Abstract
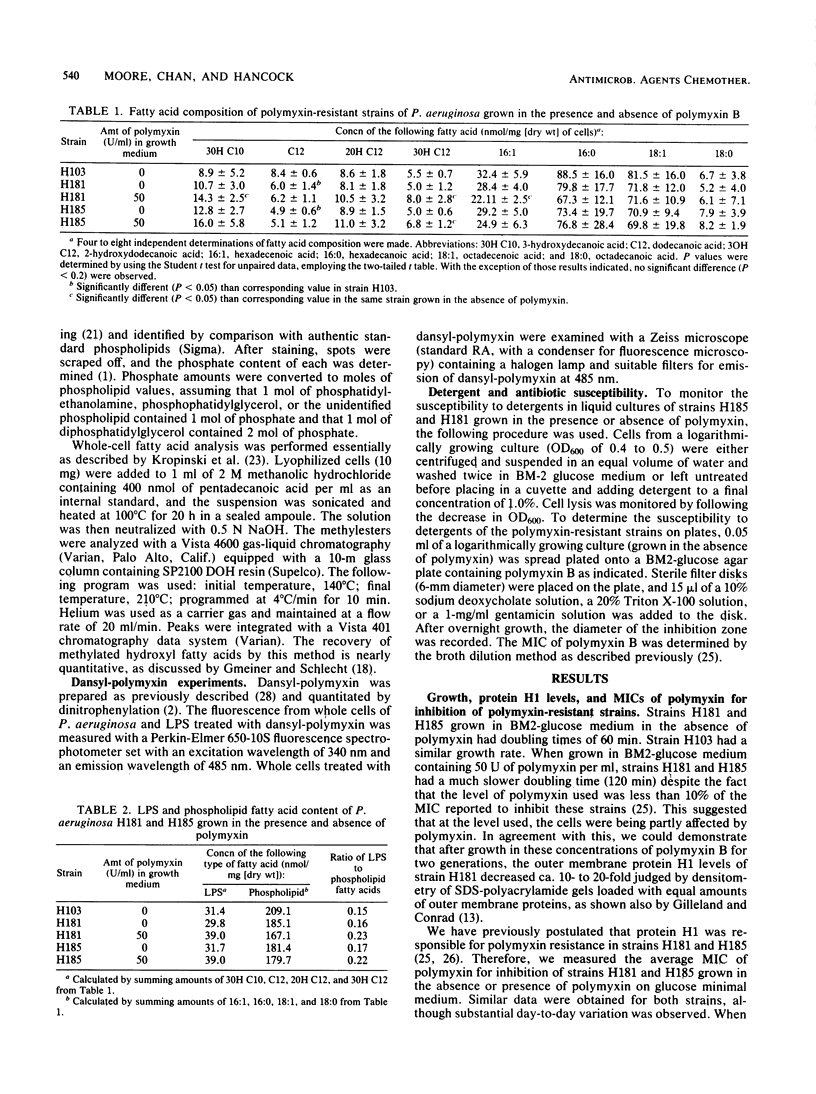
Pseudomonas aeruginosa H181 and H185 are resistant to initial exposure to polymyxin B and continue to grow in its presence. Growth of the strains in the presence of 50 U of polymyxin B per ml was characterized by a doubling time of 120 min, whereas the doubling time in the absence of polymyxin was 60 min. Growth for two generations in the presence of polymyxin caused a 23 to 31% increase in lipopolysaccharide content. In addition, a marked increase in susceptibility to the detergents sodium deoxycholate, Triton X-100, and sodium dodecyl sulfate was observed. The resistant mutants had a small but significant reduction in their levels of dodecanoic acid as compared with the parent strain; however, this was the only consistent alteration observed in levels of fatty acids or readily extractable lipids. Polymyxin was fluorescently labeled by coupling to 1-dimethylaminonaphthalene-5-sulfonyl chloride (dansyl chloride). Growth of strains H181 and H185 in the presence of dansylated polymyxin resulted in a stable association between the fluorescent antibiotic and the outer membrane. We postulate that these alterations are part of an adaptive response by the strains to the presence of polymyxin in the growth medium and reflect a resistance mechanism distinct from the mechanism affording polymyxin B resistance when these strains are initially exposed to the antibiotic.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN M. R., RICHARDS R. M. EFFECT OF POLYSORBATE (TWEEN) 80 ON THE RESISTANCE OF PSEUDOMONAS AERUGINOSA TO CHEMICAL INACTIVATION. J Pharm Pharmacol. 1964 Dec;16:SUPPL–SUPPL:5T. [PubMed] [Google Scholar]
- Bader J., Teuber M. Action of polymyxin B on bacterial membranes. 1. Binding to the O-antigenic lipopolysaccharide of Salmonella typhimurium. Z Naturforsch C. 1973 Jul-Aug;28(7):422–430. [PubMed] [Google Scholar]
- Brown M. R., Geaton E. M., Gilbert P. Additivity of action between polysorbate 80 and polymyxin B towards spheroplasts of Pseudomonas aeruginosa NCTC 6750. J Pharm Pharmacol. 1979 Mar;31(3):168–170. doi: 10.1111/j.2042-7158.1979.tb13463.x. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Winsley B. E. Synergism between polymyxin and polysorbate 80 against Pseudomonas aeruginosa. J Gen Microbiol. 1971 Nov;68(3):367–373. doi: 10.1099/00221287-68-3-367. [DOI] [PubMed] [Google Scholar]
- Champlin F. R., Gilleland H. E., Jr, Conrad R. S. Conversion of phospholipids to free fatty acids in response to acquisition of polymyxin resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1983 Jul;24(1):5–9. doi: 10.1128/aac.24.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R. S., Gilleland H. E., Jr Lipid alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J Bacteriol. 1981 Nov;148(2):487–497. doi: 10.1128/jb.148.2.487-497.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fine J. B., Sprecher H. Unidimensional thin-layer chromatography of phospholipids on boric acid-impregnated plates. J Lipid Res. 1982 May;23(4):660–663. [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Conrad R. S. Chemical alterations in cell envelopes of polymyxin-resistant mutants of Pseudomonas aeruginosa grown in the absence or presence of polymyxin. Antimicrob Agents Chemother. 1982 Dec;22(6):1012–1016. doi: 10.1128/aac.22.6.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Farley L. B. Adaptive resistance to polymyxin in Pseudomonas aeruginosa due to an outer membrane impermeability mechanism. Can J Microbiol. 1982 Jul;28(7):830–840. doi: 10.1139/m82-125. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Lyle R. D. Chemical alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J Bacteriol. 1979 Jun;138(3):839–845. doi: 10.1128/jb.138.3.839-845.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Murray R. G. Ultrastructural study of polymyxin-resistant isolates of Pseudomonas aeruginosa. J Bacteriol. 1976 Jan;125(1):267–281. doi: 10.1128/jb.125.1.267-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Eur J Biochem. 1979 Feb 1;93(3):609–620. doi: 10.1111/j.1432-1033.1979.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Meadow P. M. The extractable lipids of Pseudomonas aeruginosa. Biochim Biophys Acta. 1969 Oct 28;187(3):366–379. doi: 10.1016/0005-2760(69)90010-1. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Izaki K., Takahashi H. Degradation of phospholipid in Pseudomonas aeruginosa induced by polymyxin B. J Antibiot (Tokyo) 1975 Sep;28(9):689–695. doi: 10.7164/antibiotics.28.689. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Alteration of susceptibility to EDTA, polymyxin B and gentamicin in Pseudomonas aeruginosa by divalent cation regulation of outer membrane protein H1. J Gen Microbiol. 1983 Feb;129(2):509–517. doi: 10.1099/00221287-129-2-509. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975 Jul;8(1):95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]