Abstract
Background
An increase in the incidence of cancer among elderly people assigned to pravastatin therapy has been reported in a randomized controlled trial; however, this finding has been attributed to chance. Our aim was to assess the effect of pravastatin therapy on cancer risk and to examine whether the effect varies according to age by performing a detailed meta-analysis and meta-regression analysis of randomized controlled trials.
Methods
We performed a comprehensive literature search for relevant studies published before February 2006. Before analysis, the selected studies were evaluated for publication bias and heterogeneity. Pooled relative risk estimates with 95% confidence intervals (CIs) were calculated using fixed-and random-effects models. Meta-regression analysis was performed to examine the impact of age on the study estimates of the relative risk of cancer due to pravastatin therapy.
Results
Twelve trials that investigated the use of pravastatin therapy for cardiovascular outcomes were included in the analysis (n = 42 902). Although the overall association between pravastatin use and cancer was not statistically significant in the fixed-effects (risk ratio [RR] 1.06, 95% CI 0.99–1.13) or random-effects model (RR 1.06, 95% CI 0.97–1.14), the meta-regression analysis showed that the age of study participants significantly modified the effect of pravastatin therapy on cancer risk (p = 0.006). Specifically, this analysis showed that pravastatin therapy was associated with an increasing risk of cancer as age increased. This finding was remarkably robust in the sensitivity analysis.
Interpretation
Our findings suggest an association between pravastatin therapy and cancer in elderly patients. However, given the importance of this potential association, further verification is warranted.
Statins (3-hydroxy-3-methylglutaryl–coenzyme A [HMG–CoA] reductase inhibitors) are widely used in the treatment of lipid disorders, especially hypercholesterolemia. Clinical trials have shown that statins are beneficial in both primary and secondary prevention of coronary and cerebrovascular disease events.1,2 Statins reduce cholesterol levels by inhibiting HMG–CoA reductase, the enzyme responsible for converting HMG–CoA to mevalonate (a cholesterol precursor).3 In addition, statins improve endothelial function, stabilize plaques, reduce free radical formation and attenuate the extent of endothelial inflammation, thus providing other potential benefits for patients regardless of their cholesterol level.
However, in 2002 the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) trial reported an increase in cancer rates among study participants assigned to hydrophilic pravastatin.4 This trial, which is the only randomized placebo-controlled trial to evaluate pravastatin in a unique population of elderly patients, showed that the reduced number of deaths from coronary artery disease was associated with an increased number of cancer-related deaths. The authors suggested that the increased cancer rate was most likely due to chance.4,5 However, a significant increase in cancer rates among elderly patients assigned to pravastatin therapy was also reported in a subgroup analysis of the Long-Term Intervention with Prava-statin in Ischemic Disease (LIPID) trial.6 In contrast, several randomized controlled trials of lipophilic statin therapy have not reported an increased risk of cancer.7–9
The aim of our study was to assess the effect of pravastatin therapy on cancer risk and to examine whether the effect varies according to age by conducting a detailed meta-analysis and meta-regression analysis of randomized controlled trials published in the peer-reviewed literature.
Methods
We identified trials of interest by performing a search of the MEDLINE (1966–February 2006) and SCI Expanded (1970–February 2006) databases. The search term used was “pravastatin,” and the search was restricted to human subjects and randomized controlled trials. The titles and abstracts of the identified articles were scanned to exclude any trials that were clearly irrelevant. The full text of the remaining articles was read to determine whether it contained information on the topic of interest. The reference lists of the selected articles were reviewed for additional pertinent trials. No language restrictions were imposed.
In our analyses, we included only randomized controlled trials that evaluated pravastatin therapy compared with placebo or usual care, had a minimum duration of 1 year and reported cancer rates during the trial. We did not assess the quality of the methods used in the primary studies, because quality assessment in meta-analysis is controversial. Quality scores constructed in an ad hoc fashion may lack validity, and the results may not be associated with the quality of the studies.10,11 Instead, we performed several subgroup and sensitivity analyses.12
Both of us extracted the data independently. The following data were collected from each article: publication data (first author's last name, year of publication, country of the population studied); study design; number of subjects; population characteristics (sex, age); and interventions (drug, dose, duration). Study-level risk ratios (RRs) and 95% confidence intervals (CI) were estimated by reconstructing contingency tables based on the number of patients randomly assigned to the treatment and control groups and the number of patients with a diagnosis of cancer (intention-to-treat analysis). Non-melanoma skin cancer was not included in the analyses, because this diagnosis was neither recorded nor reported routinely in the primary studies. Differences in data extraction were resolved by consensus after referring back to the original article.
Summary relative risk estimates and corresponding 95% CIs were derived by means of the Mantel–Haenszel method13 (fixed-effects model) and the DerSimonian–Laird method14 (random-effects model). We evaluated the statistical heterogeneity between the studies using Cochran's Q test15 (0.10 level of significance) and the I2 statistic.16,17 Publication bias was assessed by means of the Begg's adjusted rank correlation test18 and Egger's regression asymmetry test.19 We also performed subgroup analyses restricted to trials that evaluated pravastatin therapy compared with placebo and to trials that had enrolled at least 3000 patients and had a minimum duration of 3 years. The publication of cancer data from large studies is less likely to depend on the magnitude and direction of the results;20 thus publication bias was assumed to be minimal.
We also conducted a meta-regression analysis21 to examine the impact of patient age on the study estimates of relative risk. This analysis is an indirect way to examine the possibility of effect modification by patient age. We converted all risk ratios by logarithmic transformation to achieve a more symmetric distribution. The natural logarithm of the risk ratio was the outcome (dependent) variable, and the mean age of the study participants was an explanatory (potential effect modifier) variable. We ran a weighted regression model to allow more precise trials (those providing risk ratio estimates with narrow confidence intervals) to have more influence in the analysis. To correspond to a random-effects meta-regression analysis, the weight assigned to each study was equal to the inverse of the sum of the within-trial variance and the residual between-trial variance.22 The estimation of the residual between-trial variance was based on a restricted maximum likelihood method.23 The meta-regression techniques described are analogous to logistic regression.
These analyses were performed according to the Quality of Reporting of Meta-analyses (QUOROM) recommendations for improving the quality of meta-analyses of randomized controlled trials.24 For all tests (except heterogeneity between studies), a probability level less than 0.05 was considered statistically significant. All statistical tests were 2-sided.
Results
The search of the MEDLINE and SCI Expanded databases identified 524 and 165 articles respectively, however, most of the abstracts did not specifically address the topic of our analysis and were excluded, which left 43 potentially relevant articles. We read the full text of these articles and checked the reference lists for other relevant articles. We also checked previous meta-analyses1,2,25–28 for references to additional relevant articles.
We identified 13 trials that investigated pravastatin therapy for cardiovascular outcomes.4,6,29–39 However, randomization had been neglected in 1 trial;39 thus this trial was considered to be an observational study and was excluded from our analysis. Twelve randomized controlled trials met our inclusion criteria.4,6,29–38 We performed a post hoc analysis of these 12 trials and calculated risk ratios for cancer in an intention-to-treat analysis. One trial6 reported subgroup data of cancer rates by age group; we decided to treat the 2 age strata as separate trials. Ten of the 12 randomized controlled trials compared the effects of pravastatin therapy with those of placebo,4,6,29,32–38 and 2 trials compared pravastatin treatment with usual care.30,31
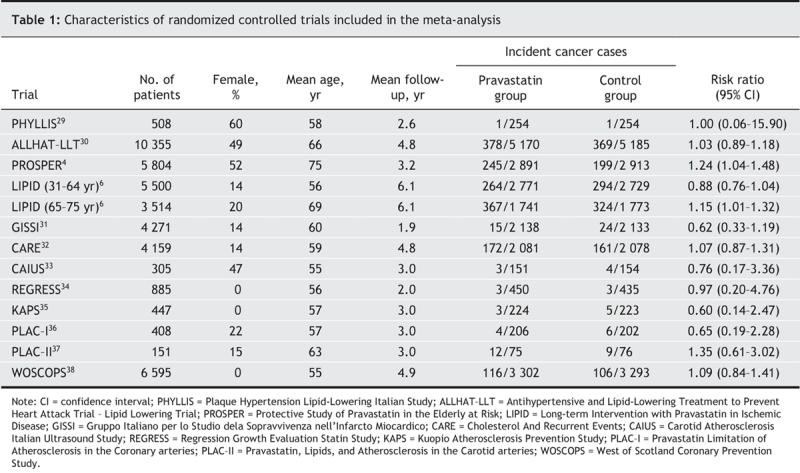
The 12 trials included in the meta-analysis were published between 1993 and 2004. Their general characteristics are described in Table 1. A total of 42 902 people (about 26% female) participated in these randomized controlled trials, 21 454 patients in pravastatin groups and 21 448 in control groups. The participants had a mean age of 63 years at enrolment and had a mean follow-up of about 4.5 years. A total experience of 190 000 person-years was reached.
Table 1
Meta-analysis
The meta-analysis of all 13 reports did not provide evidence of an association between pravastatin use and cancer risk. The overall rate of cancer was 7.4% in the pravastatin group (1583 incident cancer cases) and 7.0% in the control group (1505 incident cases, Table 1). Pravastatin use was not found to be significantly associated with cancer in either the fixed-effects model (RR 1.06, 95% CI 0.99–1.13, p = 0.10) or the random-effects model (RR 1.06, 95% CI 0.97–1.14, p = 0.20). The risk ratios and 95% CIs for each individual trial and the pooled results are shown in Fig. 1. Cochran's Q test gave a p value of 0.29 (Q 14.17, 12 degrees of freedom), and the corresponding I2 statistic was 15%, both of which indicated little variability between trials. The assumption of no publication bias was reasonable: the p values for the Begg's and Egger's tests were 0.76 and 0.35 respectively.
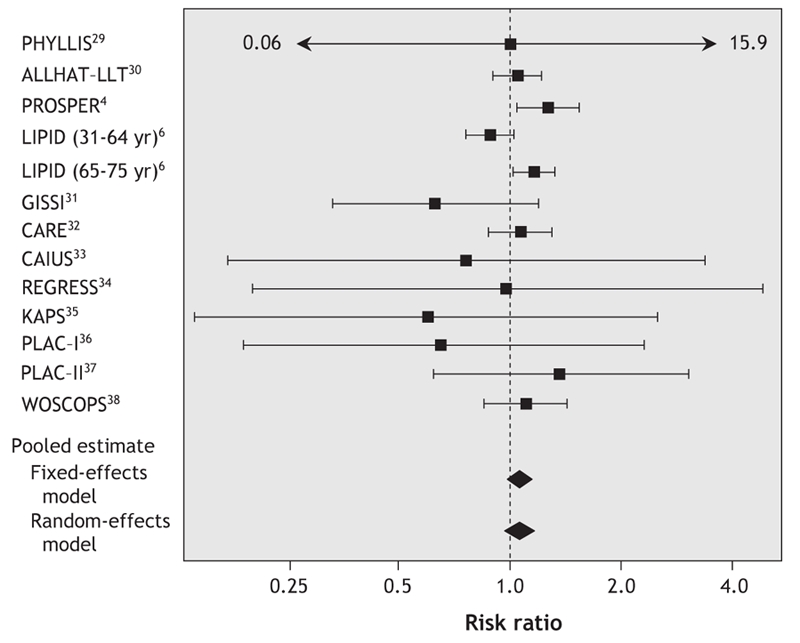
Fig. 1: Meta-analysis of pravastatin therapy and cancer risk. The risk ratios and 95% confidence intervals are displayed on a logarithmic scale. See abbreviations footnote in Table 1 for study name expansions.
When we restricted the analysis to trials that evaluated pravastatin therapy compared with placebo,4,6,29,32–38 the results changed slightly but did not reach statistical significance (fixed-effects model: RR 1.08, 95% CI 1.00–1.16, p = 0.06; random effects model: RR 1.07, 95% CI 0.98–1.17, p = 0.12; Cochran's Q test: p = 0.34, I2 11%; Begg's test: p = 0.53; Egger's test: p = 0.55).
In the meta-analysis of studies that had a minimum duration of 3 years and had enrolled at least 3000 patients, we included 5 major trials: PROSPER,4 LIPID,6 Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT),30 Cholesterol And Recurrent Events (CARE)32 and West of Scotland Coronary Prevention Study (WOSCOPS).38 The p values for the Begg's and Egger's tests for publication bias were 0.99 and 0.87 respectively. In contrast, the Cochran's Q test had a p value of 0.08 and the corresponding I2 statistic was 49%, which indicated a moderately large degree of variability across the trials. It is evident that much of the variability was due to heterogeneity between the trials rather than chance. The fixed-effects model suggested a marginally nonsignificant association between pravastatin use and cancer risk (RR 1.07, 95% CI 1.00–1.14, p = 0.06), yet the association in the random-effects model was clearly nonsignificant (RR 1.07, 95% CI 0.97–1.18, p = 0.19). On the basis of heterogeneity, the random-effects model is generally thought to be more appropriate, because it provides a more conservative estimate of the pooled effect size.40
Meta-regression analysis
To investigate the impact of age on the study-level estimate of the relative risk of cancer, we performed a random-effects meta-regression analysis. The regression included 13 data points (1 of the 12 studies provided subgroup data showing cancer incidence by age group).6 We obtained an estimate that was statistically significantly different from zero for the regression coefficient of the log risk ratio on mean age of study participants (coefficient 0.014, standard error [SE] 0.005, p = 0.006, intercept –0.854). The between-trial variation was reduced from 0.063 to 0.001 when the mean age was included as an explanatory variable in the meta-regression model. The results did not substantially change when we adjusted for the duration of follow-up (coefficient 0.015, SE 0.006, p = 0.008).
The regression coefficient is the estimated increase in the log risk ratio per unit increase in the covariate. Thus, the log risk ratio is estimated to increase by 0.014 per year increase in age. The estimated risk ratio of cancer with pravastatin therapy for a particular value of the covariate can be derived from the regression equation: log risk ratio = –0.854 + 0.014 × age. For example, a trial with a mean participant age of 60 years at enrolment would have an estimated log risk ratio of –0.014 (log risk ratio = –0.854 + 0.014 × 60). This corresponds to a risk ratio of 0.99 (exp[–0.014]). Similarly, for a trial with a mean participant age of 75 years, the estimated log risk ratio is 0.196 (log risk ratio = –0.854 + 0.014 × 75), which corresponds to a risk ratio of 1.22 (exp[0.196]). Fig. 2 shows the risk ratio estimates according to the mean age of study participants at the time of enrolment and shows that pravastatin therapy was associated with an increasing risk of cancer as patient age increased.
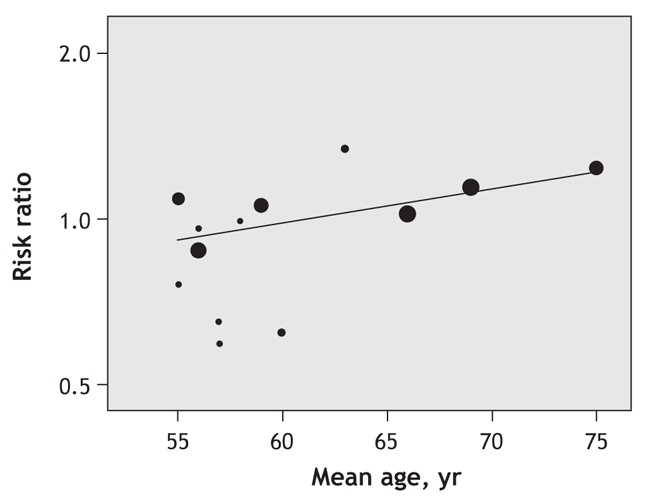
Fig. 2: Risk ratios of cancer associated with pravastatin therapy as a function of the mean age of participants at enrolment. The greater the variance of a study, the smaller the area of the circle and the less that observation contributes to the overall effect. The superimposed line is obtained by random-effects meta-regression analysis using a restricted maximum likelihood method estimate of the residual heterogeneity variance. Risk ratios are displayed on a logarithmic scale. Regression equation: log risk ratio = –0.854 + 0.014 × age.
To evaluate the stability of our findings, we performed a sensitivity analysis. We reran the regression analysis, restricting it to trials that evaluated pravastatin therapy compared with placebo (model a),4,6,29,32–38 to trials that had at least 3000 subjects and a minimum duration of 3 years (model b)4,6,30,32,38 and after excluding the PROSPER trial because of an extreme outlier risk ratio (model c).4 All 3 regression models suggested that our findings were stable, because each model indicated that the age of trial participants significantly modified the association between pravastatin use and cancer risk (model a: coefficient 0.014, SE 0.005, p = 0.007; model b: coefficient 0.013, SE 0.005, p = 0.011; model c: coefficient 0.013, SE 0.007, p = 0.045).
Interpretation
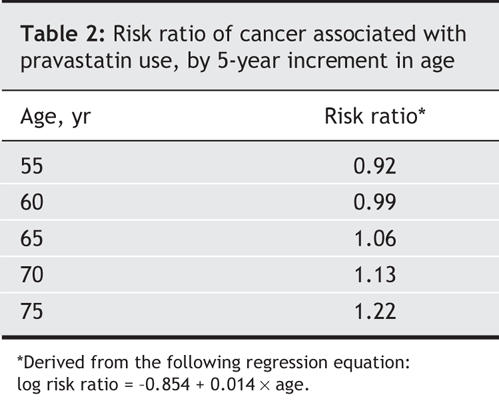
Our findings suggest that pravastatin use may be associated with an increased risk of cancer among elderly people. Although the overall association was not statistically significant in the fixed-or random-effects model, it was evident that the observed variability across the trials was due to heterogeneity rather than chance. However, the meta-regression analysis indicated that age of study participants significantly modified the association between pravastatin use and cancer risk. This analysis showed that pravastatin therapy was associated with increasing risk ratios of cancer as patient age increased. For example, the estimated risk ratios (derived from the regression equation) of cancer associated with pravastatin therapy were 0.99 and 1.22 for ages 60 and 75 respectively (Table 2). This finding was remarkably robust in the sensitivity analysis.
Table 2
When a meta-analysis of published literature is performed, it is critical to consider study bias. It is well documented that there is a publication bias in favour of statistically significant results.41–43 However, the likelihood of a substantial publication bias that would affect our results is small. The Begg's and Egger's tests revealed no relation between the estimates of relative risk and study size. Thus, we are confident that a substantial publication bias due to the preferential publication of large studies with significant findings is unlikely.
Nevertheless, our meta-analysis has several limitations. The first is cancer latency, which is a limitation beyond our control in the present study. Because the follow-up periods were about 4.5 years on average, which could be considered too short a period compared with the amount of time needed for cancer to develop, estimating cancer risk associated with longer durations of pravastatin therapy was not possible. However, the follow-up periods may have been sufficient to allow a detectable increase in the risk of cancer, if pravastatin promotes rather than causes cancer.
Second, our study cannot address whether the observed increase in cancer risk among elderly patients prescribed pravastatin therapy is unique to this specific statin. At least 10 data points (studies) are typically required for meta-regression analysis. This criterion is not met for any other individual statin; thus it is not possible at this time to apply meta-regression techniques to any other individual statin, unless individual data sets are made available.
The third limitation is that meta-regression analysis describes observational relations across studies, which are subject to confounding by other characteristics that may vary between the trials. Even though every trial is randomized, meta-regression analysis is only the study of the epidemiology of trials, and it suffers from the same disadvantages as other observational epidemiologic investigations, notably bias by confounding. Thus, any relations that are identified may not be causal.44,45 In addition, the relation of patient averages across trials may not be the same as the relation of patients within trials. This phenomenon is referred to as “aggregation bias,” “ecological bias” or “ecological fallacy,”46,47 and it cannot be investigated without individual patient data. Therefore, further verification through individual patient data sets is warranted.
Although the epidemiologic data currently available suggest that elderly patients taking pravastatin therapy may be at an increased risk of cancer, our knowledge about the mechanisms underlying this association is incomplete. Recently, Duncan and colleagues suggested that pravastatin may promote the development of cancer by inducing mevalonate synthesis in extrahepatic tissues.48 However, it is clear that laboratory investigations should be conducted to define further the mechanisms by which pravastatin may increase cancer risk.
Simulation studies comparing meta-regression analysis of summary covariates with meta-analysis of individual patient data have shown that, if the meta-regression analysis shows an effect, it is probably a large and important one.49 Given the enormous public health implications of a potential association between pravastatin use and cancer risk, especially among el-derly patients, a replication of these analyses on individual patient databases, such as the Cholesterol Treatment Trialists (CCT) Collaboration1,50,51 and the Prospective Pravastatin Pooling (PPP) Project,51,52 would be valuable. It is also important to monitor the use of pravastatin and other statins for extended follow-up periods, through national adverse event registries such as MedWatch, to identify potential long-term effects.
@ See related article page 646
Footnotes
This article has been peer reviewed.
Contributors: Stefanos Bonovas and Nikolaos Sitaras conceived the project and developed the protocol. Stefanos Bonovas conducted literature searches, selection and data extraction, performed the statistical analyses, interpreted the results and drafted the manuscript. Nikolaos Sitaras was the supervisor of the study. Both authors critically revised the article and approved the version to be published.
Competing interests: None declared.
Correspondence to: Dr. Stefanos Bonovas, Department of Pharmacology, School of Medicine, University of Athens, 75 Mikras Asias St., Athens 11527, Greece; sbonovas@med.uoa.gr
REFERENCES
- 1.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. [DOI] [PubMed]
- 2.Cheung BM, Lauder IJ, Lau CP, et al. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br J Clin Pharmacol 2004;57:640-51. [DOI] [PMC free article] [PubMed]
- 3.Sewester CS, Dombek CE, Olin BR, et al, editors. Drug facts and comparisons. St. Louis: Facts and Comparisons; 2001.
- 4.Shepherd J, Blauw GJ, Murphy MB, et al. PROSPER study group. Prospective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623-30. [DOI] [PubMed]
- 5.Shepherd J, Blauw GJ, Murphy MB. The PROSPER trial. Authors' reply. Lancet 2003;361:428.
- 6.Hunt D, Young P, Simes J, et al. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: Results from the LIPID trial. Ann Intern Med 2001;134:931-40. [DOI] [PubMed]
- 7.Strandberg TE, Pyorala K, Cook TJ, et al. 4S Group. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 2004;364:771-7. [DOI] [PubMed]
- 8.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22.12114036
- 9.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615-22. [DOI] [PubMed]
- 10.Greenland S. Invited commentary. Am J Epidemiol 1994;140:290-6. [DOI] [PubMed]
- 11.Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054-60. [DOI] [PubMed]
- 12.Friedenreich CM, Brant RF, Riboli E. Influence of methodologic factors in a pooled analysis of 13 case-control studies of colorectal cancer and dietary fiber. Epidemiology 1994;5:66-7. [DOI] [PubMed]
- 13.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed]
- 15.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29.
- 16.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed]
- 17.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- 19.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed]
- 20.Felson DT. Bias in meta-analytic research. J Clin Epidemiol 1992;45:885-92. [DOI] [PubMed]
- 21.Sharp S. Meta-analysis regression. Stata Tech Bull 1998;42:16-24.
- 22.Berkey CS, Hoaglin DC, Mosteller F, et al. A random-effects regression model for meta-analysis. Stat Med 1995;14:395-411. [DOI] [PubMed]
- 23.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693-708. [DOI] [PubMed]
- 24.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896-900. [DOI] [PubMed]
- 25.Hebert PR, Gaziano JM, Chan KS, et al. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 1997;278:313-21. [PubMed]
- 26.Bjerre LM, LeLorier J. Do statins cause cancer? A meta-analysis of large randomized clinical trials. Am J Med 2001;110:716-23. [DOI] [PubMed]
- 27.Dale KM, Coleman CI, Henyan NN, et al. Statins and cancer risk: a meta-analysis. JAMA 2006;295:74-80. [DOI] [PubMed]
- 28.Bonovas S, Filioussi K, Tsavaris N, et al. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 2005;23:8606-12. [DOI] [PubMed]
- 29.Zanchetti A, Crepaldi G, Bond MG, et al. PHYLLIS Investigators. Different effects of antihypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering by pravastatin on progression of asymptomatic carotid atherosclerosis: principal results of PHYLLIS — a randomized double-blind trial. Stroke 2004;35:2807-12. [DOI] [PubMed]
- 30.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002;288:2998-3007. [DOI] [PubMed]
- 31.GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico). Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: Do stopped trials contribute to overall knowledge? Ital Heart J 2000;1:810-20. [PubMed]
- 32.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996;335:1001-9. [DOI] [PubMed]
- 33.Mercuri M, Bond MG, Sirtori CR, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population: the Carotid Atherosclerosis Italian Ultrasound Study. Am J Med 1996;101:627-34. [DOI] [PubMed]
- 34.Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation 1995;91:2528-40. [DOI] [PubMed]
- 35.Salonen R, Nyyssonen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 1995;92:1758-64. [DOI] [PubMed]
- 36.Pitt B, Mancini GB, Ellis SG, et al. Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC-I): reduction in atherosclerosis progression and clinical events. PLAC-I investigation. J Am Coll Cardiol 1995;26:1133-9. [DOI] [PubMed]
- 37.Crouse JR III, Byington RP, Bond MG, et al. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am J Cardiol 1995;75:455-9. [DOI] [PubMed]
- 38.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995;333:1301-7. [DOI] [PubMed]
- 39.The Kyushu Lipid Intervention Study Group. A coronary primary intervention study of Japanese men: study design, implementation and baseline data. J Atheroscler Thromb 1996;3:95-104. [DOI] [PubMed]
- 40.Petitti DB. Statistical methods in meta-analysis. In: Meta-analysis, decision analysis and cost-effectiveness analysis. New York: Oxford University Press; 1994. p. 90-114.
- 41.Easterbrook PJ, Berlin J, Gopalan R, et al. Publication bias in research. Lancet 1991;337:867-72. [DOI] [PubMed]
- 42.Simes RJ. Publication bias: the case for an international registry of clinical trials. J Clin Oncol 1986;4:1529-41. [DOI] [PubMed]
- 43.Sterling TD, Rosenbaum WL, Weinkam JJ. Publication decisions revisited: the effect of the outcome of statistical tests on the decision to publish and vice versa. Am Stat 1995;49:108-12.
- 44.Sterne JA, Juni P, Schulz KF, et al. Statistical methods for assessing the influence of study characteristics on treatment effects in ‚meta-epidemiological' research. Stat Med 2002;21:1513-24. [DOI] [PubMed]
- 45.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559-73. [DOI] [PubMed]
- 46.Morgenstern H. Uses of ecological analysis in epidemiologic research. Am J Public Health 1982;72:1336-44. [DOI] [PMC free article] [PubMed]
- 47.Greenland S. Quantitative methods in the review of epidemiological literature. Epidemiol Rev 1987;9:1-30. [DOI] [PubMed]
- 48.Duncan RE, El-Sohemy A, Archer MC. Statins and cancer development. Cancer Epidemiol Biomarkers Prev 2005;14:1897-8. [DOI] [PubMed]
- 49.Lambert PC, Sutton AJ, Abrams KR, et al. A comparison of summary patient-level covariates in meta-regression with individual patient data meta-analysis. J Clin Epidemiol 2002;55:86-94. [DOI] [PubMed]
- 50.Trialists' (CTT) Collaboration. Protocol for a prospective collaborative overview of all current and planned randomized trials of cholesterol treatment regimens. Am J Cardiol 1995;75:1130-4. [DOI] [PubMed]
- 51.Simes RJ. Prospective meta-analysis of cholesterol-lowering studies: the Prospective Pravastatin Pooling (PPP) Project and the Cholesterol Treatment Trialists (CTT) Collaboration. Am J Cardiol 1995;76:122C-6C. [DOI] [PubMed]
- 52.Pfeffer MA, Keech A, Sacks FM, et al. Safety and tolerability of pravastatin in long-term clinical trials: prospective Pravastatin Pooling (PPP) Project. Circulation 2002;105:2341-6. [DOI] [PubMed]


