Abstract
Combinatorial methodologies were used for the synthesis and screening of mixed metal oxide heterogeneous catalysts. Primary screening at low reactant conversions at a throughput of greater than 10,000 catalyst compositions per month was performed by using simultaneous MS and photothermal deflection spectroscopy on spatially separated thick film catalysts with ≈200 μg per catalyst prepared by using automated liquid dispensing. Secondary screening under realistic operating conditions was performed at a throughput of greater than 3,000 catalyst compositions per month on ≈50 mg of catalyst in an array of fixed bed microreactors with gas chromatograph detection. The approach was validated by the discovery of catalysts with superior performance to those previously described for the oxidative dehydrogenation of ethane to ethylene. We show the full implementation and integration of combinatorial methodologies for synthesis, screening, discovery, and optimization of multicomponent heterogeneous catalysts.
Although combinatorial methodologies are practiced routinely for drug discovery (1, 2), this general approach is compelling in other fields where predictive abilities also are restricted. Recently, combinatorial methods have been applied within several different areas of materials science, where increasing compositional or structural complexity often results in unique or otherwise improved properties (1–12). Structural and compositional complexity may result in systems consisting of several components functioning cooperatively. In these combinations, the synergy of the multicomponent system results in performance characteristics that are particularly difficult to predict à priori. Presently, the vast majority of complex inorganic solids and multicomponent materials remain unexplored (13), in part because composition-structure-property relationships for such systems are limited. The utility of combinatorial chemistry, i.e., the ability both to prepare and to screen vast numbers of compounds in a rapid fashion, may be most productively realized within such systems. Here, we describe an integrated combinatorial program that has resulted in the discovery of improved multicomponent heterogeneous catalysts for the oxidative dehydrogenation of ethane to ethylene. Bringing combinatorial methodologies to bear on this application is particularly appealing because catalyst discovery has relied traditionally on an iterative trial-and-error synthesis and characterization strategy that is both tedious and time consuming.
The parallel synthesis of catalytic materials by the use of automation and miniaturization techniques is most efficient when preparing very small quantities (<1 mg) of catalysts. The characterization of such small amounts of catalytic materials frequently is hindered by the lack of sensitive high throughput screening methodologies, particularly for reactions of low probability such as the partial oxidation of hydrocarbons. These obstacles have been overcome in our laboratories, and an integrated combinatorial discovery program for heterogeneous catalysis has been realized. We have reported previously the preliminary implementation of such a program for CO oxidation and NO reduction by noble metals and copper (12). Here, we describe the full implementation for the oxidative dehydrogenation of ethane to ethylene, which is far more challenging from the perspective of catalyst screening caused by a lower rate of reaction and the presence of undesirable side reactions. In addition to MS, an optical detection scheme for ethylene has been developed as part of the primary screen, along with a parallel fixed bed microreactor as the secondary screen.
The development of efficient heterogeneous catalysts for the gas phase oxidative dehydrogenation of light paraffins is of particular interest because of the economic benefits of using light paraffins for the production of important base chemicals (14–18). The low-temperature oxidative dehydrogenation of C2H6 to C2H4 has been a research topic of consistent interest after the report of catalytic activity in the Mo-V-Nb-O system below 300°C in 1978 (18–22). In the work reported here using combinatorial methodologies for catalyst synthesis and screening, we demonstrate our ability both to reproduce the results in the Mo-V-Nb-O and related systems, as they have been described previously in the literature, and to identify similar but improved catalysts.
The utility of combinatorial methods applied to heterogeneous catalysis depends ultimately on the ability to prepare bulk-like materials in a high throughput manner (23). If trends measured in a combinatorial library are to correlate with bulk trends, then the identity of catalytically active sites should be the same in the two cases, assuming the reaction mechanisms do not change from low conversions as measured in the primary, high throughput screen to much higher conversions in secondary and tertiary screens. The purpose of the high throughput screen is the rapid screening of many catalysts to eliminate compositions from further examination, which allows the accelerated study of large regions of composition space with the intent of extracting relative trends that suggest continued study using focused primary screens and secondary screens.
The general strategy applied in our labs for rapid primary screening involves the creation of arrays of thick films that are spatially separated catalyst libraries of 100–200 μg per catalyst. Typically, ≈150 catalyst compositions are measured per experiment in the primary screen. These catalyst films are prepared from stabilized sol-gel precursors by automated solution deposition techniques (24).
Metal alkoxide solutions (0.5 M) were prepared by refluxing commercially available metal alkoxides in 2-methoxyethanol after which metal-specific modifiers were added. Combinatorial libraries of precursor solutions were created initially in microtiter plates by using automated liquid dispensing robots in the form of an 11-×-11-×-11 triangular matrix. Each solution was deposited into microtiter wells such that along each row of the triangle the metal ratios were decremented by 10% while maintaining constant volume. This triangular array results in individual metal alkoxide solutions in the wells mapped to each apex of the triangle, binary metal alkoxide solutions along the sides, and ternary mixtures within the interior of the triangle. These solutions (3 μl) then are transferred to a chemically and mechanically modified quartz substrate to create two duplicate 11-×-11-×-11 triangular catalyst libraries within a 12-×-12 rectangular array. Quartz substrates (disks, 76.2 mm in diameter and 1.6 mm in thickness) were chemically modified by using organosilane reagents to affect the wetting characteristics of the quartz surface. After silanization, the wafer surface was mechanically roughened to create a 12-×-12 rectangular array of matrix elements (3 mm in diameter with 4 mm spacing between element centers), and 3 μl was transferred from each microtiter well onto each corresponding matrix element of the quartz wafer array. This procedure results in ≈200 μg of catalyst material in each library element. Two redundant 11-×11-×-11 triangular catalyst libraries may be deposited within the 12-×-12 rectangular array. Precursors were allowed to gel under ambient conditions and subsequently were annealed under conditions necessary to reproduce x-ray diffraction patterns of bulk samples. Substrates were heated in air at 1°C/min and held at 120°C for 2 hr, further heated at 1°C/min and held at 180°C for 2 hr, and heated at 2°C/min and held at 400°C for 4 hr, after which the sample was allowed to cool naturally.
This process allows for a total throughput of more than 10,000 catalyst compositions per month for which trends are observed under conditions of low reactant conversions. Areas of high product yield may be re-examined in the primary screen or examined in a secondary screen. Typically, ≈50 catalyst compositions (25–50 mg) are examined per experiment in the secondary screen by using parallel fixed bed microreactors. Current operating conditions in the secondary screen allow for a total throughput of more than 3,000 catalyst compositions per month for which reliable data are collected under realistic operating conditions. Particularly interesting catalyst compositions may continue to be examined at a tertiary screen level, i.e., studies of the effects of reaction temperature, flow rate, and feed composition, using bench-scale reactors with gram quantities of catalyst.
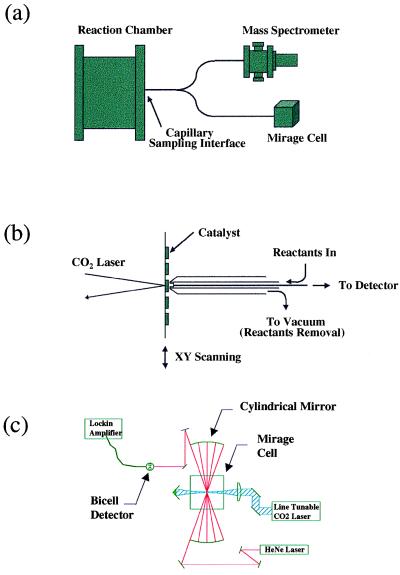
For purposes of primary catalytic screening, combinatorial arrays are loaded into a system containing a reaction chamber and an analysis chamber, see Fig. 1 (12, 25, 26). The library is placed onto a platform capable of translation in three orthogonal directions within the reaction chamber. A stationary nozzle allows the reaction gas to be delivered onto each catalyst independently, and the resulting products and unreacted gas mixture are carried through a capillary to the detection system in the analysis chamber. The temperature of a single element is controlled by using a CO2 laser for heating, a remote temperature sensor, and a feedback control loop. The composition of the product gas is measured once the gas flow, temperature, and pressure have stabilized. Single-element heating and local exposure of reactant gases prevent premature aging of catalyst elements before measurement and eliminate all contributions from neighboring catalysts.
Figure 1.
Scanning flat surface catalyst screening apparatus. (a) Schematic of scanning flat surface catalyst screening system, which is composed of three modular subsystems: reaction chamber, mass spectrometer, and PTD detector. (b) Details of the gas delivery/removal/sampling nozzle. (c) Schematic of the ethylene detector based on PTD spectroscopy.
Catalytic screening in the analysis chamber was performed by using both a mass spectrometer equipped with an electron impact (EI) ionizer and a photothermal deflection (PTD) detector (27, 28). Although EI-MS offers a universal scheme for detecting most molecules, it cannot measure the C2H4 concentration at the ppm level in the presence of high concentrations of C2H6 because the ion fragmentation pattern of C2H4 is a subset of that of C2H6. This serious problem has been solved by PTD, which relies on selective IR laser excitation of C2H4 and offers a very high discrimination factor against C2H6 (on the order of 106) and other species that are present in the product stream and are detected with EI-MS, such as CO2, CO, and H2O (27, 28). In the experimental setup, a CO2 laser, operated at a single line (10P14) to be in resonance with the ν7 mode (949.48 cm−1) of C2H4, is used as the pump laser, and a 10-mW HeNe laser is used as the probe laser. The sensitivity of the PTD detector for C2H4 is below 0.1 ppm. The product gas is introduced into both the PTD detector and the ionization zone of the mass spectrometer through a split capillary, and oxidation products C2H4 and CO2 (apart from H2O) were found. The presence of CO is masked by N2. Approximate reaction selectivities at low conversions thus are determined through the measurement of both the desired product, C2H4, and the undesired combustion side product, CO2.
Library samples were loaded into the reaction chamber and screened by PTD and MS at temperatures between 300 and 400°C. The reactant gas was a mixture of N2, C2H6, and O2 in a molar ratio of 5:4:1. The reaction chamber pressure was maintained at ≈760 Torr. After the flow and pressure were stabilized, each catalyst was heated sequentially to the reaction temperature by using a CO2 laser, and product formation was measured for 60–90 sec. Initially, a library containing multiple quantities of a single catalyst composition known to be active for C2H4 production was measured between 300°C and 400°C. The optimal reaction temperature and catalyst mass (solution concentration and volume) then were used in the synthesis of subsequent libraries to maximize the signal-to-background ratio. A library containing two identical triangular matrices of 66 elements of 0–100% molybdenum, vanadium, and niobium (resulting in 10% compositional increments per matrix element) then was prepared, and the entire library was scanned multiple times to ensure reproducibility. The resulting data reproduced trends from the literature at all temperatures, including a maximum in C2H4 production near the composition previously reported to be optimal (18), and a maximum in CO2 production in the niobium-rich areas, thus reproducing qualitatively 20 years of literature research in a single experiment. Maximum conversions of C2H6 to C2H4 as measured by PTD from this Mo-V-Nb-O library are ≈10 ppm. Thus, contrary to the pessimism expressed recently in the literature (23), there is a perfect correlation of activity and selectivity between ppm (10−6) level conversions and percent (10−2) level conversions for this catalytic system.
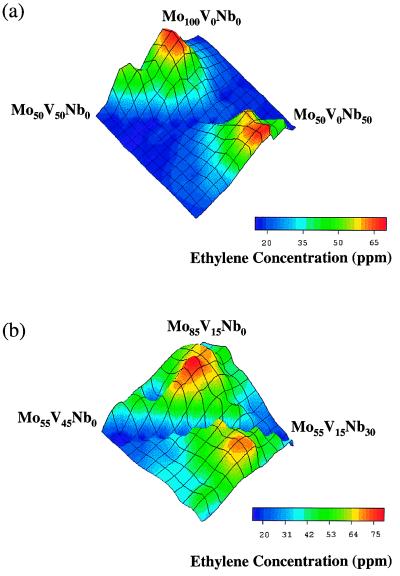
For greater compositional precision, additional libraries were prepared to focus on the most active area, see Fig. 2. Focus libraries included 50–100% molybdenum and 0–50% vanadium and niobium in 5% increments in Fig. 2a, and 55–85% molybdenum, 15–45% vanadium, and 0–30% niobium in 3% increments in Fig. 2b. As the compositional distinction between library elements decreases, the ability to differentiate between the performance of library elements becomes more difficult in this high throughput, primary screen, as may be seen in Fig. 2b. Quantitative conversion and selectivity data at higher conversions then were obtained from the library elements of interest by using miniaturized parallel fixed bed reactors, a secondary screen that mimics more realistically industrial operating conditions. The results of this screening of bulk catalyst powders in the parallel reactor in which the temperature was controlled at 300 ± 2°C are listed in Table 1. There are 48 channels in the reactor, each channel is cylindrically shaped, with an internal volume of ≈100 μl. Samples were prepared in an automated fashion on a 50-mg scale. Reactions were carried out at one atmosphere pressure by using a flow rate of 1.04 standard cm3/min per channel with 40% C2H6, 8.6% O2, and 51.4% N2. Using two gas chromatographs, each containing three identical channels, CO, CO2, C2H4, and C2H6 were separated in 3 min. In an initial examination in which the concentration of niobium was varied between 3% and 12%, compositions of maximum C2H6 conversion and C2H4 selectivity were found to decrease with increasing niobium concentration with maxima occurring along the 3% niobium row. Subsequently, another series of bulk samples was prepared with the niobium concentration varying from zero to 4%.
Figure 2.
Ethylene production in ppm for combinatorial libraries consisting of molybdenum-vanadium-niobium oxides. Ethylene production at 400°C for libraries of (a) 50–100% molybdenum and 0–50% vanadium and niobium in 5% increments, and (b) 55–85% molybdenum, 15–45% vanadium, and 0–30% niobium in 3% increments. Two identical triangular arrays exist on each wafer, differentiated by a C2 rotation operation.
Table 1.
Parallel reactor data at 300°C for oxidized Mo79-66V1-30Nb0-4
| Composition | Conversion (%) | Selectivity (%) |
|---|---|---|
| Mo79V21 | 0.51(8) | 34(10) |
| Mo76V24 | 0.86(1) | 41.8(3) |
| Mo73V27 | 0.99(1) | 43.9(2) |
| Mo70V30 | 1.2(1) | 44.0(3) |
| Mo78V21Nb1 | 8.0(1) | 83.2(1) |
| Mo75V24Nb1 | 8.3(1) | 81.9(1) |
| Mo72V27Nb1 | 6.2(1) | 79.1(1) |
| Mo69V30Nb1 | 4.4(1) | 71.0(1) |
| Mo77V21Nb2 | 11.7(1) | 77.7(1) |
| Mo74V24Nb2 | 12.6(1) | 76.7(1) |
| Mo71V27Nb2 | 11.3(1) | 76.3(1) |
| Mo68V30Nb2 | 9.4(1) | 76.8(2) |
| Mo76V21Nb3 | 11.6(1) | 74.9(1) |
| Mo73V24Nb3 | 12.8(1) | 74.4(1) |
| Mo70V27Nb3 | 11.8(1) | 70.7(1) |
| Mo67V30Nb3 | 11.8(1) | 71.9(1) |
| Mo75V21Nb4 | 10.7(1) | 74.1(1) |
| Mo72V24Nb4 | 11.5(1) | 70.9(1) |
| Mo69V27Nb4 | 10.5(1) | 69.9(1) |
| Mo66V30Nb4 | 12.1(1) | 72.1(1) |
Based on the results of Table 1, it appears that whereas the binary Mo-V catalysts will catalytically dehydrogenate C2H6 to a very limited extent, the presence of niobium dramatically increases both activity and selectivity. The addition of small amounts of Nb is most effective within the range of 1% < Nb < 4%. Based on C2H4 yield, catalysts with Nb concentrations of 2–4% are indistinguishable but are clearly better than either 1% or greater than 4% Nb. Optimal compositions of Mo and V from samples with 2% ≤ Nb ≤ 4% are within the ranges of 70% < Mo < 77% and 21% < V < 27%, whereas the most optimal composition is Mo ≈ 73%, V ≈ 24%, Nb ≈ 3%, ±1%. This compositional range and optimal composition may be compared with those described by Thorsteinson et al. (18), namely, 61% < Mo < 77%, 19% < V < 31%, 4% < Nb < 9% with an optimal composition of 73% Mo, 18% V, 9% Nb. Prepared on a gram scale or a 50-mg scale, this composition measured in our system results in 6.1% conversion with 83.2% selectivity. Our microreactor results (≈50 mg) are consistent with those of Thorsteinson (tens of grams) and have greater compositional specificity.
Based on literature examples (19, 29), the addition of antimony and calcium is expected to improve further the performance of the Mo-V-Nb-O system. When these additional elements are included, the divergence of the optimal catalyst composition from previously reported materials is even greater. Our results clearly show that the partial substitution of Sb for Nb improves the catalyst performance, as described previously. However, unlike the catalysts previously described in the literature, Sb-rich (compared with Nb) compositions display further improved catalytic performance. Although the replacement of Nb solely by Ca generally decreases the catalytic performance, the combination of Sb and Ca results in improved performance. Finally, the inclusion of Li improves the catalytic performance even further. Selected results are shown in Table 2 for 12 catalysts, all of which have performance characteristics that exceed the best catalysts heretofore reported in the literature. Superior performance is judged by selectivity improvements at similar conversions.
Table 2.
Parallel reactor dat at 300°C for oxidized Mo71V24(Nb,Sb,Ca,Li)5
| Composition | Conversion (%) | Selectivity (%) |
|---|---|---|
| Nb0.5Sb2Ca2.5 | 9.8(1) | 83.2(2) |
| Sb2.5Ca2.5 | 7.9(1) | 85.7(3) |
| Nb0.5Sb2Ca2Li0.5 | 9.2(1) | 84.4(2) |
| Sb2.5Ca2Li0.5 | 10.8(1) | 85.5(2) |
| Nb0.5Sb2Ca1.5Li1 | 7.3(6) | 86.7(6) |
| Sb2.5Ca1.5Li1.0 | 8.1(1) | 85.5(3) |
| Nb0.5Sb2Ca1Li1.5 | 9.7(5) | 85.5(9) |
| Sb2.5Ca1Li1.5 | 9.6(8) | 87(1) |
| Nb0.5Sb2Ca0.5Li1 | 8.6(2) | 87.2(3) |
| Sb2.5Ca0.5Li2 | 9.3(1) | 87.6(1) |
| Nb0.5Sb2Li2.5 | 9.8(1) | 87.9(1) |
| Sb2.5Li2.5 | 10.3(1) | 87.2(1) |
Combinatorial methodologies encompassing all synthetic and screening steps have been used to determine the optimal composition of catalysts for the oxidative dehydrogenation of C2H6 within the Mo-V-Nb-O composition space. Using primary and secondary screening techniques, the optimal composition has been determined within ±2% for all elements. The use of miniaturized, parallel fixed bed reactors on a 50-mg scale has resulted in the reproduction of literature trends using lab scale reactors on a gram scale at greater compositional precision than that originally reported. New and improved catalytic materials were discovered in the compositional space near that previously reported. The inclusion of Sb, Ca, and Li dopants improved the catalyst even further. The feasibility of combinatorial synthesis and screening techniques has been validated for their future use in catalyst discovery and optimization programs.
ABBREVIATION
- PTD
photothermal deflection
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Terrett N K, Gardner M, Gordon D W, Kobylecki R J, Steele J. Tetrahedron. 1995;30:8135–8173. [Google Scholar]
- 2.Gordon E M, Gallop M A, Patel D V. Acc Chem Res. 1996;29:144–154. [Google Scholar]
- 3.Xiang X-D, Sun X, Briceno G, Lou Y, Wang K-A, Chang H, Wallace-Freedman W G, Chen S-W, Schultz P G. Science. 1995;268:1738–1740. doi: 10.1126/science.268.5218.1738. [DOI] [PubMed] [Google Scholar]
- 4.Briceno G, Chang H, Sun X-D, Schultz P G, Xiang X-D. Science. 1995;270:273–275. doi: 10.1126/science.268.5218.1738. [DOI] [PubMed] [Google Scholar]
- 5.Hill C L, Gall R D. J Mol Cat A Chem. 1996;114:103–111. [Google Scholar]
- 6.Moates F C, Somani M, Annamalai J, Richardson J T, Luss D, Wilson R C. Ind Eng Chem Res. 1996;35:4801–4803. [Google Scholar]
- 7.Danielson E, Golden J H, McFarland E W, Reaves C M, Weinberg W H, Wu X D. Nature (London) 1997;389:944–948. [Google Scholar]
- 8.Danielson E, Devenney M, Giaquinta D M, Golden J H, Haushalter R C, McFarland E W, Poojary D M, Reaves C M, Weinberg W H, Wu X D. Science. 1998;279:837–839. doi: 10.1126/science.279.5352.837. [DOI] [PubMed] [Google Scholar]
- 9.Senkan S M. Nature (London) 1998;394:350–352. [Google Scholar]
- 10.Holzwarth A, Schmidt H-W, Maier W F. Angew Chem Int Ed. 1998;37:2644–2647. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2644::AID-ANIE2644>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Klein J, Lehman C W, Schmidt H-W, Maier W F. Angew Chem Int Ed. 1998;37:3369–3372. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3369::AID-ANIE3369>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Cong P, Doolen R D, Fan Q, Giaquinta D, Guan S, McFarland E W, Poojary D M, Self K, Turner H W, Weinberg W H. Angew Chem Int Ed. 1999;38:483–488. doi: 10.1002/(SICI)1521-3773(19990215)38:4<483::AID-ANIE483>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers J R, Villars P. Mater Res Soc Bull. 1993;18:27–29. [Google Scholar]
- 14.Grasselli R K, Oyama S T, Gaffney A M, Lyons J E, editors. Proceedings of the Third World Congress on Oxidation Catalysis. San Diego: Elsevier; 1997. [Google Scholar]
- 15.Cavani F, Trifiro F. Catal Today. 1995;24:307–313. [Google Scholar]
- 16.Hong S S, Moffat J B. Appl Catal A General. 1994;109:117–134. [Google Scholar]
- 17.Murakami Y, Otsuka K, Wada Y, Morikawa A. Bull Chem Soc Japan. 1990;63:340–346. [Google Scholar]
- 18.Thorsteinson E M, Wilson T P, Young F G, Kasai P H. J Catal. 1978;52:116–132. [Google Scholar]
- 19.Young F G, Thorsteinson E M. U.S. Patent 4,250,346. 1981. [Google Scholar]
- 20.McCain J H. Eur. Patent Appl. 0,166,438. 1986. [Google Scholar]
- 21.Burch R, Swarnakar R. Appl Catal. 1991;70:129–148. [Google Scholar]
- 22.Merzouki M, Taouk B, Tessier L, Bordes E, Courtine P. In: New Frontiers in Catalysis: Proceedings of the 10th International Congress on Catalysis. Guczi L, Solgmosi F, Teteryi P, editors. Budapest, Hungary: Elsevier; 1992. pp. 753–764. [Google Scholar]
- 23.Schlögl R. Angew Chem Int Ed. 1998;37:2333–2336. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2333::AID-ANIE2333>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Giaquinta D, Devenney M, Hall K, Goldwasser I (Symyx Technologies) U.S. Patent Pending. 1998. [Google Scholar]
- 25.Weinberg W H, McFarland E W, Cong P, Guan S (Symyx Technologies) U.S. PCT Int. Appl. WO 9815969 A2. 1998. [Google Scholar]
- 26.Cong P (Symyx Technologies) U.S. Patent Pending. 1998. [Google Scholar]
- 27.Fournier D, Boccara A C, Amer N M, Gerlach R. Appl Phys Lett. 1980;37:519–521. [Google Scholar]
- 28.de Vries H. Ph.D. thesis. The Netherlands: University of Nijmegen; 1994. [Google Scholar]
- 29.McCain J H. U.S. Patent 4,524,236. 1985. [Google Scholar]