Abstract
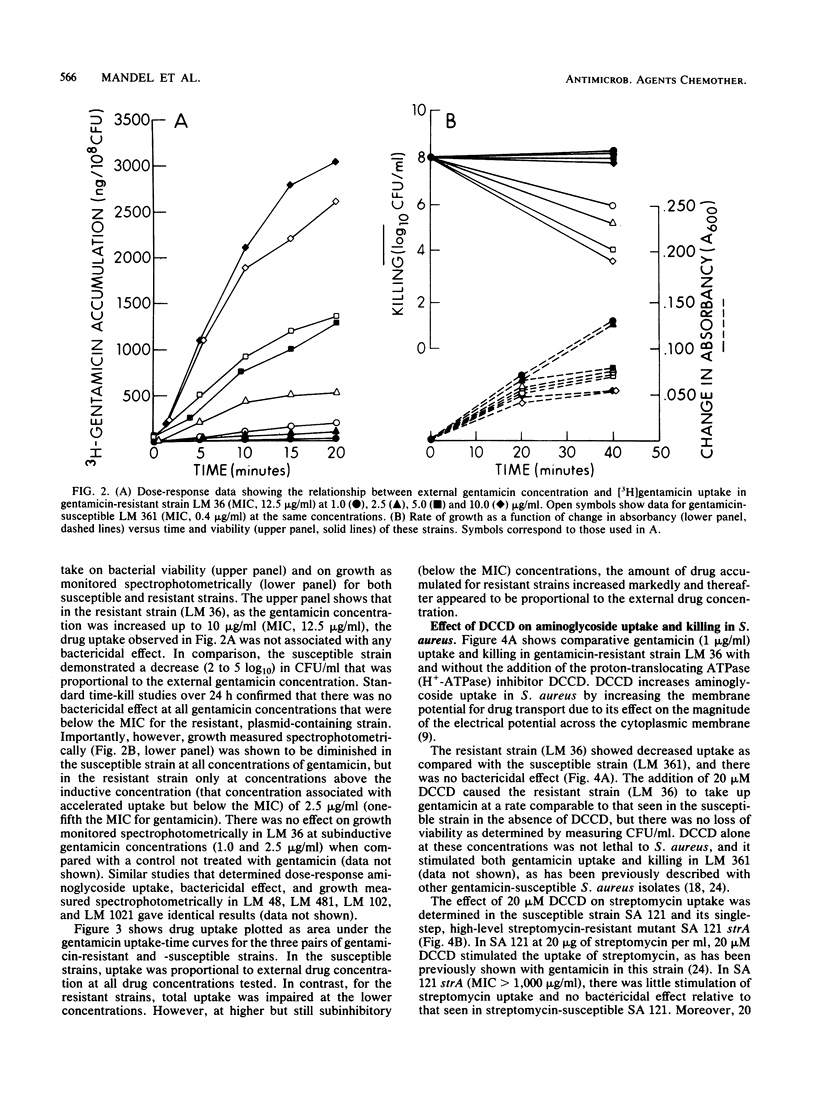
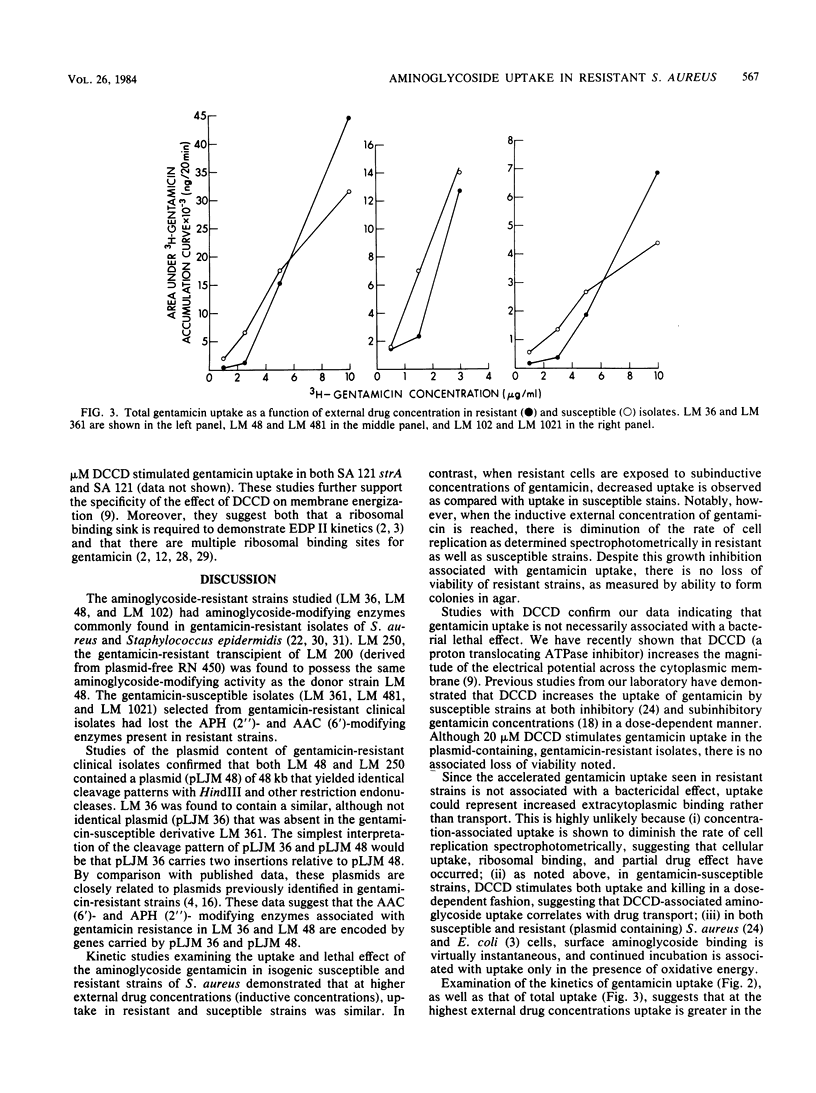
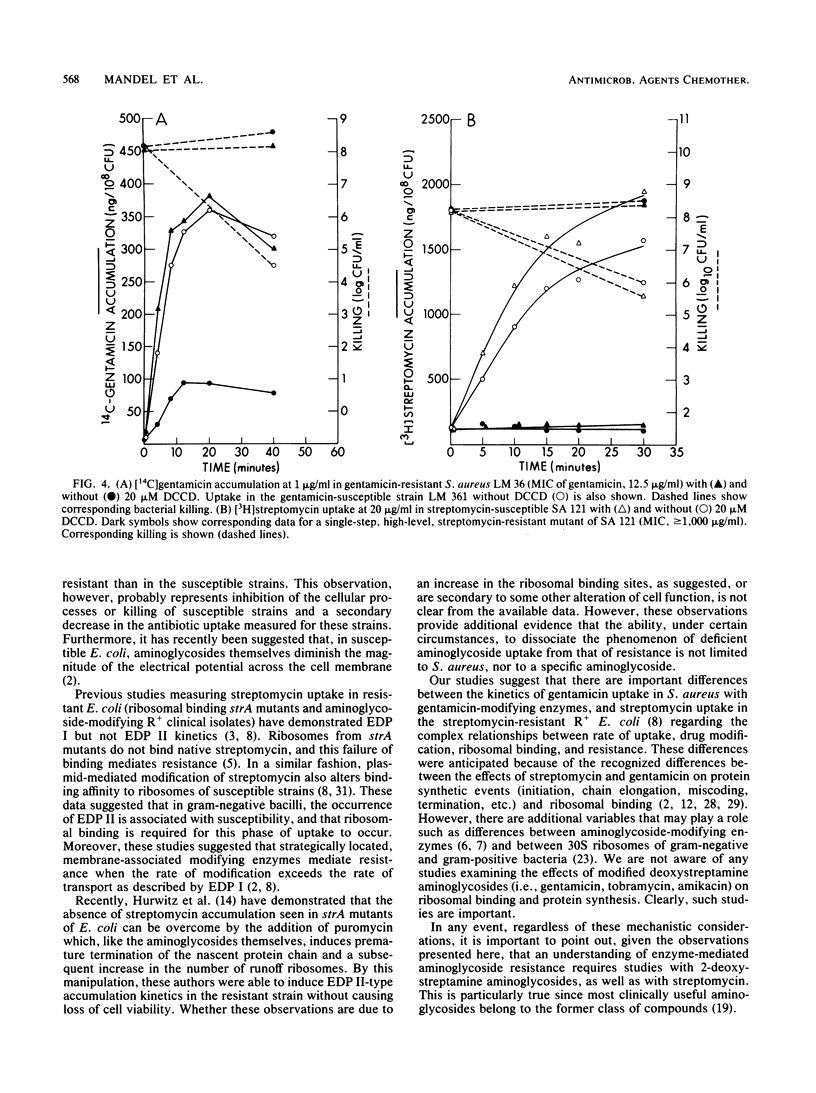
[3H]gentamicin uptake and killing were studied in three strains of gentamicin-resistant Staphylococcus aureus possessing plasmid-encoded, gentamicin-modifying enzymes and in three isogenic, enzyme-free, gentamicin-susceptible derivatives. At low (less than or equal to 2.0 micrograms/ml) concentrations of gentamicin, uptake by resistant organisms was impaired compared with that of susceptible strains, and no killing was noted. In contrast, at higher (2.5 to 10.0 micrograms/ml) concentrations (which were below the MIC for the resistant strains), rapid gentamicin uptake similar to that seen in susceptible isolates was observed. Although growth inhibition at these concentrations was apparent, there was no loss of viability in resistant strains. Consistently, the membrane H+-ATPase inhibitor N,N'-dicyclohexyl carbodiimide caused resistant strains to take up low concentrations (1.0 microgram/ml) of gentamicin at rates comparable to those seen in susceptible organisms without causing an associated loss of viability. These studies show differences between gentamicin uptake in S. aureus and streptomycin uptake in Escherichia coli (Dickie et al., Antimicrob. Agents Chemother. 14:569-580, 1978) regarding the kinetics of uptake in resistant strains with plasmid-encoded aminoglycoside-modifying enzymes. Specifically, they suggest that for 2-deoxystreptamine compounds such as gentamicin, ribosomal binding followed by accelerated uptake and subsequent interference with cell growth may occur without invariably being associated with lethal effect.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van den Elzen H. M. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jun;9(6):928–938. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Wong E. S., Falkow S. Common R-plasmids in Staphylococcus aureus and Staphylococcus epidermidis during a nosocomial Staphylococcus aureus outbreak. Antimicrob Agents Chemother. 1982 Feb;21(2):210–215. doi: 10.1128/aac.21.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J. E. STUDIES ON THE RIBOSOMES OF STREPTOMYCIN-SENSITIVE AND RESISTANT STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Apr;51:659–664. doi: 10.1073/pnas.51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. Bacterial resistance to aminoglycoside antibiotics. J Infect Dis. 1971 Dec;124 (Suppl):S7–10. doi: 10.1093/infdis/124.supplement_1.s7. [DOI] [PubMed] [Google Scholar]
- Dickie P., Bryan L. E., Pickard M. A. Effect of enzymatic adenylylation on dihydrostreptomycin accumulation in Escherichia coli carrying an R-factor: model explaining aminoglycoside resistance by inactivating mechanisms. Antimicrob Agents Chemother. 1978 Oct;14(4):569–580. doi: 10.1128/aac.14.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E. S., Mandel L. J., Kaback H. R., Miller M. H. Quantitative association between electrical potential across the cytoplasmic membrane and early gentamicin uptake and killing in Staphylococcus aureus. J Bacteriol. 1984 Mar;157(3):863–867. doi: 10.1128/jb.157.3.863-867.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action--with special reference to streptomycin and gentamicin. I. Antagonists and mutants. J Antimicrob Chemother. 1981 Oct;8(4):249–276. doi: 10.1093/jac/8.4.249. [DOI] [PubMed] [Google Scholar]
- Hurwitz C., Braun C. B., Rosano C. L. Role of ribosome recycling in uptake of dihydrostreptomycin by sensitive and resistant Escherichia coli. Biochim Biophys Acta. 1981 Jan 29;652(1):168–176. doi: 10.1016/0005-2787(81)90220-3. [DOI] [PubMed] [Google Scholar]
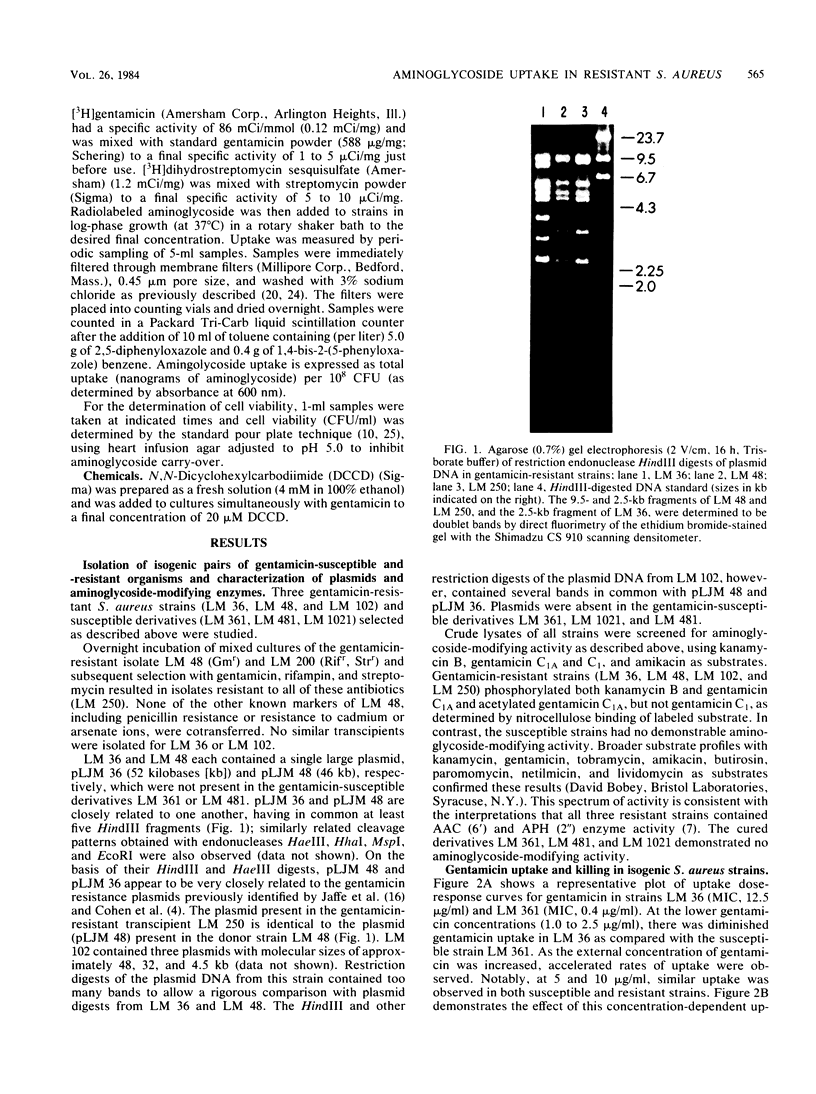
- Jaffe H. W., Sweeney H. M., Nathan C., Weinstein R. A., Kabins S. A., Cohen S. Identity and interspecific transfer of gentamicin-resistance plasmids in Staphylococcus aureus and Staphylococcus epidermidis. J Infect Dis. 1980 Jun;141(6):738–747. doi: 10.1093/infdis/141.6.738. [DOI] [PubMed] [Google Scholar]
- Jaffe H. W., Sweeney H. M., Weinstein R. A., Kabins S. A., Nathan C., Cohen S. Structural and phenotypic varieties of gentamicin resistance plasmids in hospital strains of Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1982 May;21(5):773–779. doi: 10.1128/aac.21.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühberger R., Piepersberg W., Petzet A., Buckel P., Böck A. Alteration of ribosomal protein L6 in gentamicin-resistant strains of Escherichia coli. Effects on fidelity of protein synthesis. Biochemistry. 1979 Jan 9;18(1):187–193. doi: 10.1021/bi00568a028. [DOI] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Eisenberg E. S., Simkin N. J., Miller M. H. Effect of N, N'-dicyclohexylcarbodiimide and nigericin on Staphylococcus aureus susceptibility to gentamicin. Antimicrob Agents Chemother. 1983 Sep;24(3):440–442. doi: 10.1128/aac.24.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates S. M., Eisenberg E. S., Mandel L. J., Patel L., Kaback H. R., Miller M. H. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6693–6697. doi: 10.1073/pnas.79.21.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Terry P. M., Huang T. S., Houk C. L., Davies J. Nosocomial infections with gentamicin-resistant Staphylococcus aureus: plamid analysis as an epidemiologic tool. J Infect Dis. 1979 Dec;140(6):864–872. doi: 10.1093/infdis/140.6.864. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Miller M. H., Edberg S. C., Mandel L. J., Behar C. F., Steigbigel N. H. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1980 Nov;18(5):722–729. doi: 10.1128/aac.18.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. H., Wexler M. A., Steigbigel N. H. Single and combination antibiotic therapy of Staphylococcus aureus experimental endocarditis: emergence of gentamicin-resistant mutants. Antimicrob Agents Chemother. 1978 Sep;14(3):336–343. doi: 10.1128/aac.14.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Davis B. D. Triphasic concentration effects of gentamicin on activity and misreading in protein synthesis. Biochemistry. 1979 Jan 9;18(1):193–198. doi: 10.1021/bi00568a029. [DOI] [PubMed] [Google Scholar]
- Vogel L., Nathan C., Sweeney H. M., Kabins S. A., Cohen S. Infections due to gentamicin-resistant Staphylococcus aureus strain in a nursery for neonatal infants. Antimicrob Agents Chemother. 1978 Mar;13(3):466–472. doi: 10.1128/aac.13.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]