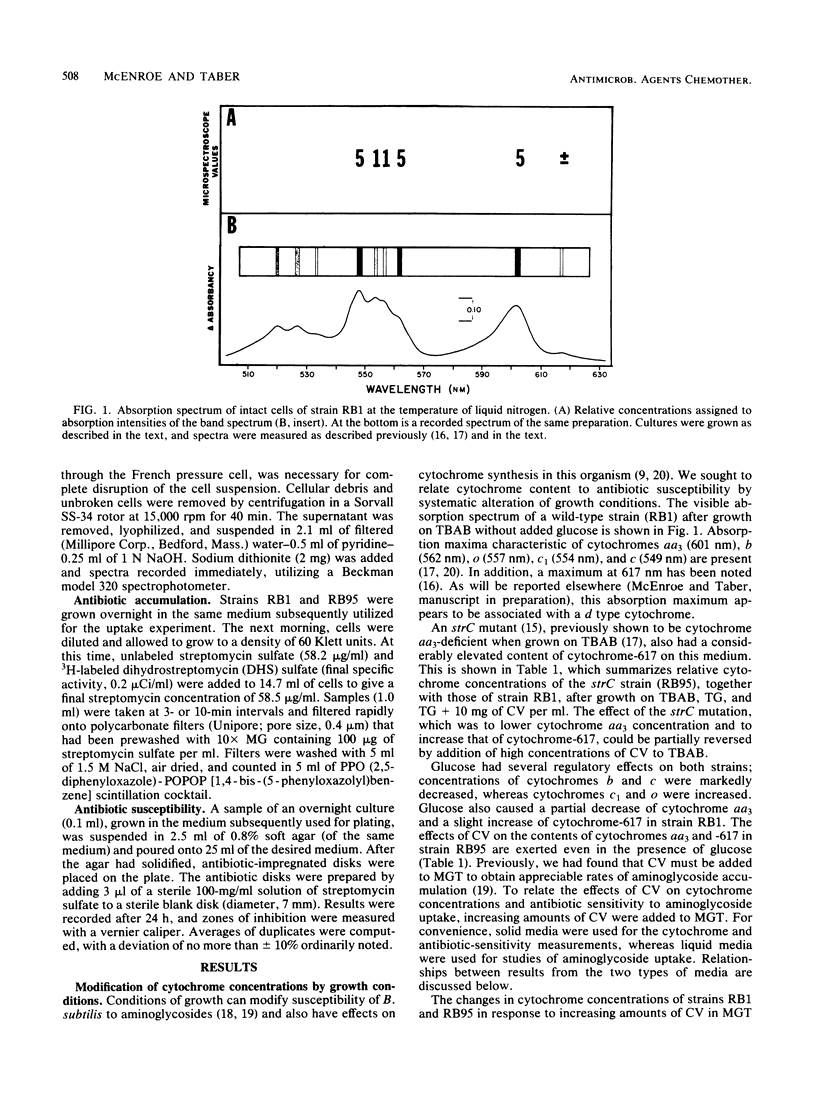
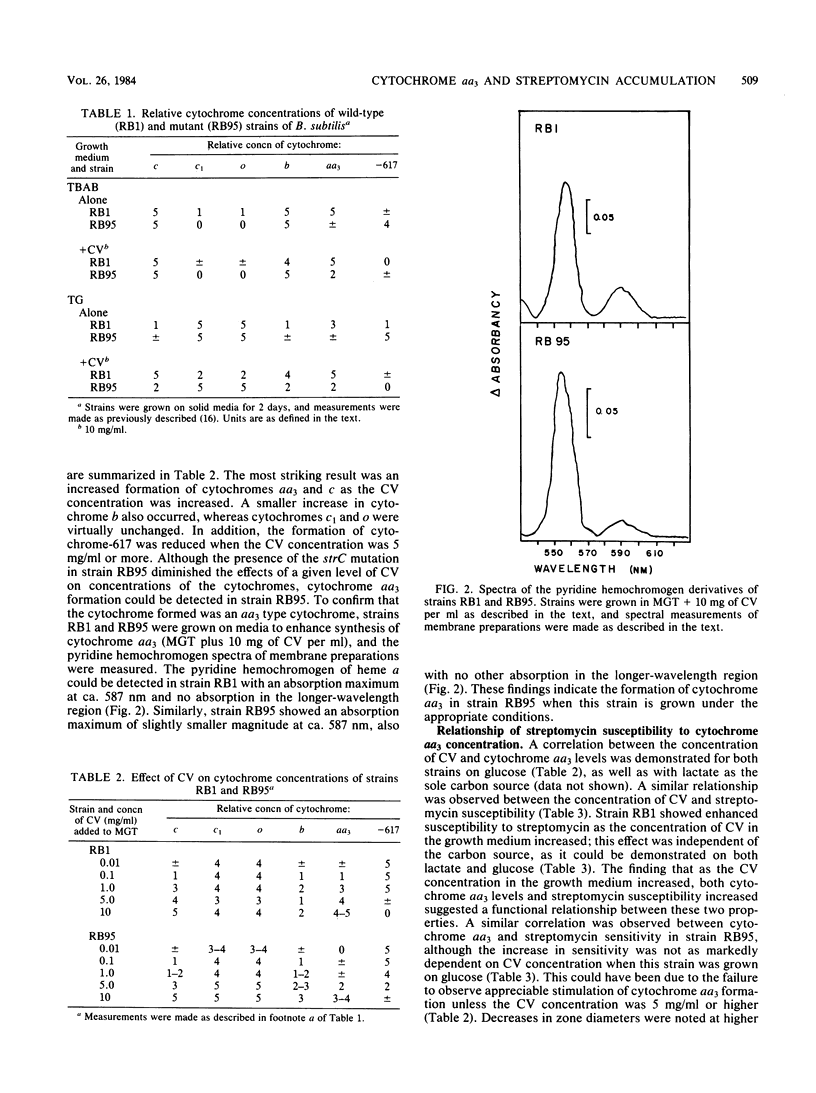
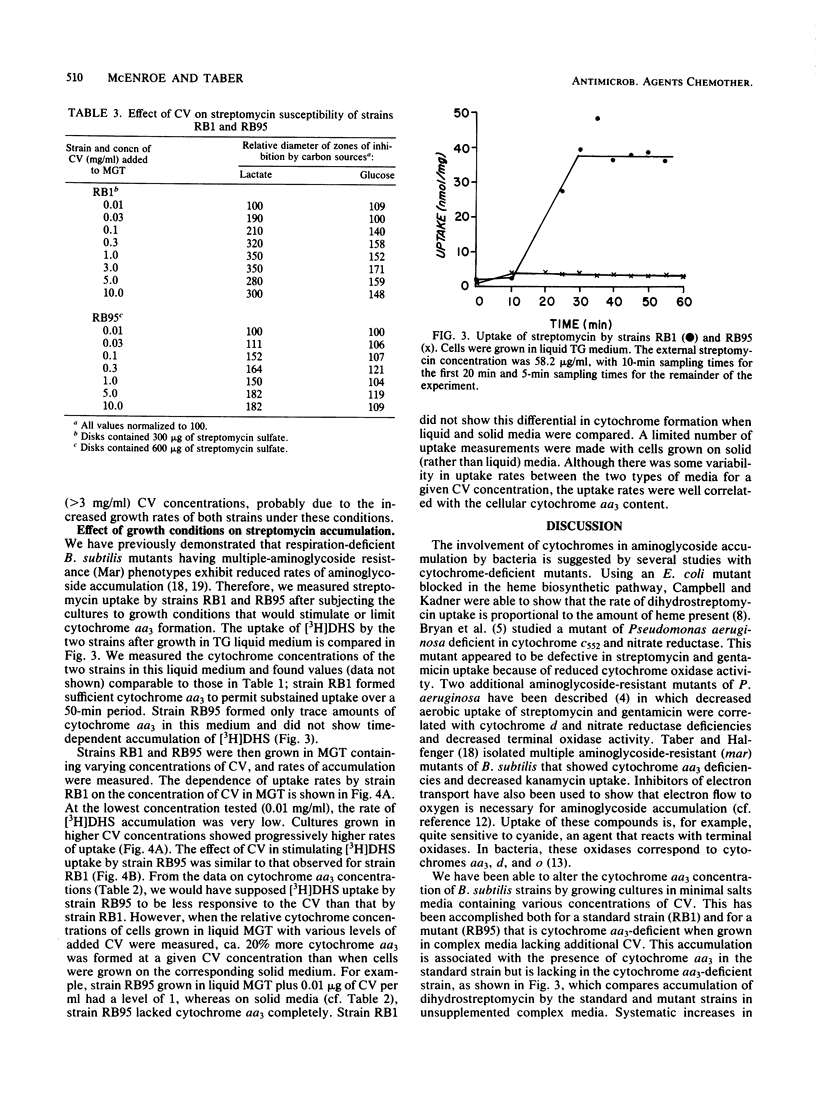
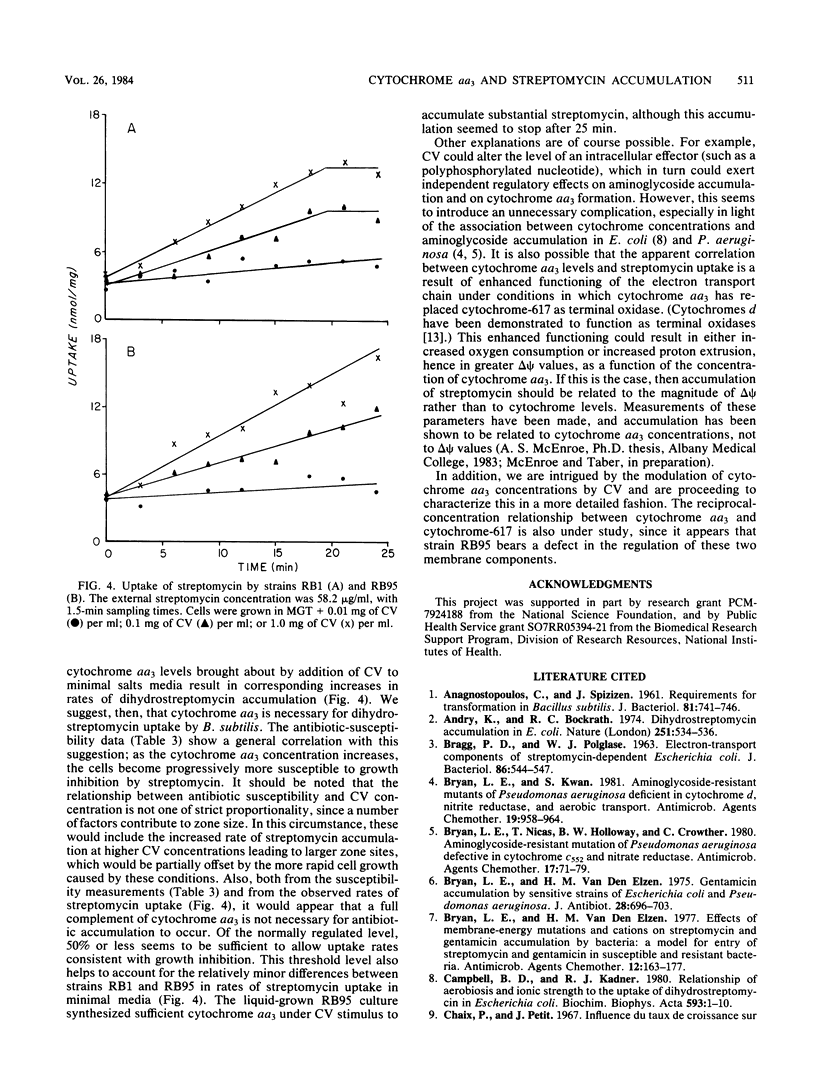
Abstract
Accumulation of aminoglycosides by Bacillus subtilis appears to require specific components of the electron transport chain. These components include cytochromes and the lipophilic quinone vitamin K2. The present study concerns the importance of cytochrome aa3, a terminal oxidase, in the uptake of streptomycin. Growth conditions have been established such that the concentration of cytochrome aa3 can be modified over a wide range; on defined minimal salts agar, the wild-type strain (RB1) and an strC mutant (RB95) synthesized cytochrome aa3 only when adequate amounts of Casamino Acids (Difco Laboratories, Detroit, Mich.) were present. A positive correlation between cytochrome aa3 levels and streptomycin accumulation was observed. The same correlation was seen when cytochrome aa3 was measured in relation to growth susceptibility. These correlations suggest that cytochrome aa3 is necessary for accumulation of streptomycin by B. subtilis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andry K., Bockrath R. C. Dihydrostreptomycin accumulation in E. coli. Nature. 1974 Oct 11;251(5475):534–536. doi: 10.1038/251534a0. [DOI] [PubMed] [Google Scholar]
- BRAGG P. D., POLGLASE W. J. ELECTRON-TRANSPORT COMPONENTS OF STREPTOMYCIN-DEPENDENT ESCHERICHIA COLI. J Bacteriol. 1963 Sep;86:544–547. doi: 10.1128/jb.86.3.544-547.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Aminoglycoside-resistant mutants of Pseudomonas aeruginosa deficient in cytochrome d, nitrite reductase, and aerobic transport. Antimicrob Agents Chemother. 1981 Jun;19(6):958–964. doi: 10.1128/aac.19.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Nicas T., Holloway B. W., Crowther C. Aminoglycoside-resistant mutation of Pseudomonas aeruginosa defective in cytochrome c552 and nitrate reductase. Antimicrob Agents Chemother. 1980 Jan;17(1):71–79. doi: 10.1128/aac.17.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Gentamicin accumulation by sensitive strains of Escherichia coli and Pseudomonas aeruginosa. J Antibiot (Tokyo) 1975 Sep;28(9):696–703. doi: 10.7164/antibiotics.28.696. [DOI] [PubMed] [Google Scholar]
- CHAIX P., PETIT J. F. Influence du taux de croissance sur la constitution du spectre hématinique de B. subtilis. Biochim Biophys Acta. 1957 Sep;25(3):481–486. doi: 10.1016/0006-3002(57)90517-6. [DOI] [PubMed] [Google Scholar]
- Campbell B. D., Kadner R. J. Relation of aerobiosis and ionic strength to the uptake of dihydrostreptomycin in Escherichia coli. Biochim Biophys Acta. 1980 Nov 5;593(1):1–10. doi: 10.1016/0005-2728(80)90002-x. [DOI] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Physiological effects of menaquinone deficiency in Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1035–1044. doi: 10.1128/jb.115.3.1035-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANCOCK R. Uptake of 14C-streptomycin by Bacillus megaterium. J Gen Microbiol. 1962 Jul;28:503–516. doi: 10.1099/00221287-28-3-503. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action--with special reference to streptomycin and gentamicin. I. Antagonists and mutants. J Antimicrob Chemother. 1981 Oct;8(4):249–276. doi: 10.1093/jac/8.4.249. [DOI] [PubMed] [Google Scholar]
- Miller M. H., Edberg S. C., Mandel L. J., Behar C. F., Steigbigel N. H. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1980 Nov;18(5):722–729. doi: 10.1128/aac.18.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P., Hoch J. A. Conditional dihydrostreptomycin resistance in Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):202–207. doi: 10.1128/jb.110.1.202-207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H. W., Sugarman B. J., Halfenger G. M. Involvement of menaquinone in the active accumulation of aminoglycosides by Bacillus subtilis. J Gen Microbiol. 1981 Mar;123(1):143–149. doi: 10.1099/00221287-123-1-143. [DOI] [PubMed] [Google Scholar]
- Taber H., Halfenger G. M. Multiple-aminoglycoside-resistant mutants of Bacillus subtilis deficient in accumulation of kanamycin. Antimicrob Agents Chemother. 1976 Feb;9(2):251–259. doi: 10.1128/aac.9.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H. Isolation and properties of cytochrome a deficient mutants of Bacillus subtilis. J Gen Microbiol. 1974 Apr;81(2):435–444. doi: 10.1099/00221287-81-2-435. [DOI] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


