 0.001 for both, respectively). After retransfusion of the cardiotomy blood, plasma TAT concentrations rose significantly in the systemic circulation (from 5.1 μg/L to 37.5 μg/L, P = 0.01). Thereafter, the plasma TAT levels remained significantly elevated until 2 hours after the operation (P < 0.05). Moreover, close correlation was measured between the amount of retransfused cardiotomy blood and the rise of plasma TAT levels in the systemic circulation (r = 0.90, P = 0.001). No correlation was detected between the circulating TAT levels and factor VIIa levels, Figure 5 (r = 0.12, P = 0.32).
Figure 4.
Thrombin-antithrombin plasma concentrations TAT plasma concentrations with time in patients undergoing CPB (●), and in the cardiotomy blood (□). Blood samples were taken at the various time points as described in the "Methods" section. Data are presented as mean ± SEM. Asterisks indicate a statistically significant difference compared with the sample before heparin administration (P < 0.05 by paired Student's t test); double asterisk indicate a statistically significant difference between the simultaneous samples of the cardiotomy blood and the systemic circulation (P < 0.001 by paired Student's t test); cross signs indicate a statistically significant difference compared with the sample before retransfusion of the cardiotomy blood (P < 0.05 by paired Student's t test).
Figure 5.
Correlation between factor VIIa and TAT Correlation between circulating factor VIIa levels and TAT levels in patients undergoing CPB.
Discussion
To diminish allogeneic blood transfusions and volume loss in the extracorporeal circulation, retransfusion of suctioned blood from the thoracic cavities during CPB is considered a salutary technique. However, recent reports have highlighted the quality of this blood and its relative role on the activation of the extrinsic coagulation pathway [23,25,26]. In the present study we found evidence that blood aspirated from the pericardium and pleural space is highly thrombogenic, because of an excellent correlation between the amount of retransfused blood and the increased levels of TAT and F1+2. The origin of ongoing thrombin generation is most likely caused by the presence of TF rather than factor VIIa in the blood aspirated from the pericardium and pleural space [27].
Triggering of the in vivo coagulation is thought to require complex formation between TF and factor VIIa to form an activation complex that converts factor IX and X into the serine proteases factor IXa and Xa, respectively. Factor Xa is the enzyme that ultimately generates thrombin [28]. Chung and associates [21] showed that blood mononuclear cells taken from the pericardium express twice the level of TF compared with cells taken simultaneously from the systemic circulation. In addition, in vivo thrombin generation is at least partly mediated by cell-derived microparticles containing TF [29]. In this study we found that blood aspirated from the thoracic cavities had significantly higher levels of factor VIIa compared to the simultaneous samples from the systemic circulation. Because TF is an essential cofactor for factor VIIa generation [28], high factor VIIa levels in blood aspirated from the pericardium and pleura space reflects the presence of significant amounts of TF.
Accordingly to the presence of TF in the blood aspirated from the thoracic cavities, corresponding elevations in markers of direct thrombin generation were observed. In addition, this study is the first to provide direct evidence that the amount of retransfused cardiotomy blood volume contributes to the level of thrombin generation during CPB. Close correlation was established between the amount of retransfused cardiotomy blood and the rise of plasma TAT levels and F1+2 levels in the systemic circulation. Retransfusion of an amount as minimal as 200 mL cardiotomy blood already resulted in a systemic increase of 8% in plasma TAT concentrations and 0.4% in plasma F1+2 concentrations. These results confirm the suggestions and findings of others that the general practice of retransfusion of cardiotomy blood is a potential source of activated hemostatic components [20-23,25,26]. A possible explanation for the high activation marker levels in the blood aspirated from the thoracic cavities might be poor local anticoagulation. It has been reported that pericardial blood had lower levels of heparin than corresponding perfusate samples, and the levels of heparin in samples from the thoracic cavities correlated inversely with those of thrombin generation markers and factor VIIa [23]. Alternatively, it is tempting to speculate that the concentration of the natural inhibitor of the TF/factor VIIa complex, tissue factor pathway inhibitor (TFPI), in the suctioned blood is greatly reduced, which in turn greatly amplifies thrombin generation. Additional anticoagulation of the cardiotomy blood seems thus required. The notion that anticoagulation must be focused on inhibiting TF-driven blood coagulation is also supported by the observation that local administration of aprotinin, an inhibitor of the intrinsic blood coagulation, in the thoracic cavities did not prevent generation of highly procoagulant mediators in blood aspirated from these cavities [30].
Conclusions
In conclusion, we demonstrated that blood aspirated from the pericardium and pleural space is highly thrombogenic, and retransfusion of this blood may promote systemic thrombin generation during CPB. Therefore, as been pointed out by others [19,20,22,25,26], this blood should be discarded or processed with a cell salvage system prior to retransfusion.
Competing interests
None declared.
Authors' contributions
PW was responsible for the study design, performed the perfusions, analyzed and interpreted the data, and wrote the manuscript. TL participated in the design of the study, carried out the immunoassays, and reviewed the manuscript. NC contributed to the planning of study design, performed the perfusions, and aided with blood sampling. DJ participated in the design of the study and reviewed the manuscript. All authors read and approved the final manuscript.
Contributor Information
Patrick W Weerwind, Email: P.Weerwind@thorax.umcn.nl.
Theo Lindhout, Email: T.Lindhout@BIOCH.unimaas.nl.
Nicole EH Caberg, Email: nca@scpc.azm.nl.
Dick S de Jong, Email: ddj@scpc.azm.nl.
References
- Brister SJ, Ofosu FA, Buchanan MR. Thrombin generation during cardiac surgery: heparin the ideal anticoagulant? Thromb Haemost. 1993;70:259–262. [PubMed] [Google Scholar]
- Boisclair MD, Lane DA, Philippou H, Sheikh S, Hunt B. Thrombin production, inactivation and expression during open heart surgery measured by assays for activation fragments including a new ELISA for prothrombin fragment F1+2. Thromb Haemost. 1993;70:253–258. [PubMed] [Google Scholar]
- Wachtfogel WT, Harpel PC, Edmunds LH, Jr, Colman RW. Formation of C1s-C1-inhibitor, and plasmin-α2-plasmin inhibitor complexes during cardiopulmonary bypass. Blood. 1989;73:468–471. [PubMed] [Google Scholar]
- Irvine L, Sundaram S, Courtney JM, Taggart DP, Wheatley DJ, Lowe GDO. Monitoring of factor XII activity and granulocyte elastase release during cardiopulmonary bypass. ASAIO Trans. 1991;37:569–571. [PubMed] [Google Scholar]
- Boisclair MD, Lane DA, Philippou H, Esnouf MP, Sheikh S, Hunt B, Smith KJ. Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood. 1993;82:3350–3357. [PubMed] [Google Scholar]
- Boisclair MD, Philippou H, Lane DA. Thrombogenic mechanisms in the human: fresh insights obtained by immunodiagnostic studies of coagulation markers. Blood Coagul Fibrinolysis. 1993;4:1007–1021. [PubMed] [Google Scholar]
- Philippou H, Adami A, Boisclair MD, Lane DA. An ELISA for factor X activation peptide: application to the investigation of thrombogenesis in cardiopulmonary bypass. Br J Haematol. 1995;90:432–437. doi: 10.1111/j.1365-2141.1995.tb05170.x. [DOI] [PubMed] [Google Scholar]
- Burman JF, Chung HI, Lane DA, Philippou H, Adami A, Lincoln JCR. Role of factor XII in thrombin generation and fibrinolysis during cardiopulmonary bypass. Lancet. 1994;344:1192–1193. doi: 10.1016/S0140-6736(94)90509-6. [DOI] [PubMed] [Google Scholar]
- Weerwind PW, Maessen JG, van Tits LJH, Stad RK, Fransen EJ, de Jong DS, Penn OCKM. Influence of Duraflo II heparin-treated extracorporeal circuits on the systemic inflammatory response in patients having coronary bypass. J Thorac Cardiovasc Surg. 1995;110:1633–1641. doi: 10.1016/S0022-5223(95)70024-2. [DOI] [PubMed] [Google Scholar]
- Korn RL, Fisher CA, Livingston ER, Stenach N, Fishman SJ, Jeevanandam V, Addonizio VP. The effects of Carmeda bioactive surface on human blood components during simulated extracorporeal circulation. J Thorac Cardiovasc Surg. 1996;111:1073–1084. doi: 10.1016/s0022-5223(96)70384-7. [DOI] [PubMed] [Google Scholar]
- Yii M, Gourlay T, Fleming J, Matata B, Taylor KM. Evaluation of Carmeda bioactive surface (CBAS), Duraflo II and a novel nonspecific protease-modified surface using a new in vitro model simulating cardiopulmonary bypass. Perfusion. 1996;11:229–240. doi: 10.1177/026765919601100308. [DOI] [PubMed] [Google Scholar]
- Hsu LC. Biocompatibility in cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:376–382. doi: 10.1016/s1053-0770(97)90108-7. [DOI] [PubMed] [Google Scholar]
- Baksaas ST, Videm V, Pedersen T, Karlsen H, Mollnes TE, Brosstad F, Svennevig JL. Comparison of three oxygenator-coated and one total-circuit-coated extracorporeal devices. Perfusion. 1999;14:119–127. doi: 10.1177/026765919901400205. [DOI] [PubMed] [Google Scholar]
- Weerwind PW, Reutelingsperger CPM, Lindhout T, Hamulyak K, Schauwaert A, de Jong DS, Hemker HC, Penn OCKM. Clinical evaluation of Duraflo II heparin-treated extracorporeal circuits on the activation of the kinin and coagulation system. Proc Am Acad Cardiovasc Perfusion. 1994;15:62–69. [Google Scholar]
- Ernofsson M, Thelin S, Siegbahn A. Thrombin generation during cardiopulmonary bypass using heparin-coated or standard circuits. Scand J Thorac Cardiovasc Surg. 1995;29:157–165. doi: 10.3109/14017439509107224. [DOI] [PubMed] [Google Scholar]
- Gorman RC, Ziats NP, Rao AK, Gikakis N, Sun L, Khan MM, Stenach N, Sapatnekar S, Chouhan V, Gorman JH, 3rd, et al. Surface-bound heparin fails to reduce thrombin formation during clinical cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;111:1–12. doi: 10.1016/s0022-5223(96)70395-1. [DOI] [PubMed] [Google Scholar]
- Wagner WR, Johnson PC, Thompson KA, Marrone GC. Heparin-coated cardiopulmonary bypass circuits: hemostatic alterations and postoperative blood loss. Ann Thorac Surg. 1994;58:734–741. doi: 10.1016/0003-4975(94)90736-6. [DOI] [PubMed] [Google Scholar]
- Gu YJ, van Oeveren W, van der Kamp KWHJ, Akkerman C, Boonstra PW, Wildevuur CRH. Heparin-coating of extracorporeal circuits reduces thrombin formation in patients undergoing cardiopulmonary bypass. Perfusion. 1991;6:221–225. [Google Scholar]
- Tabuchi N, de Haan J, Boonstra PW, van Oeveren W. Activation of fibrinolysis in the pericardial cavity during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;106:828–833. [PubMed] [Google Scholar]
- de Haan J, Boonstra PW, Monnink SH, Ebels T, van Oeveren W. Retransfusion of suctioned blood during cardiopulmonary bypass impairs hemostasis. Ann Thorac Surg. 1995;59:901–907. doi: 10.1016/0003-4975(95)00012-A. [DOI] [PubMed] [Google Scholar]
- Chung JH, Gikakis N, Rao K, Drake TA, Colman RW, Edmunds LH., Jr Pericardial blood activates the extrinsic coagulation pathway during clinical cardiopulmonary bypass. Circulation. 1996;93:2014–2018. doi: 10.1161/01.cir.93.11.2014. [DOI] [PubMed] [Google Scholar]
- de Haan J, Boonstra PW, Tabuchi N, van Oeveren W, Ebels T. Retransfusion of thoracic wound blood during heart surgery obscures biocompatibility of the extracorporeal circuit. J Thorac Cardiovasc Surg. 1996;111:272–275. doi: 10.1016/S0022-5223(96)70427-0. [DOI] [PubMed] [Google Scholar]
- Philippou H, Davidson SJ, Mole T, Pepper JR, Burman JF, Lane DA. Two-chain factor VIIa generated in the pericardium during surgery with cardiopulmonary bypass: relationship to increased thrombin generation and heparin concentration. Arterioscler Thromb Vasc Biol. 1999;19:248–254. doi: 10.1161/01.atv.19.2.248. [DOI] [PubMed] [Google Scholar]
- Salemink I, Franssen J, Willems GM, Hemker HC, Li AG, Wun TC, Lindhout T. Factor Xa cleavage of tissue factor pathway inhibitor is associated with loss of anticoagulant activity. Thromb Haemost. 1998;80:273–280. [PubMed] [Google Scholar]
- Aldea GS, Soltow LO, Chandler WL, Triggs CM, Vocelka CR, Crockett GI, Shin YT, Curtis WE, Verrier ED. Limitation of thrombin generation, platelet activation, and inflammation by elimination of cardiotomy suction in patients undergoing coronary artery bypass grafting treated with heparin-bonded circuits. J Thorac Cardiovasc Surg. 2002;123:742–755. doi: 10.1067/mtc.2002.120347. [DOI] [PubMed] [Google Scholar]
- De Somer F, Van Belleghem Y, Caes F, François K, Van Overbeke H, Arnout J, Taeymans Y, Van Nooten G. Tissue factor as the main activator of the coagulation system during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2002;123:951–958. doi: 10.1067/mtc.2002.120334. [DOI] [PubMed] [Google Scholar]
- van 't Veer C, Golden NJ, Mann KG. Inhibition of thrombin generation by the zymogen factor VII: implications for the treatment of hemophilia A by factor VIIa. Blood. 2000;95:1330–1335. [PubMed] [Google Scholar]
- Mann KG. Biochemistry and physiology of blood coagulation. Thromb Haemost. 1999;82:165–174. [PubMed] [Google Scholar]
- Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PG, ten Have K, Eijsman L, Hack CE, Sturk A. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96:3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- Maquelin KN, Nieuwland R, Lentjes EGWM, Böing AN, Mochtar B, Eijsman L, Sturk A. Aprotinin administration in the pericardial cavity does not prevent platelet activation. J Thorac Cardiovasc Surg. 2000;120:552–557. doi: 10.1067/mtc.2000.108530. [DOI] [PubMed] [Google Scholar]