Abstract
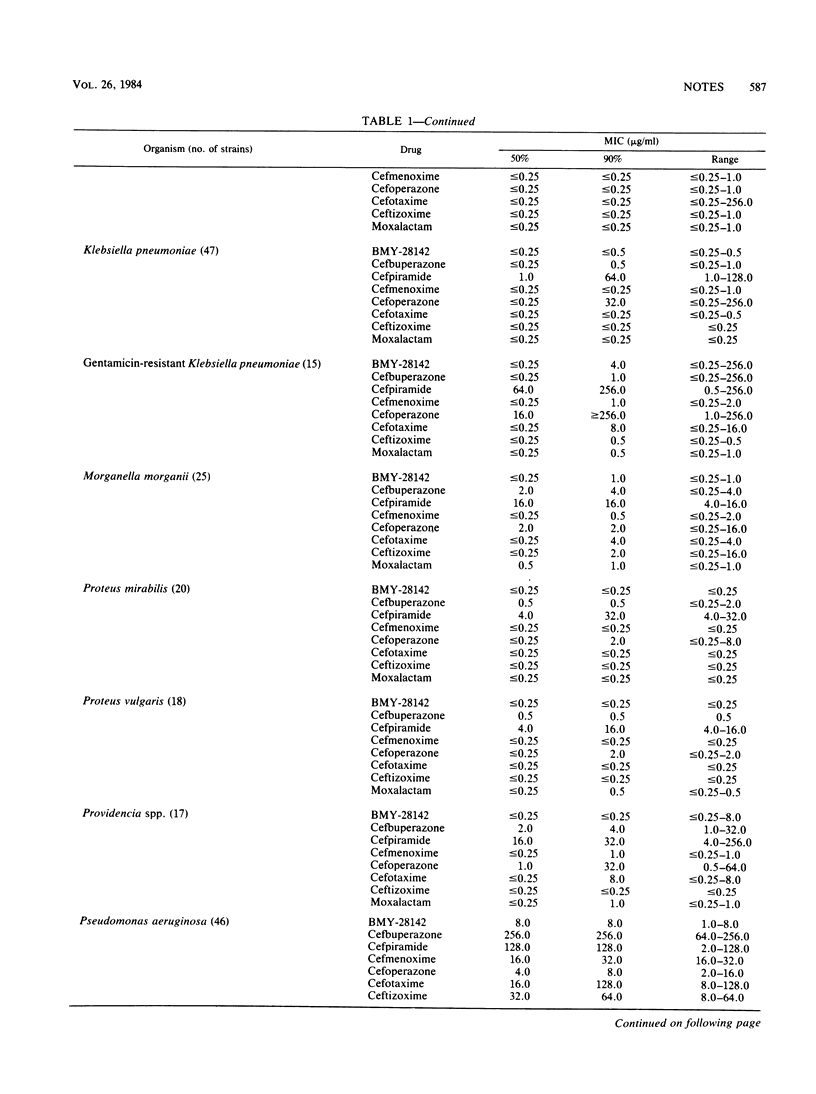
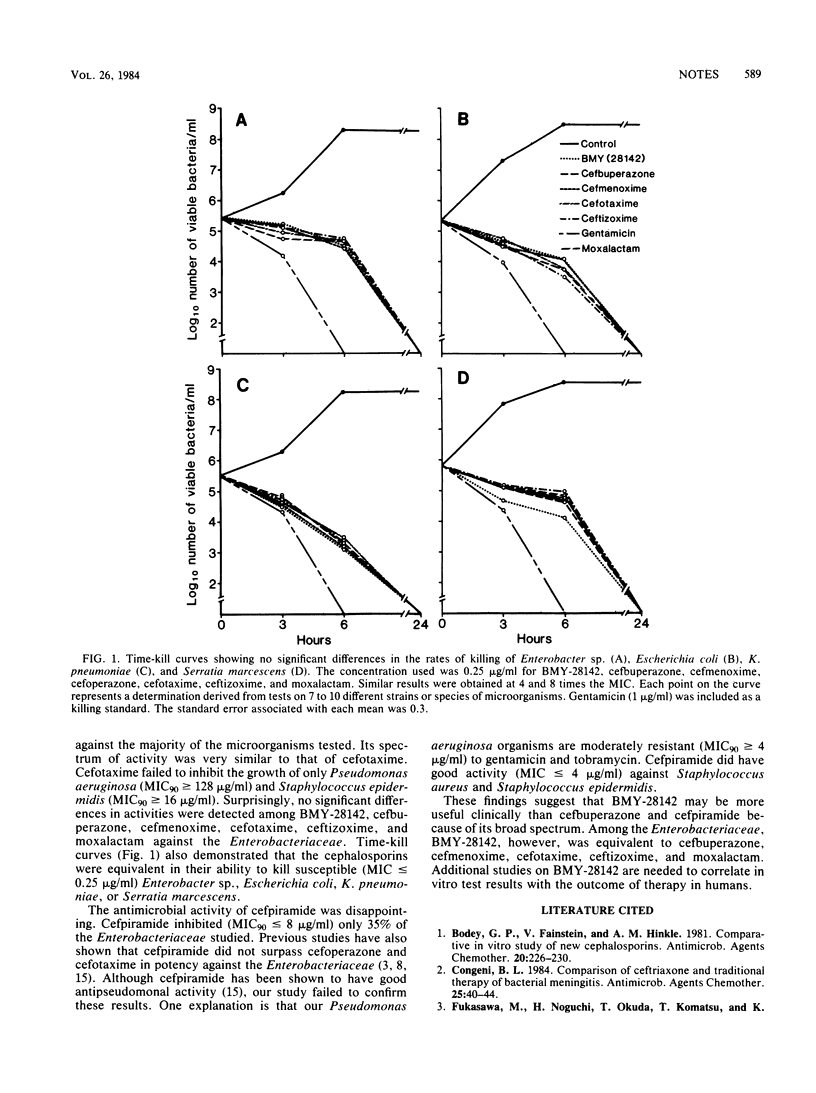
The antimicrobial activities of BMY-28142, cefbuperazone (BMY-25182; formerly T-1982), and cefpiramide (WY-44635; formerly SM-1652) were compared with those of cefmenoxime, cefoperazone, cefotaxime, ceftizoxime, and moxalactam. BMY-28142 was the most active cephalosporin against the majority of aerobic and facultatively anaerobic microorganisms studied. Its spectrum of activity was very similar to that of cefotaxime. However, BMY-28142, cefbuperazone, cefmenoxime, cefotaxime, ceftizoxime, and moxalactam were equivalent in activity and rate of killing against members of the family Enterobacteriaceae. Cefpiramide was considerably less active than the other cephalosporins against the Enterobacteriaceae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P., Fainstein V., Hinkle A. M. Comparative in vitro study in new cephalosporins. Antimicrob Agents Chemother. 1981 Aug;20(2):226–230. doi: 10.1128/aac.20.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congeni B. L. Comparison of ceftriaxone and traditional therapy of bacterial meningitis. Antimicrob Agents Chemother. 1984 Jan;25(1):40–44. doi: 10.1128/aac.25.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa M., Noguchi H., Okuda T., Komatsu T., Yano K. In vitro antibacterial activity of SM-1652, a new broad-spectrum cephalosporin with antipseudomonal activity. Antimicrob Agents Chemother. 1983 Feb;23(2):195–200. doi: 10.1128/aac.23.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzone P., Lyon J., Yu V. L. Third-generation and investigational cephalosporins: I. Structure-activity relationships and pharmacokinetic review. Drug Intell Clin Pharm. 1983 Jul-Aug;17(7-8):507–515. doi: 10.1177/106002808301700703. [DOI] [PubMed] [Google Scholar]
- Garzone P., Lyon J., Yu V. L. Third-generation and investigational cephalosporins: II. Microbiologic review and clinical summaries. Drug Intell Clin Pharm. 1983 Sep;17(9):615–622. doi: 10.1177/106002808301700901. [DOI] [PubMed] [Google Scholar]
- Imasaki H., Enjoji Y., Matsui H., Kawai R., Kawamura S., Okuda T. Metabolic fate of [14C]SM-1652, a new antipseudomonal cephalosporin, after parenteral administration to rats. Antimicrob Agents Chemother. 1983 Jul;24(1):42–47. doi: 10.1128/aac.24.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Inoue M., Mitsuhashi S. Antibacterial activities of SM-1652 compared with those of other broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1982 Nov;22(5):721–727. doi: 10.1128/aac.22.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz T. O., Winston D. J., Hindler J. A., Young L. S., Hewitt W. L., Martin W. J. Comparative in vitro activity of moxalactam, cefotaxime, cefoperazone, piperacillin, and aminoglycosides against gram-negative bacilli. Antimicrob Agents Chemother. 1980 Oct;18(4):645–648. doi: 10.1128/aac.18.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. D., Edwards D. J., Durack D. T. Comparison of cefoperazone, cefotaxime, and moxalactam (LY127935) against aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1980 Mar;17(3):488–493. doi: 10.1128/aac.17.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFrock J. L., Prince R. A., Leff R. D. Mechanism of action, antimicrobial activity, pharmacology, adverse effects, and clinical efficacy of cefotaxime. Pharmacotherapy. 1982 Jul-Aug;2(4):174–184. doi: 10.1002/j.1875-9114.1982.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The new beta-lactamase-stable cephalosporins. Ann Intern Med. 1982 Sep;97(3):408–419. doi: 10.7326/0003-4819-97-3-408. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Niles A. C., Murray P. R. In vitro antibacterial activity of cefpiramide. Antimicrob Agents Chemother. 1984 Mar;25(3):368–372. doi: 10.1128/aac.25.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., LeFrock J. L., Smith B. R., Francisco M. In vitro antimicrobial activity of ceftizoxime. Chemotherapy. 1983;29(6):408–414. doi: 10.1159/000238228. [DOI] [PubMed] [Google Scholar]
- Schell R. F., Smith B. R., LeFrock J. L., Francisco M. A. Antimicrobial activity of cefmenoxime compared with those of other cephalosporins. Antimicrob Agents Chemother. 1983 May;23(5):774–777. doi: 10.1128/aac.23.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert G., Limbert M., Winkler I., Dick T. The Antibacterial activity in vitro and beta-lactamase stability of the new cephalosporin HR 810 in comparison with five other cephalosporins and two aminoglycosides. Infection. 1983 Sep-Oct;11(5):275–279. doi: 10.1007/BF01641262. [DOI] [PubMed] [Google Scholar]
- Smith B. R., LeFrock J., Carr B. B. Cefmenoxime penetration into gallbladder bile and tissue. Antimicrob Agents Chemother. 1983 Jun;23(6):941–943. doi: 10.1128/aac.23.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai M., Fukuoka Y., Yotsuji A., Kumano K., Takahata M., Mikami H., Yasuda T., Saikawa I., Mitsuhashi S. In vitro and in vivo antibacterial activity of T-1982, a new semisynthetic cephamycin antibiotic. Antimicrob Agents Chemother. 1982 Nov;22(5):728–734. doi: 10.1128/aac.22.5.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H., Carter W. T., Harris B., Finegold S. M. Comparative in vitro activities of cefpiramide and apalcillin against anaerobic bacteria. Antimicrob Agents Chemother. 1984 Feb;25(2):162–164. doi: 10.1128/aac.25.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]



