Abstract
Background
Information about antibiotic use and resistance patterns of common microorganisms are lacking in hospitals in Western Nepal. Excessive and inappropriate use of antibiotics contributes to the development of bacterial resistance. The parameter: Defined daily dose/100 bed-days, provides an estimate of consumption of drugs among hospital in-patients. This study was carried out to collect relevant demographic information, antibiotic prescribing patterns and the common organisms isolated including their antibiotic sensitivity patterns.
Methods
The study was carried out over a 3-month period (01.04.2002 to 30.06.2002) at the Manipal Teaching Hospital, Western Nepal. The median number of days of hospitalization and mean ± SD cost of antibiotics prescribed during hospital stay were calculated. The use of antibiotics was classified for prophylaxis, bacteriologically proven infection or non-bacteriologically proven infection. Sensitivity patterns of the common organisms were determined. Defined daily dose/100 bed-days of the ten most commonly prescribed antibiotics were calculated.
Results
203 patients were prescribed antibiotics; 112 were male. Median duration of hospitalization was 5 days. 347 antibiotics were prescribed. The most common were ampicillin, amoxicillin, metronidazole, ciprofloxacin and benzylpenicillin. Mean ± SD cost of antibiotics was 16.5 ± 13.4 US$. Culture and sensitivity testing was carried out in 141 patients. The common organisms isolated were H. influenzae, E. coli, K. pneumoniae and S. aureus.
Conclusions
Antibiotic resistance is becoming a problem in the Internal Medicine ward. Formulation of a policy for hospital antibiotic use and an educational programme especially for junior doctors is required.
Keywords: Antibiotics, Drug utilization, Internal Medicine ward
Background
Quality of life can be improved by enhancing standards of medical treatment at all levels of the health care delivery system. Setting standards and assessing the quality of care through performance review should become part of everyday clinical practice [1]. The study of prescribing patterns seeks to monitor, evaluate and suggest modifications in practitioners' prescribing habits so as to make medical care rational and cost effective.
Information about antibiotic use patterns is necessary for a constructive approach to problems that arise from the multiple antibiotics available [2]. In developing countries the cost of health care is a matter of major concern [3]. This is especially true for Nepal, a developing country in South Asia. Excessive and inappropriate use of antibiotics in hospitals, health care facilities and the community contributes to the development of bacterial resistance. In India reports on antibiotic utilization at an institutional level include both cross-sectional [3] and longitudinal studies [4,5] of prescribing patterns.
The ATC (anatomic-therapeutic-chemical) classification assigns code letters and numbers to drugs [6,7]. The defined daily dose (DDD) concept was developed to overcome objections against traditional units of drug consumption [6]. The DDD for a given drug is established on the basis of an assumed average use per day of the drug for its main indication in adults [6]. DDD will be assigned only for drugs that already have an ATC code. DDD is a unit of measurement and may not reflect the prescribed daily dose, however they provide a fixed unit of measurement independent of price and formulation and enable the researcher to perform comparisons between population groups. DDD/100 bed-days provides a rough estimate of consumption of drugs among hospital in-patients.
Information on antibiotic use patterns, the illnesses for which antibiotics are prescribed and the DDD/100 bed-days of commonly used antibiotics in hospitals in Western Nepal are lacking. Hence the present study was carried out over 3 months (01.04.02 to 30.06.02) at the Manipal Teaching Hospital, a tertiary care hospital attached to the Manipal College of Medical Sciences, Pokhara, Nepal.
The objectives of the study were to:
1) collect relevant demographic information and information on duration of hospitalization of patients admitted to the Internal Medicine ward and prescribed antibiotics during the study.
2) obtain information on the antibiotic prescribing pattern and the disease conditions for which antibiotics were prescribed.
3) obtain information on the common organisms isolated during culture and sensitivity testing and their antibiotic sensitivity patterns.
4) apply the ATC classification to the commonly used antibiotics and to calculate their DDDs/100 bed-days.
5) calculate the mean ± SD cost of antibiotics per prescription.
Methods
The study was carried out over a three-month period at the Manipal Teaching Hospital, Pokhara, Nepal. Patients admitted to the Internal Medicine ward who were prescribed antibiotics were included in the study and were identified manually. This may have the potential for bias and errors. Quantitative estimation of the bias has not been carried out in this study. Computerization of the Medical Records Department is in progress and there is a proposal for storing the hospital records in a computerized format. This may help in reducing any potential bias.
The age and sex of the patients, clinical diagnosis, duration of hospitalization, antibiotic information (name, dose and frequency) and the results of culture and sensitivity testing were entered into the patient indicator form (PIF) [see Additional file 1]. The antibiotics prescribed for parenteral use and indications for their use were also analyzed separately. Antibiotics were used for: prophylaxis, bacteriologically proven infection (BPI) or non-bacteriologically proven infection (non-BPI). Details of antibiotic use in BPI were noted.
The mean cost of antibiotics prescribed during the hospital stay and on discharge from the hospital was noted. The cost of antibiotics prescribed during the hospital stay was determined using the price list supplied by the hospital pharmacy.
Patients with a monthly family income less than 2000 Nepalese rupees (1 US$ = 78 Nepalese rupees) were taken as belonging to low socioeconomic status; those with a monthly income between 2000 to 4000 rupees and more than 4000 rupees were taken as belonging to middle and high socioeconomic status respectively. The socioeconomic classification was worked out by Mr. Theodore of the Community Medicine department of our institution with input from the Statistics department of the Green Pasture hospital, Pokhara. The average per capita income of Nepal was the major consideration while preparing the classification which had been previously tested during the field visits by the Community Medicine department. Housing, the extent of land holdings, and material possessions owned by the respondents were examined in these field visits. Respondents were also asked about their family income when interviewed in the ward, (the limitation being that we were entirely dependent on the respondents answer and had no means to cross check the same, as in the field visits.)
The percentage of antibiotics prescribed from the Essential drug list of Nepal [8] and from the WHO list of Essential drugs [9] and the DDD/100 bed-days of the 10 most commonly prescribed antibiotics in the Internal Medicine ward, was calculated. The DDD/100 bed-days of the individual antibiotics were added together to get the total antibiotic consumption. Ciprofloxacin has two DDDs, one for oral use of the antibiotic and the other for parenteral use. The 2 DDDs/100 bed-days were calculated separately and then were added to measure the total consumption of ciprofloxacin in DDDs/100 bed-days. DDD/100 bed-days was calculated by the formula:
![]()
The institutional review board of the Manipal College of Medical Sciences, Pokhara, Nepal, approved the study.
Results
Two hundred and three of the 687 patients admitted to the Internal Medicine ward were prescribed antibiotics during the study period. Out of the total of 203 patients, 112 (55.2%) were male. One hundred and four patients (51.2%) were above the age of 59 years. Regarding the ethnic composition, 56 patients (27.6%) were Gurungs while 50 patients (24.6%) were Brahmins. The other common ethnic/caste groups in the study were Chettris [43 patients (21.2%)], Bishwakarmas [15 patients (7.4%)] and Newars [11 patients (5.4%)]. One hundred and eighteen patients (58.1%) belonged to an urban area while the remaining were from rural areas.
Figure 1 shows the number of antibiotics prescribed during the period of hospital stay. Ninety-eight patients were prescribed a single antibiotic, while 75 patients were prescribed two antibiotics. Twenty-three patients were prescribed 3 antibiotics, while 4 and 5 antibiotics were prescribed to 5 and 2 patients respectively.
Figure 1.
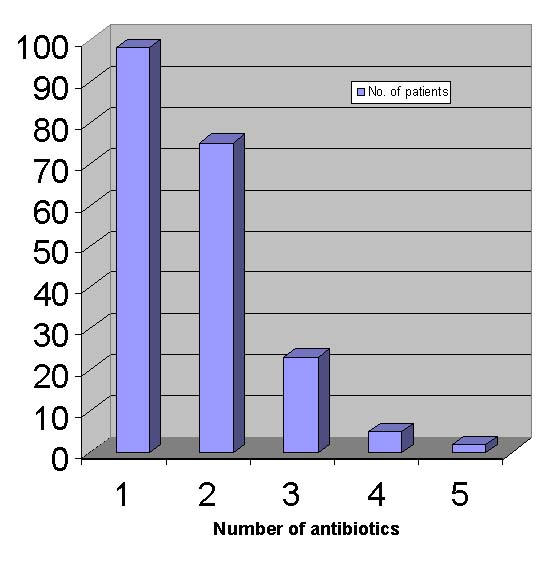
Number of antibiotics prescribed during hospital stay. Detailed legend: The majority of patients were prescribed either one or two antibiotics during the period of hospital stay. A single antibiotic was prescribed in 98 patients while two antibiotics were prescribed in 75 patients. Three antibiotics were prescribed in 23 patients while four and five antibiotics were prescribed in 5 and 2 patients respectively.
The duration of hospitalization of the 203 patients was recorded. One hundred and one patients (49.7%) were hospitalized for a time period ranging from 4 to 7 days. Median duration of hospitalization was 5 days and the interquartile range was 3 days. The corresponding figures for hospitalized patients who were not prescribed an antibiotic were 3 and 2 days respectively.
The most common condition for which an antibiotic was prescribed was chronic obstructive pulmonary disease (COPD) (22.7%). The other common conditions were lower respiratory tract infections (LRTI) (15.8%), urinary tract infections (UTI) (13.3%) and pneumonia (8.9%). One hundred and seventy three patients were discharged, 7 patients died, 8 patients were discharged at request and 5 were transferred to the Intensive Care Unit (ICU). Six patients left against medical advice while 4 patients were referred to other hospitals.
Sixty-eight patients (33.5%) belonged to the lower socioeconomic group while 112 patients (55.2%) and 23 patients (11.3%) were from the middle and high socioeconomic groups respectively. Three hundred and forty seven patient antibiotics were prescribed. Table 1 shows the frequency of prescribing of the 5 most commonly prescribed antibiotics. One hundred and seventy six patient antibiotics (51%) were prescribed parenterally.
Table 1.
Antibiotics prescribed during hospital stay
| Name of antibiotic | Number of prescriptions (percentage) |
| Ampicillin | 86 (24.8) |
| Amoxicillin | 58 (16.7) |
| Metronidazole | 46 (13.2) |
| Ciprofloxacin | 40 (11.5) |
| Crystalline penicillin | 28 (8.1) |
| Others | 89 (25.7) |
| Total | 347 |
Antibiotics were used for BPI in 65 patients, non-BPI in 126 patients and for prophylaxis in 12 patients. Mean ± SD cost of antibiotics prescribed during the hospital stay was 1236 ± 1002 Nepalese rupees (16.5 ± 13.4 US$). Parenteral antibiotics accounted for 80.3% of the total cost. Antibiotics were prescribed at the time of discharge in 145 patients with amoxicillin (55 patients) being most commonly prescribed. The other commonly prescribed antibiotics on discharge were ciprofloxacin (18 patients), ampicillin (11 patients) and metronidazole (10 patients).
Of the 347 patient antibiotics prescribed, 233 (67.1%) were prescribed from the Essential drug list of Nepal [8] and 226 (65.1%) from WHO Essential drug list [9]. One hundred and twelve patient antibiotics (32.3%) were used in BPI. Ampicillin (24.8%), amoxicillin (16.7%), metronidazole (13.3%) ciprofloxacin (11.5%) and crystalline penicillin (8.1%) were the predominant antibiotics. Other antibiotics (25.6%) included norfloxacin, cephalexin, cotrimoxazole and cefotaxime. Sixty-three (56.2%) of the 112 patient antibiotics used in BPI were prescribed by the parenteral route.
Table 2 shows the ATC codes and the DDDs/100 bed days of the 10 most commonly used antibiotics in the Internal Medicine ward. The study was carried out for a period of 90 days, the number of beds in the Internal Medicine ward was 60 and the average occupancy index during the study period was 0.7.
Table 2.
ATC codes and DDD/100 bed-days of the ten most commonly used antibiotics in the Internal Medicine ward
| Name of antibiotic | ATC code | DDD/100 bed-days |
| Ampicillin | J01CA01 | 27.5 |
| Amoxicillin | J01CA04 | 17.5 |
| Metronidazole | J01XD01 | 10.43 |
| Ciprofloxacin* | J01MA02 | 3.42, 2.73 |
| Crystalline penicillin | J01CE01 | 17.88 |
| Gentamicin | J01GB03 | 5.14 |
| Co-amoxiclav | J01CR02 | 7.87 |
| Ceftriaxone | J01DA13 | 4.56 |
| Norfloxacin | J01MA06 | 0.75 |
| Cloxacillin | J01CF02 | 3.18 |
* Two DDDs have been defined one for oral use and the other for parenteral use
Culture and sensitivity testing was carried out in 141 patients (69.4%) and a total of 154 specimens were sent for testing. One hundred and twenty eight patients had single specimen sent for culture and sensitivity testing, 10 patients had two specimens, and 2 patients, three specimens. Sputum was the most frequent specimen followed by urine and blood. The results were negative in 47 cases while in 41 cases normal flora was grown. A total of 163 organisms were isolated. The predominant organisms isolated were H. influenzae (n = 16), E. coli (n = 14), K. pneumoniae (n = 11), S. aureus (n = 9) and S. pneumoniae (n = 9). Table 3 shows the antibiotic resistance patterns of the commonly isolated organisms. Table 4 shows the number of antibiotics to which the commonly isolated organisms were resistant.
Table 3.
Resistance patterns of commonly isolated organisms from the internal medicine ward*
| Organism | No. of specimens resistant/ No. of specimens tested (% resistant) | ||||||||
| Ampicillin | Cotrimoxazole | Chloramphenicol | Gentamicin | Ciprofloxacin | Norfloxacin | Erythromycin | Co-amoxiclav | C. Penicillin | |
| H. influenzae | 3/13 (23) | 2/3 (66) | 2/11 (18.2) | nt | nt | nt | 5/7 (71.4) | 1/10 (10) | nt |
| E. coli | 5/7 (71) | 5/9 (55) | 2/2 (100) | 4/9 (44) | 5/9 (55) | 4/8 (50) | nt | 7/8 (87) | nt |
| K. pneumoniae | 1/6 (17) | 4/9 (44) | nt | 1/10(10) | nt | nt | nt | 2/3 (66) | 1/1 (100) |
| S. aureus | 2/5 (40) | 2/2 (100) | 1/1 (100) | 2/8 (25) | 2/8 (25) | nt | nt | 4/5 (80) | 2/2 (100) |
| S. pneumoniae | nt | 4/8 (50) | 2/5 (40) | nt | nt | nt | nt | nt | nt |
| S. paratyphi | nt | nt | nt | nt | nt | nt | nt | nt | nt |
| Acinetobacter | nt | nt | nt | nt | 1/2 (50) | 1/1 (100) | nt | nt | 1/1 (100) |
nt = Not tested * 151 specimens were collected for culture and sensitivity testing from the 141 patients in whom the test was carried out
Table 4.
Number of antibiotics to which the commonly isolated organisms were resistant
| Organism | Number of antibiotics to which the organism is resistant | ||||
| 0 (No. of organisms) | 1 (No. of organisms) | 2–4 (No. of organisms) | 5–8 (No. of organisms) | >8 (No. of organisms) | |
| H. influenzae (n = 16) | 6 | 6 | 3 | 0 | 0 |
| E. coli (n = 14) | 2 | 2 | 4 | 1 | 5 |
| K. pneumoniae (n = 11) | 2 | 5 | 4 | 0 | 0 |
| S. aureus (n = 9) | 3 | 2 | 2 | 2 | 0 |
| S. pneumoniae (n = 8) | 5 | 3 | 0 | 0 | 0 |
| S. paratyphi (n = 2) | 2 | 0 | 0 | 0 | 0 |
| Acinetobacter (n = 1) | 1 | 0 | 0 | 0 | 0 |
The most frequent organism isolated in the sputum was H. influenzae (n = 16). These isolates were resistant to tetracyclines in 17% of cases, to chloramphenicol in 18.2% of cases, amoxicillin in 20% of cases, cotrimoxazole in 66% of cases and erythromycin in 71.4% of cases. In the urine E. coli was the most frequent organism isolated, accounting for 52.2% of total organisms from the urine. The resistance patterns of E. coli were as follows: gentamicin (44%), norfloxacin (50%), ciprofloxacin (55%) and ampicillin (71%). However, 25% of E. coli isolates were resistant to most antibiotics but were sensitive to cotrimoxazole. The blood cultures were negative in most of the instances.
Discussion
Antibiotic resistance among pathogenic microorganisms is a matter of worldwide concern. Selective pressure by antimicrobial drugs is by far the most important driving force for the development of such resistance. Antibiotics are among the most commonly prescribed drugs in hospitals and in developed countries around 30% of the hospitalized patients are treated with these drugs [10]. The present study documents that 29.5%of the patients were prescribed antibiotics. This is very similar to the reports from developed countries [10]. Antibiotic prescription was studied over 3 months, the major disadvantage being that seasonal variations in antibiotic prescribing could not be taken into consideration over this short period. The number of samples which were sent for culture and sensitivity testing were small which may also affect the validity of the conclusions drawn about antibiotic resistance.
Among patients admitted to the Internal Medicine ward, there was a preponderance of those above the age of 59 years. This factor may have influenced antibiotic prescribing as older patients are more likely to be sick and to have more serious illnesses. Also paediatric patients (< 12 years) were not included in the analysis as they are admitted to the Paediatric Medicine ward.
Brahmins and Gurungs were the 2 major ethnic groups encountered in our study. The data on the ethnic composition of patients gives information on the utilization of the hospital by the different ethnic groups in Kaski and the neighbouring districts. A substantial number of Gurungs are ex-servicemen and our hospital has tied up with the Gorkha Welfare Society of the British Gorkhas. A Gorkha Welfare Scheme (GWS) has been introduced for these ex-servicemen and their dependents. Seven of the 56 Gurungs were benefited by GWS.
Sixty-eight patients (33.5%) belonged to low socioeconomic groups. The Manipal Teaching Hospital is a private sector health care provider where most of the patients have to pay for their treatment and medicines. We have created a Poor Patients Fund (PPF) in our hospital to help the economically deprived patients. Partial or sometimes even full concession in hospital charges may be offered to these patients. Thirty-eight of the 203 patients (18.7%) benefited from the PPF at our hospital, 32 of these patients belonged to a low socioeconomic group.
The median duration of hospitalization was 5 days and the interquartile range was 3 days. The majority of patients were hospitalized for a time period between 4 to 7 days. In a previous study [11] in the Intensive Care Unit (ICU) of our hospital, the mean ± SD period of hospitalization was 3.84 ± 3.14 days and economic constraints were a major reason for seeking an early discharge from the ICU. In this study median duration of hospitalization for patients receiving systemic antibiotics was significantly higher compared to patients not receiving antibiotics. This is similar to the results observed in a Hungarian study [12].
COPD, LRTI and UTI were the most frequent clinical conditions for which an antibiotic was prescribed. The morbidity profile of antibiotic use broadly corresponds to that observed in an Indian study [13]. In another study from Israel [14], antibiotics were most commonly used in respiratory tract infection and UTI followed by sepsis and intra-abdominal infections. However, the authors had looked at the entire hospital patient population[14].
Sixty-seven percent of antibiotics were prescribed from the Essential drug list of Nepal [8] and 65.1% were prescribed from the WHO Essential drug list [9]. The Manipal Teaching Hospital is a tertiary care provider in the Pokhara valley and due to various reasons it is sometimes necessary to use antibiotics which may not be covered in the Essential drug lists. The Essential drug lists are mainly for primary health care and their utility in our study is more limited.
The average number of drugs per prescription is an important index of a prescription audit. It is preferable to keep the number of drugs per prescription as low as possible to minimize the risk of drug interactions, development of bacterial resistance and hospital costs [15]. We did not look at the co-prescribed drugs here, but concentrated only on antibiotics. The average number of antibiotics prescribed during the hospital stay was 1.7, which is similar to the observation in an Indian study [5]. The antibiotics most frequently prescribed together were ampicillin and gentamicin. Three or more antibiotics were prescribed to patients in whom the antibiotics were changed either after reviewing the culture and sensitivity results or due to lack of improvement in the clinical condition. Three or more antibiotics were started together in seriously ill patients.
Mean ± SD cost of antibiotics prescribed during the hospital stay was 16.5 ± 13.4 US$. The medicines were purchased by the patients from the hospital pharmacy, the cost price of drugs in the hospital pharmacy being less than the open market price,(the pharmacy buying medicines in bulk and passing the discount on to patients). The difference in cost varied from 2 to 4% for different drugs. The total cost incurred by the patients during the period of hospital stay was 33.4 ± 16.2 US$. The cost incurred on hospital and nursing charges, other co-prescribed drugs and laboratory and other investigations were included in the total cost.
Fifty-one percent of the patients were prescribed antibiotics by the parenteral route. In a study reported from South India [2], 36% of antibiotics were prescribed by the parenteral route. In the Israeli study [14], 64% of antibiotics were prescribed parenterally. Parenteral antibiotics in the study were prescribed for a median duration of 5 days and the interquartile range was 3 days. The duration of use of parenteral antibiotics may be decreased and the patients switched over earlier to oral antibiotics.
In Canada a route conversion program on the prescribing of antimicrobials succeeded in reducing the frequency of use of parenteral antibiotics [16]. Parenteral antibiotics are costly and the cost of drugs is a major factor influencing treatment in a poor country like Nepal. In our hospital, patients are usually discharged once the antibiotics are changed from the parenteral to the oral route. One hundred and eighteen patients were from urban areas and their houses were accessible by all weather roads, these patients can either return to the hospital or visit a neighbouring health care facility in case of problems after being discharged. Early switchover to oral antibiotics may be more difficult for rural patients as they may have difficulty in accessing medical care in case of problems. The patients are generally unwilling to stay in the hospital after injectable drugs have been stopped. Economic considerations may be partly responsible for the desire to continue further treatment at home. The patients can be educated about the need for remaining under observation in the hospital even after the stoppage of parenteral drugs, thus a programme similar to the one in the Canadian study could be implemented in our hospital for cost-effective use of parenteral antibiotics.
Antibiotics were used for BPI in 65 patients. One hundred and twelve patient antibiotics were prescribed. The antibiotics were started in BPI after sending specimens for culture and sensitivity testing. In 42 patients the empirical use of the antibiotic conformed to the culture and sensitivity patterns of organisms isolated and hence the antibiotic was continued. In 15 patients the antibiotic was changed after reviewing the sensitivity report. In 8 patients though the organism was resistant to the antibiotic, the patient improved clinically and the use of the antibiotic was continued. Delay in receiving the sensitivity reports was a problem encountered and may have delayed switching over the patient to the sensitive antibiotic. Steps which could be taken to quicken the report availability are:
a) immediate transport and processing of the specimen after collection
b) constant monitoring of the culture systems to detect growth
c) identification of the organism and antibiotic sensitivity testing (AST) to be done at 16 h
d) reading of the AST to be taken 16 h after putting the antibiotic disc
e) immediate dispatch, collection and interpretation of the results.
In our hospital due to logistical problems these steps cannot be followed strictly within the specified time period, hence the results are delayed by about 12 to 24 h.
In case of blood culture, the biphasic culture system is used in which growth is observed, either by detection of turbidity in the liquid media or by growth in the solid media. However, a continuous automated monitoring system such as BACTALERT could save a substantial amount of time. This system is not being used at present as the sample load is less and hence it is not cost effective.
Antibiotics were used in non-BPI in 62% of the cases. In our previous study in ICU patients [11] 84.5% of the antibiotics were prescribed without bacteriological support. In an Indian study [2] 62% of prescriptions were therapeutic, of which 36% were therapeutic prescriptions without bacteriological support while 59% were on a bacteriological basis.
Ampicillin, amoxicillin, metronidazole, ciprofloxacin and crystalline penicillin were the 5 most commonly prescribed antibiotics. In many patients treatment was started with parenteral ampicillin which later changed to oral amoxicillin once the condition of the patient improved. The DDD/100 bed-days of ampicillin was 27.5 while that of amoxicillin was 17.5. These figures are higher than those reported by Mikic et al [17]. In their study the DDD/100 bed-days of aminopenicillins was 5.1; ampicillin was used in 37.6% of patients and amoxicillin in 13.1%. The antibiotic use in our study was 108.5 DDDs/100 bed-days. We have no data on the long trends of antibiotic utilization in our hospital. Castro et al [18] reported that the use of antibiotics in a Brazilian hospital had increased from 83.8 DDDs/100 bed-days in 1990 to 124.6 DDDs/100 bed-days in 1996. The introduction of a hospital antibiotic policy helped to reduce antibiotic utilization at a university hospital from 45.9 DDDs/100 bed-days to 32.9 DDDs/100 bed-days [19].
The use of cephalosporins in our study was less than that reported in the literature [20,21]. However, direct comparisons of cephalosporin utilization may not be appropriate as one of the studies [20] was carried out both inside and outside of health care facilities in Norway while the other study [21] was carried in a rural hospital. In the Norwegian study, cephalosporins represented 16% of the use of antibacterials in hospitals, while in the study carried out at a rural hospital, ceftriaxone and cefuroxime together accounted for 30.8% of the total antibiotic days. Antibiotic utilization in different specialties may have been considered in the above studies [20,21] while our study was confined to the Internal Medicine ward. The isolated organisms are becoming resistant to the commonly used antibiotics and cephalosporins may have to be prescribed in resistant cases. Cephalosporins are available in the hospital pharmacy but more data on sensitivity patterns is required before they can be more frequently prescribed.
A total of 163 organisms were isolated from the specimens sent for culture and sensitivity testing. The small number of specimens may, however, limit conclusions about antibiotic resistance. Carrying out the study for a longer period of time could partly overcome this lacuna. H. influenzae, E. coli, K. pneumoniae, S. aureus and S. pneumoniae were the commonest organisms isolated and were resistant to cotrimoxazole, chloramphenicol and co-amoxiclav in a significant number of cases. This maybe, is one of the reasons for the decreased use of sulfonamides in the Internal Medicine ward. Urine was the second most common specimen sent for culture and sensitivity testing. In a study reported from Trinidad [22], E. coli was the most frequent isolate from urine samples and was mostly resistant to tetracycline, trimethoprim and cotrimoxazole. The low number of samples in our study makes it difficult to draw firm conclusions but an increasing resistance of E. coli and S. aureus to commonly used antibiotics is observed.
Antibiotic resistance is an increasingly encountered problem in the Internal Medicine ward of our hospital. We are in the process of formulating an antibiotic use policy and at present, only have antibiotic use guidelines for the department of Internal Medicine. We are working to develop a system to recognize and report trends in antibiotic resistance within our institution. However, a system to rapidly detect and report resistant organisms in individual patients should be in place to ensure a rapid response by caregivers. Prevention and control of the spread of antibiotic-resistant organisms will require increased adherence to basic infection control policies and procedures, incorporation of antimicrobial resistance strategies into institutional goals and development of a plan to deal with patients colonized with resistant organisms [23].
In a recent study [24], a vast majority of physicians (97%) believed that widespread and inappropriate use of antimicrobials was an important cause of resistance. However, only 60% favoured restricting the use of broad-spectrum antibiotics [25]. Also it has been found that the public health concern of contributing to the problem of antibiotic resistance does not exert a strong impact on physician prescribing decisions for community acquired pneumonia. All these factors may have to be taken into consideration while developing a programme to reduce the use of antibiotics.
Conclusions
The high rate of prescription of parenteral antibiotics is a matter of concern. Decreasing the prescribing of parenteral antibiotics and an early switch to oral antibiotics will significantly reduce the expenditure incurred. An intravenous to oral antibiotic conversion program can be instituted. Quickening the availability of culture and sensitivity reports will enable the treatment to have a sound bacteriological basis. Antibiotic resistance is becoming a problem and formulation of a hospital antibiotic use policy is a matter of urgent concern. An educational programme and an antibiotic order form may be useful initiatives to reduce antibiotic use. Guidelines for antibiotic use in the community and restricting the level of health care practitioners who can prescribe antibiotics are required.
Competing interests
None declared.
Author's contributions
PRS planned the study, collected the data, analyzed the data, compiled the results and wrote the manuscript. PP helped in planning the study, collecting the data and writing the manuscript. NS helped in planning the study and analyzing the data and working out the cost of antibiotic therapy. JME helped in collecting the data, analyzing the culture and sensitivity results and writing the manuscript. KNB helped in overall planning of the study, analyzing the culture and sensitivity data and writing the manuscript.
Supplementary Material
Patient indicator form. Description of data: Format used to enter information about the patients
Acknowledgments
Acknowledgements
We are grateful to Bente Martinsen of the WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway for sending us a free copy of the 'Guidelines for ATC Classification and DDD assignment' and an updated ATC index. The help of Dr. Lill Fevang Harr and Dr. Hege Salvesan Blix of the WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway is gratefully acknowledged. We are grateful to Dr. Angela Panther and Dr. Denys Wheatley of the Manuscript Presentation Service, University of Aberdeen for copyediting the manuscript and kindly agreeing to waive the copyediting charges. We are grateful to Dr. Christine Nathaniel, Registrar in respiratory medicine, Scunthorpe General Hospital, UK for going through the manuscript and suggesting modifications in English usage.
Contributor Information
Ravi Pathiyil Shankar, Email: pathiyilravi@hotmail.com.
Praveen Partha, Email: praveen_partha@hotmail.com.
Nagesh Kumar Shenoy, Email: shenoynagesh@rediffmail.com.
Joshy Maducolil Easow, Email: jesjosh@hotmail.com.
Kottallur Narayanan Brahmadathan, Email: knb1948@hotmail.com.
References
- Patterson HR. The problems of audit and research. J R Coll Gen Pract. 1986;36:196. [PMC free article] [PubMed] [Google Scholar]
- Srishyla MV, Naga Rani MA, Venkataraman BV. Drug utilization of antimicrobials in the in-patient setting of a tertiary hospital. Indian J Pharmacol. 1994;26:282–287. [Google Scholar]
- Kuruvilla A, George K, Rajaratnam A, John KR. Prescription patterns and cost analysis of drugs in a base hospital in South India. Natl Med J India. 1994;7:167–168. [PubMed] [Google Scholar]
- Uppal R, Khanna S, Sharma SK, Sharma PL. Antimicrobial drug use in urology. Int J Clin Pharmacol Ther Toxicol. 1991;9:366–368. [PubMed] [Google Scholar]
- Sharma D, Reeta Kh, Badyal DK, Garg SK, Bhargava VK. Antimicrobial prescribing pattern in an Indian tertiary hospital. Indian J Physiol Pharmacol. 1998;42:533–537. [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology Guidelines for ATC classification and DDD assignment. Oslo. 2002.
- WHO Collaborating Centre for Drug Statistics Methodology ATC index with DDDs 2002. Oslo. 2002.
- Department of drug administration List of Essential drugs. Drugs Bulletin of Nepal. 2002;13:7–10. [Google Scholar]
- World Health Organization WHO model list of essential drugs. WHO Drug Information. 1999;13:249–262. [Google Scholar]
- Van der Meer JW, Gyssens IC. Quality of antimicrobial drug prescription in hospital. Clin Microbiol Infect. 2001;Suppl 7:12–15. doi: 10.1046/j.1469-0691.2001.00079.x. [DOI] [PubMed] [Google Scholar]
- Shankar PR, Partha P, Shenoy N, Brahmadathan KN. Investigation of antimicrobial use pattern in the intensive care unit of a teaching hospital in western Nepal. Am J Infect Control. [DOI] [PubMed]
- Almasi I, Ternak G. Simple parameters of antibiotic utilization and diagnostic background of antimicrobial therapy in Hungarian hospitals in 1995. Orv-Hetil. 1997;138:473–478. [PubMed] [Google Scholar]
- Das AK, Roy K, Kundu KK, Das N, Islam CN, Ram AK, Banerjee SN, Chaudhuri SB, Dutta S, Munshi S. Study of rational utilisation and cost analysis of antimicrobials in a government teaching hospital. Indian J Pharmacol. 2002;34:59–61. [Google Scholar]
- Raveh D, Levy Y, Schlesinger Y, Greenberg A, Rudensky B, Yinnon AM. Longitudinal surveillance of antibiotic use in the hospital. QJM. 2001;94:141–152. doi: 10.1093/qjmed/94.3.141. [DOI] [PubMed] [Google Scholar]
- Stratton CW, Ratner H, Johnston PE, Schaffner W. Focused microbiological surveillance by specific hospital unit: practical application and clinical utility. Clin Ther. 1993;15 Suppl A:12–20. [PubMed] [Google Scholar]
- Zamin HT, Pitre MM, Conly JM. Development of an intravenous-to-oral route conversion program for antimicrobial therapy at a Canadian tertiary health care facility. Ann Pharmacother. 1997;31:564–570. doi: 10.1177/106002809703100507. [DOI] [PubMed] [Google Scholar]
- Mikic SS, Sabo A, Jakovljevic V, Fabri M, Stefan Z, Vukadinovic I, Dulejic V. Use of aminopenicillins in hospitals and outpatient facilities. Med Pregl. 2001;45:547–551. [PubMed] [Google Scholar]
- Castro MS, Pilger D, Ferreira MB, Kopittke L. Trends in antimicrobial utilization in a university hospital, 1990–96. Rev Saude Publica. 2002;36:553–558. doi: 10.1590/s0034-89102002000600003. [DOI] [PubMed] [Google Scholar]
- Vlahovic-Palcevski V, Morovic M, Palcevski G. Antibiotic utilization at the university hospital after introducing an antibiotic policy. Eur J Clin Pharmacol. 2000;56:97–101. doi: 10.1007/s002280050727. [DOI] [PubMed] [Google Scholar]
- Blix HS. Utilization of antibiotics in and outside of health facilities in Norway in 1998. Tidsskr Nor Laegeforen. 2000;120:1731–1734. [PubMed] [Google Scholar]
- Mylotte JM, Weislo P. Antibiotic use and cost indicators at a rural hospital: a pilot project. Am J Infect Control. 2000;28:415–420. doi: 10.1067/mic.2000.109910. [DOI] [PubMed] [Google Scholar]
- Orrett FA, Shurland SM. The changing patterns of antimicrobial susceptibility of urinary pathogens in Trinidad. Singapore Med J. 1998;39:256–259. [PubMed] [Google Scholar]
- Goldmann DA, Weinstein RA, Wenzel RP, Tablan OC, Duma RJ, Gaynes RP, Schlosser J, Martone WJ. Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms in hospitals. A challenge to hospital leadership. JAMA. 1996;275:234–240. doi: 10.1001/jama.275.3.234. [DOI] [PubMed] [Google Scholar]
- Wester CW, Durairaj L, Evans AT, Schwartz DN, Husain S, Martinez E. Antibiotic resistance: a survey of physicians perceptions. Arch Intern Med. 2002;162:2210–2216. doi: 10.1001/archinte.162.19.2210. [DOI] [PubMed] [Google Scholar]
- Metlay JP, Shea JA, Crossette LB, Asch DA. Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients. J Gen Intern Med. 2002;17:89–94. doi: 10.1046/j.1525-1497.2002.10711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient indicator form. Description of data: Format used to enter information about the patients


