Abstract
This report describes the integration of laser-scanning fluorometric cytometry and nonseparation ligand-binding techniques to provide new assay methods adaptable to miniaturization and high-throughput screening. Receptor-bound, cyanine dye-labeled ligands, [Cy]ligands, were discriminated from those free in solution by measuring the accumulated fluorescence associated with a receptor-containing particle. To illustrate the various binding formats accommodated by this technique, saturation- and competition-binding analyses were performed with [Cy]ligands and their cognate receptors expressed in CHO cells or as fusion proteins coated on polystyrene microspheres. We have successfully applied this technique to the analysis of G protein-coupled receptors, cytokine receptors, and SH2 domains. Multiparameter readouts from ligands labeled separately with Cy5 and Cy5.5 demonstrate the simultaneous analysis of two target receptors in a single well. In addition, laser-scanning cytometry has been used to assay enzymes such as phosphatases and in the development of single-step fluorescent immunoassays.
The surge in identification of disease-related genes (1) and the combinatorial expansion of compound collections used in “drug discovery” (2) have compelled both the biotechnology and pharmaceutical industries to seek more efficient bioassays and screening methods to search for and develop new medicines. The measurement of binding interactions forms the basis of many assays and typically requires the physical separation of free ligand, L [e.g., [125I]corticotropin (ACTH)], from receptor-bound ligand, R⋅L (e.g., ACTH receptor⋅[125I]ACTH), a procedure that often compromises the efficiency and feasibility of the assay, e.g., low-affinity interactions often cannot be filtered.
Recently, various nonseparation formats have been described to address this issue (3–7). In this paper, the application of a novel strategy, based on laser-scanning cytometry (8, 9) or laser-scanning imaging (LSI) coupled to ligand-binding assays, is introduced. In LSI, as in flow cytometry, “positive” events are identified through the detection of emitted light from laser excitation of fluorophores associated with cells or particles. However, unlike the hydrodynamic requirements of a flow cytometer, these particles do not travel in a fluid stream but, rather, remain stationary. Thus, LSI is ideally suited for the measurement of populations of cells or particles in two-dimensional arrays, for example, a microtiter plate.
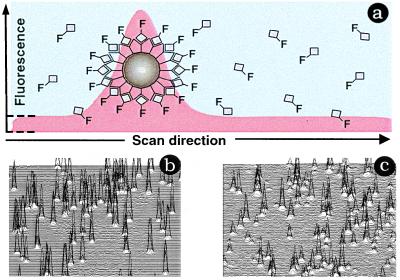
Because the effective concentration of fluorescent ligands clustered on a receptor-containing cell or particle can be greater than that for an equivalent area of surrounding buffer, light emitted from a fluorophore-coated cell or particle can be optically discriminated from fluorophore free in buffer (Fig. 1a). Therefore, LSI assays do not require physical separation of bound and free ligand, so that these assays have some features in common with the scintillation proximity assay (SPA; ref. 3). Although both techniques exploit selective binding of labeled ligands to particles in the sample, LSI uses nonradioactive fluorescence detection of the bound ligand, whereas SPA relies on light derived from scintillant-impregnated particles in proximity to a radiolabeled ligand. Thus, the need for physical separation of bound from free ligand in a standard receptor-binding assay is replaced, in LSI, by solid–liquid phase partitioning by using a cell or inert particles (Fig. 1 b and c) and optical detection afforded by novel instrumentation (9).
Figure 1.
(a) Cross-section fluorescence scan of a labeled bead or cell. As the laser beam moves into the sample, bulk or free fluorescent ligand is measured (red area bracketed by dotted line). Above this plateau is the fluorescence associated with particle-bound ligand. Measurement of bound and free ligand thus is accomplished simultaneously, achieving the “separation” required for a receptor-binding assay using optical methods. (b) Raster scans from mm2 sectors of a single well of a 96-well microtiter plate. Shown is a scan from a well containing Cy5-labeled ligand associated with 6-μm beads (b) or cells (c).
LSI overcomes several limitations of conventional receptor-binding assay methods, such as the need for wash steps, and can accommodate many of the reagents and strategies that have been developed commercially for fluorescence-activated cell sorter analysis and ELISA. The LSI device used here, capable of scanning microtiter plates, is based on the Imagn2000, a “volumetric cytometer” designed for the clinical analysis of CD4+ lymphocytes in blood (9). Here we show that the LSI concept, the optical separation of bound and free fluorescent molecules, is a versatile strategy applicable to the rapid and efficient development of nonseparation screening assays for G protein-coupled receptors (GPCRs), cytokine receptors, SH2 domains, as well as some enzymes.
MATERIALS AND METHODS
Cy5 and Cy5.5 N-hydroxysuccinimide (NHS) esters were purchased from Amersham Pharmacia, and Cy5 iodoacetamide was prepared according to published procedures (10). Europium-chelate of N1-(p-isothiocyanatobenzyl)-diethylenetriamine N1,N2,N3,N3-tetraacetic acid (DTTA) was purchased from Wallac (Gaithersburg, MD). Sphero streptavidin polystyrene particles (6.2 μm) were obtained from Spherotech (Libertyville, IL). Yersinia phosphatase (YOP) was purchased from New England Biolabs. The pY-IRS1893–899 peptide, MDYKDDDDK(caproic linker)-GNpYVNIE[NH2], and the phosphoFLAG peptide, DpYKDEAGGK(biotin)[NH2], were prepared by Princeton Biomolecules (Columbus, OH), the pY-Shc310–324 peptide, [Ac]-ELFDDPSpYVNVQNLDK[NH2], was prepared by Chiron, and the pY-ObR981–992 (leptin receptor) RQPFVKpYATLISN, and the pY-EpoR450–461, CPHLKpYLYLVVSD, were prepared by Research Genetics (Huntsville, AL). The CXCR2-expressing CHO cell line and the IL8S72C mutant were prepared as described previously (11). Recombinant human IL6Rα-Fc and IL-6 were the generous gift of Neil Stahl (Regeneron Pharmaceuticals, Tarrytown, NY). Recombinant human leptin (methionyl form) and ObR-Fc were from R&D Systems. Anti-FLAG M1 was from Sigma, antiphosphotyrosine (PY20) was from Upstate Biotechnology (Lake Placid, NY), and IL-8, IL-6, and leptin ELISA kits were from R&D Systems. Fluorescence readings were made on a custom laser-scanning imager prototype 100 series (Biometric Imaging, Mountain View, CA).
Laser-Scanning Imager Prototype 100 Series.
The scanning imager technology is based on Biometric Imaging’s Imagn2000 (9). The system utilizes a HeNe laser for excitation (633 nM), standard microscope lens, a galvanometer for scanning, and two photo-multiplier tubes for the collection of emitted light (665 nm for Cy5 and 695 nm for Cy5.5). Two-color detection is accomplished as follows (9). Fluorescent emission returning from the sample passes through a long-pass edge filter having a 655-nm cutoff. A 45° dichroic beam splitter divides the emission at 685 nm to allow optimal separation of the Cy5 and Cy5.5 spectra. Signal processing uses the known spectral overlap, and system response to each dye permits compensation for the effect of crosstalk between detectors. In addition, an x-y-z stage is incorporated to move and focus the individual wells of a 96-, 384-, or 1,536-well microtiter plate. The scan produces a fluorescence topographical map from a sector of each well. For 96-well plates, the system scans a 1-mm2 area consisting of 250 lines across the center of each well and collects fluorescence at 250 pixels per line (resolution is 4 μm/pixel). For a 1,536-well plate, the system scans a 0.6-mm2 area.
Analysis software identifies events within a well and calculates various parameters for each event including peak signal above background, peak area, and particle size, among others (9). These data are evaluated by software that extracts specified data for each well over an entire plate and outputs the data in a tab-delimited format (S.S., unpublished data). fmat software for the analysis of two-color events was kindly provided by PE Biosystems.
Chemokine Receptor Ligand [Cy5]IL8S72C.
To 50 nmol Cy5-IA was added 20 nmol (169 μg) IL8S72C in 50 mM sodium phosphate, pH 6.5/300 mM NaCl. The solution was allowed to stand in the dark at ambient temperature for 24 hr. The labeled chemokine was purified by HPLC by using a reverse-phase column (particle size, 5 μm; stationary phase, C5; pore size, 300A; 250 × 4.6 mm). The concentration of the [Cy5]IL8S72C was determined by IL-8 ELISA and Cy5-labeling stoichiometry by measuring Cy5 absorbance at 649 nm (ɛ649 = 250,000 M−1⋅cm−1).
Cytokine Receptor Ligands [Cy5]IL-6 and [Cy5]leptin.
To 50 nmol Cy5 or Cy5.5-NHS ester was added 10 nmol cytokine in 50 mM sodium phosphate, pH 7.6/150 mM NaCl. The solution was allowed to stand in the dark at 16°C for 18 hr, and the product was purified by FPLC size-exclusion chromatography on a Superdex 75 column (0.5 ml/min, Tris-buffered saline, pH 8.0). The concentration and dye stoichiometry of cytokines were determined by either IL-6 or leptin ELISA and UV absorbance, respectively.
Antibodies [Cy5]anti-IL-8, [Cy5]anti-FLAG M1, and [Cy5]PY-20.
A one-milliliter solution of antibody (1 mg/ml) was exhaustively dialyzed against 50 mM sodium bicarbonate, pH 8.5/150 mM NaCl at 4°C and then added to a vial containing 300 nmol Cy5-NHS ester. The reaction was allowed to stand at ambient temperature in the dark overnight. The product was purified by FPLC and analyzed as described above.
Phosphopeptide [Cy5]IRS-1 pY.
To a tube containing 100 nmol Cy5 NHS ester was added 300 nmol IRS-1 pY in 100 μl of coupling buffer. The reaction was allowed to stand at ambient temperature overnight, followed by reverse-phase HPLC purification. For Electrospray-MS, [Cy5]IRS-1 pY895 was 2,763.9 atomic mass units (amu) (calculated) and 2,764.6 amu (observed).
Cell-Based Assay.
CHO cells expressing CXCR2 were grown to near confluency, trypsinized, and washed in Hanks’ Balanced salt solution +0.1% BSA buffer immediately before use. For each data point, 15,000 cells per well were used.
Bead-Based Assay.
For Fc-fusion proteins, streptavidin-derivatized microspheres (6–8 μm, 107 beads) were incubated with biotinylated anti-human Fc (10 μl of 2.4 mg/ml) in 200 μl of TBS/1% BSA for 2 hr and then washed extensively with TBS/1% BSA. These beads then were used to immobilize IL6Rα-Fc or ObR-Fc (10 μg protein) under similar binding conditions. Other biotinylated proteins or peptides were bound directly to the streptavidin-derivatized beads. For each data point, 17,000 beads per well were used.
Ligand-Binding Assays.
Binding reactions were performed in a volume of 100 μl in 96-well microtiter plates at ambient temperature, in the dark, for 1 hr on a plate shaker. After incubation the plates were counted on the prototype imager. For CXCR2-binding assays, [Cy5]IL8S72C was incubated at designated concentrations with CXCR2-expressing CHO cells in the presence of varying concentrations of IL-8, growth-related cytokine α (GROα), or regulated on activation, normal T cell, expressed and secreted (RANTES). For IL6Rα-binding assays, indicated concentrations of [Cy5]IL-6 were incubated with IL6Rα-labeled microspheres in the presence of varying concentrations of IL-6.
Phosphatase Assay.
The reaction was carried out in 40 μl of assay buffer (25 mM Bis-Tris-Propane, pH 6.5/50 mM NaCl/1 mM EDTA/10 mM 2-mercaptoethanol). Approximately 75,000 beads (6.2 μm sphero streptavidin polystyrene particles; Spherotech) coated with pY-FLAG-biotin were incubated with 5 nM YOP (New England Biolabs) for 2 hr at ambient temperature. After this time, 7 μg/ml [Cy5]anti-pY or 1.3 μg/ml [Cy5]anti-M1-FLAG was added to the indicated wells and incubated for 1 hr at ambient temperature and then counted on the scanning imager. For the time course of dephosphorylation, varying concentrations of YOP were incubated in a 60-μl assay volume with substrate beads in the presence of 5 μg/ml [Cy5]anti-M1-FLAG. Plate wells were rescanned at the indicated time intervals.
Mass Spectroscopy.
Mass spectra were obtained on a Finnigan MAT LCQ spectrophotometer by injecting 5 μl of a 12.5-μM solution (60 pmol) sample in ≈50% ACN/50%H2O containing 0.5% trifluoroacetic acid.
Data Analysis.
Ligand-binding data were analyzed by nonlinear, least-squares regression by using prizm (GraphPad, San Diego). Saturation-binding data were fitted to a rectangular hyperbola, and competition data were fitted to a sigmoidal curve with a slope of unity. Data points were expressed as average fluorescence, determined by dividing the total fluorescence by the number of cells or beads counted. Fluorescent events are the number of beads counted at a defined emission wavelength.
RESULTS
GPCR-Binding Assays as Cell-Based Systems for LSI Analysis.
GPCRs, characterized by a serpentine transmembrane topology and comprising nearly 2,000 members, interact with ligands diverse in nature to regulate many aspects of physiology and pharmacology (12–14). The architecture of the ligand-binding pocket is circumscribed by extracellular and transmembrane regions of the receptor and precludes the isolation of a soluble “ligand-binding domain.” Binding measurements on GPCRs therefore are made directly on receptor-containing membranes or intact cells expressing target receptors; the later assay strategy often is not compatible with SPA.
As an example, IL-8, a 72-aa chemokine (15) modified by the addition of a carboxyl-terminal cysteine mutation, was derivatized with Cy5-iodoacetamide to generate [Cy5]IL8S72C (10, 11). Chinese hamster ovary cells expressing the recombinant human chemokine receptor for IL-8, CXCR2, were incubated with increasing concentrations of the labeled chemokine, [Cy5]IL8S72C, and the consequent increase in Cy5 fluorescence displayed saturation binding. The data, fit to the rectangular hyperbola shown in Fig. 2a, gave a KD of 1 nM, similar to that reported when using [125I]IL-8 or [Eu3+]IL8S72C in standard filter-binding assays (ref. 11 and references therein). IL-8 and another CXCR2 ligand, growth-related cytokine α, as well as the small-molecule antagonist, PS 769292, but not the CCR1 ligand, RANTES, competed for [Cy5]IL8S72C binding with the expected potencies (Fig. 2a Inset), demonstrating the feasibility of cell-based competition-binding assays using LSI.
Figure 2.
Evaluation of binding parameters for GPCR and cytokine receptors by LSI. (a) Saturation binding of CXCR2 expressed on CHO cells with [Cy5]IL8S72C, KD = 0.91 ± 0.45 nM. (Inset) Competition of [Cy5]IL8S72C by IL-8 (○), growth-related cytokine α (●), PS76292 (♦), or RANTES (⋄). [[Cy5]IL8S72C] = 1 nM; Ki(IL-8) = 1.2 ± 0.42 nM; Ki(growth-related cytokine α) = 2.5 ± 0.8 nM, Ki(PS76292) = 101 ± 2 nM. (b) Saturation binding of bead-immobilized IL6Rα-Fc with [Cy5]IL-6, KD= 3.4 ± 1.5 nM. (Inset) Competition of [Cy5]IL-6 by IL-6 (●) and leptin (○). [[Cy5]IL-6] = 5 nM; Ki (IL-6) = 4.1 ± 3.4 nM.] (c) Saturation binding of bead-immobilized ObR-Fc with [Cy5]leptin, KD= 0.5 ± 0.6 nM. (Inset) Competition of [Cy5]leptin by leptin (●) and IL-6 (○). [[Cy5]leptin] = 2.5 nM; Ki (leptin) = 0.42 ± 0.14 nM.] (d) Dissociation of [Cy5]leptin from bead-immobilized ObR-Fc, t1/2 = 35 min. (Inset) Reversibility of ObR-Fc and IL6Rα-Fc receptor ligand complexes; signal from R⋅L complex at equilibrium (open bars) and after addition of 10 μM cytokine (solid bars). All data shown represent averages of three to five experiments.
Cytokine Receptor Assays as Bead-Based Systems for LSI Analysis.
Cytokine receptors function as immunoregulators as well as in cell growth and differentiation (16). Many of these receptors are activated through ligand-induced dimerization resulting in activation of an associated tyrosine kinase. Members of this receptor class include the various interleukin receptors as well as the receptor for the satiety factor leptin.
As a consequence of the nonessential nature of the single membrane-spanning segment of cytokine receptors in ligand binding, the extracellular ligand-binding domain (ECD) of several receptors have been expressed as soluble Fc-fusion proteins (17). Here, the ECD-Fc fusion proteins that bind IL-6 and leptin, IL6Rα-Fc and ObR-Fc, respectively, were coated onto 6-μm anti-Fc microsphere conjugate beads. The dissociation constants of [Cy5]IL-6 and [Cy5]leptin were determined by incubating increasing concentrations of these ligands with the coated microspheres (Fig. 2 b and c). The values obtained, KD([Cy5]IL-6) = 3.4 ± 1.5 nM and KD([Cy5]leptin) = 0.5 ± 0.6 nM, were in agreement with values reported in the literature (17, 18). The specificity of these interactions was demonstrated by competition of Cy5-labeled cytokine only by the corresponding unlabeled cytokine (Fig. 2 b and c Insets). Leptin receptor number per microsphere was measured directly by lanthanide-based, time-resolved fluorometry by using ObR-Fc labeled with the europium-chelate, [Eu3+]DTTA (4). Thus, by using saturating levels of [Cy5]leptin, the instrument was determined to have a lower detection limit of ≈20,000 receptors per bead (data not shown).
The [Cy5]cytokine–receptor complex was freely reversible as demonstrated by a fluorescence signal decrease after addition of excess cytokine subsequent to an established equilibrium (Fig. 2d Inset). The off-rate of leptin was slow enough that it could be measured (Fig. 2d), displaying a half-life of 30 min. Because assay wells can be measured repeatedly without sample manipulation, this method is well suited to kinetic measurements (vida infra).
The differential labeling of leptin and IL-6 with dyes that have distinct emission profiles, EMmax(Cy5.5) = 695 nm or EMmax(Cy5) = 665 nm (10), permitted simultaneous detection of the binding of both cytokines to their respective receptors on common or distinct beads within the same assay well. This “multiplexed target” strategy improves the efficiency of screening and allows direct ratiometric quantification of drug effects on multiple targets within a sample well. In one experiment, both receptors, ObR-Fc and IL6Rα-Fc, are coated onto the same particles. Fluorescently labeled ligands for these receptors, [Cy5.5]leptin and [Cy5]IL-6, were incubated separately (Fig. 3, wells 1 and 2) or together (Fig. 3, well 3). These beads gave rise to specific measurable events depending on the labeled cytokine added, as shown in Fig. 3 b and c. If both labeled cytokines were added, only dual-positive beads are detected (Fig. 3a, well 3). After addition and incubation of a saturating concentration of IL-6 to beads incubated with both Cy5 and Cy5.5-labeled cytokines (Fig. 3 a– c, well 3), the dual-positive signal decreased to background as a result of a decrease in the [Cy5]IL-6⋅IL6Rα population. In addition, events attributable only to [Cy5.5]leptin are now measurable (Fig. 3b, well 3). Consequently, a specific competitor of the [Cy5]IL-6⋅IL6Rα equilibrium is detectable both as the decrease in signal resulting from the disruption of the specific complex and the concurrent appearance of a signal from the nondisrupted complex.
Figure 3.
Receptor multiplex assays. (Left) Different receptors on common particles. Cy5.5+ and Cy5+ (a), Cy5.5+, (b) or Cy5+ (c) fluorescence events detected for indicated labeled ligands bound to ObR-Fc and IL6Rα-Fc on common beads (solid bars) and in the presence of 10 μM unlabeled IL-6 (hatched bars). (Right) Different receptors on different particles. Cy5.5+ (d) or Cy5+ (e) fluorescence events detected for indicated labeled ligands bound to ObR-Fc and IL6Rα-Fc on separate beads (solid bars) and in the presence of 10 μM unlabeled IL-6 (hatched bars).
In another application, receptors coated on distinct particles can be measured as independent events in the same well (Fig. 3 d and e, wells 4–6). In this case, signals are measured independently of one another. Thus, two independent binding equilibria (leptin⋅ObR or IL-6⋅IL6Rα) are detectable in the same well on common or distinct particles, allowing the design of assays having internal specificity controls.
Phosphotyrosine-Mediated Protein Interactions Measured with LSI Analysis.
Intracellular protein–protein binding events, such as those that occur between the SH2 domain and tyrosine-phosphorylated motifs, are mediated by a variety of modular protein domains (19). The SH2 domain of Grb2, an adapter protein that links receptor tyrosine kinases to the ras-signaling pathway, displays low micromolar affinity for phosphopeptides with Asn occupying the pY+2 position (20–22). A Cy5-labeled, phosphorylated peptide sequence derived from the insulin receptor substrate ([Cy5]pY-IRS1893–899), a Grb2 target, was used to measure phosphopeptide binding to the SH2 domain of Grb2. The interaction specificity was measured by the ability of various phosphopeptide sequences to displace [Cy5]pY-IRS1 from biotinylated glutathione S-transferase (GST)-Grb2 anchored to avidin-coated microspheres. Phosphotyrosyl peptides derived from regions surrounding Tyr896-Val-Asn in IRS-1 or Tyr317-Val-Asn in Shc competed effectively at low micromolar concentrations with [Cy5]pY-IRS1893–899; sequences having nonconsensus residues (e.g., Thr or Tyr) at the pY+2 position were ineffective at concentrations up to 100 μM, whereas phenyl phosphate, a phosphotyrosine mimic, competed weakly at millimolar concentrations (Fig. 4a).
Figure 4.
Phosphopeptide–SH2 domain interactions. (a) Displacement of [Cy5]pY-IRS1893–899 from Grb2 by consensus (circles) and nonconsensus (diamonds) phosphopeptides; pY-IRS1893–899 (○); pY-Shc310–324 (●); pY-ObR981–992 (⋄); pY-EpoR450–461(♦); and phenylphosphate (■). [Cy5]pY-IRS1893–899 = 15 nM. (b) Avidity effects. [Cy5]pY-IRS1893–899 (20 nM) displacement from bead-tethered GST-Grb2 with excess pY-IRS1893–899 (60 μM) (solid bars). [Cy5]GST-Grb2 (3 nM; open bars) displacement from bead-tethered pY-IRS1893–899 by excess GST-Grb2 (1 μM).
It is of interest that although this system showed reversible binding (Fig. 4b), an opposing assay configuration, i.e., incubating a Cy5-labeled GST-fusion protein of Grb2 with phosphopeptide tethered to a bead, resulted in only partial reversibility (Fig. 4b). This effect has been observed in comparable systems (23, 24) and is thought to originate from enhanced avidity arising from a bivalent interaction between the GST-fusion protein, a dimer in solution, and the polyvalent bead surface. Thus configured, low-affinity protein–protein interactions can be studied in dynamic equilibrium by using LSI, potentially yielding new insights into these molecular interactions.
Phosphatase Activity Measured with LSI.
The activity of enzymes catalyzing the formation of immunoreactive products is measurable by LSI. As an example, for the assay of protein phosphatases we have designed phosphopeptide substrates that reveal an antibody epitope upon dephosphorylation (Fig. 5a). In this case, to measure the activity of the Yersinia PTPase, YOP, the FLAG epitope Asp-Tyr-Lys-Asp-Glu (25) was “masked” as the tyrosine-phosphorylated peptide Asp-phosphoTyr-Lys-Asp-Glu-Ala-Ala-Gly-Lys(Nɛ-biotin) containing a biotin and spacer peptide for the purpose of linking the phosphoFLAG peptide to avidin-coated microspheres. These phosphopeptide microspheres were detected easily by using [Cy5]antiphosphotyrosine (Fig. 5b); however, [Cy5]anti-FLAG was unreactive toward the “masked” FLAG sequence (Fig. 5b). Subsequent to incubation with YOP, detectable phosphotyrosine decreased with a reciprocal increase in the detection of the FLAG epitope. By scanning a well repeatedly over time, reaction progress can be followed (Fig. 5c). The time interval between measurements is limited by the scan time per well (≈4 sec) and the current software package. Further, detection limits (see above) impose a minimum FLAG-bead⋅[Cy5]anti-FLAG antibody complex concentration below which the signal is not detected. The formation of this complex, a second-order process, is dependent on the individual concentrations of these components. This is illustrated by the time lag in detectable product formation with decreasing enzyme concentration (Fig. 5c).
Figure 5.
Phosphatase assay. (a) Schematic of unmasking assay. (b) PhospoFLAG peptide-coated microspheres incubated with YOP or without YOP. Phosphotyrosine (solid bars) and FLAG peptide (open bars) were detected with [Cy5]antiphosphotyrosine and [Cy5]anti-FLAG, respectively. (c) Time course of dephosphorylation. Various concentrations of YOP, 0 nM (♦), 1.5 nM (▾), 3 nM (▴), 6 nM (■), or 25 nM (●), were incubated with phosphoFLAG peptide-coated microspheres in the presence of [Cy5]anti-FLAG and scanned repeatedly at the indicated time intervals.
DISCUSSION
GPCRs and cytokine receptors represent two large families of cell-surface receptors. These receptors, and the signaling networks they coordinate, involve many pharmaceutically important disease targets (26, 27). Using LSI, many of the interacting components of these pathways can be measured in a nonseparation format. Although similar in concept to SPA, LSI, a nonradioactive alternative, has the added capability of measuring ligand binding on intact cells and is adaptable to target multiplexing by virtue of multiparameter readouts. Although different emission fluorescence profiles were used here to resolve signals from different ligands, other parameters such as particle size can be used to discern distinct binding events in a common assay well. Assay multiplexing permits internal specificity controls or ratiometric analysis for individual microplate wells and the single-step addition of receptor–ligand complexes to compound library plates, a significant factor in assay miniaturization.
LSI has been applied to the detection of enzymatic activities. For example, PTPase activity was detected by using an “epitope unmasking” assay, where peptide dephosphorylation is coupled to formation of a [Cy5]antipeptide–peptide complex. This provides a desirable increase-in-signal readout with reaction progress as opposed to the decease-in-signal observed in measuring phosphotyrosine depletion with antiphosphotyrosine. From a practical standpoint, [Cy5]antipeptide can accompany the addition of PTPase and phosphopeptide, allowing all components to be present simultaneously, an arrangement not possible when using phosphotyrosine antibodies that react with pY-containing substrates or enzyme.
The wide application profile and versatility of LSI will allow simplification of many routine laboratory assays. For example, ELISAs are easily converted to “no-wash” fluoroimmunoassays (data not shown). The ability to multiplex targets and standardize formats is a benefit LSI offers toward improving the efficiency of receptor–ligand binding and antibody-based assays.
Acknowledgments
We thank Drs. P. Samama, V. D. Fitzpatrick, D. Dunn, and M. Webb for critical reading of the manuscript.
ABBREVIATIONS
- Cy
cyanine dye
- LSI
laser-scanning imaging
- SPA
scintillation proximity assay
- ILRα
IL-6 receptor α subunit
- ObR
leptin receptor
- GPCR
G protein-coupled receptor
- YOP
Yersinia phosphatase
- GST
glutathione S-transferase
References
- 1.Debouck C, Goodfellow P N. Nat Genet. 1999;21:48–50. doi: 10.1038/4475. [DOI] [PubMed] [Google Scholar]
- 2.Chabala J C. In: Comb. Chem. Mol. Diversity Drug Discovery. Gordon E M, Kerwin J F Jr, editors. New York: Wiley–Liss; 1998. pp. 3–15. [Google Scholar]
- 3.Hart H E, Greenwald E B. Mol Immunol. 1979;16:265–267. doi: 10.1016/0161-5890(79)90065-8. [DOI] [PubMed] [Google Scholar]
- 4.Hemmila I, Webb S. Drug Discovery Today. 1997;2:373–381. [Google Scholar]
- 5.Tsien R Y, Bacskai B J, Adams S R. Trends Cell Biol. 1993;3:242–245. doi: 10.1016/0962-8924(93)90124-j. [DOI] [PubMed] [Google Scholar]
- 6.Auer M, Moore K J, Meyer-Almes F J, Guenther R, Pope J, Stoeckli K A. Drug Discovery Today. 1998;3:457–465. [Google Scholar]
- 7.Lynch B A, Loiacono K A, Tiong C L, Adams S E, MacNeil I A. Anal Biochem. 1997;247:77–82. doi: 10.1006/abio.1997.2042. [DOI] [PubMed] [Google Scholar]
- 8.Kamentsky L A, Kamentsky L D. Cytometry. 1991;12:381–387. doi: 10.1002/cyto.990120502. [DOI] [PubMed] [Google Scholar]
- 9.Dietz L J, Dubrow R S, Manian B S, Sizto N L. Cytometry. 1996;23:177–186. doi: 10.1002/(SICI)1097-0320(19960301)23:3<177::AID-CYTO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Ernst L A, Gupta R K, Mujumdar R B, Waggoner A S. Cytometry. 1989;10:3–10. doi: 10.1002/cyto.990100103. [DOI] [PubMed] [Google Scholar]
- 11.Inglese J, Samama P, Patel S, Burbaum J, Stroke I L, Appell K C. Biochemistry. 1998;37:2372–2377. doi: 10.1021/bi972161u. [DOI] [PubMed] [Google Scholar]
- 12.Ji T H, Grossmann M, Ji I. J Biol Chem. 1998;273:17299–17302. doi: 10.1074/jbc.273.28.17299. [DOI] [PubMed] [Google Scholar]
- 13.Watson S, Arkinstall S. The G Protein Linked Receptor Facts Book. London: Academic; 1994. [Google Scholar]
- 14.Luttrell L M, Daaka Y, Lefkowitz R J. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 15.Baggiolini M, Dewald B, Moser B. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 16.Nicola N A. Guidebook to Cytokines and Their Receptors. New York: Oxford Univ. Press; 1994. [Google Scholar]
- 17.Brown S J, Becherer K A, Blumeyer K, Kautzer C, Axelrod F, Le H, McConnell S J, Whalley A, Spinella D G. Protein Expression Purif. 1998;14:120–124. doi: 10.1006/prep.1998.0940. [DOI] [PubMed] [Google Scholar]
- 18.Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, et al. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 19.Pawson T. Nature (London) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 20.Lowenstein E J, Daly R J, Batzer A G, Li W, Margolis B, Lammers R, Ullrich A, Skolnik E Y, Bar-Sagi D, Schlessinger J. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 21.Burke T R J, Smyth M S, Otaka A, Nomizu M, Roller P P, Wolf G, Case R, Shoelson S E. Biochemistry. 1994;33:6490–6494. doi: 10.1021/bi00187a015. [DOI] [PubMed] [Google Scholar]
- 22.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, et al. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 23.Greenlund A C, Morales M O, Viviano B L, Yan H, Krolewski J, Schreiber R D. Immunity. 1995;2:677–687. doi: 10.1016/1074-7613(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 24.Ladbury J E, Lemmon M A, Zhou M, Green J, Botfield M C, Schlessinger J. Proc Natl Acad Sci USA. 1995;92:3199–3203. doi: 10.1073/pnas.92.8.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopp T P, Prickett K S, Price V L, Libby R T, March C J, Cerretti D P, Urdal D L, Conlon P J. Bio/Technology. 1988;6:1204–1210. [Google Scholar]
- 26.Spiegel A M. Annu Rev Physiol. 1996;58:143–170. doi: 10.1146/annurev.ph.58.030196.001043. [DOI] [PubMed] [Google Scholar]
- 27.Lamb P, Tapley P, Rosen J. Drug Discovery Today. 1998;3:122–130. [Google Scholar]