Abstract
Background
Identification of the genes and pathways associated with the activation of endothelial cells (ECs) could help uncover the role of ECs in wound healing, vascular permeability, blood brain barrier function, angiogenesis, diabetic retinopathy, atherosclerosis, psoriasis, and growth of solid tumors.
Design
Herein, we embedded ECs in 3D type I collagen gel, left unstimulated or stimulated with VEGF165, and subjected to suppression subtractive hybridization followed by differential display (SSHDD). Gene fragments obtained from SSHDD were subjected to DNA sequence analysis. Database search with nucleotide sequence were performed using the BLAST algorithm and expression of candidate genes determined by northern blot analysis.
Results
A total of ~32 cDNA fragments, including known regulators of angiogenesis, and a set of genes that were not reported to be associated with activation of ECs and angiogenesis previously were identified. We confirmed the mRNA expression of KDR, α2 integrin, Stanniocalcin, including a set of 11 candidate genes. Western immunoblotting results indicated that KDR, α2 integrin, MMP-1, MMP-2, and VE-cadherin genes were indeed active genes.
Conclusion
We have identified a set of 11 VEGF-responsive endothelial cell candidate genes. Their expression in endothelial cell is confirmed by northern blot analyses. This preliminary report forms as a foundation for functional studies to be performed to reveal their roles in EC activation and pathophysiological events associated with the vasculature including tumor growth.
Background
Identification of vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF)-responsive genes could potentially help elucidate the detail molecular mechanisms of endothelial cells (EC) activation. EC activation is a program of gene-expression that could affect angiogenesis, permeability, and inflammation that are closely associated with a large number of vascular diseases. Recent studies show that priming of quiescent ECs with appropriate cytokines could switch-on the EC transcription machinery, the genes and protein products of which could activate ECs. Mediators known to activate ECs include inflammatory cytokines, growth factors such as VEGF/VPF and basic fibroblast growth factor (bFGF), extracellular matrix (ECM) proteins such as collagen and fibronectin, and proteases such as MMPs [1,2]. In vitro and in vivo assays indicate that VEGF signaling promotes increased permeability, cell migration, proliferation, and differentiation, functioning through two EC-specific tyrosine kinase receptor such as Kinase domain receptor (KDR) [1-3]. In the majority of solid tumors, VEGF is upregulated in response to hypoxia, inducing the expression of pro-angiogenic genes that promote tumor angiogenesis, growth and eventual metastasis [3-5]. The expression of both positive and negative factors must be tightly and specifically regulated in a coordinated manner. Uncontrolled expressions of these factors could activate the EC transcription machinery, which could promote pathological consequences including tumor growth and metastasis [3-6].
Thus, the identification and dissection of the cellular and molecular pathways associated with EC activation may reveal novel clues which could provide further understanding of biological processes such as wound healing, vascular permeability, and angiogenesis among others. A number of in vitro model systems have thus been developed to study EC activation that employs either one or more combinations of ECM molecules including fibrin, fibronectin, collagens, laminins, and Matrigel® matrices together with PMA and FGF [7-9]. When placed in a 3D type I collagen matrix and stimulated with PMA, VEGF, or bFGF, ECs undergo rapid morphological changes and differentiate into capillary-like tubular networks. Transcriptional inhibitors block this in vitro tube formation, suggesting that the angiogenesis requires EC activation and gene expression [8,9]. Indeed, a recent review describes approaches used to identify such angiogenesis-related genes [10]. Although many angiogenesis-related soluble factors, ECM components, and intracellular signaling pathways have been identified and elucidated, their subsequent targets and their mode of action still remain to be determined.
Despite extensive studies, for example, the molecular mechanisms underlying endothelial cell activation and EC differentiations are not entirely understood. To better understand molecular mechanisms and pathways of EC activation, we embedded ECs in 3D type I collagen in presence or absence of human recombinant VEGF165. RNAs isolated from these cultures were then subjected to suppression subtractive hybridization and differential display (SSHDD). Through this method, we identified a set of 11 new induced genes previously not reported to be associated with the processes of EC activation. We confirmed expressions of candidate genes in ECs by northern and western blot analyses. There is a large number of literature showing microarray and gene-expression profiling analysis of EC transcriptome. However, this preliminary study forms the basis for the future experiments involving analyses of signaling pathways associated with EC activation that are crucial for many pathophysiological conditions including angiogenesis, cardiovascular diseases and growth of solid tumors.
Results
Identification of VEGF-responsive endothelial cell genes using SSHDD
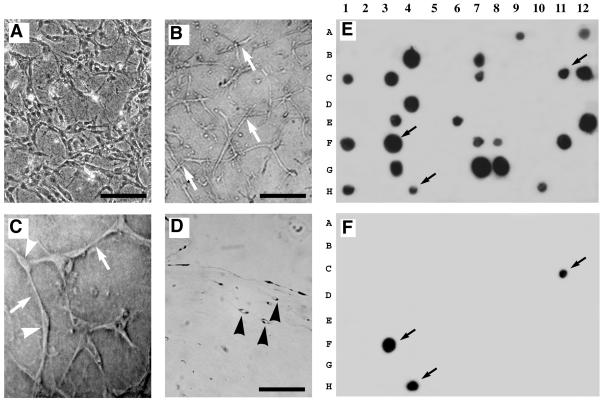
Angioblasts and ECs are the precursor cells that can form functional blood vessels in response to specific angiogenic stimuli. To study the molecular mechanisms underlying capillary morphogenesis in ECs, we used a well-established in vitro model of angiogenesis. Model system employed in our study closely resembles with those that have been described previously [7-9]. Either human microvascular endothelial cells (HMECs) or human umbilical vein endothelial cells (HUVECs) were seeded between two thin layers of type I collagen gels, and overlayed with complete media supplemented with VEGF165, as described in methods. This typically induced cytoplasmic projections, spikes and extensions, elongations of ECs within 4 to 8 hr. These processes were considered to represent a precursor to cell elongation, interconnections of cells, and eventual tubule formation. A small population of ECs that failed to elongate, did not form such interconnections. This model system closely approximates ECM proteolysis, cell migration, proliferation, and capillary formation. Representative photomicrographs of VEGF-induced capillary morphogenesis in ECs after 8 hr and 16 hr are shown in Figs. 1A and 1B, respectively. Figure 1C shows ECs forming (at 200X magnification) elaborate interconnections and vacuole formation at 36 hr. Although control (un-stimulated) ECs formed interconnections at the end of 24 hr, but fail to form any capillaries (data not shown). Eosin staining of these structures demonstrated that the capillaries were surrounded by at least three to five ECs (Fig. 1D). To identify genes associated with activation of ECs, we used the method of SSHDD. By employing forward- and reverse-subtraction, we identified both common (indicated by arrows) and differentially expressed genes (Fig. 1E &1F). The addition of VEGF into ECs embedded in the 3D type I collagen matrix (forward-subtraction) generated more than 450 cDNA fragments. To eliminate the identification of artifactual clones, we analyzed only those cDNA fragments that were detected at least 4 times. Based on this criterion, only 311 cDNA fragments (clones) out of 450 sequences (clones) were retained for further evaluation. From this 311, a total number of 32 induced fragments were identified and sequenced (Table I). Database search with these nucleotide sequences using the BLAST algorithm revealed many known positive or negative regulators of angiogenesis, including proteases and cell surface receptors. Importantly, we identified 11 VEGF-responsive candidate genes associated with the activation of endothelial cells.
Figure 1.
VEGF induced capillary morphogenesis of endothelial cells and dot blot screening. HUVECs (3 × 105 cells/ml) were grown human recombinant VEGF165 (100 ng/ml), as described in Experimental Procedures. (A) Elongation and morphogenic changes of ECs after 8 hr exposure to VEGF; (B) ECs formed a mosaic of interconnecting networks after 16 hr of exposure to VEGF; (C) High resolution phase contrast phomicrograph of interconnected ECs developing vacuoles after 24 hr exposure to VEGF, as indicated by white arrowheads; and (D) cross section of an eosin-stained fixed gel, reveals the formation of capillary-like structures after 36 hr exposure to VEGF (indicated by black arrowheads). White arrows indicate cell-contacts. Magnification 100X. Bar, 50 μM. E, F) Escherichia coli cultures transformed with plasmids containing enriched cDNAs, were spotted from a 96-well dish onto duplicate nylon membranes. The membranes were hybridized with (E) forward-subtracted and (F) reverse-subtracted cDNA 32P-radiolabeled probes, as described in the "Methods". Black arrows indicate common genes detected by both probes.
Table I.
Endothelial cell genes (ECG) detected by SSHDD. BLAST searches revealed similarity to the accession # provided on the right most column. Genbank accession number in bold letter denotes complete cDNA sequence deposited by the authors.
| Class of genes | Gen Bank Accession Number | Reference |
| Known Genes associated with the activation of endothelial cells | ||
| Matrix metalloproteinases-1 (MMP-1) | X54925 | 3,25,26 |
| Matrix metalloproteinases-2 (MMP2) | NM_ 004530 | 3,25,26 |
| Stromelysin (MMP-3) | X05232 | 2,3,25 |
| Type IV collagenase (MMP9) | J05070 | 2,3,26 |
| Myeloblastin | M75154 | 25,26 |
| Cathepsin B | M14221 | 28 |
| Calpastatin | E02261 | 29 |
| Urokinase plasminogen activator surface receptor (uPAR) | X51675 | 2,30 |
| Vascular endothelial growth factor receptor (VEGFR1/FLT-1) | AF063657 | 4,5 |
| Vascular endothelial growth factor receptor-2(VEGFR-2/KDR) | X61656 | 4,5,6 |
| Vascular cell adhesion molecule-1 (VCAM-1) | X53051 | 2,3 |
| α2 integrin subunit | X17033 | 2,20 |
| VE-cadherin | X79981 | 2,3,21 |
| Prostaglandin endoperoxidase (Cox) | E03346 | 2,32 |
| Metabolic and miscellaneous genes | ||
| Ketohexokinase | P50053 | - |
| Adenosine deaminase | X02994 | - |
| Spermidine synthase | M64231 | - |
| Adenylyl cyclase | X74210 | 33 |
| Platelet factor 4 (PF4) | M25897 | 34 |
| Stanniocalcin | U25997 | 35,36 |
| Candidate genes associated with the activation of endothelial cells | ||
| Clone 7D Kinesin-heavy chain (KHC) | X65873 | 37 |
| Clone 17E Epiregulin (Epireg) | NM_001432 | 38 |
| Clone 22G Similar to transcription factor BTF3 | XM_038290 | 40 |
| Clone 33A Phosphatidic acid phosphatase-2b (PAP2b/VCIP) | AB000889/AF480883 | 40,41 |
| Clone 37F Synaptojanin-2 (similar to KIAA0348 protein) | AB002346 | 42,43 |
| Clone 48A Similar to GPCR kinase-interactor-2 (GIT-2) | NM_014776 | 44 |
| Clone 54C Smg GDS-associated protein (SMAP) | AI401257/U59919 | 45 |
| Clone 77D Angiopoietin related protein (AngRP) | AF169312 | 46 |
| Clone 94H Similar to Ribosomal protein L19 (RibL19) | AB019566/BC013016 | 47,48 |
| Clone 263F Eukaryotic translation EF-1 alpha l(EF-lal) | BC028674 | 49 |
| Clone 309C Stabilin-1 | NM_015136 | 50 |
Confirmation of Differentially Expressed Genes by Northern Blot
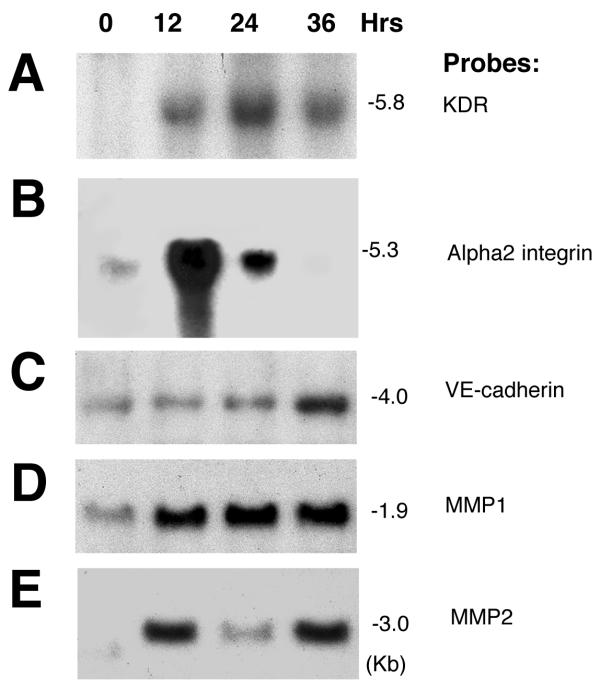
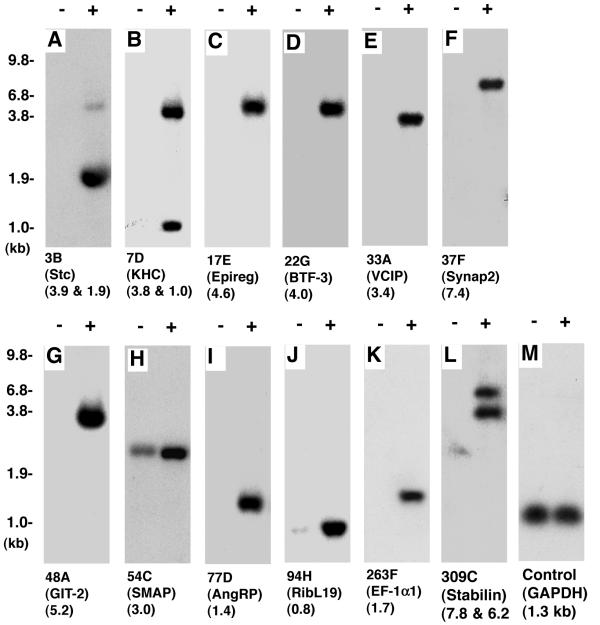
To confirm the differential expression of genes identified by SSHDD, we performed Northern blot analysis of total RNA isolated from ECs grown in 3D collagen matrices. The expression of 5 known regulators of angiogenesis, KDR and α2 integrin, VE-cadherin, MMP1, and MMP2, as well as 11 candidate genes (Figs. 2, 4; Table I) identified were analyzed. Consistent with previous reports, the expression of KDR/VEGFR-2 increased by ~2.5 fold at 12 hours, ~2 fold at 24 hrs, and ~1.5 fold at 36 hours, in response to VEGF165 treatment (Fig. 2A), whereas the expression of α2 integrin subunit was increased by ~5 fold at 12 hours, by ~2 fold at 24 hrs, and was barely detectable at 36 hrs (Fig. 2B) [11,12]. Expression of VE-cadherin was minimal at the earlier time points, but significantly increased at the end of 36 hours (Fig. 2C). MMP1 expression remained robustly active from 12 to 36 hours (Fig. 2D). While expression of MMP2 appeared to be biphasic, highest expressions were detected at 12 and 36 hrs (Fig. 2E). Equal loading of RNAs was confirmed by probing membrane with a β-actin cDNA probe (data not shown). Northern analyses of clones 7D, 3B, 17E, 22G, 33A, 37F, 48A, 54C, 77D, 94H, 263F, and 309C were performed at 24 hrs following VEGF165 treatment (Fig. 3A,3B,3C,3D,3E,3F,3G,3H,3I,3J,3K,3L). Northern blot analysis did not support the differential expression of clone 54C (Fig. 3H). Clone 7D, 3B, and 309C probes each detected 2 transcripts, which could be either alternatively spliced products or related genes. Clone 17E, 22G, 33A, 37F, 48A, 54C, 77D, 94H, 263F probes detected single transcripts (Fig. 3C,3D,3E,3F,3G,3H,3I,3J,3K). Of particular note, probe 3B (corresponding to Stanniocalcin) detected primarily the lower isoform (1.9 kb) in ECs. While probe 309C (corresponding to Stabilin) is known to produce three isoforms. Most likely, ECs primarily express Stabilin-1 and -2 that are 7.8 and 6.2 kbs isoforms respectively. The GAPDH probe was included as a control for the quantity and integrity of the loaded RNA, detecting a single transcript of ~1.3 kb. All data shown are representative of those obtained in at least three separate experiments, with similar results.
Figure 2.
Northern confirmation of KDR, α2 integrin subunit, VE-cadherin, MMP1, and MMP2 during activation of ECs. ECs were cultured in 3D collagen matrices with VEGF165. At various time points (indicated), total RNA was isolated and analyzed by Northern blot, as described in the "Methods". Blots were hybridized with indicated 32P-radiolabeled probes. PCR primers used to generate probes are shown in Table II. Data shown are representative of those obtained in two or three separate experiments, with similar results.
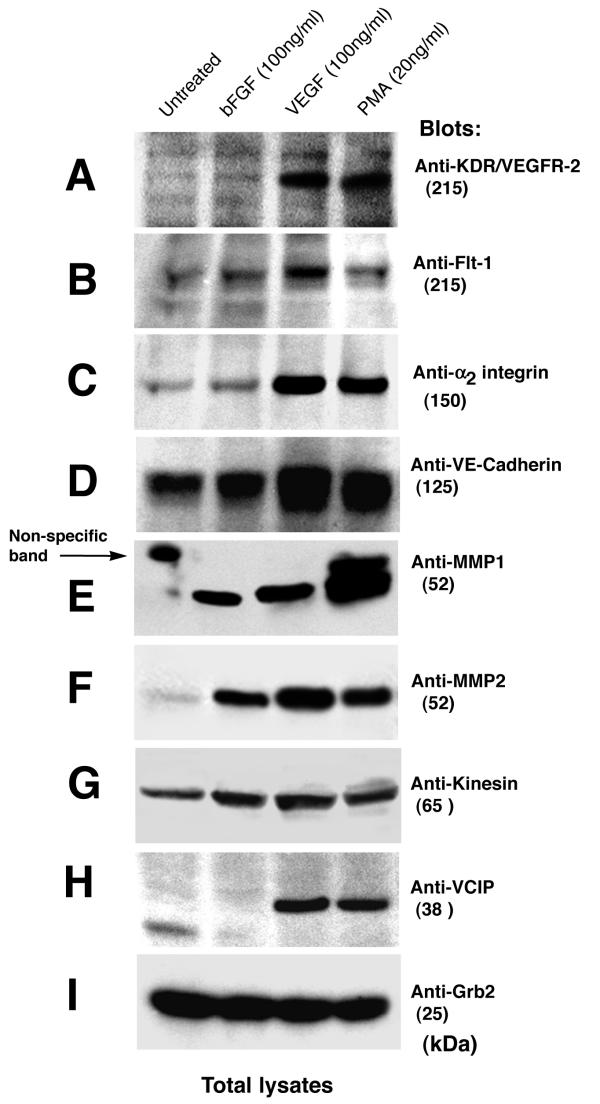
Figure 4.
Confirmation of gene expression as determined by Western blot analysis. EC monolayers were either left untreated (lane 1) or were treated with 100 ng/ml of bFGF (lane 2), VEGF (lane 3), or 20 ng/ml PMA (lane 4) for 48 hours. Total cellular protein was extracted and analyzed by Western blot, as described in the "Methods". Blots were incubated with: (A) anti-KDR; (B) anti-F1t-1; (C) anti-α2 integrin subunit; (D) anti-VE-cadherin; (E) anti-MMPl; (F) anti-MMP2; (G) anti-Kinesin; (H) anti-VCIP; and (I) anti-Grb2 antibodies. The molecular mass of each protein detected is indicated (kiloDaltons, kDa). Blots are representative of those obtained in two or three separate experiments, with similar results.
Figure 3.
Northern blot confirmation of VEGF-responsive genes. ECs were cultured in 3D collagen matrices with (+) or without (-) VEGF165. After 24 hours, total RNA was isolated and analyzed by Northern blot, as described in the "Methods". The 32P-radiolabelled probes used and their corresponding GenBank accession numbers are indicated, and abbreviated gene names are given in perentheses (also see Table I). The GAPDH probe was included as a control for the integrity and loading of the total RNA. Blots are representative of those obtained in two or three separate experiments, with similar results.
Confirmation of Differentially Expressed Genes by Western Blot
To confirm that the genes identified by SSHDD encode proteins, we performed Western blot analysis of total cellular protein isolated from ECs grown in monolayers. The addition of bFGF, VEGF, or PMA to the monolayers ECs induced cellular elongation over a period of 8 to 24 hr. In contrast, unstimulated ECs remained proliferative and retained a cobblestone appearance (data not shown). Western blot analysis revealed that VEGF and PMA, but not bFGF, induced the expression of KDR, Flt-1, and the α2 integrin subunit at 48 hr (Fig. 4A,4B,4C). Likewise, only VEGF and PMA upregulated the expression of VE-Cadherin (Fig. 4D). As expected, all 3 cytokines induced the expression of MMP-1 and MMP-2 (Fig. 4E and 4F). These cytokines also induced expression of Kinesin (Fig. 4G), albeit less appreciably. Furthermore, we found dramatic increase in expression of VCIP/PAP2b in ECs that were stimulated with VEGF and PMA (Fig. 4H, lane 3 and 4), in contrast, in bFGF stimulated cells (Fig. 4, lane 2) or in control (Fig. 4, lane 1) its expression remained undetectable. Anti-Grb2 mAb was included as control for protein loading (Fig. 4I). These data suggest that the cDNA fragments detected by SSHDD were indeed active genes that produce proteins that could activate endothelial cells. Similar studies will be required to test whether remaining novel genes produce functional proteins or not and to establish their exact role in EC activation. For Western immunoblotting experiment, monolayer ECs as opposed to cells grown in 3D gel were used. Because, it was difficult to solubilize ECs embedded in 3D gel. Cold cell extraction buffer containing 1.25% Triton X-100 and 0.1% SDS did not effectively solubilize solidified 3D collagen, - warm (37°C) cell extraction buffer was incompatible because it activated endogenous proteases. It remains possible that different genes may be expressed in ECs cultured as monolayer as opposed to 3D.
Discussion
In adult human, mature ECs usually remain quiescent for 3–7 years. However, ECs become activated in response to inflammatory cytokines and growth factors and infections. Increased accumulation of these cytokines including VEGF in the vasculature could activate the transcription machinery of ECs. Despite large number of studies activation of ECs and pathway that drive angiogenic phenotype of adult or tumor angiogenesis remain largely unknown [1-3]. Herein, we report identification of a set of 11 candidate genes associated with the activation of ECs in vitro (Table I). Other investigators have used cDNA arrays and representational differential display methods to catalogue pattern of gene expression in ECs that were stimulated with or without VEGF, bFGF, and PMA [13-15]. We also provide evidence that these EC gene fragments are induced in response to VEGF treatment in 3D collagen gel, as illustrated by northern and western blot analysis.
Known Regulators of Angiogenesis
Of the known regulators of EC activation identified in our study, the role of MMPs has been well established. Matrix digestion by MMPs, including MMP-1, MMP-2, and MMP-9, is a pre-requisite for EC activation, differentiation, and tumor induced angiogenesis [16,17]. The growth of mesenchymal cells in a 3D type I collagen matrix induces the processing and activation of pro-MMP-2 by MMP-1. Consistent with this, incubation of ECs with angiogenic cytokines induced the expression of MMP-1 and MMP-2 (Figs. 2 &4).
We also identified 4 indirect regulators of EC activation and angiogenesis, i.e., myeloblastin, cathepsin B, calpastatin, and urokinase plasminogen activator surface receptor (u-PAR). The myeloblastin protein displays anti-proliferative properties and is also a key protease involved in factor-independent growth of hematopoietic cells [18]. Overexpression of myeloblastin has been linked to multifunctional cytokine transforming growth factor-β (TGF-β) a known regulator of angiogenesis. We postulate that myeloblastin in our model system may negatively regulate EC activation, thereby signaling to end EC morphogenic differentiation. Cathepsin B is a lysosomal cysteine protease, the expression of which is increased in different tumors, including human brain, lung, colon, and breast tumors [19]. Calpastatin is a physiological protease inhibitor that acts specifically on calpain, a calcium-dependent cysteine protease. The cleavage of calpastatin by caspases initiates the apoptotic cascade in certain neurological disorders [20]. It is possible that increased expression of calpastatin may control calpain activity thereby limiting extent of proteolysis during capillary sprouting. In contrast, uPAR has been shown to regulate plasminogen-mediated extracellular proteolysis during angiogenesis [21].
The identification of VEGFR-1 and VEGFR-2 (KDR) was consistent with the observation that the stimulation of ECs with VEGF increases the expression of VEGFR-2, promoting myocardial revascularization [22]. We also detected the cell adhesion molecules VCAM-1, VE-Cadherin, and the α2 integrin subunit, all known regulators of angiogenesis. In the present study, we confirmed the expression of KDR, α2 integrin, VE-cadherin, MMP1, and MMP2 in activated ECs by northern and western blot analysis (Figs. 2 and 4).
Finally, we identified a known modulator of angiogenesis, the prostaglandin endoperoxidase-H synthase, also known as cyclooxygenase (COX). The COX-1 isoform is constitutively expressed in blood vessels, while expression of the COX-2 isoform is induced in new blood vessels. In many angiogenic tumors COX-2 colocalizes with VEGF and TGF-β, and increased expression of COX-2 and VEGF has been correlated with increased tumor microvascular density [23].
Metabolic genes and Miscellaneous genes
Although metabolic genes such as ketohexokinase, adenosine deaminase, and spermidine synthase detected by SSHDD assay per se do not directly activate of EC, it is likely that they could represent a component of the "angiogenic switch". Among the miscellaneous genes identified in our study were adenylyl cyclase and platelet factor 4 (PF4). Intracellular signaling machinery of adenylyl cyclase is required for cytoskeletal organization, vessel maturation, and vessel integrity [24]. PF4 is a chemokine that binds to FGF and is a known inhibitor of EC proliferation and migration [25]. Thus, the upregulation of PF4 might limit the degree of activation of ECs and morphogenic differentiation. It is noteworthy that Stanniocalcin (Stc; accession # U25997; Figs. 3A) was also identified by GeneCalling (a mRNA profiling technique) and in situ hybridization demonstrated that Stc is expressed in the vasculature of a subset of squamous cell carcinomas [26]. Stc is a secreted protein known to regulate calcium and phosphate homeostasis, potential role of this protein in EC activation and permeability is not clear [27]. Since most ECM-degrading enzymes are metal ion dependent, Stc may act as a sensor to maintain a steady-state level of metal ions during EC activation.
Candidate genes associated with the activation of ECs
In addition to the genes discussed above that have previously been implicated in EC activation and differentiation, we also identified 11 candidate genes (Table I).
Kinesin heavy chain (KHC; accession# X65873; Figs. 3B and 4G), mediates organelle movement towards the plus ends of microtubules in the presence of ATP and functions in cell differentiation and axonal guidance [28]. To our knowledge, increased expression of KHC during EC differentiation has not been previously reported. Although the specific role of KHC in the differentiation process is unclear, this protein might be important for the maintenance of the plasticity and/or polarity of ECs, which may be a prerequisite for the formation of capillary ends.
Epiregulin (Fig. 3C) is a member of epidermal growth factor (EGF) family of mitogens [29]. Expression of epiregulin has been reported to be highest in placenta and in a subset of carcinoma cells. Epiregulin activates COX-2, therefore, epiregulin may serve as an upstream inflammatory component of COX-2 signaling pathway. The function of epiregulin in the activation of ECs has not been investigated.
A general transcription factor BTF3 (Fig. 3D, clone 22G, accession #AA130020) protein is thought to be involved in precise transcription by RNA polymerase II, but fails to interact with DNA. Disruption of gene encoding BTF3 protein in the mouse caused postimplantation lethality around embryonic day 6.5 [30]. Detailed studies have not been reported in the literature. We believe, this is the first report of identification of BTF3 as one of the induced genes in the activation of ECs.
As a first step towards elucidating the molecular mechanisms and pathways associated with activation of ECs, we cloned the full-length cDNA corresponding to the Clone 33A called VCIP, also known as phosphatidic acid phosphatase type 2b (PAP2b). PAP2b shares similarity with Drosophila polarity gene Wunen-2 [31,32]. PAP2b/VCIP encodes 311 amino acid residues, contains a single N-glycosylation site, a consensus lipid phosphatase motif (KPSXXXRPH), an RGD cell attachment motif, six-transmembrane channel-like structure, and displays Mg++ independent lipid phosphatase activity in transfected 293T cells [32]. Both N- and C-terminus segments of PAP2b/VCIP are located inside the cytoplasm. Consistent with previously published report our data suggest that PAP2b/VCIP is cell surface protein and the expression of VCIP is highly enriched in vascularized tissues. In addition to its known lipid phosphatase activity, PAP2b/VCIP also mediate cell-cell interactions [31].
Synaptojanin-2 (Figure 4F) is also known as a polyphosphoinositide phosphatase-2. This family of proteins is distant homolog of yeast protein SacI [33]. The SacI homology domain is most notably found at the amino terminal of the inositol 5'-phosphatase synaptojanin. Synaptojanin-2 (lipid phosphatase) activities may be regulated by cell-cell and cell matrix interactions through RAFTs 'morphogenetic' platforms [34].
G protein-coupled receptor (GPCR) kinase-interactor-2 (Fig. 3G) interacts with G protein-coupled receptor kinases, and exhibits ADP-ribosylation factor (ARF) GTPase-activating protein (GAP) activity. It is known have several isoforms, alternatively sliced products, however, we primarily detected a single transcript of 5.2 kb size (EC specific isoform?). Because GIT-2 localizes with a subset of paxillin, therefore, makes it a potential candidate signaling protein that may collaborate with focal adhesion signaling machinery [35]. Thus, GIT-2 may play a role in cell adhesion, spreading, and motility.
SMAP, an Smg GDS-associating protein (Fig. 3H, Clone 54C, accession# AI401257), contains 9 Armadillo' repeats and interacts with the smg GDS protein through these repeats. This protein is a v-Src substrate, phosporylation affects the affinity of the protein for smg GDS. The small G protein GDP dissociation stimulator (smg GDS) acts on a group of small G proteins including the Rho and Rap1 family members and Ki-Ras. Smg GDS exhibits dual activity. One is to stimulate their GDP/GTP exchange reactions, and the other is to inhibit their interactions with membranes [36].
Angiopoietin related protein (AngRP) (Fig. 3I) is chiefly expressed by the hepatic cells. AngRP contain the characteristic coiled-coil and fibrinogen-like domains that are conserved in angiopoietins, however, do not interact with Tie-1 and Tie-2 receptor [37]. AngRP act as a survival factor for ECs, but its role in angiogenesis is not clear. It will be interesting to examine if there is a specific receptor for this molecule in ECs or other vascular cells.
Probe 94H (Fig. 3J) detected 0.8 kb transcript, amino acid sequence analysis suggest that this protein is likely to be member of ribosomal protein L19 [38]. In eukaryotes, ribosome consists of a 60S large subunit and a 40S small subunit [39]. In mammalian cells, ribosomal proteins accounts for up to 12% of the total cellular protein.
The alpha subunit of Translational elongation factor-1 (Fig. 3K) mediates binding of aminoacyl-tRNAs to 80S ribosomes. This process is driven by hydrolysis of GTP into GDP. Translational elongation factor-1 interacts with guanine nucleotides, 80S ribosomes, and aminoacyl-tRNAs. In addition, Translational elongation factor-1 binds with the β subunit of Translational elongation factor-1 to exchange bound GDP for GTP [41].
Finally, clone 309C (Fig. 3L) DNA sequence was identical with stabilin-1. Stabilin has been described as an endothelial-macrophage member of the fasciclin domain containing protein. Stabilin-1 and -2 are homologous transmembrane proteins showing 7 fasciclin-like adhesion domains, 18–20 EGF domains, 1 X-link domain and 3–6 B-X(7)-B hyaluronan-binding motifs [40]. Stabilin-1 and stabilin-2 are likely to play a role in cell-cell and cell-matrix interactions in vascular cells [40].
Several articles describe the identification of genes associated with the activation of ECs and angiogenesis, as well as the repertoire of genes expressed in normal versus tumor-associated endothelium [42-45]. Because EC activation is a complex process, accompanied by alterations in many aspects of EC physiology, it was not surprising that the genes identified in these studies were in several functional classes. However, the genes identified in the present study are significantly different from those reported by others, which may reflect differences in the approaches used and types of tissues analyzed [42-45]. This preliminary study serves as groundwork for our laboratory, and we believe, it will be worthwhile to examine their role in ECs. Detail study of these candidate genes will entail generation of recombinant cDNA constructs, generation of cell lines, antibodies, fusion proteins, and perform in-situ hybridization among others. Detailed analysis of each of the differentially expressed genes identified in this report is ongoing and is beyond the scope of this article.
Methods
Materials
HUVECs were purchased from Clonetics (CA). Most cell culture reagents were purchased from InVitrogen Corp. Oligonucleotide primers were purchased from Sigma-Genosys; recombinant human VEGF (VEGF165), bFGF, anti-VEGF (MAB293), and anti-MMP1 (MAB900) from R&D Systems; adult human serum-AB, from Gemini Bioproducts; and bovine skin-derived type I collagen, from Cohesion Inc. Affinity-purified anti-α2β1 integrin (MAB1988), anti-αvβ3 integrin (LM609), anti-MMP2 (MAB13405), and anti-VE-cadherin (MAB1989) monoclonal antibodies (mAbs) were purchased from Chemicon Intl; anti-Flt-1 (sc-316), anti-KDR (sc-6251), anti-Kinesin heavy chain (sc-13359), anti-Stanniocalcin (sc-14352), and anti-Grb2 were obtained from Santa Cruz Biotechnology Inc., and anti-β1 integrin subunit mAbs (4B4), from Coulter Inc. Rabbit anti-α2 integrin subunit polyclonal antibodies (pAbs) were a kind gift from Dr. Guido Tarone (University of Torino Pavia, Italy). Anti-MHC class II (W6/32) was purchased from Sigma. Anti-VCIP/PAP2b-RGD antibody was generated by immunizing rabbit with a synthetic peptide modeled after 20 amino acid residues of VCIP/PAP2b (EGYIQNYRCRGDDSKVQEAR) [31].
Cell Culture
Monolayer cell culture was performed as previously described [46,47]. To induce capillary formation, unstarved proliferating HUVECs (passage 4) were gently resuspended (at 6 × 105 cells/ml) in a 3D collagen matrix that was prepared by mixing 7 ml of 3.0 mg/ml type I collagen solution with 1 ml of 10X M199 medium at 4°C, adjusting the pH to 7.5 with 0.1 N sodium hydroxide, adding 0.1 ml of 100X ITS, and the final volume to 10 ml with sterile distilled water. The cells were then seeded in 24-well tissue culture dishes and the matrix was allowed to polymerize for 30 minutes at 37°C. The matrix was subsequently layered with M199 medium containing 20% adult human serum-AB, 4 mM L-glutamine, 1X ITS, and 100 ng/ml VEGF165. The growth medium was supplemented with fresh VEGF165 every 6 hours. The old growth medium was removed and fresh medium + VEGF added every 24 hours.
Suppression Subtractive Hybridization and Differential Display (SSHDD)
HUVECs were cultured in a 3D collagen matrix for 0, 12, 24, 36, 48, and 72 hours, with or without VEGF165 and total RNA was extracted with TRIzol® Reagent (Invitrogen Corp). Integrity of the RNA was determined by 1.2% agarose-formaldehyde gel electrophoresis. Poly(A)+ mRNA was isolated from ~800 μg of total RNA using the FastTrack® 2.0 mRNA Isolation Kit (Invitrogen Corp) and cDNA was subsequently generated using random hexamers as primers and SUPERSCRIPT II™ RNase H- Reverse Transcriptase (Invitrogen Corp). The PCR-Select cDNA Subtraction and PCR-Select Differential Screening Kits (Clontech Laboratories Inc.) were used to enrich for and identify differentially expressed genes in the driver and tester cDNAs [48,49]. Both forward- and reverse-subtraction were performed; untreated HUVEC cDNA served as driver and VEGF-treated HUVEC cDNA served as tester in the former, whereas untreated HUVEC cDNA served as tester and VEGF-treated HUVEC cDNA served as driver in the latter [48,49]. Target cDNA fragments were amplified using the Marathon™ cDNA Amplification Kit, subcloned into pCR®2.1 using the TA Cloning® Kit and transformed into TOP 10 One Shot™ competent cells (all from Invitrogen Corp). DNA sequencings were performed by Applied Biosystems Model 373A (Lonestar Lab).
Northern Blot Analysis
HUVECs were cultured in a 3D collagen matrix for 0, 12, 24, and 36 hours, with or without VEGF165, as described above. Northern blot analysis was performed as previously described [31]. The KDR probe was amplified from full-length KDR cDNA (available in our laboratory). The other probes were amplified from untreated HUVEC cDNA which was generated from total RNA by RT-PCR, as described above. Oligonucleotide primers used for probe amplification from cDNA are shown in Table II.
Table II.
Oligonucleotides used for generating PCR-probes. Genbank accession and their corresponding EST sequence numbers are given in parentheses. F and R denote forward and reverse primers.
| Genes (accession number) | Primers | |
| α2 integrin (X17033 /NM_002203) | (F) | 5'-CTGTAGTAATGTTACCTGCTGGTT-3' |
| (R) | 5'-GGTCTCATCAATCTCATCTGGATT-3' | |
| KDR/VEGFR-2 (X61656) | (F) | 5'-GGTTCTGAGTCCGTCTCATGGAATTG-3' |
| (R) | 5'-GTGTAATTTCCTGTGTCTCTTTCACTC-3' | |
| VE-cadherin(X79981) | (F) | 5'-GATGTTCCCGGAGATCAGAAGACGTC-3' |
| (R) | 5'-TGACTGATGCC ACTTCTCCAAGGTGTG-3' | |
| MMP1 (X54925/NM_002421) | (F) | 5'-CTCTAGAGTCACTGATAC AC AG-3' |
| (R) | 5'-CAGGGTGAC ACCAGTGACTGCAC-3' | |
| MMP2 (NM_004530) | (F) | 5'-GC AGCCGTGCCTTCAGCTCTAC AG-3' |
| (R) | 5'-GAAAGGAGAAGAGCCTGAAGTGT-3' | |
| Clone 3B (U25997) | (F) | 5'-GGAC ACTGCCTTAGCCTCTTGGA-3' |
| (R) | 5'-ATGC AAACTGGTCTAGGTCAGCC-3' | |
| Clone 7D (X65873) | (F) | 5'-GGCATTCTGCACAGATTGCTAAAC-3' |
| (R) | 5'-CCTAAGATGCC AAAATTGCACTC-3' | |
| Clone 12F (BE468199/EST hz69e06.xl/) | (F) | 5'-GGACCAGAGCAGAAGGCCGAGCG-3' |
| (R) | 5'-GGAAGGCCTCGTTAATATCCCGC-3' | |
| Clone 22G (AA130020/EST zo40c12.rl) | (F) | 5'-GCTTCAGATTTACCAACAGCATG-3' |
| (R) | 5'-GGAAGC ATTTCTGTGATTGGTTT-3' | |
| Clone 33A (T35116/AF480883/EST80550) | (F) | 5'GGAGGATCCCTCGCGCCGCAGCCAGCGCCA-3' |
| (R) | 5'-GTGGCACCTAC ATCATGTTGTGGTG-3' | |
| Clone37F (XM_029746) | (F) | 5'-GGTTGATTAAATA ATCTTGACAATG-3' |
| (R) | 5'-CCTTGAACTTC AACAACGTTAAAC-3' | |
| Clone 48A (NM_014776) | (F) | 5'-GGCAGAGGTGCACTTTATGAAACT-3' |
| (R) | 5'-ACGTTACCTTCAACCAGGACAAGG-3' | |
| Clone 54C (AI401257/ESTtg86g04.xl) | (F) | 5'-GC AGTGTTTGTTTTCC AGTCTAG-3' |
| (R) | 5'-GGCTATGGATCTTGATAAAGTAT-3' | |
| Clone 77D (AF169312) | (F) | 5'-AGGACACGGCCTATAGCCTGCAG-3' |
| (R) | 5'-AGAGGCGGCTCTTGGCGCAGTT-3' | |
| Clone 94H (BC013016) | (F) | 5'-GGTC ACATGGATGAGGAGAATGA-3' |
| (R) | 5'-TTGGATAAAGTCTTGATGATCTCC-3' | |
| Clone 263F (BC028674) | (F) | 5'-GTATTGGATTGCCACACGGCTC A-3' |
| (R) | 5'-CGATGCATTGTTATCATTAACCAG-3' | |
| Clone 309 C (NM_015 136) | (F) | 5'-GCTTTGTGGAC AACATGACGCTGA-3' |
| (R) | 3'-CC AAGCCAAGC AGTGCTCC AGCGGC-3' | |
| GAPDH(M33197) | (F) | 5'-GGTCTCCTCTGACTTC AACAGCG-3' |
| (R) | 5'-GGTACTTTATTGATGGTACATGAC-3' |
Western Immunoblot Analysis
HUVEC monolayers were either left untreated or were treated with 100 ng/ml of VEGF or bFGF, or with 20 ng/ml of PMA every 6 hours for 48 hours. Monolayers were then washed with cold PBS, pH 7.5, and solubilized in 1.0 ml of cell extraction buffer (50 mM HEPES, pH 7.5, 1% Triton X-100, 0.1% SDS, 0.25% deoxycholate, 150 mM sodium chloride, 1 mM EDTA, and protease inhibitors. Equal quantities of clarified proteins were resolved by SDS-PAGE under reducing conditions and transferred to a nitrocellulose membrane. Antigen-antibody complexes were detected as described previously [46,47].
Abbreviations
EC, endothelial cell; MMP, matrix metalloproteinases; 3D, three-dimensional; KDR, kinase domain receptor; SSHDD, suppression subtractive hybridization followed by differential display; VE-cadherin, vascular endothelial-cadherin; PMA, phorbol 12-myristate 13-acetate; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor.
Authors' contributions
KKW was responsible for preparation of SSHDD, northern blot, data analyses, and manuscript preparation. GDT was responsible for DNA subcloning, northern blot analysis, DNA sequencing, and BLAST algorithm analysis. JOH performed RT-PCR and Western immunoblotting assays. JY performed RT-PCR, northern and DNA sequencing analysis. All authors read and approved the manuscript.
Acknowledgments
Acknowledgment
Part of this study was made possible by new IBT faculty start-up fund. We thank Dr Magnus Höök and Mingyao Liu for their helpful discussions.
Contributor Information
Kishore K Wary, Email: kwary@ibt.tamu.edu.
Geeta D Thakker, Email: gthakker@lexgen.com.
Joseph O Humtsoe, Email: jhumtsoe@ibt.tamu.edu.
Jun Yang, Email: jyang@ibt.tamu.edu.
References
- Folkman J. Tumor angiogenesis: role in regulation of tumor growth. Symp Soc Dev Biol. 1974;30:43–52. [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Terman BI, Dougher-Vermazen M. Biological properties of VEGF/VPF receptors. Cancer Metastasis Rev. 1996;15:159–163. doi: 10.1007/BF00437468. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358–1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- Madri JA, Williams SK. Capillary endothelial cells: phenotypic modulation by matrix components. J Cell Biol. 1983;97:153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Orci L. Tumor promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42:469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Form DM, Pratt BM, Madri JM. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986;55:521–530. [PubMed] [Google Scholar]
- Peale FV, Gerritsen ME. Gene profiling techniques and their application in angiogenesis and vascular development. J Pathol. 2001;195:7–19. doi: 10.1002/path.888. [DOI] [PubMed] [Google Scholar]
- Lee LY, Patel SR, Hackett NR, Mack CA, Polce DR, El-Sawy T, Hachamovitch R, Zanzonico P, Sanborn TA, Parikh M, Isom OW, Crystal RG, Rosengart TK. Focal angiogen therapy using intramyocardial delivery of an adenovirus vector coding for vascular endothelial growth factor. Ann Thorac Surg. 2000;69:14–23. doi: 10.1016/S0003-4975(99)01102-9. [DOI] [PubMed] [Google Scholar]
- Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through α1β1 and α2β1 integrin. Proc Natl Acad Sci U S A. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- Henderson AM, Wang SJ, Taylor AC, Aitkenhead M, Hughes CC. The basic helix-loop-helix transcription factor HESR1 regulates endothelial cell tube formation. J Biol Chem. 2001;276:6169–6176. doi: 10.1074/jbc.M008506200. [DOI] [PubMed] [Google Scholar]
- Aitkenhead M, Wang SJ, Nakatsu MN, Mestas J, Heard C, Hughes CC. Identification of Endothelial Cell Genes Expressed in an in Vitro Model of Angiogenesis: Induction of ESM-1, βig-h3, and NrCAM. Microvasc Res. 2002;63:159–171. doi: 10.1006/mvre.2001.2380. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Tumor progression: defining the soil round the tumor seed. Curr Biol. 2001;11:R25–27. doi: 10.1016/S0960-9822(00)00038-5. [DOI] [PubMed] [Google Scholar]
- Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS. 1999;107:11–18. doi: 10.1111/j.1699-0463.1999.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Lutz PG, Moog-Lutz C, Coumau-Gatbois E, Kobari L, Di Gioia Y, Cayre YE. Myeoblastin is a granulocyte colony-stimulating factor responsive gene conferring factor-independent growth to hematopoietic cells. Proc Nat Acad Sci U S A. 2000;97:1601–1606. doi: 10.1073/pnas.97.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin IM, Sloane BF. Cathepsin B expression in human tumors. Adv Exp Med Biol. 1996;389:281–294. doi: 10.1007/978-1-4613-0335-0_35. [DOI] [PubMed] [Google Scholar]
- Wang KK. Calpain and caspase: can you tell the difference. Trends Neurosci. 2000;23:20–22. doi: 10.1016/S0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- Mignatti P, Rifkin DB. Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzyme Protein. 1996;49:117–137. doi: 10.1159/000468621. [DOI] [PubMed] [Google Scholar]
- Hamawy AH, Lee LY, Crystal RG, Rosengart TK. Cardiac angiogenesis and gene therapy: a strategy for myocardial revascularization. Curr Opin Cardiol. 1999;14:515–522. doi: 10.1097/00001573-199911000-00012. [DOI] [PubMed] [Google Scholar]
- Fosslien E. Molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci. 2001;31:325–348. [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine 1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Control of angiogenesis by heparin and other sulphated polysaccharides. Adv Exp Med Biol. 1992;313:355–364. doi: 10.1007/978-1-4899-2444-5_34. [DOI] [PubMed] [Google Scholar]
- Kahn J, Mehrabahn F, Ingle G, Xin X, Bryant JE, Vehar G, Schoenfeld J, Grimaldi CJ, Peale F, Draksharapu A, Lewin DA, Gerritsen ME. Gene expression profiling in an in vitro model of angiogenesis. Am J Pathol. 2000;156:1887–1900. doi: 10.1016/S0002-9440(10)65062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filvaroff EH, Guillet S, Zlot C, Bao M, Ingle G, Steinmetz H, Hoeffel J, Bunting S, Ross J, Carano RA, Powell-Braxton L, Wagner GF, Eckert R, Gerritsen ME, French DM. Stanniocalcin-1 alters muscle and bone structure and function in transgenic mice. Endocrinology. 2002;143:3681–3690. doi: 10.1210/en.2001-211424. [DOI] [PubMed] [Google Scholar]
- Goldstein LS. Kinesin molecular motors: transport pathways, receptors, and human disease. Proc Natl Acad Sci U S A. 2001;98:6999–7003. doi: 10.1073/pnas.111145298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Komurasaki T, Uchida D, Morimoto S. Distribution of mRNA for human epiregulin, a differentially expressed member of the epidermal growth factor family. J Biochem. 1997:69–75. doi: 10.1042/bj3260069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JM, Behringer RR. An insertional mutation in the BTF3 transcription factor gene leads to an early postimplantation lethality in mice. Transgenic Res. 1995;4:264–269. doi: 10.1007/BF01969120. [DOI] [PubMed] [Google Scholar]
- Humtsoe JO, Feng S, Thakker GD, Yang J, Hong J, Wary KK. Regulation of cell-cell interactions by phosphatidic acid phosphatase 2b/VCIP. EMBO J. 2003;22:1539–1554. doi: 10.1093/emboj/cdg165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M, Wada I, Imai S, Sakane F, Kanoh H. Cloning and characterization of two human isozymes of Mg2+-independent phosphatidic acid phosphatase. J Biol Chem. 1997;272:24572–24578. doi: 10.1074/jbc.272.39.24572. [DOI] [PubMed] [Google Scholar]
- Hughes WE, Cooke FT, Parker PJ. Sac phosphatase domain proteins. Biochem J. 2000;350:337–352. doi: 10.1042/0264-6021:3500337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P. Actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaki Y, Hashimoto S, Okawa K, Tsubouchi A, Nakamura K, Yagi R, Yano H, Kondo A, Iwamatsu A, Mizoguchi A, Sabe H. An ADP-ribosylation factor GTPase-activating protein Git2-short/KIAA0148 is involved in subcellular localization of paxillin and actin cytoskeletal organization. Mol Biol Cell. 2001;12:645–662. doi: 10.1091/mbc.12.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Kawabe H, Minami S, Honda T, Takaishi K, Shirataki H, Takai Y. SMAP, an Smg GDS-associating protein having arm repeats and phosphorylated by Src tyrosine kinase. J Biol Chem. 1996;271:27013–27017. doi: 10.1074/jbc.271.43.27013. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346:603–610. doi: 10.1042/0264-6021:3460603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B, Fried M. The L19 ribosomal protein gene (RPL19): gene organization, chromosomal mapping, and novel promoter region. Genomics. 1995;25:372–380. doi: 10.1016/0888-7543(95)80036-L. [DOI] [PubMed] [Google Scholar]
- Doudna JA, Rath VL. Structure and function of the eukaryotic ribosome: the next frontier. Cell. 2002;109:153–156. doi: 10.1016/s0092-8674(02)00725-0. [DOI] [PubMed] [Google Scholar]
- Brands JH, Maassen JA, van Hemert FJ, Amons R, Moller W. The primary structure of the alpha subunit of human elongation factor 1. Structural aspects of guanine-nucleotide-binding sites. Eur J Biochem. 1986;155:167–171. doi: 10.1111/j.1432-1033.1986.tb09472.x. [DOI] [PubMed] [Google Scholar]
- Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P, Kannicht C, Kzhyshkowska J, Longati P, Velten FW, Johansson S, Goerdt S. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J. 2002;362:155–164. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croix BS, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins G, Lengauer C, Vogelstein V, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, Croix BS, Romans KE, Choti MA, Lengauer C, Kinzler KW, Vogelstein B. A phosphatase associated with metastasis of colorectal cancer. Science. 2001;294:1343–1346. doi: 10.1126/science.1065817. [DOI] [PubMed] [Google Scholar]
- Abe M, Sato Y. cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis. 2001;4:289–298. doi: 10.1023/A:1016018617152. [DOI] [PubMed] [Google Scholar]
- Weston CG, Haviv I, Rogers PA. Microarray analysis of VEGF-responsive genes in myometrial endothelial cells. Mol Hum Reprod. 2002;8:855–863. doi: 10.1093/molehr/8.9.855. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couple a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999;274:10002–10007. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, Siebert SD. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci U S A. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchenko L, Lukyanov S, Lau YF, Siebert PD. Suppression subtractive hybridization: a versatile method for identifying differentially expressed genes. Methods Enzymol. 1999;303:349–380. doi: 10.1016/s0076-6879(99)03022-0. [DOI] [PubMed] [Google Scholar]