Abstract
Testosterone and estrogen are no longer considered male only and female only hormones. Both hormones are important in both sexes. It was known as early as the 1930's that developmental exposure to a high dose of estrogen causes malformation of the male reproductive tract, but the early formative years of reproductive biology as a discipline did not recognize the importance of estrogen in regulating the normal function of the adult male reproductive tract. In the adult testis, estrogen is synthesized by Leydig cells and the germ cells, producing a relatively high concentration in rete testis fluid. Estrogen receptors are present in the testis, efferent ductules and epididymis of most species. However, estrogen receptor-α is reported absent in the testis of a few species, including man. Estrogen receptors are abundant in the efferent ductule epithelium, where their primary function is to regulate the expression of proteins involved in fluid reabsorption. Disruption of the α-receptor, either in the knockout (αERKO) or by treatment with a pure antiestrogen, results in dilution of cauda epididymal sperm, disruption of sperm morphology, inhibition of sodium transport and subsequent water reabsorption, increased secretion of Cl-, and eventual decreased fertility. In addition to this primary regulation of luminal fluid and ion transport, estrogen is also responsible for maintaining a differentiated epithelial morphology. Thus, we conclude that estrogen or its α-receptor is an absolute necessity for fertility in the male.
Introduction
It was known as early as the 1930's that the developing testis was responsive to the "female" hormone [[1], also reviewed by [2]]. It was also known in the 1930's and 40's that developmental exposure to high doses of estrogens could induce malformation of the male reproductive tract [3-6]. Thus, during the formative years of reproductive biology as a discipline it was suggested that estrogen might be important in the male; however, even in the early 1990's many scientists considered estrogen receptor presence in the adult male reproductive tract to be a remnant from the indifferent sex stage of embryological differentiation [7].
Reference to estrogen production by the testis was more of a curiosity at first, as efforts were made to determine the various metabolites of testosterone being produced [8-11]. During the 1970's, the prediction of an estrogen receptor in testis and epididymis became a reality as estradiol binding was discovered [12-15]. However, it was clear from subsequent publications that most scientists did not consider estrogen to be a major steroid hormone in the male reproductive tract, in the adult [16-19]. The potential importance of estrogen during development of the male reproductive system was made popular by the report that diethylstilbestrol (DES) treatment during pregnancy induced cryptorchidism and epididymal cysts in male mice [20]. This discovery opened the door to numerous investigations into the long-term effects of developmental exposure to estrogenic compounds on male reproduction, an inquiry that continues today [21,22]. Although estrogen effects in the developing male are important, such studies have not actually proven that estrogen has a role in the adult male reproductive organs. At best, it was thought that an estrogen binding ability was left over from developmental processes and that estrogen played only a small role in the adult male [7,23,24].
Most interesting was the discovery that cytochrome P450 aromatase, which is capable of converting androgens into estrogens, is present in the testis [25-39]. During this same period of discovery, others were using the radioimmunoassay to identify steroids present in body fluids and estrogen concentrations were found to be relatively high in seminal and rete testis plasma [40-48]. Thus, up to the 1990's it appears that most scientific inquiry into estrogen's presence in the male remained a curiosity, as well as a worry that estrogen exposure during development was harmful. Then, in the decade of the 90's new discoveries in the male led to the hypothesis that estrogen not only has important functions in the adult male reproductive tract, but that estrogen and its α-receptor are "essential" for normal fertility. This new paradigm for estrogen's role in the male began with the discovery that testicular germ cells and epididymal sperm contain aromatase and synthesize estrogen [49]. This discovery explained the presence of a high concentration of estradiol in rete testis of the rat [41] and provided a source of estrogen for the high concentration of receptors that were subsequently found to populate the head of the male reproductive tract [50-55]. However, an estrogen function was not uncovered until the ERα knockout (αERKO) was produced. The αERKO mouse, originally generated by Dennis Lubahn and colleagues [56], showed for the first time that ERα is essential for fertility in the male [56-58]. This animal model was further developed to show that estrogen provides a physiological function in regulating fluid dynamics in the male reproductive tract, a function that is "essential" for normal reproductive performance [59-66].
Estrogen in the male tract
Estrogen is produced in sizable quantities in the testis, as well as the brain [67]. It is also present in very high concentrations in the semen of several species [40-48]. Table 1 shows the reported locations for estrogen synthesis in the adult male reproductive system from several species. Early studies reported that the primary source of estrogen in the immature male was the Sertoli cell [68]. In the adult testis, Leydig cells express aromatase (P450arom) and actively synthesize estradiol at a rate much greater than that seen in the adult Sertoli cell [31,32,38,69-72]. Currently, a growing body of evidence indicates that germ cells also synthesize estrogen, and possibly serve as the major source of this steroid in the male reproductive tract [see review by [72]]. In 1993, in collaboration with the laboratories of Bahr and Bunick [49], we reported for the first time that P450arom is present in testicular germ cells of the adult male mouse. The enzyme was localized in the Golgi of round spermatids and throughout the cytoplasm of elongating and late spermatids. Its presence was confirmed by Western and Northern blot analysis of isolated germ cells. Its activity in germ cells was equal to or exceeded the activity found in the interstitial cells. More recently, Carreau and others [72,73] have shown aromatase expression and activity in the human sperm.
Table 1.
P450 Aromatase in the adult male reproductive system.
| Species | Leydig cells | Sertoli cells | Germ cells | Spermatozoa | References |
| Mouse1 | + | + | + | [49,74] | |
| Rat1 | + | + | + | [31,32,34,36,38,69,75,77,81,151-154] | |
| Rooster | + | + | [76] | ||
| Bear2 | + | + | + | [155] | |
| Boar | + | [156] | |||
| Ram | + | [157] | |||
| Stallion | + | + | + | [158-160] | |
| Bank vole | + | + | + | [79,161,162] | |
| Rainbow trout fish | + | [163] | |||
| Dogfish shark | + | [164,165] | |||
| Marmoset | + | [151] | |||
| Rhesus | + | + | [166] | ||
| Human | + | + | [72,73,80,151] |
1 Early work showed only Leydig cells being positive for Aromatase in the adult testis 2 Location depended upon the season[155].
The presence of P450arom in male germ cells has now been demonstrated in several species, including mouse, rat, brown bear, the bank vole, rooster, and man [49,52,73-80]. The enzyme is located in cytoplasmic droplets of the sperm tail, but the staining becomes less intense as sperm traverse the epididymis [73,75]. Its presence in germ cells and spermatozoa was recently confirmed and shown to represent approximately 62% of the total testicular aromatase [69,70,81]. Testicular germ cells in the boar, ram and stallion have not been shown to be aromatase-positive. It is unclear whether this is due to differences in antibodies used or if some species simply do not generate estradiol by the germ cell pathway. It would be interesting to determine if aromatase is expressed in the epididymal tract of those species lacking germ cell expression. Others have shown the absence of aromatase in the mouse epididymis [82]; thus, the conversion of androgens to estrogens by sperm remains the primary source of estrogen in the lumen of the reproductive tract of this species. This observation raises new and exciting hypotheses regarding the potential for estrogen to regulate functions in the efferent ductules, epididymis and vas deferens.
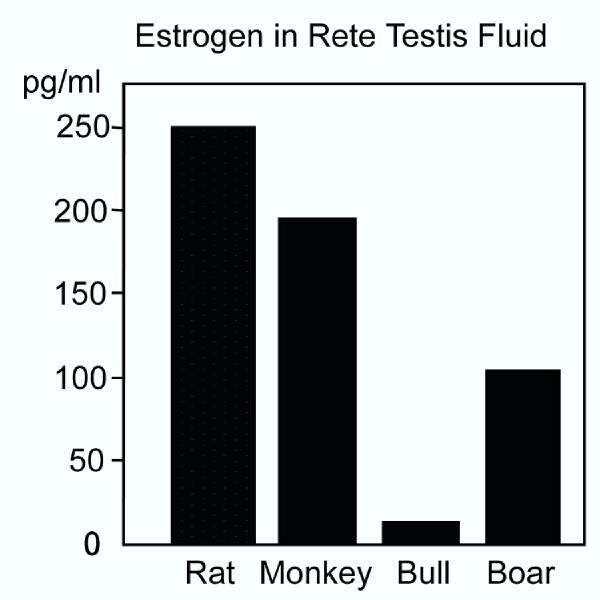
The concentration of estrogens in peripheral blood is typically low in the male, but ranges from 2–180 pg/ml depending upon the species [40,42-47,83-88]. The horse is an exception, where estrone sulfate is found as high as 2,447 pg/ml [40,88]. Estrone-sulfate concentration is 900 ng/ml in testicular lymph in the horse, suggesting that intra-testicular estrogens can be rather high [89]. Estrogen concentrations are typically higher in the testicular vein and lymph than in the general circulation. Also, in the reproductive tract, estrogen can reach relatively high concentrations (Fig. 1). In one report, estrogen concentration in rete testis fluid of the rat was approximately 250 pg/ml [41], which is higher than the average serum concentration of estradiol in the female [83,90]. Estrogens are also abundant in semen and depending upon the species, their concentrations can range from 14 to nearly 900 pg/ml [42-46]. Estrone-sulfate is found as high as 4,000 pg/ml in the horse [40].
Figure 1.
Estrogen in rete testis fluid. Mean concentrations (pg/ml) for estradiol or total estrogens in four species, rat [41], monkey [44], bull [42] and boar [46].
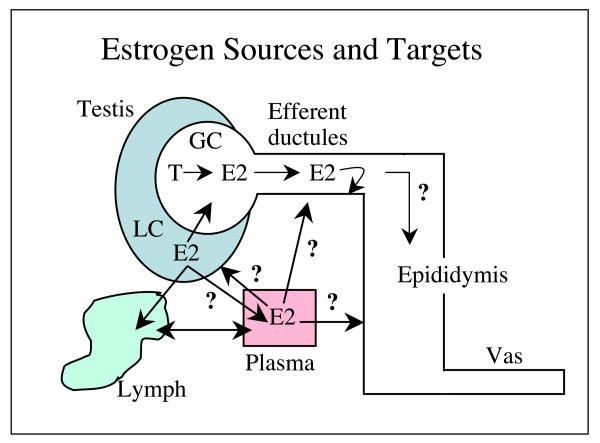
The potential sources of estrogen in the male reproductive tract are illustrated in Fig. 2. Although the concentration of estradiol is known for various compartments of the male tract (Fig. 1), the relative amounts of estradiol that are derived from the different sources are not known. For many years it was assumed that most of the testicular estrogen was derived from Leydig cells (Table 1). However, with the discovery that germ cells also synthesize estrogen, the Leydig cell is no longer required as a source for estrogen in the reproductive tract lumen. Actually, it is more likely that Leydig cell derived estradiol would move toward the lymphatics, because the cells lie adjacent to endothelial cells of the lymphatic system and estrogens are reported to be in very high concentration within testicular lymphatics [47,88]. Because blood estrogens are in low concentrations in the male, we would assume that this source would provide limited endocrine activity in the reproductive tract. In the efferent ductules, the blood source would likely have even less effect than in the remainder of the reproductive tract, as these ductules are responsible for reabsorption of over 90% of the luminal fluids [91] and thus display an overwhelming luminal to basal orientation, which could limit the movement of substances from basement membrane into the cell cytoplasm. Although this hypothesis has not been tested directly, there are studies suggesting that this region of the male tract does not respond to exogenous androgens following castration [92].
Figure 2.
Estrogen sources and targets in the male reproductive tract. Estradiol 17β (E2) is produced in peripheral tissues and delivered via the plasma, but is also synthesized by Leydig cells (LC) in the testicular interstitium. The contribution of E2 from testis to plasma and from the vasculature to the testis is unknown, but it is assumed that most of the lymphatic E2 would be derived from LC. LC and germ cells (GC) contain p450 aromatase in the adult testis. LC may also contribute to the E2 concentrations in the rete testis fluid, but it is more likely that germ cell production of E2 provides the estrogen that will target the efferent ductule epithelium, the region that contains the highest concentration of ER. Less is known of E2 function and targets in the epididymis and vas deferens.
Estrogen receptors in the male tract
It has been known for at least 25 years that an estrogen receptor-like protein exists in epididymal tissues [12]. However, those early studies lead to the conclusion that estrogen was more important during development of the epididymis than in adult function [17]. Estrogen binding in epididymal tissues has been noted in many species, including the dog [93,94], human [95], turtle [96], monkey [97,98], ram [99], guinea pig [100], and the rat [101]. Autoradiography was also used to show estrogen binding throughout the male reproductive system [55,102]. Schleicher and coworkers [102] found very strong labeling of the efferent ductules and initial segment epididymis, with lesser binding in the distal tract. However, binding assays do not differentiate between ERα and β. Therefore, other methods, such as immunocytochemistry (ICC), in situ hybridization and Northern blot analysis, have been used to separate the two ER subtypes. However, these techniques do not always provide identical results, and there are disagreements between laboratories and between species. Using ICC, ER has been localized primarily in the epithelium of efferent ductules [53,55,98,103-108]. However, in the goat and monkey, only nonciliated cells of the efferent ductal epithelium stained ER positive [54,98]. After the discovery of ER subtypes and the production of specific antibodies, ERα localization in the epididymis has also given confusing results [53,59,103-105,109,110]. In the mouse at 90 days of age, the efferent ductule epithelium was strongly positive for ERα immunostaining, using the H222 antibody [51]. Other epithelia along the epididymis were only slightly positive. Using a different antibody, the mouse epididymis showed strong ERα staining in principal cells and other cell types, but in a region specific manner [110]. This immunostaining is somewhat similar to the autoradiography data previously shown by Schleicher [102].
In the testis, ERβ is the more abundant receptor and is typically found in nearly every cell type of the interstitium and the seminiferous tubule, except for the elongated spermatids [108-121]. In contrast, ERα is found only in the interstitium of the testis in most species examined [51,53,109,110,122,123]. In some species both Leydig and peritubular myoid cells are ERα positive but the testis of the goat, monkey and human are reportedly devoid of ERα [98,104,108]. The ERβ knockout mouse [124,125] shows no testicular phenotype and the αERKO and double ERαβ knockout mice [56-58,125,126] show no testicular phenotype during early development, suggesting that these receptors are not essential for normal development of sperm in the testis.
Transplantation of germ cells from the αERKO mouse testis into normal testis (made devoid of sperm) results in normal spermatozoa capable of fertilization and the production of offspring [126], suggesting that testicular ERα has no influence on spermatogenesis. However, loss of estrogen synthesis in the aromatase knockout mouse [127,128] results in decreased fertility with aging. Another study in the mouse also suggests that estrogen may have a testicular function, acting through the Leydig cells. It has been suggested that testosterone concentrations are elevated in the αERKO male [57], but it was generally concluded that this increase was due to the disruption in feedback regulation at the hypothalamus. However, a more recent study found that Leydig cells isolated from the αERKO testis had increased production of testosterone and that normal Leydig cells when treated with the pure ER inhibitor ICI 182,780 also showed increased steroidogenesis [129]. Therefore, ER in the testis, although not necessarily essential for spermatogenesis, does appear to have a subtle function in the Leydig cells.
In the rat, ERα localization has been more controversial. In one study, using a mouse monoclonal antibody (6F11) against the A/B region of the human ERα, positive staining was found only in epithelial cells of the efferent ductules [53]. The epididymal tissues were negative. Our laboratory repeated this study using the 6F11 antibody (Novocastra, UK) and the data are in complete agreement with the Fisher study, showing staining only in epithelia of the efferent ductules [130]. In another study using frozen sections and the ER21 antibody, which is made against a peptide containing the first 21 amino acids of the rat and human ERα (does not cross-react with ERβ), we also found predominant staining in efferent ductules [59], as shown for all species examined to date. However, the initial segment epididymis was also strongly positive and the remaining regions of the epididymis were moderately positive. This study was repeated, but using antigen retrieval methods instead of frozen sections, and the results differed only slightly [130]. The major difference was in staining that was observed in the epithelium of the vas deferens, which was negative using frozen sections. This difference in staining in the rat between the two antibodies, 6F11 and ER21, raises serious questions regarding the literature's description of ER localization in the male reproductive tract using ICC alone. Autoradiography and estradiol binding assays indicate that ER is present in the rat epididymis. RT-PCR data also show that ERα is present in epididymal tissues [59,108]. Therefore, future studies should focus on in situ hybridization methods for localizing the mRNA in specific regions and cell types of the epididymis.
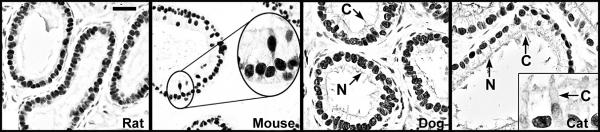
Although there are reported differences in ERα localization in the epididymis of various species, its presence in efferent ductule epithelium has remained constant across species (Fig. 3). ERα protein is abundant in epithelial cells of the efferent ductule, with intense immunohistochemical staining of the nonciliated cell nucleus and the ciliated cells showing considerable variability in staining. The presence of an abundance of ERα protein in efferent ductule tissue is supported by an elevated expression of its mRNA. A previous study by our laboratory reported that ERα mRNA expression in efferent ductules of the rat is 3.5 fold greater than in the uterus [55]. Thus, in comparison to the well-recognized estrogen-responsive female tissue, the efferent ductules of the male reproductive tract are also a major target for estrogen action. Several laboratories [95,108,131,132] have reported evidence for ER in the human efferent ductules and epididymis. However, in some cases the principal cells were negative, while the basal cells and stromal cells were positive. The epididymis in nonhuman primates is also ER positive by RT-PCR, but there was no distinction between the α and β subtypes [133].
Figure 3.
Estrogen receptor-α immunohistochemistry in the efferent ductules. ERα is abundant in the ductules of most species examined. Represented here are ductules from the rat, mouse, dog and cat [109,110,130]. Ciliated (C) and nonciliated (N) cells are strongly positive in all these species, except the cat, where ciliated cells show weak staining. Bar = 25 μm.
The discovery of a second form of ER (ERβ) further complicates the interpretation of earlier data from estrogen binding studies. ERβ has now been found in testis, efferent ductules, epididymis and prostate [55,101,108,119,124,134-137]. However, a function for ERβ in the male reproductive tract awaits further investigation, as the ERβ knockout mouse has been shown to be fertile and appears to have a normal testis and epididymis [124]. ERβ is more widely distributed in the male tract than ERα [130]. ERβ has strong reactivity in efferent ductules, similar to ERα. In the remainder of the tract, ERβ appears to be weaker in initial segment epididymis but stronger in the corpus, cauda and vas deferens. The stromal tissue cells also stain strongly positive for ERβ throughout the male reproductive tract. Thus, there is a large potential for estrogen binding in the epididymis and vas deferens through ERβ.
Estrogen function in testis
There is limited direct evidence that estrogen has a major role in adult testicular function [see review by [127]], other than the recent paper by Hardy and colleagues [129], in which the antiestrogen ICI 182,780 inhibited in vitro Leydig cell production of testosterone. Estradiol alone was unable to stimulate Leydig cell steroidogenesis. In the developing testis, estrogen has significant activity in establishing Sertoli cell function [127] and potentially even in establishing Sertoli-germ cell adhesion [138,139]. However, in the total absence of estrogen synthesis, the ArKO male shows normal spermatogenesis at the beginning of puberty and only with aging does the testis begin to develop lesions associated with the round spermatids [127,140]. This is not entirely surprising in light of the fact that ERα is not present within the seminiferous epithelium [109,110] and although ERβ is found in Sertoli cells and nearly all germ cells [108-110,141,142], the ERβ knockout (β ERKO) male testis appears normal and the males are fertile [58,124,125].
Indirect evidence of estrogen's influence on spermatogenesis comes from animal models such as the hpg mouse, which is deficient in gonadotropin releasing hormone (GnRH). Ebling and colleagues [143] found that estradiol implants in the hpg mouse stimulated a 4-5-fold increase in seminiferous tubular volume, in the absence of measurable levels of androgens. Although it is possible that this effect was due to the slightly elevated levels of FSH, an alternative hypothesis put forward was direct effects of estrogen on cells of the testis. This hypothesis appears plausible when the ArKO mouse data are taken into consideration. The ArKO testis is normal at first, but with aging shows decreases in testis weight, seminiferous epithelium, and germ cell numbers [144]. When the ArKO male is maintained on a soy-free diet, these effects are accelerated and enhanced [127,140]. Thus, soy based phytoestrogens likely protected the testis somewhat in the ArKO mouse, suggesting that small amounts of estrogen do have testicular effects independent of effects due to FSH or LH. This role of estrogen in the testis will most likely be found in the germ cells, as they express ERβ abundantly [108-110,142] and genistein has a higher affinity for ERβ than for ERα [145]. Finally, although the Sertoli cell does not express ERα, it is interesting that in the αERKO testis there is significantly less seminiferous tubular secretion than in the wild-type testis [59]. The same effect was suggested for the ArKO testis, as seminiferous tubule luminal volume and tubular length was decreased [140]. Thus overall, estrogen does appear to have subtle functions in the testis, not only at the Leydig cell but also possibly targeting the seminiferous epithelium, too.
Estrogen function in efferent ductules
Efferent ductules are a major site for estrogen function in the male reproductive tract, across numerous species. These ductules are a series of tubules that connect rete testis to the epididymis (Fig. 4). One-third or more of the head of the epididymis in man and other mammals contains these ducts and it was once thought that they simply transported sperm from testis to the epididymis. However, it is now known that efferent ductules have an important function in the reabsorption of over 90% of the rete testis fluid and thereby concentrate sperm prior to entering the epididymal lumen [91]. Nonciliated cells of the epithelium are reabsorptive, similar to proximal tubules of the kidney, having a brush border of microvilli connecting in the apical cytoplasm to a profusion of apical canaliculi, vesicles, tubules and membrane-bound bodies, which constitutes an elaborate endocytotic/lysosomal system [146]. In the basal region, rough endoplasmic reticulum, mitochondria and lipid droplets are common [147]. The efferent ductules express an abundance of both androgens and estrogen receptors [109,110,130].
Figure 4.
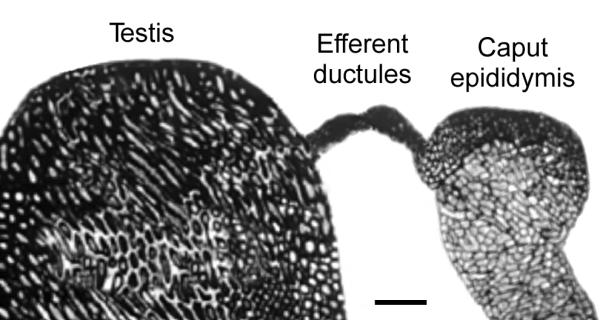
Testis, efferent ductules and epididymis. The surrounding fat pad was dissected away to show the efferent ductules that lie between the testis and caput epididymis. Bar = 2 mm.
Much of what we know about estrogen's function in efferent ductules has been derived from the study of the αERKO mouse and the use of antiestrogen treatment models. The male αERKO mouse was found to be infertile [56], raising the possibility that ERα is required for normal function of the male reproductive system. Although the αERKO testis appeared normal before puberty, after the onset of spermatogenesis, the testis began to degenerate and eventually became atrophic [57]. By 150 days, cauda sperm from the αERKO male were abnormal and sperm concentrations were significantly reduced [57], suggesting that the reproductive tract was also abnormal. A later study by Eddy's lab showed that αERKO germ cells transplanted into a normal testis (treated with busulphan to remove native germ cells) were capable of fertilization [148]. That study clearly pointed to extra-testicular regions, such as the efferent ductules and epididymis, being the major source of pathological alterations in αERKO males [57,59].
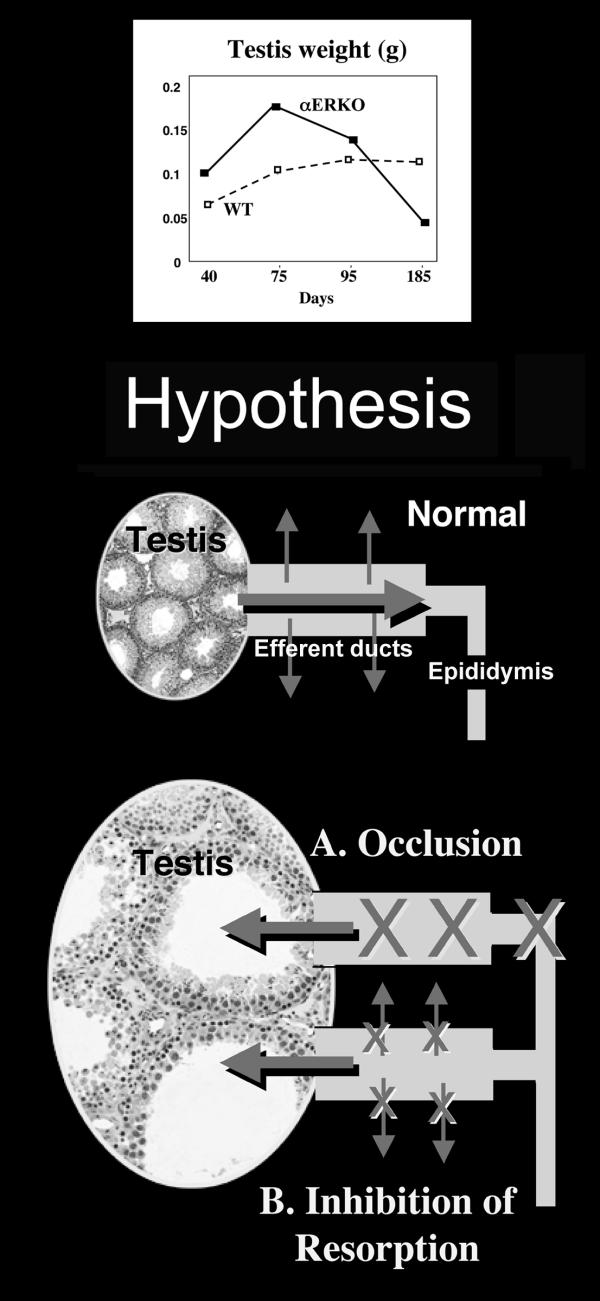
The rete testis in αERKO males is dilated and protrudes into the testis [57,59]. Based upon this data, we hypothesized that the efferent ductules were either a) occluded due to excessive reabsorption, or b) dilated due to an inhibition of fluid reabsorption (Fig. 5). After careful examination, we found the second hypothesis to be true, as the efferent ductule lumen was dilated markedly [59]. There appeared to be an inhibition of fluid reabsorption and possibly a net inward flux of water into the ductal lumen. Thus, the excessive accumulation of fluid in the lumen was overloading the funnel-like ductal system found in the rodent. As predicted, the accumulation of fluid caused a transient increase in testis weight in αERKO males between 32–81 days of age and then a steady decrease in weight out to 185 days of age, when total atrophy was observed. These data suggested that long-term atrophy of testes in the knockout mouse was caused by backpressure of the accumulating luminal fluids, a well-recognized pathogenesis found after exposure to various toxicants [59,149]. However, atrophy was not induced by antiestrogen treatment in adult mice (unpublished data), suggesting that in the αERKO mouse, this pathological event is due to a developmental anomaly.
Figure 5.
Hypothesis to account for testicular weight increase in the αERKO mouse. The αERKO mouse testis was shown to increase in weight from day 40 to 75 days of age, and then the weight declined until the testis was atrophied by day 185 [59]. Two hypotheses were proposed to account for mechanisms that could explain the transient increase in testis weight prior to regression. In the normal testis, efferent ductules receive low concentrations of sperm from the rete testis. Approximately 95% of this fluid is reabsorbed by the efferent ductule epithelium, which increases the concentration of sperm that enter the epididymis. Disruption of ERα causes testicular swelling through one of two possible mechanisms: A. the efferent ductules become occluded, or B. the fluid reabsorption pathways are inhibited. Both mechanisms will result in fluid accumulation in the seminiferous tubules and backing up of fluids into the testis. Atrophy occurs by an unknown mechanism that inhibits spermatogenesis.
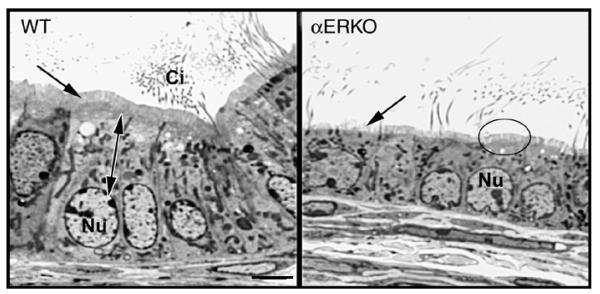
In the αERKO efferent ductule epithelium (Fig. 6), the endocytotic apparatus was nearly lost and other cytoplasmic organelles appeared reduced and scattered randomly [59,60,62,63,149]. The endocytotic pathway includes apical vesicles and PAS+ lysosomal granules, which are prominent in nonciliated cells of normal efferent ductules [91,147,150]. The αERKO epithelium was also flattened and the microvillus border was shortened and even absent in some cells. All of these changes are consistent with a decrease in fluid reabsorption, which was observed in the αERKO male [59]. Thus, in the absence of a functional ERα, the apical surface of this reabsorbing epithelium appeared to be transformed into a non-absorbing structure.
Figure 6.
Histology of the efferent ductule epithelium in αERKO mouse. The wild-type (WT) ductule epithelium is columnar in shape with nonciliated cells that contain large spherical to oblong shaped nuclei (Nu) and extensive apical cytoplasm (double arrow). The nonciliated cell has a tall microvillus brush border (arrow) and extensive endocytotic apparatus. The ciliated cells have motile cilia (Ci) that extend into the lumen. The αERKO efferent ductule epithelium has a low cuboidal shape, with the apical cytoplasm reduced in size and the nucleus (Nu) also smaller. Microvilli are sparse on some cells (arrow) and reduced in height in other cells (circle). Bar = 10 μm.
The αERKO mouse provided the first strong evidence that estrogen, or more specifically, a functional ERα, is involved in the regulation of fluid transport in the male reproductive tract, and responsible for increasing the concentration of sperm as they enter the epididymis. Subsequent studies have shown that the major Na+ transporter in the efferent ductule epithelium (NHE3) is down regulated in the αERKO male reproductive tract. Both the mRNA and NHE3 protein were decreased substantially in αERKO tissue, and Na+ uptake by the epithelial cell in vitro was negligible [63]. However, the αERKO mouse lacks a functional ERα throughout development. Therefore, the morphological and physiological abnormalities observed could represent developmental defects, rather than adult dysfunction. To test this hypothesis, adult mice were treated with a pure antiestrogen, ICI 182,780 (AstraZeneca, Macclesfield, Cheshire, UK). This collaborative study with David Bunick and Janice Bahr showed conclusively that ERα is important for adult function of the efferent ductules, as ICI induced pathological changes that were nearly identical to those seen in the αERKO mouse [60]. A second species, the adult male rat, also responds in a similar manner to ICI treatment over a 125-day period [65,66]. The two major response variables, dilation of efferent ductule lumen and decreased expression of NHE3, show identical responses in rats and mice [63,65]. Although the rats became infertile, they did show greater variation in response overall than was seen in the ICI-treated mice. Long-term treatment in the rat resulted in a transient increase in testicular weight, eventual testicular atrophy at the time of infertility, whereas in the ICI-treated mouse there was no change in testicular weight. After ICI treatment, the rat efferent ductule epithelium also showed a transient increase and redistribution of PAS-positive lysosomal granules in the nonciliated cells [65,66]. However, with continued treatment the rat epithelium showed a decrease in the number of lysosomes to nearly undetectable levels [59], similar to αERKO and mice treated with ICI. Lysosomes are more numerous in the rat than in the mouse efferent ductules [147]; therefore, this intriguing interspecies difference in response to the antiestrogen must be examined in future studies involving other species. Overall, it was shown that ICI promotes adult dysfunctional changes in rat efferent ductules similar to those of αERKO and ICI treated mice, with luminal dilation, decreases in epithelial height, loss of cytoplasmic organelles and decreases in the expression of NHE3 protein and mRNA [65,66].
Summary and Conclusions
Estrogen is important in the regulation of the male reproductive tract, with clear evidence pointing to a direct effect on the function of Leydig cells and the efferent ductule epithelium, but potential effects also on germ cells (Fig. 7). Estrogen is synthesized by the germ cells, producing a relatively high concentration in rete testis fluid. Estrogen receptors are abundant throughout the male reproductive tract, but ERα is primarily localized in the efferent ductule epithelium, where its expression is more abundant than even the female reproductive tract. Estrogen's primary function in the male tract appears to be the regulation of fluid reabsorption in the efferent ductules via the ERα. Disruption of the receptor, either in the knockout (αERKO) or by treatment with a pure antiestrogen, results in dilution of cauda epididymal sperm, disruption of sperm morphology, inhibition of sodium transport and subsequent water reabsorption, increased secretion of Cl-, and eventual decreased fertility. In addition to this primary regulation of luminal fluids and ions, estrogen is also responsible for maintaining a differentiated epithelial morphology. Thus, we conclude that estrogen or its receptor is an absolute necessity for fertility in the male.
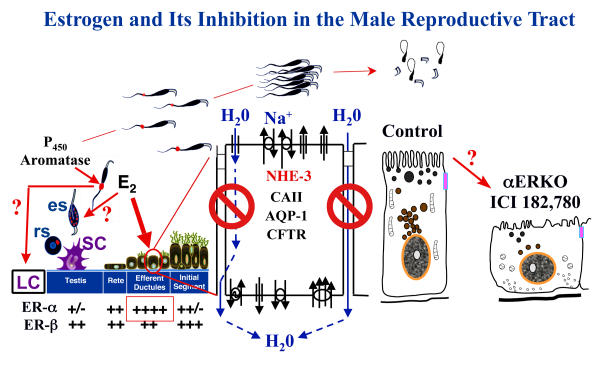
Figure 7.
Estrogen and its inhibition in the male reproductive tract: a summary. In adult males, germ cells, as well as Leydig cells (LC) contain P450 aromatase and actively synthesize estrogen (E2), which produces a relatively high concentration in rete testis fluid. This luminal estrogen targets estrogen receptors that are abundant throughout the male reproductive tract, but particularly ERα that is localized in the efferent ductule epithelium, where its expression is more abundant than even the female reproductive tract. In the testis, E2 may also feedback to influence the function of LC and spermatids, either round spermatids (rs) or elongated spermatids (es). Estrogen's primary function in the male tract is the regulation of fluid reabsorption in the efferent ductules via ERα, which increases the concentration of sperm prior to entering the epididymis. Disruption of ERα, either in the knockout (αERKO) or by treatment with a pure antiestrogen ICI 182,780, results in a decrease in Na+ transport from lumen to interstitium and thus a decrease in water (H2O) and fluid reabsorption. This inhibition is mediated by a decrease in the expression of NHE3 mRNA and protein and also decreases in carbonic anhydrase II (CAII) and aquaporin I (AQP-1) proteins. There is also an increase in cystic fibrosis transmembrane conductance regulator protein and mRNA, which adds to the NHE3 effect by secreting Cl- into the lumen by the cystic fibrosis transmembrane conductance regulator (CFTR) [64]. This inhibition of fluid reabsorption results in the dilution of cauda epididymal sperm, disruption of sperm morphology, and eventual decreased fertility. In addition to this primary regulation of luminal fluids and ions, estrogen is also responsible for maintaining a differentiated epithelial morphology through an unknown mechanism.
Acknowledgments
Acknowledgments
I would like to acknowledge recent students of my laboratory whose work has helped to shape our understanding of estrogen function in the male: Masaaki Nakai, Rong Nie, Qing Zhou and Cleida Oliveira. The excellent technical support of Kay Carnes is always appreciated. Supported by grants from NIH # HD35126 and CONRAD.
References
- Wolff E, Ginglinger A. Sur la transformation des Poulets males en intersexues par injection d'hormone femelle (folliculine) aux embryons. Archs Anat Histol Embryol. 1935;20:219–278. [Google Scholar]
- Weniger JP. Aromatase activity in fetal gonads of mammals. J Dev Physiol. 1990;14:303–306. [PubMed] [Google Scholar]
- Burrows H. Pathological conditions induced by oestrogenic compounds in the coagulating gland and prostate of the mouse. Am J Cancer. 1935;23:490–512. [Google Scholar]
- Greene RR, Burrill MW, Ivy AC. Experimental intersexuality. Am J Anat. 1940;67:305–345. [Google Scholar]
- Arai Y, Mori T, Suzuki Y, Bern H. Long-term effects of perinatal exposure to sex steroids and diethylstilbestrol on the reproductive system of male mammals. Int Rev Cytol. 1983;84:235–265. doi: 10.1016/s0074-7696(08)61019-0. [DOI] [PubMed] [Google Scholar]
- McLachlan JA. Transplacental effects of diethylstilbestrol in mice. Natl Cancer Inst Monogr. 1979:67–72. [PubMed] [Google Scholar]
- Greco TL, Duello TM, Gorski J. Estrogen receptors, estradiol, and diethylstilbestrol in early development: the mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev. 1993;14:59–71. doi: 10.1210/er.14.1.59. [DOI] [PubMed] [Google Scholar]
- Baggett B, Engel LL, Balderas L, lanman G, Savard K, Dorfman RI. Conversion of C14-androgens to C14-estrogenic steroids by endocrine tissues. Endocrinology. 1959;64:600–608. doi: 10.1210/endo-64-4-600. [DOI] [PubMed] [Google Scholar]
- Bedrak E, Samuels LT. Steroid biosynthesis by the equine testis. Endocrinology. 1969;85:1186–1195. doi: 10.1210/endo-85-6-1186. [DOI] [PubMed] [Google Scholar]
- Staffieri JJ, Badano H, Celoria G. [Study of testicular estrogenic production in normal individuals and in patients with various alterations of the seminiferous tube] Rev Iber Endocrinol. 1965;12:85–93. [PubMed] [Google Scholar]
- Weniger JP, Zeis A. [Induction of estrogen production in the embryonic chicken testicle by dihydrotestosterone] Biochimie. 1973;55:1163–1164. doi: 10.1016/s0300-9084(73)80456-0. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Eller BC, Judy LA, Trautman JR, Orgebin-Crist MC. Estradiol binding in cytosol from epididymides of immature rabbits. Mol Cell Endocrinol. 1975;2:91–105. doi: 10.1016/0303-7207(75)90051-9. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Wolfe MS, Curry JB. The presence of an estradiol binding component in cytosol from immature rat epididymides. Mol Cell Endocrinol. 1977;6:271–279. doi: 10.1016/0303-7207(77)90101-0. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Sutton W, Eller BC, Danzo BJ, Wolfe MS, Curry JB. Analysis of [3H]estradiol binding to nuclei prepared from epididymides of sexually immature intact rabbits: The presence of an estradiol binding component in cytosol from immature rat epididymides. Mol Cell Endocrinol. 1978;9:291–301. doi: 10.1016/0303-7207(78)90071-0. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Eller BC. The presence of a cytoplasmic estrogen receptor in sexually mature rabbit epididymides: comparison with the estrogen receptor in immature rabbit epididymal cytosol. Endocrinol. 1979;105:1128–1134. doi: 10.1210/endo-105-5-1128. [DOI] [PubMed] [Google Scholar]
- Hendry W. J. d, Eller BC, Orgebin-Crist MC, Danzo BJ. Hormonal effects on the estrogen receptor system in the epididymis and accessory sex organs of sexually immature rabbits. J Steroid Biochem. 1985;23:39–49. doi: 10.1016/0022-4731(85)90258-4. [DOI] [PubMed] [Google Scholar]
- Danzo BJ. A protease acting on the estrogen receptor may modify its action in the adult rabbit epididymis. J Steroid Biochem. 1986;25:511–519. doi: 10.1016/0022-4731(86)90396-1. [DOI] [PubMed] [Google Scholar]
- Toney TW, Danzo BJ. Developmental changes in and hormonal regulation of estrogen and androgen receptors present in the rabbit epididymis. Biol Reprod. 1988;39:818–828. doi: 10.1095/biolreprod39.4.818. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Eller BC, Hendry W. J. d. Identification of cytoplasmic estrogen receptors in the accessory sex organs of the rabbit and their comparison to the cytoplasmic estrogen receptor in the epididymis. Mol Cell Endocrinol. 1983;33:197–209. doi: 10.1016/0303-7207(83)90167-3. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock B. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science. 1975;190:991–992. doi: 10.1126/science.242076. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Rivas A, Walker M, McKinnell C, Fisher JS. Effect of neonatal treatment of rats with potent or weak (environmental) oestrogens, or with a GnRH antagonist, on Leydig cell development and function through puberty into adulthood. Int J Androl. 2003;26:26–36. doi: 10.1046/j.1365-2605.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- Wistuba J, Brinkworth MH, Schlatt S, Chahoud I, Nieschlag E. Intrauterine bisphenol A exposure leads to stimulatory effects on Sertoli cell number in rats. Environ Res. 2003;91:95–103. doi: 10.1016/S0013-9351(02)00019-1. [DOI] [PubMed] [Google Scholar]
- Greco TL, Furlow JD, Duello TM, Gorski J. Immunodetection of estrogen receptors in fetal and neonatal female mouse reproductive tracts. Endocrinology. 1991;129:1326–1332. doi: 10.1210/endo-129-3-1326. [DOI] [PubMed] [Google Scholar]
- Greco TL, Furlow JD, Duello TM, Gorski J. Immunodetection of estrogen receptors in fetal and neonatal male mouse reproductive tracts. Endocrinology. 1992;130:421–429. doi: 10.1210/en.130.1.421. [DOI] [PubMed] [Google Scholar]
- Weniger JP, Zeis A. [Aromatization of testosterone by the rat embryo testis] C R Seances Acad Sci III. 1983;296:293–296. [PubMed] [Google Scholar]
- Dorrington JM, Fritz B, Armstrong DT. Control of testicular estrogen synthesis. Biol Reprod. 1978;18:55–64. doi: 10.1095/biolreprod18.1.55. [DOI] [PubMed] [Google Scholar]
- Tcholakian RK, Chowdhury M, Steinberger E. Time of action of oestradiol-17beta on luteinizing hormone and testosterone. J Endocrinol. 1974;63:411–412. doi: 10.1677/joe.0.0630411. [DOI] [PubMed] [Google Scholar]
- Pomerantz DK. Effects of in vivo gonadotropin treatment on estrogen levels in the testis of the immature rat. Biol Reprod. 1979;21:1247–1255. doi: 10.1095/biolreprod21.5.1247. [DOI] [PubMed] [Google Scholar]
- Valladares LE, Payne AH. Induction of testicular aromatization by luteinizing hormone in mature rats. Endocrinol. 1979;105:431–436. doi: 10.1210/endo-105-2-431. [DOI] [PubMed] [Google Scholar]
- Nozu K, Dehejia A, Zawistowich L, Catt KJ, Dufau ML. Gonadotropin-induced desensitization of Leydig cells in vivo and in vitro: estrogen action in the testis. Ann N Y Acad Sci. 1982;383:212–230. doi: 10.1111/j.1749-6632.1982.tb23170.x. [DOI] [PubMed] [Google Scholar]
- Rommerts FF, de Jong FH, Brinkmann AO, van der Molen HJ. Development and cellular localization of rat testicular aromatase activity. J Reprod Fertil. 1982;65:281–288. doi: 10.1530/jrf.0.0650281. [DOI] [PubMed] [Google Scholar]
- Rommerts FF, Brinkman AO. Modulation of steroidogenic activities in testis Leydig cells. Mol Cell Endocrinol. 1981;21:15–28. doi: 10.1016/0303-7207(81)90026-5. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Aquilano DR, Dufau ML. Gonadotropic regulation of aromatase activity in the adult rat testis. Endocrinology. 1985;116:31–37. doi: 10.1210/endo-116-1-31. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Carreau S, Szerman-Joly E, Drosdowsky MA, Dehennin L, Scholler R. Rat testis 17b-estradiol: identification by gas chromatography-mass spectrometry and age related cellular distribution. J Steroid Biochem. 1986;24:1211–1216. doi: 10.1016/0022-4731(86)90385-7. [DOI] [PubMed] [Google Scholar]
- Carreau S, Papadopoulos V, Drosdowsky MA. Stimulation of adult rat Leydig cell aromatase activity by a Sertoli cell factor. Endocrinology. 1988;122:1103–1109. doi: 10.1210/endo-122-3-1103. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris C-H, Aquilano DR, Dufau ML. Gonadotropic regulation of aromatase activity in the adult rat testis. Ann NY Acad Sci. 1984;438:666–669. doi: 10.1111/j.1749-6632.1984.tb38368.x. [DOI] [PubMed] [Google Scholar]
- Kurosumi M, Ishimura K, Fujita H, Osawa Y. Immunocytochemical localization of aromatase in rat testis. Histochemistry. 1985;83:401–404. doi: 10.1007/BF00509199. [DOI] [PubMed] [Google Scholar]
- Payne AH, Perkins LM, Georgiou M, Quinn PG. Intratesticular site of aromatase activity and possible function of testicular estradiol. Steroids. 1987;50:435–448. doi: 10.1016/0039-128X(87)90030-4. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Knox GF, Dufau ML. Acquisition of hormone-mediated mechanisms regulating testicular steroidogenesis during development. Ann N Y Acad Sci. 1987;513:40–57. doi: 10.1111/j.1749-6632.1987.tb24997.x. [DOI] [PubMed] [Google Scholar]
- Claus R, Dimmick MA, Gimenez T, Hudson LW. Estrogens and prostaglandin F2a in the semen and blood plasma of stallions. Theriogenology. 1992;38:687–693. doi: 10.1016/0093-691X(92)90031-L. [DOI] [PubMed] [Google Scholar]
- Free MJ, Jaffe RA. Collection of rete testis fluid from rats without previous efferent duct ligation. Biol Reprod. 1979;20:269–278. doi: 10.1095/biolreprod20.2.269. [DOI] [PubMed] [Google Scholar]
- Ganjam VK, Amann RP. Steroids in fluids and sperm entering and leaving the bovine epididymis, epididymal tissue, and accessory sex gland secretions. Endocrinology. 1976;99:1618–1630. doi: 10.1210/endo-99-6-1618. [DOI] [PubMed] [Google Scholar]
- Bujan L, Mieusset R, Audran F, Lumbroso S, Sultan C. Increased oestradiol level in seminal plasma in infertile men. Hum Reprod. 1993;8:74–77. doi: 10.1093/oxfordjournals.humrep.a137878. [DOI] [PubMed] [Google Scholar]
- Waites GM, Einer-Jensen N. Collection and analysis of rete testis fluid from macaque monkeys. J Reprod Fertil. 1974;41:505–508. doi: 10.1530/jrf.0.0410505. [DOI] [PubMed] [Google Scholar]
- Eiler H, Graves CN. Oestrogen content of semen and the effect of exogenous oestradiol-17a on the oestrogen and androgen concentration in semen and blood plasma of bulls. J Reprod Fert. 1977;50:17–21. doi: 10.1530/jrf.0.0500017. [DOI] [PubMed] [Google Scholar]
- Claus R, Schopper D, Hoang-Vu C. Contribution of individual compartments of the genital tract to oestrogen and testosterone concentrations in ejaculates of the boar. Acta Endocrinol. 1985;109:281–288. doi: 10.1530/acta.0.1090281. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Laurie MS, Flint AP, Heap RB. Transport of free and conjugated steroids from the boar testis in lymph, venous blood and rete testis fluid. J Endocrinol. 1983;96:127–136. doi: 10.1677/joe.0.0960127. [DOI] [PubMed] [Google Scholar]
- Adamopoulos D, Lawrence DM, Vassilopoulos P, Kapolla N, Kontogeorgos L, McGarrigle HH. Hormone levels in the reproductive system of normospermic men and patients with oligospermia and varicocele. J Clin Endocrinol Metab. 1984;59:447–452. doi: 10.1210/jcem-59-3-447. [DOI] [PubMed] [Google Scholar]
- Nitta H, Bunick D, Hess RA, Janulis L, Newton SC, Millette CF, Osawa Y, Shizuta Y, Toda K, Bahr JM. Germ cells of the mouse testis express P450 aromatase. Endocrinology. 1993;132:1396–1401. doi: 10.1210/en.132.3.1396. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Young P, Hess RA, Cunha GR. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology. 1991;128:2874–2879. doi: 10.1210/endo-128-6-2874. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Uesugi Y, Sato T, Ohta Y, Takasugi N. Developmental pattern of estrogen receptor expression in male mouse genital organs. Mol Androl. 1991;6:109–119. [Google Scholar]
- Hess RA, Bunick D, Bahr JM. Sperm, a source of estrogen. Environ Health Perspect. 1995;103 Suppl 7:59–62. doi: 10.1289/ehp.95103s759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Millar MR, Majdic G, Saunders PT, Fraser HM, Sharpe RM. Immunolocalisation of oestrogen receptor-alpha within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. J Endocrinol. 1997;153:485–495. doi: 10.1677/joe.0.1530485. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Bartol FF, Wiley AA, Khalil MK, Chiu J, Vig MM. Immunolocalization of androgen receptor and estrogen receptor in the developing testis and excurrent ducts of goats. Anat Rec. 1997;249:54–62. doi: 10.1002/(SICI)1097-0185(199709)249:1<54::AID-AR7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL. Estrogen receptor (a & b) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997;18:602–611. [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinol. 1996;137:4796–4805. doi: 10.1210/en.137.11.4796. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors a (ER a) and b (ER b) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod. 2000;63:1873–1880. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- Hess RA, Nakai M. Histopathology of the male reproductive system induced by the fungicide benomyl. Histol Histopathol. 2000;15:207–224. doi: 10.14670/HH-15.207. [DOI] [PubMed] [Google Scholar]
- Nakai M, Bouma J, Nie R, Zhou Q, Carnes K, Jassim E, Lubahn DB, Hess RA. Morphological analysis of endocytosis in efferent ductules of estrogen receptor-alpha knockout male mouse. Anat Rec. 2001;263:10–18. doi: 10.1002/ar.1071. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: Roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci U S A. 2001;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Finnigan-Bunick C, Bahr J, Bunick D. Estrogen Regulation of Ion Transporter Messenger RNA Levels in Mouse Efferent Ductules Are Mediated Differentially Through Estrogen Receptor (ER) alpha and ERbeta. Biol Reprod. 2001;65:1534–1541. doi: 10.1095/biolreprod65.5.1534. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Zhou Q, Carnes K, Nie R, Kuehl DE, Jackson GL, Franca LR, Nakai M, Hess RA. ER Function in the Adult Male Rat: Short- and Long-Term Effects of the Antiestrogen ICI 182,780 on the Testis and Efferent Ductules, without Changes in Testosterone. Endocrinology. 2002;143:2399–2409. doi: 10.1210/en.143.6.2399. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, Franca LR, Hess RA. Infertility and testicular atrophy in the antiestrogen-treated adult male rat. Biol Reprod. 2001;65:913–920. doi: 10.1095/biolreprod65.3.913. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–357. doi: 10.1016/S0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- van der Molen HJ, Brinkmann AO, de Jong FH, Rommerts FF. Testicular oestrogens. J Endocrinol. 1981;89:33P–46P.. [PubMed] [Google Scholar]
- Levallet J, Bilinska B, Mittre H, Genissel C, Fresnel J, Carreau S. Expression and immunolocalization of functional cytochrome P450 aromatase in mature rat testicular cells. Biol Reprod. 1998;58:919–926. doi: 10.1095/biolreprod58.4.919. [DOI] [PubMed] [Google Scholar]
- Carreau S, Genissel C, Bilinska B, Levallet J. Sources of oestrogen in the testis and reproductive tract of the male. Int J Androl. 1999;22:211–223. doi: 10.1046/j.1365-2605.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/S0039-128X(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S. Aromatase expression and role of estrogens in male gonad : a review. Reprod Biol Endocrinol. 2003;1:35. doi: 10.1186/1477-7827-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rago V, Bilinska B, Palma A, Ando S, Carpino A. Evidence of aromatase localization in cytoplasmic droplet of human immature ejaculated spermatozoa. Folia Histochem Cytobiol. 2003;41:23–27. [PubMed] [Google Scholar]
- Janulis L, Hess RA, Bunick D, Nitta H, Janssen S, Asawa Y, Bahr JM. Mouse epididymal sperm contain active P450 aromatase which decreases as sperm traverse the epididymis. J Androl. 1996;17:111–116. [PubMed] [Google Scholar]
- Janulis L, Bahr JM, Hess RA, Bunick D. P450 aromatase messenger ribonucleic acid expression in male rat germ cells: detection by reverse transcription-polymerase chain reaction amplification. J Androl. 1996;17:651–658. [PubMed] [Google Scholar]
- Kwon S, Hess RA, Bunick D, Nitta H, Janulis L, Osawa Y, Bahr JM. Rooster testicular germ cells and epididymal sperm contain P450 aromatase. Biol Reprod. 1995;53:1259–1264. doi: 10.1095/biolreprod53.6.1259. [DOI] [PubMed] [Google Scholar]
- Janulis L, Bahr JM, Hess RA, Janssen S, Osawa Y, Bunick D. Rat testicular germ cells and epididymal sperm contain active P450 aromatase. J Androl. 1998;19:65–71. [PubMed] [Google Scholar]
- Hess RA, Bunick D, Bahr J. Oestrogen, its receptors and function in the male reproductive tract - a review. Mol Cell Endocrinol. 2001;178:29–38. doi: 10.1016/S0303-7207(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Kotula-Balak M, Slomczynska M, Fraczek B, Bourguiba S, Tabarowski Z, Carreau S, Bilinska B. Complementary approaches demonstrate that cellular aromatization in the bank vole testis is related to photoperiod. Eur J Histochem. 2003;47:55–62. doi: 10.4081/807. [DOI] [PubMed] [Google Scholar]
- Lambard S, Galeraud-Denis I, Bouraima H, Bourguiba S, Chocat A, Carreau S. Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Mol Hum Reprod. 2003;9:117–124. doi: 10.1093/molehr/gag020. [DOI] [PubMed] [Google Scholar]
- Levallet J, Carreau S. In vitro gene expression of aromatase in rat testicular cells. C R Acad Sci III. 1997;320:123–129. doi: 10.1016/S0764-4469(97)85003-2. [DOI] [PubMed] [Google Scholar]
- Schleicher G, Khan S, Nieschlag E. Differentiation between androgen and estrogen receptor mediated effects of testosterone on FSH using androgen receptor deficient (Tfm) and normal mice. J Steroid Biochem. 1989;33:49–51. doi: 10.1016/0022-4731(89)90356-7. [DOI] [PubMed] [Google Scholar]
- Overpeck JG, Colson SH, Hohmann JR, Applestine MS, Reilly JF. Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J Toxicol Environ Health. 1978;4:785–803. doi: 10.1080/15287397809529700. [DOI] [PubMed] [Google Scholar]
- de Jong FH, Hey AH, van der Molen HJ. Effect of gonadotrophins on the secretion of oestradiol-17b and testosterone by the rat testis. J Endocrinol. 1973;57:277–284. doi: 10.1677/joe.0.0570277. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- Kumari GL, Allag IS, Das RP, Datta JK. Regional differences in steroidogenesis and hormone levels in the epididymis and vas deferens of adult rats. Int J Androl. 1980;3:267–281. doi: 10.1111/j.1365-2605.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Melnyk PM, Sanford LM, Robaire B. Moderate increases in peripheral blood estradiol concentration in the adult ram do not directly inhibit testosterone secretion. Can J Physiol Pharmacol. 1992;70:1384–1391. doi: 10.1139/y92-194. [DOI] [PubMed] [Google Scholar]
- Setchell BP. The flow and composition of lymph from the testes of pigs with some observations on the effect of raised venous pressure. Comp Biochem Physiol A. 1982;73:201–205. doi: 10.1016/0300-9629(82)90056-1. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Cox JE. Secretion of free and conjugated steroids by the horse testis into lymph and venous blood. J Reprod Fertil Suppl. 1982;32:123–127. [PubMed] [Google Scholar]
- Robaire B, Fan X. Regulation of apoptotic cell death in the rat epididymis. J Reprod Fertil Suppl. 1998;53:211–214. [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA, Man SY. Fluid and electrolyte reabsorption in the ductuli efferentes testis. J Reprod Fertil Suppl. 1998;53:1–14. [PubMed] [Google Scholar]
- Fawcett DW, Hoffer AP. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod. 1979;20:162–181. doi: 10.1095/biolreprod20.2.162. [DOI] [PubMed] [Google Scholar]
- Younes M, Evans BA, Chaisiri N, Valotaire Y, Pierrepoint CG. Steroid receptors in the canine epididymis. J Reprod Fertil. 1979;56:45–52. doi: 10.1530/jrf.0.0560045. [DOI] [PubMed] [Google Scholar]
- Younes MA, Pierrepoint CG. Estrogen steroid-receptor binding in the canine epididymis. Andrologia. 1981;13:562–572. doi: 10.1002/pros.2990020203. [DOI] [PubMed] [Google Scholar]
- Murphy JB, Emmott RC, Hicks LL, Walsh PC. Estrogen receptors in the human prostate, seminal vesicle, epididymis, testis, and genital skin: a marker for estrogen-responsive tissues? J Clin Endocrinol Metab. 1980;50:938–948. doi: 10.1210/jcem-50-5-938. [DOI] [PubMed] [Google Scholar]
- Dufaure JP, Mak P, Callard IP. Estradiol binding activity in epididymal cytosol of the turtle, Chrysemys picta. Gen Comp Endocrinol. 1983;51:61–65. doi: 10.1016/0016-6480(83)90097-7. [DOI] [PubMed] [Google Scholar]
- Kamal N, Agarwal AK, Jehan Q, Setty BS. Biological action of estrogen on the epididymis of prepubertal rhesus monkey. Andrologia. 1985;17:339–345. doi: 10.1111/j.1439-0272.1985.tb01016.x. [DOI] [PubMed] [Google Scholar]
- West NB, Brenner RM. Estrogen receptor in the ductuli efferentes, epididymis, and testis of rhesus and cynomolgus macaques. Biol Reprod. 1990;42:533–538. doi: 10.1095/biolreprod42.3.533. [DOI] [PubMed] [Google Scholar]
- Tekpetey FR, Amann RP. Regional and seasonal differences in concentrations of androgen and estrogen receptors in ram epididymal tissue. Biol Reprod. 1988;38:1051–1060. doi: 10.1095/biolreprod38.5.1051. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, St. Raymond PA, Davies J. Hormonally responsive areas of the reproductive system of the male guinea pig. III. Presence of cytoplasmic estrogen receptors. Biol Reprod. 1981;25:1159–1168. doi: 10.1095/biolreprod25.5.1159. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinol. 1997;138:863–870. doi: 10.1210/en.138.3.863. [DOI] [PubMed] [Google Scholar]
- Schleicher G, Drews U, Stumpf WE, Sar M. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. An autoradiographic study. Histochemistry. 1984;81:139–147. doi: 10.1007/BF00490107. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Bartol FF, Wiley AA, Khalil MK, Williams CS, Vig MM. Regulation of androgen and estrogen receptors in male excurrent ducts of the goat: an immunohistochemical study. Anat Rec. 1998;250:164–171. doi: 10.1002/(SICI)1097-0185(199802)250:2<164::AID-AR6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Bartol FF, Wiley AA, Neff CW. Immunolocalization of receptors for androgen and estrogen in male caprine reproductive tissues: unique distribution of estrogen receptors in efferent ductule epithelium. Biol Reprod. 1997;56:90–101. doi: 10.1095/biolreprod56.1.90. [DOI] [PubMed] [Google Scholar]
- Kwon S, Hess RA, Bunick D, Kirby JD, Bahr JM. Estrogen receptors are present in the epididymis of the rooster. J Androl. 1997;18:378–384. [PubMed] [Google Scholar]
- Ergun S, Ungefroren H, Holstein AF, Davidoff MS. Estrogen and progesterone receptors and estrogen receptor-related antigen (ER-D5) in human epididymis. Mol Reprod Dev. 1997;47:448–455. doi: 10.1002/(SICI)1098-2795(199708)47:4<448::AID-MRD12>3.3.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Sato T, Chiba A, Hayashi S, Okamura H, Ohta Y, Takasugi N, Iguchi T. Induction of estrogen receptor and cell division in genital tracts of male mice by neonatal exposure to diethylstilbestrol. Reprod Toxicol. 1994;8:145–153. doi: 10.1016/0890-6238(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Sharpe RM, Williams K, Macpherson S, Urquart H, Irvine DS, Millar MR. Differential expression of oestrogen receptor alpha and beta proteins in the testes and male reproductive system of human and non-human primates. Mol Hum Reprod. 2001;7:227–236. doi: 10.1093/molehr/7.3.227. [DOI] [PubMed] [Google Scholar]
- Nie R, Zhou Q, Jassim E, Saunders PT, Hess RA. Differential expression of estrogen receptors a and b in the reproductive tracts of adult male dogs and cats. Biol Reprod. 2002;66:1161–1168. doi: 10.1095/biolreprod66.4.1161. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–881. [PubMed] [Google Scholar]
- Bilinska B, Schmalz-Fraczek B, Sadowska J, Carreau S. Localization of cytochrome P450 aromatase and estrogen receptors alpha and beta in testicular cells--an immunohistochemical study of the bank vole. Acta Histochem. 2000;102:167–181. doi: 10.1078/S0065-1281(04)70026-4. [DOI] [PubMed] [Google Scholar]
- Makinen S, Makela S, Weihua Z, Warner M, Rosenlund B, Salmi S, Hovatta O, Gustafsson JK. Localization of oestrogen receptors alpha and beta in human testis. Mol Hum Reprod. 2001;7:497–503. doi: 10.1093/molehr/7.6.497. [DOI] [PubMed] [Google Scholar]
- Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H. Expression and Cellular Localization of Estrogen Receptors alpha and beta in the Human Fetus. J Clin Endocrinol Metab. 2001;86:2258–2262. doi: 10.1210/jc.86.5.2258. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM. Comparison of androgen receptor and oestrogen receptor beta immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction. 2001;122:419–429. doi: 10.1530/reprod/122.3.419. [DOI] [PubMed] [Google Scholar]
- Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol. 2000;15:1261–1270. doi: 10.14670/HH-15.1261. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Fisher JS, Sharpe RM, Millar MR. Expression of oestrogen receptor beta (ER beta) occurs in multiple cell types, including some germ cells, in the rat testis. J Endocrinol. 1998;156:R13–7. doi: 10.1677/joe.0.156r013. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Majdic G, Parte P, Millar MR, Fisher JS, Turner KJ, Sharpe RM. Fetal and perinatal influence of xenoestrogens on testis gene expression. Adv Exp Med Biol. 1997;424:99–110. doi: 10.1007/978-1-4615-5913-9_19. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG, van der Burg B, van der Saag PT, Gustafsson JA, Kuiper GG. Ontogeny of estrogen receptor-beta expression in rat testis. Endocrinology. 1999;140:478–483. doi: 10.1210/en.140.1.478. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Ganjam VK, Taylor JA, Yuan X, Stiehr JR, Hardy MP, Lubahn DB. Transcription and translation of estrogen receptor-beta in the male reproductive tract of estrogen receptor-alpha knock-out and wild-type mice. Endocrinology. 1998;139:2982–2987. doi: 10.1210/en.139.6.2982. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Expression of Estrogen Receptor beta Is Developmentally Regulated in Reproductive Tissues of Male and Female Mice. Biol Reprod. 2000;62:310–317. doi: 10.1095/biolreprod62.2.310. [DOI] [PubMed] [Google Scholar]
- Echeverria OM, Maciel AG, Traish AM, Wotiz HH, Ubaldo E, Vazqueznin GH. Immuno-electron microscopic localization of estradiol receptor in cells of male and female reproductive and non-reproductive organs. Biol Cell. 1994;81:257–265. doi: 10.1016/0248-4900(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor alpha, oestrogen receptor beta and androgen receptors in the rat reproductive organs. J Endocrinol. 2000;165:359–370. doi: 10.1677/joe.0.1650359. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM. Estrogen receptor-alpha is required by the supporting somatic cells for spermatogenesis. Mol Cell Endocrinol. 2001;178:57–63. doi: 10.1016/S0303-7207(01)00410-5. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/er.22.3.289. [DOI] [PubMed] [Google Scholar]
- Robertson KM, Simpson ER, Lacham-Kaplan O, Jones ME. Characterization of the fertility of male aromatase knockout mice. J Androl. 2001;22:825–830. [PubMed] [Google Scholar]
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144:84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- Hess RA, Zhou Q, Nie R. The Role of Estrogens in the Endocrine and Paracrine Regulation of the Efferent Ductules, Epididymis and Vas deferens. In: Robaire B and Hinton B T, editor. The Epididymis: from Molecules to Clinical Practice. New York, Kluwer Academic/Plenum Publishers; 2002. pp. 317–338. [Google Scholar]
- Misao R, Fujimoto J, Niwa K, Morishita S, Nakanishi Y, Tamaya T. Immunohistochemical expressions of estrogen and progesterone receptors in human epididymis at different ages--a preliminary study. Int J Fertil Womens Med. 1997;42:39–42. [PubMed] [Google Scholar]
- Palacios J, Regadera J, Paniagua R, Gamallo C, Nistal M. Immunohistochemistry of the human ductus epididymis. Anat Rec. 1993;235:560–566. doi: 10.1002/ar.1092350408. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O, Mahony MC, Gordon K, Hsiu JG, Hodgen GD, Gibbons WE. Estrogen and progesterone receptor mRNA are expressed in distinct pattern in male primate reproductive organs. J Assist Reprod Genet. 1995;12:198–204. doi: 10.1007/BF02211799. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/en.138.5.1801. [DOI] [PubMed] [Google Scholar]
- Prins GS, Marmer M, Woodham C, Chang W, Kuiper G, Gustafsson JA, Birch L. Estrogen receptor-beta messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139:874–883. doi: 10.1210/en.139.3.874. [DOI] [PubMed] [Google Scholar]
- MacCalman CD, Getsios S, Farookhi R, Blaschuk OW. Estrogens potentiate the stimulatory effects of follicle-stimulating hormone on N-cadherin messenger ribonucleic acid levels in cultured mouse Sertoli cells. Endocrinology. 1997;138:41–48. doi: 10.1210/en.138.1.41. [DOI] [PubMed] [Google Scholar]
- MacCalman CD, Blaschuk OW. Gonadal-steroids regulate N-cadherin messenger-RNA levels in the mouse testis. ENDOCRINE. 1994;2:157–163. [Google Scholar]
- Robertson KM, O'Donnell L, Simpson ER, Jones ME. The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact significantly on testis function. Endocrinology. 2002;143:2913–2921. doi: 10.1210/en.143.8.2913. [DOI] [PubMed] [Google Scholar]
- Scobie GA, Macpherson S, Millar MR, Groome NP, Romana PG, Saunders PT. Human oestrogen receptors: differential expression of ERalpha and beta and the identification of ERbeta variants. Steroids. 2002;67:985–992. doi: 10.1016/S0039-128X(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, Sharpe RM, Scobie GA. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab. 2002;87:2706–2715. doi: 10.1210/jc.87.6.2706. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Brooks AN, Cronin AS, Ford H, Kerr JB. Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology. 2000;141:2861–2869. doi: 10.1210/en.141.8.2861. [DOI] [PubMed] [Google Scholar]
- Robertson KM, O'Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci U S A. 1999;96:7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/en.139.10.4252. [DOI] [PubMed] [Google Scholar]
- Hermo L, Oko R, Morales CR. Secretion and endocytosis in the male reproductive tract: a role in sperm maturation. Int Rev Cytol. 1994;154:106–189. [PubMed] [Google Scholar]
- Ilio KY, Hess RA. Structure and function of the ductuli efferentes: a review. Microsc Res Tech. 1994;29:432–467. doi: 10.1002/jemt.1070290604. [DOI] [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM. Spermatogenic cells do not require estrogen receptor-alpha for development or function [see comments] Endocrinology. 2000;141:1273–1276. doi: 10.1210/en.141.3.1273. [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. J Androl. 2000;21:107–121. [PubMed] [Google Scholar]
- Hermo L, de Melo V. Endocytic apparatus and transcytosis in epithelial cells of the vas deferens in the rat. Anat Rec. 1987;217:153–163. doi: 10.1002/ar.1092170207. [DOI] [PubMed] [Google Scholar]
- Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, Groome NP, Sharpe RM, Fraser HM, Saunders PT. Development and validation of a new monoclonal antibody to mammalian aromatase. J Endocrinol. 2002;172:21–30. doi: 10.1677/joe.0.1720021. [DOI] [PubMed] [Google Scholar]
- Levallet J, Mittre H, Delarue B, Carreau S. Alternative splicing events in the coding region of the cytochrome P450 aromatase gene in male rat germ cells. J Mol Endocrinol. 1998;20:305–312. doi: 10.1677/jme.0.0200305. [DOI] [PubMed] [Google Scholar]
- Lanzino M, Catalano S, Genissel C, Ando S, Carreau S, Hamra K, McPhaul MJ. Aromatase messenger RNA is derived from the proximal promoter of the aromatase gene in Leydig, Sertoli, and germ cells of the rat testis. Biol Reprod. 2001;64:1439–1443. doi: 10.1095/biolreprod64.5.1439. [DOI] [PubMed] [Google Scholar]
- Carpino A, Pezzi V, Rago V, Bilinska B, Ando S. Immunolocalization of cytochrome P450 aromatase in rat testis during postnatal development. Tissue Cell. 2001;33:349–353. doi: 10.1054/tice.2001.0186. [DOI] [PubMed] [Google Scholar]
- Tsubota T, Howell-Skalla L, Nitta H, Osawa Y, Mason JI, Meiers PG, Nelson RA, Bahr JM. Seasonal changes in spermatogenesis and testicular steroidogenesis in the male black bear Ursus americanus. J Reprod Fertil. 1997;109:21–27. doi: 10.1530/jrf.0.1090021. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Corbin CJ, Hinshelwood MM, Liu Z, Simpson ER, Ford JJ, Harada N. Functional aromatase expression in porcine adrenal gland and testis. Biol Reprod. 1996;54:497–505. doi: 10.1095/biolreprod54.2.497. [DOI] [PubMed] [Google Scholar]
- Schmalz B, Bilinska B. Immunolocalization of aromatase and estrogen receptors in ram Leydig cells. Ginekol Pol. 1998;69:512–516. [PubMed] [Google Scholar]
- Lemazurier E, Sourdaine P, Nativelle C, Plainfosse B, Seralini G. Aromatase gene expression in the stallion. Mol Cell Endocrinol. 2001;178:133–139. doi: 10.1016/S0303-7207(01)00435-X. [DOI] [PubMed] [Google Scholar]
- Eisenhauer KM, McCue PM, Nayden DK, Osawa Y, Roser JF. Localization of aromatase in equine Leydig cells. Domest Anim Endocrinol. 1994;11:291–298. doi: 10.1016/0739-7240(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Sipahutar H, Sourdaine P, Moslemi S, Plainfosse B, Seralini GE. Immunolocalization of aromatase in stallion Leydig cells and seminiferous tubules. J Histochem Cytochem. 2003;51:311–318. doi: 10.1177/002215540305100306. [DOI] [PubMed] [Google Scholar]
- Fraczek B, Bourguiba S, Carreau S, Bilinska B. Immunolocalization and activity of aromatase in the bank vole testes. Folia Histochem Cytobiol. 2001;39:315–319. [PubMed] [Google Scholar]
- Bilinska B, Schmalz-Fraczek B, Kotula M, Carreau S. Photoperiod-dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohistochemistry. Mol Cell Endocrinol. 2001;178:189–198. doi: 10.1016/S0303-7207(01)00427-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nakamura M, Kajiura-Kobayashi H, Young G, Nagahama Y. Immunolocalization of steroidogenic enzymes (P450scc, P450c17, P450arom, and 3beta-HSD) in immature and mature testes of rainbow trout (Oncorhynchus mykiss) Cell Tissue Res. 1998;292:573–577. doi: 10.1007/s004410051086. [DOI] [PubMed] [Google Scholar]
- Callard GV, Pudney JA, Mak P, Canick JA. Stage-dependent changes in steroidogenic enzymes and estrogen receptors during spermatogenesis in the testis of the dogfish, Squalus acanthias. Endocrinology. 1985;117:1328–1335. doi: 10.1210/endo-117-4-1328. [DOI] [PubMed] [Google Scholar]
- Betka M, Callard GV. Negative feedback control of the spermatogenic progression by testicular oestrogen synthesis: insights from the shark testis model. Apmis. 1998;106:252–7; discussion 257-8. doi: 10.1111/j.1699-0463.1998.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Pereyra-Martinez AC, Roselli CE, Stadelman HL, Resko JA. Cytochrome P450 aromatase in testis and epididymis of male rhesus monkeys. Endocrine. 2001;16:15–19. doi: 10.1385/ENDO:16:1:15. [DOI] [PubMed] [Google Scholar]