Abstract
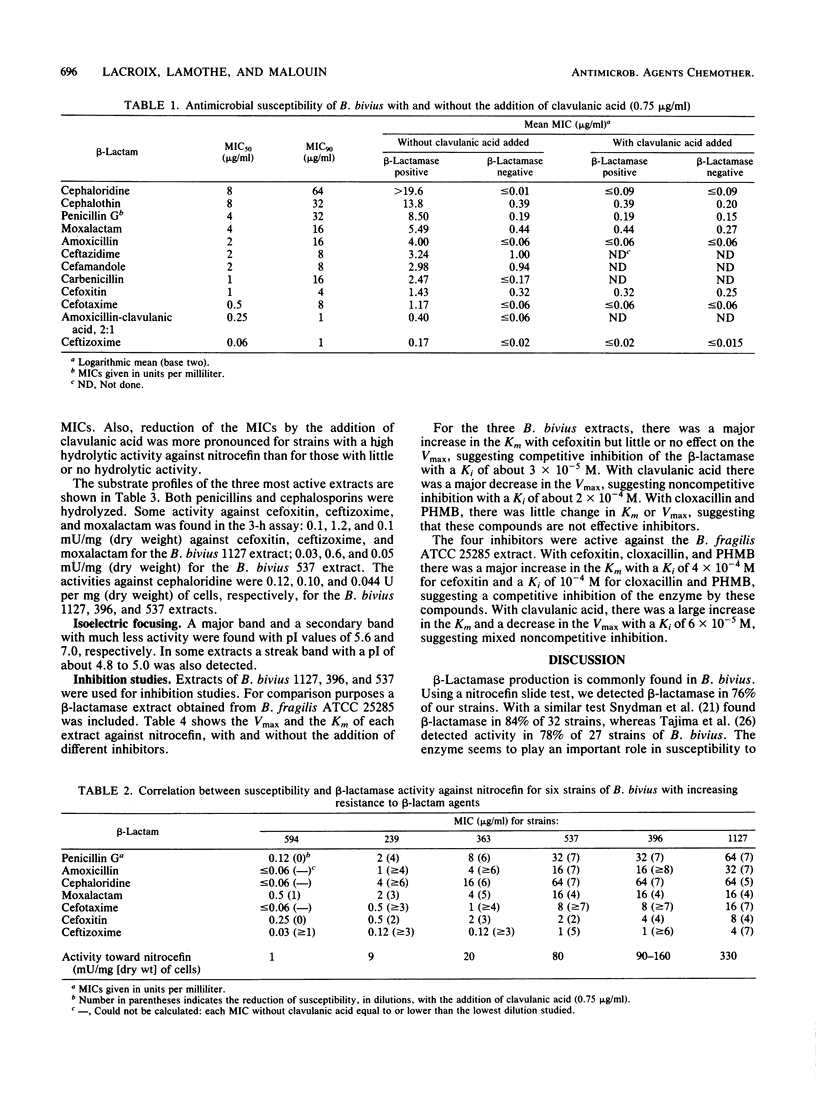
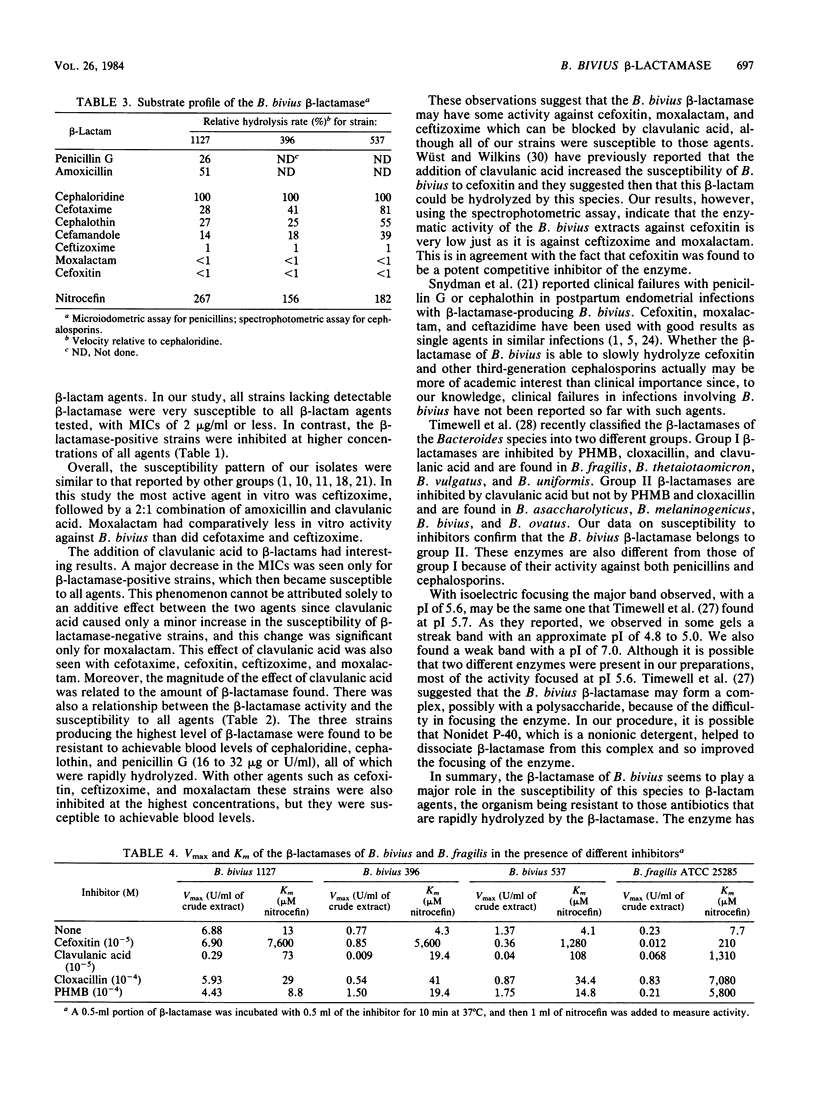
The susceptibility of 46 clinical isolates of Bacteroides bivius to amoxicillin, cefotaxime, cefoxitin, ceftizoxime, cephaloridine, cephalothin, moxalactam, penicillin G, amoxicillin plus clavulanic acid in a ratio of 2:1, carbenicillin, cefamandole, and ceftazidime was determined by an agar dilution technique. For the first eight agents susceptibility testing was also done with the addition of clavulanic acid (0.75 microgram/ml). For all agents, beta-lactamase-positive strains (35, using a nitrocefin slide test) were inhibited at higher concentrations than beta-lactamase-negative strains. Clavulanic acid reduced the susceptibility of the beta-lactamase-positive strains to the level of the beta-lactamase-negative strains to all agents. We prepared crude extracts of beta-lactamase from six strains. Activity against nitrocefin was directly related to their susceptibilities. The beta-lactamase had a mixed-substrate profile, hydrolyzing both penicillins and cephalosporins. Our results suggest a slow inactivation of cefoxitin, ceftizoxime, and moxalactam by the beta-lactamase. Clavulanic acid and cefoxitin inhibited the enzyme, whereas p-hydroxymercuribenzoate and cloxacillin did not. Thus, there was a clear relationship between beta-lactamase activity and susceptibility to beta-lactams, including cefoxitin and third-generation cephalosporins. The substrate and inhibition profiles of the B. bivius beta-lactamase were different from those of enzymes found in the "B. fragilis group."
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanco J. D., Gibbs R. S., Duff P., Castaneda Y. S., St Clair P. J. Randomized comparison of ceftazidime versus clindamycin-tobramycin in the treatment of obstetrical and gynecological infections. Antimicrob Agents Chemother. 1983 Oct;24(4):500–504. doi: 10.1128/aac.24.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgault A. M., Rosenblatt J. E. Characterization of anaerobic gram-negative bacilli by using rapid slide tests for beta-lactamase production. J Clin Microbiol. 1979 Jun;9(6):654–656. doi: 10.1128/jcm.9.6.654-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I., Calhoun L., Yocum P. Beta-lactamase-producing isolates of Bacteroides species from children. Antimicrob Agents Chemother. 1980 Jul;18(1):164–166. doi: 10.1128/aac.18.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall S. A., Kohan A. P., Ayers O. M., Hughes C. E., Addison W. A., Hill G. B. Intravenous metronidazole or clindamycin with tobramycin for therapy of pelvic infections. Obstet Gynecol. 1981 Jan;57(1):51–58. [PubMed] [Google Scholar]
- Gibbs R. S., Blanco J. D., Duff P., Castaneda Y. S., St Clair P. J. A double-blind, randomized comparison of moxalactam versus clindamycin-gentamicin in treatment of endomyometritis after cesarean section delivery. Am J Obstet Gynecol. 1983 Aug 1;146(7):769–772. doi: 10.1016/0002-9378(83)91075-x. [DOI] [PubMed] [Google Scholar]
- Hirai K., Iyobe S., Inoue M., Mitsuhashi S. Purification and properties of a new beta-lactamase from Pseudomonas cepacia. Antimicrob Agents Chemother. 1980 Mar;17(3):355–358. doi: 10.1128/aac.17.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye D., Kobasa W., Kaye K. Susceptibilities of anaerobic bacteria to cefoperazone and other antibiotics. Antimicrob Agents Chemother. 1980 Jun;17(6):957–960. doi: 10.1128/aac.17.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby B. D., George W. L., Sutter V. L., Citron D. M., Finegold S. M. Gram-negative anaerobic bacilli: their role in infection and patterns of susceptibility to antimicrobial agents. I. Little-known Bacteroides species. Rev Infect Dis. 1980 Nov-Dec;2(6):914–951. doi: 10.1093/clinids/2.6.914. [DOI] [PubMed] [Google Scholar]
- Levison M. E., Corman L. C., Carrington E. R., Kaye D. Quantitative microflora of the vagina. Am J Obstet Gynecol. 1977 Jan 1;127(1):80–85. doi: 10.1016/0002-9378(77)90318-0. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S., Yotsuji A., Inoue M., Mitsuhashi S. Induction of beta-lactamase by various beta-lactam antibiotics in Enterobacter cloacae. Antimicrob Agents Chemother. 1980 Sep;18(3):382–385. doi: 10.1128/aac.18.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Muggleton P. W., Ross G. W. Effects of beta-lactamase from gram-negative organisms on cephalosporins and penicillins. Antimicrob Agents Chemother (Bethesda) 1968;8:57–63. [PubMed] [Google Scholar]
- Ohm-Smith M. J., Hadley W. K., Sweet R. L. In vitro activity of new beta-lactam antibiotics and other antimicrobial drugs against anaerobic isolates from obstetric and gynecological infections. Antimicrob Agents Chemother. 1982 Oct;22(4):711–714. doi: 10.1128/aac.22.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Nord C. E., Wadström T. Formation of beta-lactamase in Bacteroides fragilis: cell-bound and extracellular activity. Antimicrob Agents Chemother. 1976 May;9(5):727–735. doi: 10.1128/aac.9.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautter R. L., Brown W. J. Sequential vaginal cultures from normal young women. J Clin Microbiol. 1980 May;11(5):479–484. doi: 10.1128/jcm.11.5.479-484.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snydman D. R., Tally F. P., Knuppel R., Landrigan J., Gorbach S. L., Bartlett J. G. Bacteroides bivius and Bacteroides disiens in obstetrical patients: clinical findings and antimicrobial susceptibilities. J Antimicrob Chemother. 1980 Jul;6(4):519–525. doi: 10.1093/jac/6.4.519. [DOI] [PubMed] [Google Scholar]
- Sweet R. L., Ledger W. J. Cefoxitin: single-agent treatment of mixed aerobic-anaerobic pelvic infections. Obstet Gynecol. 1979 Aug;54(2):193–198. [PubMed] [Google Scholar]
- Sweet R. L. Treatment of mixed aerobic-anaerobic infections of the female genital tract. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):105–114. doi: 10.1093/jac/8.suppl_d.105. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Nordström K. Microiodometric determination of beta-lactamase activity. Antimicrob Agents Chemother. 1972 Feb;1(2):94–99. doi: 10.1128/aac.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M., Sawa K., Watanabe K., Ueno K. The beta-lactamases of genus Bacteroides. J Antibiot (Tokyo) 1983 Apr;36(4):423–428. doi: 10.7164/antibiotics.36.423. [DOI] [PubMed] [Google Scholar]
- Timewell R. M., Phillips I., Söderholm J., Nord C. E. Isoelectric focusing of Bacteroides melaninogenicus group beta-lactamases. Antimicrob Agents Chemother. 1981 May;19(5):700–704. doi: 10.1128/aac.19.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timewell R., Taylor E., Phillips I. The beta-lactamases of Bacteroides species. J Antimicrob Chemother. 1981 Feb;7(2):137–146. doi: 10.1093/jac/7.2.137. [DOI] [PubMed] [Google Scholar]
- Wilkins T. D., Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1976 Dec;10(6):926–928. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst J., Wilkins T. D. Effect of clavulanic Acid on anaerobic bacteria resistant to Beta-lactam antibiotics. Antimicrob Agents Chemother. 1978 Jan;13(1):130–133. doi: 10.1128/aac.13.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]



