Abstract
Intracellular pathogens have evolved a wide array of mechanisms to invade and co-opt their host cells for intracellular survival. Apicomplexan parasites such as Toxoplasma gondii employ the action of unique secretory organelles named rhoptries for internalization of the parasite and formation of a specialized niche within the host cell. We demonstrate that Toxoplasma gondii also uses secretion from the rhoptries during invasion to deliver a parasite-derived protein phosphatase 2C (PP2C-hn) into the host cell and direct it to the host nucleus. Delivery to the host nucleus does not require completion of invasion, as evidenced by the fact that parasites blocked in the initial stages of invasion with cytochalasin D are able to target PP2C-hn to the host nucleus. We have disrupted the gene encoding PP2C-hn and shown that PP2C-hn-knockout parasites exhibit a mild growth defect that can be rescued by complementation with the wild-type gene. The delivery of parasite effector proteins via the rhoptries provides a novel mechanism for Toxoplasma to directly access the command center of its host cell during infection by the parasite.
Toxoplasma gondii is an obligate intracellular parasite in the phylum Apicomplexa that causes severe central nervous system disorders of immunocompromised (AIDS/transplant/lymphoma) individuals and birth defects in congenitally infected neonates worldwide (16). Toxoplasma infects a wide range of mammalian hosts and is capable of infecting virtually any nucleated cell type from these organisms. The parasite actively invades its host cell, establishing a specialized parasitophorous vacuole (PV) within the host cytoplasm (22). This vacuole fails to fuse with the host endocytic or exocytic pathways, thus avoiding lysosomal destruction, and provides a residence in which parasites can replicate within the host cell (29, 37). The processes of invasion and vacuole formation therefore establish an intimate yet separate association between the parasite and its host cell.
Host cell invasion and PV formation are mediated in part by the action of the rhoptries, specialized secretory organelles that release their contents at the onset of invasion (32). The club-shaped rhoptries are composed of two suborganellar domains, the bulbous rhoptry bodies and the duct-like rhoptry necks. These domains appear to carry out very different roles in host cell invasion and establishment of the intracellular niche for survival. Proteins secreted from the rhoptry necks have recently been shown to be released into the moving junction, a ring-shaped structure that forms the intersection between the invading parasite and the host plasma membrane (1, 6). Rhoptry neck proteins in the moving junction likely serve to filter host transmembrane proteins from the nascent PV during invasion, a process that contributes to the nonfusogenic nature of the vacuole within the host cell. Rhoptry proteins from the other subcompartment, the rhoptry bodies, are secreted into the nascent PV, where they are destined to remain within the vacuole or are targeted to the vacuolar membrane, where they interact with the host cytoplasm (32, 38). Thus, the rhoptries are thought to play key roles in invasion, PV formation, and modification of the vacuole for survival within the host cell.
In order to enter and survive within its host cells, Toxoplasma actively subverts host defenses and co-opts host cell processes. While the host response is understood to some extent, little is known about the specific parasite proteins that modulate host cell processes. Candidate host-modulating effectors include parasite surface antigens as well as proteins secreted from the Apicomplexan-specific secretory organelles: the rhoptries, micronemes, and dense granules. Recently, a cyclophilin-like protein secreted from the dense granules has been shown to bind the chemokine receptor CCR5 on dendritic cells and to stimulate interleukin-12 production (2). The rhoptries may also provide proteins that can modulate host cell functions. During invasion, the rhoptries release their constituents in a burst of secretion into the nascent PV. This process results in the formation of so-called “evacuoles” in the cytoplasm of the host near the forming PV (14). It is still unclear whether evacuoles are membrane delimited, but they clearly contain abundant internal membranous whorls and both soluble and membrane-associated rhoptry proteins. Following invasion, the evacuoles associate with host organelles and then appear to fuse with the parasite-containing vacuole or disappear. This process of rhoptry-mediated injection of parasite proteins into the host cell represents an opportunity for parasite effectors to directly access the host cell. Access to other compartments within the host cell would require only the appropriate targeting information, although no such parasite proteins have been identified previously.
We have identified a novel, rhoptry-localized protein phosphatase 2C (PP2C) that is secreted during invasion and targeted to the host nucleus. Significantly, this is the first Toxoplasma protein that has been shown to reach the host cell nucleus. Targeted disruption of the PP2C results in a mild growth defect that can be complemented by the wild-type gene, indicating that it plays a role in some aspect of the parasite's lytic cycle. Thus, in addition to roles in invasion and PV formation, we show here that the rhoptries have a role in delivering parasite proteins to the host cell nucleus, providing yet another means, this time direct, by which the parasite can interact with its host cell.
MATERIALS AND METHODS
Parasite and host cell culture.
T. gondii strain RHΔhpt (parental) and the resulting modified strains were maintained in confluent monolayers of human foreskin fibroblast (HFF) host cells as described elsewhere (12).
Production of MAb 9D6 and IFA analysis.
Monoclonal antibodies (MAbs) were prepared against a highly purified rhoptry preparation from T. gondii (P. J. Bradley et al., unpublished data). For immunization, ∼100 μg of purified rhoptries (6) was injected in RIBI adjuvant into a BALB/c mouse. Following four injections, the spleen was isolated, hybridoma lines were prepared, and supernatants from individual clones were screened for antibody reactivity to the rhoptries. Immunofluorescence assay (IFA) analysis of T. gondii-infected host cells was performed as described previously (6). Evacuoles were prepared as described previously using cytochalasin D-treated parasites (14) and polyclonal ROP2 antisera at a 1:1,000 dilution (generously provided by Jean-Francois Dubremetz) (3). Images were collected as previously described (6).
Immunoaffinity purification with MAb 9D6 and identification of PP2C-hn.
For immunoaffinity chromatography with antibody 9D6, the antibody was first dimethylpimelimidate cross-linked to protein G-Sepharose (Amersham) as described previously (15). For immunoisolation, 5 × 109 RH strain tachyzoites were lysed in radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 0.5% NP-40, 0.5% sodium deoxycholate) and the insoluble material pelleted at 10,000 × g for 30 min. The solubilized proteins were incubated with the 9D6-protein G-Sepharose beads, washed in radioimmunoprecipitation assay buffer, and eluted at a high pH (100 mM triethylamine, pH 11.5). The eluate was separated by SDS-polyacrylamide gel electrophoresis (PAGE) and the resulting 47-kDa band identified by Coomassie staining. Identification of the protein (named PP2C-hn, for protein phosphatase 2C-host nuclear) was carried out by Stanford University Mass Spectrometry (see the supplemental material). A search for similarity to known proteins was performed by BLAST analysis, and signal peptide prediction was carried out using Signal P (13).
Generation of polyclonal antisera against ROP13 and against residues 24 to 109 of PP2C-hn.
To generate polyclonal antisera against ROP13, recombinant ROP13 protein (6) was used to immunize rabbits by using a commercial vendor (Animal Pharm). The resulting antisera colocalize with mouse anti-ROP13 (data not shown). For production of PP2C-hn antisera for Western blot analysis, residues 24 to 109 of PP2C-hn were expressed as a six-His fusion by using the pET28a vector. The region was amplified from cDNA by using primers P1 and P2 (all oligonucleotide sequences and restriction sites are listed in Table S1 in the supplemental material) and subcloned into pET28a. The recombinant protein was expressed in Escherichia coli BL21(DE3) cells and purified by nickel-nitrilotriacetic acid (Ni-NTA) agarose chromatography under denaturing conditions as recommended by the manufacturer (QIAGEN). The purified protein was dialyzed against phosphate-buffered saline, and ∼30 μg was injected into a BALB/c mouse on a 21-day immunization schedule. The resulting polyclonal mouse antisera (anti-PP2C-hn; 1:500) stain the rhoptries and host nucleus similarly to 9D6 and are also reactive by Western blot analysis.
Generation of recombinant PP2C-hn and PP2C2 for phosphatase activity assays.
For recombinant expression of PP2C-hn, the region encoding amino acids 20 to 418 of PP2C-hn was amplified and subcloned into pET161GW-D-TOPO (Invitrogen) using primers P3 and P4. For PP2C2 (TgTwinscan_7301, available at http://www.toxodb.org/toxo/home.jsp), the sequence encoding residues 92 to 537 was amplified and subcloned into pET28a using primers P5 and P6. Plasmids were sequenced to verify the junctions of the vector and insert and then transformed into E. coli BL21(DE3) cells for expression. For expression under native conditions, bacteria containing the expression constructs were grown to an A600 of 0.6, placed at 4°C for 30 min, and induced with 0.05 mM isopropyl-1-thio-d-galactopyranoside for 3 h at 25°C. Following induction, the bacteria were pelleted and resuspended in native lysis buffer (10 mM Tris-HCl [pH 7.5], 0.5 M NaCl, 3 mM MgCl2, 100 μg/ml hen egg white lysozyme, 1× Roche protease inhibitor cocktail). Resuspended bacteria were then lysed by sonication and insoluble material removed by centrifugation at 12,000 × g for 30 min. The soluble fraction was applied to Ni-NTA agarose, and the recombinant protein was allowed to bind for 3 h at 25°C. The Ni-NTA agarose was washed three times in wash buffer (10 mM Tris-HCl [pH 7.5], 0.5 M NaCl, 3 mM MgCl2, 20 mM imidizole) and eluted in elution buffer (10 mM-Tris HCl [pH 7.5], 0.5 M NaCl, 3 mM MgCl2, 350 mM imidizole). Purified proteins were separated by SDS-PAGE, and their purity was examined by Coomassie staining.
In vitro phosphatase assays.
Hydrolyzed and partially dephosphorylated casein (Sigma) was phosphorylated using [γ-32P]ATP (3,000 Ci/mmol) and the catalytic subunit of protein kinase A in a buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, and 2 mM dithiothreitol. The phosphorylation reaction was allowed to proceed overnight at 30°C. Phosphorylated casein was separated from [γ-32P]ATP using a Sephadex G-25 PD-10 column (Amersham Pharmacia). The activities of PP2C-hn and PP2C2 were assayed using purified enzymes as described previously (28). The standard phosphatase assay mixture (50 μl) contained 5 mM Tris-HCl (pH 7.5), 0.1 mM EGTA, 2 mM dithiothreitol, 0.01% Brij 35 (wt/vol), 4 to 10 mM MnCl2 or MgCl2, ∼1 μg recombinant PP2C-hn or PP2C2, and ∼1 × 105 cpm radiolabeled casein. Phosphatase assay samples were incubated at 30°C for 1 h, a 10-μl sample was removed for determination of total [32P]phosphate, and the reaction was terminated by the addition of 7 μl of 100% cold trichloroacetic acid (wt/vol). After 1 h on ice, the samples were centrifuged, and 10 μl of the supernatant was removed for liquid scintillation counting. Phosphatase activity was expressed as the percentage of dephosphorylation of the 32P-labeled casein (free [32P]phosphate/total [32P]phosphate). Similar assays were performed using myelin basic protein as a substrate.
Demonstration that PP2C-hn is a soluble protein.
To assess whether PP2C-hn is glycosylphosphatidylinositol (GPI) anchored, phosphatidylinositol (PI)-phospholipase C (PLC) cleavage of parasite lysates and detection of immunoprecipitated PP2C-hn with anti-cross-reactive determinant (anti-CRD) antibodies were performed as described previously by using SAG1 as a control (31). Immunoprecipitated PP2C-hn was separated by SDS-PAGE and probed with anti-CRD antibodies. For Triton X-114 (TX-114) phase partitioning, 2 × 107 parasites were hypotonically lysed in 10 mM Tris-HCl (pH 7.5)-5 mM NaCl, and the lysate was partitioned as described previously (34).
Gene disruption of PP2C-hn.
Generation of the PP2C-hn knockout construct utilized the pMini-GFP.hh knockout vector (21), which contains the selectable marker hypoxanthine-xanthine-guanine phosphoribosyltransferase (HPT) driven by the dihydrofolate reductase (DHFR) promoter and the downstream marker green fluorescent protein (GFP) driven by the GRA1 promoter. The PP2C-hn 5′ flank (5,198 bp) was amplified from RH strain genomic DNA using primers P7 and P8. The PP2C-hn 3′ flank (4,907 bp) was amplified using primers P9 and P10. The 5′ flank was cloned into the pMini-GFP.hh vector upstream of the HPT gene, and the 3′ flank was cloned downstream of the HPT gene. The resulting vector (pKO-PP2C-hn) was linearized with KpnI, and 30 μg of DNA was transfected into RHΔhpt parasites. The transfected parasites were selected for HPT using 50 μg/ml mycophenolic acid and 50 μg/ml xanthine. Eight days posttransfection, the stably selected populations were cloned by limiting dilution. Clones were screened for GFP by fluorescence microscopy, and GFP-negative clones were assessed by IFA using MAb 9D6. Western blot analysis of whole-parasite lysates of parental and RHΔpp2c-hn+HPT strains was performed using anti-PP2C-hn and anti-AMA1 antibodies as described previously (6).
For removal of HPT, the pKO-PP2C-hn knockout vector was digested with NheI and XhoI, blunted, and recircularized. The plasmid lacking HPT was linearized with KpnI and transfected into RHΔpp2c-hn+HPT parasites as described above. Transfected parasites were selected for the absence of HPT using 200 μg/ml 6-thioxanthine (Sigma) for 3 weeks and cloned. The resulting clones were assessed to be GFP negative by fluorescence microscopy, indicating homologous recombination. GFP-negative clones were then tested for the lack of ability to grow in mycophenolic acid and xanthine. Several clones were selected, and one, which lacks both PP2C-hn and HPT, was named RHΔpp2c-hn.
Reconstitution of PP2C-hn.
To reintroduce PP2C-hn into RHΔpp2c-hn parasites, the PP2C-hn coding region was amplified with primers P11 and P12. PP2C-hn was cloned into the pGRA4GFP vector from which GFP had been removed and to which HPT driven by the DHFR promoter had been added (23). The GRA1 promoter was replaced with the PP2C-hn promoter, which was provided by a 1.4-kb fragment of the 5′ flanking genomic region that had been amplified with primers P13 and P14. The resulting plasmid was linearized, transfected into RHΔpp2c-hn parasites, and selected with mycophenolic acid and xanthine as described above. Stable transfectants were cloned and screened for PP2C-hn expression by IFA. Clones that gave approximately wild type levels of rhoptry and host nuclear staining by IFA were selected.
Competition growth rate assays.
Equal numbers (106 parasites) of competing strains (e.g., parental and RHΔpp2c-hn [see Fig. 5D]) were mixed and used to infect confluent HFF monolayers. The percentage of each strain in the population was assessed by IFA. All parasites were stained using rabbit anti-ROP13 antibodies, and MAb 9D6 was used to differentiate parental from RHΔpp2c-hn parasites. For each time point in the assay, the percentage of each strain in the population was determined by counting at least 200 parasite vacuoles.
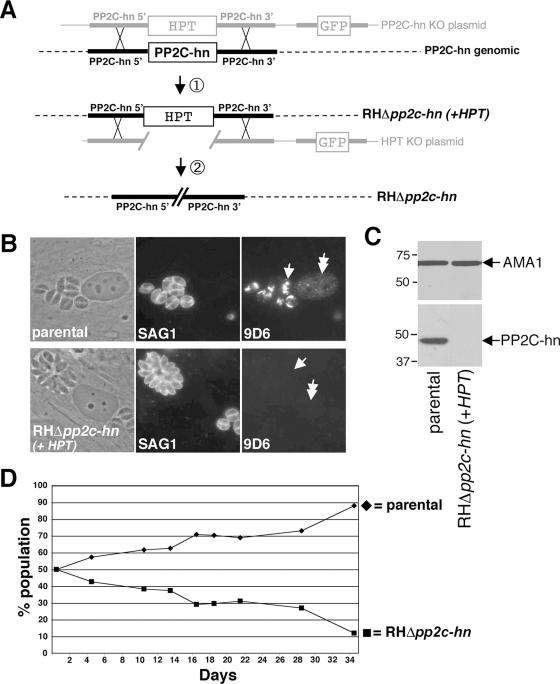
FIG. 5.
Targeted disruption of PP2C-hn. (A) Schematic of the knockout approach. Homologous recombination at the PP2C-hn locus results in replacement of the PP2C-hn gene with HPT and loss of the downstream marker GFP. A second round of homologous recombination is used to remove HPT in order to exclude polar effects and/or effects from expression of the selectable marker. (B) IFA analysis of parental and RHΔpp2c-hn+HPT parasites shows no 9D6 staining in either the rhoptries (arrow) or the infected host nuclei (double-headed arrow) of RHΔpp2c-hn parasites. A polyclonal anti-SAG1 antibody was used to identify parasites and as a control for staining. (C) Western blot analysis of parental and RHΔpp2c-hn+HPT parasites with an antibody to recombinant PP2C-hn protein shows the absence of PP2C-hn in RHΔpp2c-hn+HPT parasites. Antibodies against the microneme protein AMA1 were used as a loading control. (D) RHΔpp2c-hn parasites show a mild growth defect. A competition experiment using a mixed population of parental and RHΔpp2c-hn parasites shows increasing percentages of parental parasites over time and demonstrates the subtle growth defect in RHΔpp2c-hn parasites.
Other procedures.
For details regarding mass spectrometry of PP2C-hn and for data not shown here, including microarray analysis, apoptosis assays, and in vivo infections, see the supplemental material.
RESULTS
Monoclonal antibody 9D6 stains the rhoptries and the host nuclei in infected cells.
As a complementary approach to our recent proteomic analysis of the T. gondii rhoptries (6), we raised a panel of MAbs against a highly purified rhoptry fraction. One of the resulting MAbs (named 9D6) was strikingly different from the others in that it stained both the rhoptries and the host nuclei of infected cells (Fig. 1A); it was chosen for further study. Localization to the rhoptries within the parasite was confirmed by costaining using anti-ROP13 antibodies (Fig. 1B). The colocalization with ROP13 demonstrates that the suborganellar localization is within the bulbous body portion of the rhoptries as opposed to the duct-like rhoptry necks (6).
FIG. 1.
MAb 9D6 stains the rhoptries in Toxoplasma and the host nuclei in infected cells. (A) 9D6 stains an apical location within the parasite (arrow) and also stains the infected host nuclei (double-headed arrow). Note that the nucleus of the uninfected cell does not stain with 9D6 (arrowhead) and that the intensity of the host nuclear staining is dependent on the number of infections per cell. (B) Rhoptry localization within the parasite was confirmed by colocalization using antibodies to the rhoptry protein ROP13. ROP13 colocalization also demonstrates that staining is within the bulbous body of the organelle as opposed to the duct-like rhoptry necks. (C) 9D6 staining is detected in the host nucleus shortly after invasion, as shown here at 30 min after addition of parasites to host cells (arrows as in panel A). (D) 9D6 staining is not seen in evacuoles formed by cytochalasin D-treated parasites. The evacuoles are identified by ROP2 staining (arrow) and do not stain with 9D6, which can be detected in the nucleus (double-headed arrow) (shown here at 15 min after beginning of evacuole formation; little to no host nuclear staining can be seen at earlier time points).
Staining with MAb 9D6 in the host nucleus is seen only in infected cells (Fig. 1A), strongly suggesting that the monoclonal antibody is indeed detecting a rhoptry protein delivered to the host nucleus as opposed to detecting a cross-reactive host protein. The intensity of the staining within the infected host cell nucleus is dependent on the number of infections in a single cell (i.e., the number of PVs per cell), with more infections resulting in brighter staining (Fig. 1A). Host nuclear localization occurs early during Toxoplasma infection, as evidenced by the fact that 9D6 staining is detected within 15 to 30 min following host cell invasion (Fig. 1C). No host nuclear staining is seen in parasites trapped in the act of invasion by using a temperature shift assay (in this assay [5], parasites are allowed to settle but not invade host cells at 4°C, briefly warmed to allow invasion to commence, and fixed in the early stages of invasion and vacuole formation [data not shown]). This is most likely due to an inability to detect the diffuse amounts of protein in the host cytoplasm as it transits to the host nucleus (microinjection studies suggest a transit time of ∼5 to 10 min for nuclear targeting [43]). However, formation of the nascent vacuolar membrane surrounding the invading parasite is not required for host nuclear localization; staining can be seen for cytochalasin D-treated parasites, which can release the contents of the rhoptries and form evacuoles but cannot invade (Fig. 1D). Intriguingly, 9D6 staining is not seen in evacuoles (which are readily detected with anti-ROP2 [Fig. 1D]), suggesting that either the host nuclear import pathway can selectively and very rapidly remove the protein recognized by 9D6 from the cytoplasmic evacuoles or introduction is simultaneous with, but independent of, evacuoles. Staining of the host nucleus can be seen throughout the lytic cycle; however, in many cells, the host nuclear staining appears reduced later in infection, consistent with data indicating that release from the rhoptries occurs exclusively during the initial stages of invasion and vacuole formation (8, 14).
Identification of the Toxoplasma protein recognized by antibody 9D6.
To identify the protein recognized by antibody 9D6, we cross-linked antibody 9D6 to protein G-Sepharose for immunoaffinity chromatography from Toxoplasma lysates. Immmunoaffinity isolation was carried out on lysates from extracellular parasites, and the eluted protein was separated by SDS-PAGE and visualized by Coomassie staining. As shown in Fig. 2A, a ∼47-kDa protein was specifically purified using antibody 9D6. To identify the Toxoplasma gene encoding the 9D6 protein, the stained band was cut from the gel and digested with trypsin, and the tryptic fragments were identified by mass spectrometry. Six tryptic fragments were identified, all in a single Toxoplasma open reading frame (Fig. 2B). A combination of rapid amplification of 5′ cDNA ends and cDNA sequencing was used to determine the complete coding sequence of the protein recognized by 9D6. The gene is composed of a maximum of 13 exons interrupted by 12 introns (Fig. 2B) and encodes a 445-amino-acid protein. Notably, several of the exons in the N-terminal portion of the protein are extremely short (exon 3 consists of only 1 codon and exons 2, 4, and 5 each consist of only 4 codons). Examination of Toxoplasma expressed sequence tags shows that differential splicing likely results in the removal of one or more of these short exons in many transcripts (data not shown). As expected from a rhoptry protein, the protein sequence contains a predicted N-terminal signal peptide for entry into the secretory pathway (Fig. 2B). The protein also contains a putative bipartite nuclear localization sequence that could enable targeting from the host cytoplasm to the host nucleus. An unusually hydrophobic region, similar to hydrophobic sequences seen in GPI-anchored proteins, exists at the extreme C terminus.
FIG. 2.
Immunoaffinity purification and identification of PP2C-hn. (A) Coomassie-stained SDS-PAGE gel showing a ∼47-kDa protein eluted from the 9D6 affinity column. The protein band was excised from the gel and digested with trypsin, and the tryptic fragments were analyzed by mass spectrometry. (B) Predicted amino acid sequence of PP2C-hn. The six tryptic peptides identified by mass spectrometry are boxed. A predicted signal peptide is underlined, and a putative bipartite nuclear localization sequence is marked by a double underline. The dashed underline indicates a C-terminal hydrophobic region. The positioning of the 12 introns in the PP2C-hn gene is indicated by inverted triangles, highlighting several short exons in the N-terminal region of the protein.
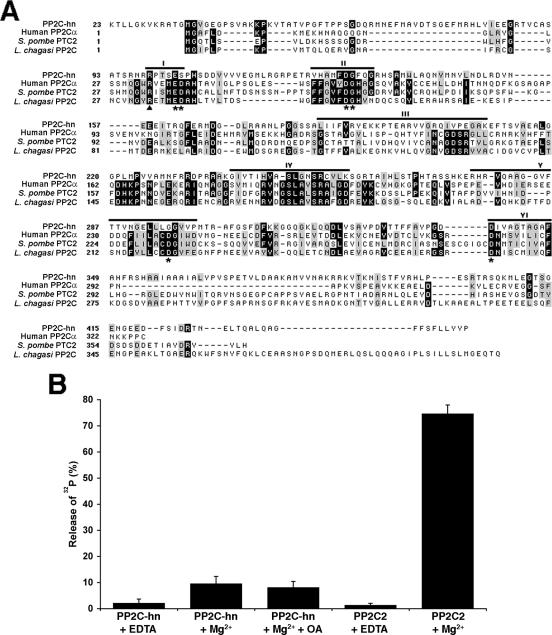
BLAST analysis of the protein sequence revealed the presence of a PP2C domain, and the protein was thus named PP2C-hn. PP2C domains are most common to PP2C proteins but are also found in other proteins in the PP2C superfamily, including pyruvate dehydrogenase phosphatase and adenylate cyclase (4, 9). PP2C proteins are monomeric serine/threonine phosphatases that are distinguished by their dependence on metals (Mn2+ and/or Mg2+) and their resistance to inhibitors (e.g., okadaic acid) of type 1, 2A, and 2B serine/threonine phosphatases. Alignment of PP2C-hn with known PP2C proteins shows significant homology in six motifs previously shown to be common to bona fide PP2C proteins (Fig. 3A). A hallmark of PP2C proteins is the presence of 6 conserved residues that play a role in metal binding, including 4 aspartic acid residues that coordinate metal ions in the active sites of these proteins (10, 24). Figure 3A shows that PP2C-hn contains 4 of the 6 conserved residues but lacks 2 of the 4 aspartic acid residues known to be involved in metal binding.
FIG. 3.
PP2C-hn contains a PP2C domain and exhibits metal-dependent phosphatase activity in vitro. (A) Alignment of PP2C-hn with human PP2Cα (GenBank accession no. AAB21784), Schizosaccharomyces pombe PTC2 (CAA20880), and Leishmania chagasi PP2C (AAA02864). Bars above the residues indicate six conserved motifs of PP2C proteins. Residues in PP2C proteins involved in metal coordination are marked with asterisks and phosphate binding with a triangle. PP2C-hn lacks two of the four aspartic acid residues involved in metal binding. (B) Recombinant PP2C-hn has low metal-dependent phosphatase activity when [32P]casein is used as a substrate. PP2C-hn activity is shown in the presence of 4 mM Mg2+. The activity is inhibited by EDTA and insensitive to 10 μM okadaic acid, characteristics of PP2C proteins. As a positive control, an additional Toxoplasma PP2C protein (PP2C2) was expressed; it shows robust activity in the presence of 10 mM Mg2+.
PP2C-hn exhibits low, metal-dependent phosphatase activity in vitro.
The lack of the conserved aspartic acid residues raises the question of whether PP2C-hn can act as a metal-dependent protein phosphatase. To address this question, we expressed recombinant six-His-tagged PP2C-hn in E. coli and examined phosphatase activity in vitro. Residues 20 to 418 of PP2C-hn, which contain the entire PP2C domain but lack the predicted signal peptide and C-terminal hydrophobic region, were chosen for expression in E. coli. As a positive control, we similarly expressed residues 92 to 537 of a second distinct Toxoplasma PP2C (TgTwinscan_7301) that was originally identified in our proteomic analysis of purified rhoptries (6). This control protein contains all six of the conserved residues typically found in PP2C proteins and was named PP2C2. Both PP2C-hn and PP2C2 were expressed using native conditions in E. coli and purified by nickel agarose chromatography, and activity was assessed in vitro using radiolabeled casein as a substrate (Fig. 3B) (28). As expected, the control PP2C2 had robust phosphatase activity in which approximately 75% of the radiolabeled phosphate could be removed from casein. PP2C2 activity was inhibited in the presence of the metal chelator EDTA. In contrast, PP2C-hn exhibited low but reproducible phosphatase activity on phosphorylated casein (Fig. 3B). The phosphatase activity was shown to be PP2C type activity, since it is inhibited by EDTA and insensitive to 10 μM okadaic acid, which inhibits type 1, 2A, and 2B protein phosphatases (46). Similar activity was seen using manganese as a cofactor and myelin basic protein as a second artificial PP2C substrate (data not shown). No PP2C activity was detected for control bacterial lysates passed over nickel agarose columns or for other (nonphosphatase) recombinant Toxoplasma proteins similarly prepared. We conclude that PP2C-hn exhibits low, metal-dependent, and okadaic acid-insensitive phosphatase activity in vitro by using the artificial substrates casein and myelin basic protein and thus can be designated a PP2C.
PP2C-hn is not tightly associated with a membrane.
The extreme C terminus of PP2C-hn contains a stretch of 11 hydrophobic amino acids that resemble a GPI anchor addition sequence. Two computer algorithms for GPI prediction disagree on whether PP2C-hn is predicted to be GPI anchored: the DGPI program (http://129.194.185.165/dgpi/index_en.html) predicts a GPI anchor, whereas the big-PI Predictor (http://mendel.imp.ac.at/gpi/gpi_server.html) does not (13). Since GPI-anchored proteins have been identified in the rhoptries of Plasmodium spp. and are suggested to be present in the Toxoplasma rhoptries (6, 44), we examined the PP2C-hn protein for membrane association via a GPI anchor. Many GPI-anchored proteins can be detected by cleavage of the anchor with PI-PLC and detection with anti-CRD antibodies that recognize the cleaved product (18). PI-PLC cleavage of parasite lysates and anti-CRD detection of immunoprecipitated PP2C-hn failed to show anti-CRD reactivity, whereas anti-CRD detection of PI-PLC-cleaved SAG1 was readily detected (data not shown). Since some GPI anchors cannot be cleaved with PI-PLC (18), we assessed whether PP2C-hn was soluble or membrane associated by using an independent assay, TX-114 phase partitioning (34). As shown in Fig. 4, PP2C-hn partitions with soluble proteins in the aqueous phase, while the GPI-anchored surface protein SAG1 partitions exclusively with membrane proteins in the detergent phase. A portion of the PP2C-hn protein was not solubilized in TX-114, but this was due to incomplete solubilization of the sample, since the soluble rhoptry protein ROP1 was partitioned similarly under these conditions. These results demonstrate that PP2C-hn is a soluble rhoptry protein and is not GPI anchored.
FIG. 4.

PP2C-hn is a soluble protein. TX-114 partitioning was used to separate parasite lysates into a detergent (membrane-associated) phase and an aqueous (soluble) phase. Western blot analysis of the TX-114 phase separation shows that PP2C-hn fractionates to the aqueous phase with the soluble protein ROP1. GPI-anchored proteins such as SAG1 partition exclusively to the detergent (membrane) fraction.
Targeted gene disruption of PP2C-hn.
To investigate the function of PP2C-hn and to study its targeting to the host nucleus, we disrupted the PP2C-hn gene by homologous recombination (Fig. 5). The construct used for the knockout contains the selectable marker HPT flanked by ∼5 kb of the PP2C-hn upstream and downstream genomic regions (Fig. 5A). The plasmid also contains GFP as a downstream marker to distinguish homologous from heterologous recombinants (i.e., homologous recombinants will be HPT+ GFP−). The knockout construct was transfected into RHΔhpt (parental) parasites (12), and stably transfected GFP-negative clones were screened for PP2C-hn knockout parasites by IFAs. Seven of eight GFP-negative clones did not stain with antibody 9D6 in either the rhoptries or the infected host nucleus, indicating successful targeting of the knockout construct (Fig. 5B, RHΔpp2c-hn+HPT). Immunoblotting with a polyclonal mouse serum raised against an N-terminal portion of recombinant PP2C-hn confirmed that the knockout parasites lack PP2C-hn (Fig. 5C) (the original MAb 9D6 lacks reactivity in immunoblots, so this additional polyclonal antibody was raised, which gives identical rhoptry and host nuclear staining by IFA [data not shown]). These results demonstrate that PP2C-hn can be disrupted and is not essential for in vitro propagation of T. gondii.
To exclude polar effects of the knockout on nearby genes and effects from the expression of HPT itself, we took advantage of the fact that HPT can be used as both a positive and a negative selectable marker and carried out a second round of homologous recombination to remove the HPT gene from RHΔpp2c-hn+HPT parasites (Fig. 5A). For this purpose, the HPT gene was deleted from the original knockout plasmid, the resulting construct was transfected into RHΔpp2c-hn+HPT knockout parasites, and parasites were selected for homologous recombinants that lack HPT. The resulting knockout parasites lack the entire coding region of PP2C-hn, resulting in the 5′ and 3′ flanks abutting one another, as shown in Fig. 5A. PCR was used to confirm the deletion at the PP2C-hn locus (data not shown). The PP2C-hn knockout strains were distinguished by naming the original knockout RHΔpp2c-hn+HPT, whereas the knockout strain lacking HPT was named RHΔpp2c-hn.
RHΔpp2c-hn parasites display a subtle growth defect.
RHΔpp2c-hn parasites do not display a severe defect in the invasion of host cells or intracellular growth. To determine more subtle effects of the knockout, parasite growth was assessed using a competition growth assay (Fig. 5D) (7). In this assay, equal quantities of parental and RHΔpp2c-hn parasites were mixed and the percentage of each in the population was measured over time by IFA. The parental strain gradually became a larger proportion of the population, demonstrating a subtle growth defect in RHΔpp2c-hn parasites. While manipulation of Toxoplasma by incorporation of selectable markers can result in mild growth changes, the growth defect seen here is not likely to be the result of these manipulations, since both the parental and the RHΔpp2c-hn parasites lack the HPT gene. The defect in growth kinetics of RHΔpp2c-hn parasites indicates that PP2C-hn plays a role in some aspect of the parasite's lytic cycle in human fibroblasts.
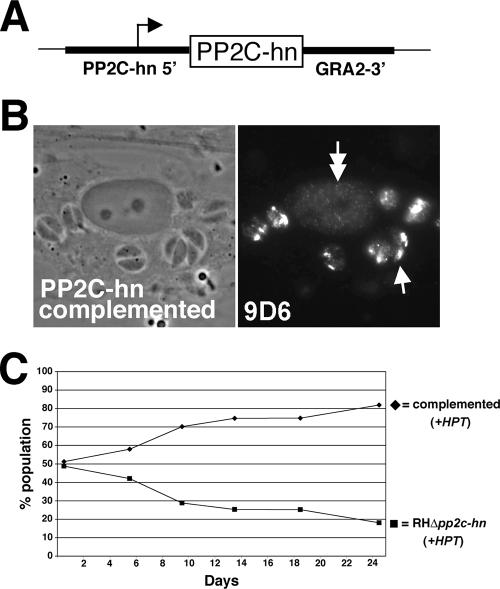
Reconstitution of PP2C-hn restores rhoptry and host nuclear targeting and complements the growth defect.
For functional analyses of PP2C-hn, we reconstituted RHΔpp2c-hn parasites with the wild-type PP2C-hn gene. To ensure appropriate levels and timing of expression, the coding region was driven by its endogenous promoter, which was provided by a 1.4-kb 5′ genomic flanking region (Fig. 6A). A heterologous 3′ flanking region from the GRA2 gene was used, since 3′ elements have so far appeared to be largely interchangeable for expression of most Toxoplasma genes. HPT was included in the construct for selection of transfected parasites (data not shown). The construct was transfected into RHΔpp2c-hn parasites, and stably transfected parasite clones were examined by IFA. As expected, expression of the PP2C-hn transgene restores MAb 9D6 staining of parasite rhoptries and nuclei of infected host cells (Fig. 6B). To assess whether complementation rescues the growth defect, a competition growth assay was performed using complemented and knockout parasites (to exclude effects on growth due to the presence of HPT, strains containing HPT were used for the competition assay). As expected, the complemented strain outcompeted the PP2c-hn knockout (Fig. 6C) with similar kinetics to that seen for the parental strain in Fig. 5D, demonstrating that reintroduction of wild-type PP2C-hn can rescue the growth phenotype seen in the knockout.
FIG. 6.
Reconstitution of PP2C-hn. (A) Schematic of the construct used for reconstitution of the RHΔpp2c-hn parasites. The coding region of PP2C-hn (box) is driven by its endogenous promoter, contained in a 1.4-kb 5′ genomic flanking region. The GRA2 3′ region provides 3′ elements for expression. The construct also contains the selectable marker HPT (not shown). (B) IFA analysis of RHΔpp2c-hn parasites stably transfected with PP2C-hn demonstrates that both rhoptry localization and infected host nuclear localization are restored. (C) Restoration of PP2C-hn results in complemented parasites outcompeting knockout parasites in a competition growth assay. Both strains in the assay contain HPT to control for effects of the selectable marker.
Functional and in vivo studies using RHΔpp2c-hn parasites.
Because PP2C-hn is targeted to the host nucleus during infection, we reasoned that its target is likely also nuclear and that PP2C-hn function could be assessed by analyzing the host transcriptional response to infection using wild-type and knockout parasites. To test this, we infected HFF cells with parental and RHΔpp2c-hn parasites and used Affymetrix human microarrays to assess the host response to infection. We chose to analyze the host response at 1, 4, and 9 h postinfection using a multiplicity of infection of ∼5 parasites per cell. These conditions allowed for sufficient levels of PP2C-hn targeted to the host nucleus and >95% infection of host cells. While the microarrays were remarkably consistent, no differences were seen in the host transcriptional response to parental versus RHΔpp2c-hn parasites in 2 to 4 biological replicates (see the supplemental material). The lack of detectable differences in the host response may be due either to the presence of parasite proteins with functions that are redundant with those of PP2C-hn or to experimental conditions including host cell type, parasite life cycle stage, infection level, and timing of infection. Alternatively, the effect of PP2C-hn on its host cell may be posttranscriptional and therefore not detectable by microarray analyses.
One of the most striking examples of the ability of Toxoplasma to subvert host cell functions is the ability to block host cell apoptosis (39). This process helps to maintain a host cell for productive replication of the parasite. To test if PP2C-hn is necessary for this process, we infected BALB/c 3T3 cells with parental and RHΔpp2c-hn parasites, induced apoptosis, and assessed the ability of each strain to block host cell apoptosis. As assessed by Western blot analysis of caspase 3 activation, parental and RHΔpp2c-hn parasites were equally able to block tumor necrosis factor-induced apoptosis (data not shown). We conclude that PP2C-hn is not essential for the overall ability of Toxoplasma to block host cell apoptosis.
Finally, we assessed the fitness and virulence of the RHΔpp2c-hn strain using in vivo infections in mice. The type I RH strain used for these studies is highly virulent in mice: intraperitoneal infections initiated with even a single parasite always result in fatality by 8 to 9 days, regardless of the mouse strain used. In experiments using a low (2 to 4 parasites) or medium (∼30 parasites) dose, we saw no difference in virulence between parental and RHΔpp2c-hn parasites (data not shown). Thus, PP2C-hn appears dispensable in vivo, at least in terms of a basic virulence assay performed with RH strain parasites. While the highly virulent RH strain used for the knockout is excellent for its in vitro cultivation and high transfection efficiency, it is not ideal for in vivo studies. In addition to its virulence, this strain also has been passed in tissue culture for many years and has developed a reduced capacity to form cysts, resulting in a loss of oral infectivity (45). The precise biological function of PP2C-hn will likely best be resolved by performing the knockout for a less virulent and more biologically relevant strain of T. gondii.
DISCUSSION
Toxoplasma gondii subverts numerous host cell processes to establish and maintain an intracellular niche. Host cell subversion begins almost immediately upon invasion, with secretion from the rhoptries playing dual roles in assisting invasion and circumventing host cell defenses (1, 32). During this burst of secretion, rhoptry proteins have been shown to be introduced into host cells in the form of membranous evacuoles, which likely contribute to vacuolar formation and/or maturation (14). We show here that Toxoplasma can use this burst of rhoptry release to also deliver a rhoptry-localized PP2C protein (PP2C-hn) to the cytosol and thence to the host nucleus during infection. This process is unprecedented among protozoan parasites bounded by a vacuolar membrane and presents a novel means by which Toxoplasma can access its host cell.
Examination of the primary sequence of PP2C-hn shows homology to PP2C proteins. PP2C proteins have been shown to regulate a wide variety of cellular functions, including signaling, splicing, the cell cycle, and development (25). Most PP2C studies, however, have focused on cytosolic PP2C proteins, and little is known about the function of nuclear family members. Alignment of the PP2C-hn phosphatase domain with other PP2C proteins shows an absence of two conserved aspartic acid residues that are important for metal binding and typical PP2C activity. In spite of the absence of these residues, we were able to demonstrate that recombinant PP2C-hn displayed low but reproducible metal-dependent phosphatase activity on artificial substrates in vitro. This low activity of PP2C-hn may simply reflect the substrate specificity of the enzyme. Similar low levels of in vitro activity have been observed for other PP2C proteins, indicating that the artificial substrates used in these assays are not ideal substrates and/or that the correct conditions have not been determined (24, 47). The host nuclear localization suggests that the PP2C-hn substrate will also be in the nucleus, but the protein may also have targets within the rhoptries. Interestingly, a cytoplasmic Toxoplasma PP2C has been identified that interacts with the actin-binding protein Toxofilin in parasite lysates (11). Since Toxofilin has recently been shown to be a rhoptry protein (6), it seems unlikely that it interacts with a cytosolic PP2C; thus, it could instead interact with the rhoptry-localized PP2C-hn or the putative rhoptry protein PP2C2. However, we see no evidence of interacting Toxoplasma proteins in the stringent PP2C-hn immunoprecipitation shown in Fig. 2.
Another explanation for the low activity of PP2C-hn is that the in vitro activity shown here is not physiologically relevant. Mutagenesis studies show that alteration of conserved aspartic acid residues in PP2C family members dramatically reduces the phosphatase activity of these proteins (20). It is thus possible that PP2C-hn does not function as a phosphatase but may instead bind to host cell substrates, making them unavailable for modulation by host or parasite phosphatases. The Toxoplasma PP2C2 shown here to have robust phosphatase activity may represent a PP2C that is modulated by PP2C-hn, although its localization to the rhoptries has not been confirmed (6). Interestingly, a partner switching pathway in Chlamydia trachomatis is proposed to be regulated by CtRsbU and Ct589, a pair of proteins containing PP2C domains of which Ct589 is similarly missing key aspartic acid residues (19). Signaling via Ct589 is proposed to occur through the phosphatase activity of Ct589 itself, by the regulation of the second phosphatase, CtRsbU, or by the binding of other substrates. Whether a similar interplay occurs between PP2C-hn and other host or parasite PP2C proteins awaits identification of the host or parasite factors that interact with PP2C-hn upon host cell invasion.
While all Apicomplexans are obligate intracellular parasites, subtle differences in their intracellular lifestyles have resulted in significant differences in their mechanisms of delivery of parasite proteins to the host cell. In Theileria spp., the parasite targets proteins to the nucleus of the host cell, where these proteins are believed to play a role in transforming the infected cell, resulting in uncontrolled proliferation (41, 42). However, Theileria is unusual in that it is taken up into a PV and then escapes from the vacuole and resides in the host cytoplasm. Thus, Theileria does not have to contend with a delimiting vacuolar membrane, such as is typically seen in Apicomplexans, and secreted constituents have direct access to the host cell. Interestingly, a recent bioinformatic analysis of the Theileria genome that mined for potential host-modulating proteins failed to find either phosphatases or kinases that are likely to be trafficked into the host cell or to the parasite surface, indicating that Theileria uses other types of proteins to modulate host signaling pathways (36). Disruption of the vacuolar membrane appears to occur during rhoptry release by Theileria (35), possibly allowing an opportunity for Theileria rhoptry proteins to access the cytoplasm or other compartments of the host cell. However, few rhoptry proteins have been identified in Theileria, and little is known regarding their ultimate destination following secretion.
Plasmodium spp. reside in a parasitophorous vacuole during the infection of erythrocytes and have developed specialized mechanisms for targeting to their host cells proteins that are central to the virulence and pathogenesis of these organisms. Plasmodium proteins targeted to the host cell contain a conserved export signal known as a Plasmodium export element, or host targeting signal, which is present in the N-terminal portion of exported proteins ∼15 to 20 amino acids downstream of the predicted signal peptide (17, 27). Proteins containing the export signal clearly cross the vacuolar membrane, although the detailed mechanism of transport is not known and the protein translocation machinery involved in transport across the vacuolar membrane has not been identified. Regardless of the precise mechanism, constitutively secreted proteins can be delivered to the host cell and do not require a burst from the rhoptries for delivery. While this process appears to be distinct from the rhoptry-mediated process described here, it is possible that Plasmodium could also exploit the rhoptries for delivery of proteins to the host cell. As in Toxoplasma, rhoptry release in Plasmodium coincides with the initial stages of invasion and vacuolar formation, allowing for potential delivery to the host cell. The infection of hepatocytes by the sporozoite stage of the parasite also presents a nucleated cell in which the parasite may deliver effector proteins to the host nucleus. Further identification and characterization of Plasmodium and Toxoplasma rhoptry proteins will undoubtedly reveal whether these parasites employ common mechanisms for controlling their infected host cells.
The delivery of rhoptry proteins to the host cytoplasm by Toxoplasma shares intriguing similarities with bacterial type III secretion systems, used for delivery of effectors to the host cell (30). Instead of using a needle apparatus, Toxoplasma appears to use the secretory rhoptries to directly inject proteins into the cytoplasm during invasion. Patch-clamp experiments examining parasites in the process of infecting host cells show a transient breach in conductivity at the plasma membrane that appears to coincide with rhoptry release (40). This breach may reflect the moment when the rhoptry proteins are released into the host cytoplasm. When PP2C-hn reaches the cytoplasm, its delivery is likely to be aided by its putative nuclear localization sequence and by the host nuclear trafficking machinery, although its size is below the ∼50-kDa cutoff of the nuclear pore (26), and thus, it could also reach the nucleus by diffusion and retention by nuclear substrates.
While the direct injection of rhoptry proteins into the cytosol agrees with the timing of the release from the rhoptries and evacuole formation (8, 14), we cannot exclude the possibility that PP2C-hn is first released into the vacuole and then somehow translocated across the vacuolar membrane by unknown transporters. Strongly arguing for the direct injection model, however, is the fact that parasites that are blocked from invasion and vacuole formation by cytochalasin D still secrete evacuoles and are able to deliver PP2C-hn to the host nucleus (Fig. 1D). In such parasites, the vacuole is barely (if at all) formed, yet transport to the nucleus appears to be fully competent.
We demonstrate here that PP2C-hn is released and targeted to the host nucleus during invasion. Host cell localization of the other rhoptry body proteins that are known to be released from the rhoptries (e.g., ROP1, ROP2, ROP4, subtilisin) is not apparent outside of the evacuoles. The delivery of PP2C-hn to the host nucleus indicates that access of rhoptry proteins to the host cytoplasm during the burst of rhoptry secretion is not merely a minor consequence of vacuole formation but a specific method for delivery of proteins to the host cell. It seems likely that other rhoptry proteins may also be delivered to the cytoplasm by using this mechanism but that these proteins have not been detected previously simply because, as diffuse cytosolic proteins, they are below the level of detection by antibody staining. Our detection of PP2C-hn is almost certainly aided by the fact that it is concentrated in the host nucleus. The scenario of multiple rhoptry proteins entering the host cell presents new possibilities for how Toxoplasma coopts the host cell for its own purposes. While detection of many of these proteins in the host cell may be problematic, assessment of the host response by using parasite gene knockout and overexpression approaches may well shed light on their functions. In agreement with this notion, we have recently identified a second rhoptry protein, in this case a protein kinase, which reaches the host cell nucleus with similar kinetics to PP2C-hn (33). Hence, the phenomenon reported here may be a general mechanism for the interaction of Toxoplasma with its host cell.
Supplementary Material
Acknowledgments
We thank Cathy Sohn for assistance in cloning the PP2C-hn promoter. Mass spectrometry was carried out by Allis Chien at the Stanford Mass Spectrometry Facility, and the hybridoma fusion was carried out by Phuoc Vo at the Stanford FACS/antibody core facility.
This work was supported by a Banos Undergraduate Research Scholarship award to L.A.G., a Microbial Pathogenesis Training grant (T32-AI07323) to J.M.T., and National Institutes of Health grants RO1AI 21423 (to J.C.B.) and 1R01AI064616 (to P.J.B.).
Footnotes
Published ahead of print on 3 November 2006.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Alexander, D. L., J. Mital, G. E. Ward, P. Bradley, and J. C. Boothroyd. 2005. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 1:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliberti, J., J. G. Valenzuela, V. B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J. M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485-490. [DOI] [PubMed] [Google Scholar]
- 3.Beckers, C. J., J. F. Dubremetz, O. Mercereau-Puijalon, and K. A. Joiner. 1994. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J. Cell Biol. 127:947-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bork, P., N. P. Brown, H. Hegyi, and J. Schultz. 1996. The protein phosphatase 2C (PP2C) superfamily: detection of bacterial homologues. Protein Sci. 5:1421-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley, P. J., C. L. Hsieh, and J. C. Boothroyd. 2002. Unprocessed Toxoplasma ROP1 is effectively targeted and secreted into the nascent parasitophorous vacuole. Mol. Biochem. Parasitol. 125:189-193. [DOI] [PubMed] [Google Scholar]
- 6.Bradley, P. J., C. Ward, S. J. Cheng, D. L. Alexander, S. Coller, G. H. Coombs, J. D. Dunn, D. J. Ferguson, S. J. Sanderson, J. M. Wastling, and J. C. Boothroyd. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 280:34245-34258. [DOI] [PubMed] [Google Scholar]
- 7.Camps, M., G. Arrizabalaga, and J. Boothroyd. 2002. An rRNA mutation identifies the apicoplast as the target for clindamycin in Toxoplasma gondii. Mol. Microbiol. 43:1309-1318. [DOI] [PubMed] [Google Scholar]
- 8.Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114-123. [PubMed] [Google Scholar]
- 9.Cohen, P. 1989. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 58:453-508. [DOI] [PubMed] [Google Scholar]
- 10.Das, A. K., N. R. Helps, P. T. Cohen, and D. Barford. 1996. Crystal structure of the protein serine/threonine phosphatase 2C at 2.0 Å resolution. EMBO J. 15:6798-6809. [PMC free article] [PubMed] [Google Scholar]
- 11.Delorme, V., X. Cayla, G. Faure, A. Garcia, and I. Tardieux. 2003. Actin dynamics is controlled by a casein kinase II and phosphatase 2C interplay on Toxoplasma gondii Toxofilin. Mol. Biol. Cell 14:1900-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donald, R. G., D. Carter, B. Ullman, and D. S. Roos. 1996. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J. Biol. Chem. 271:14010-14019. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhaber, B., P. Bork, and F. Eisenhaber. 1999. Prediction of potential GPI-modification sites in proprotein sequences. J. Mol. Biol. 292:741-758. [DOI] [PubMed] [Google Scholar]
- 14.Hakansson, S., A. J. Charron, and L. D. Sibley. 2001. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20:3132-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlow, E., and D. Lane. 1988. Immunoaffinity purification, p. 511-533. In E. Harlow and D. Lane (ed.), Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 16.Hill, D. E., S. Chirukandoth, and J. P. Dubey. 2005. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 6:41-61. [DOI] [PubMed] [Google Scholar]
- 17.Hiller, N. L., S. Bhattacharjee, C. van Ooij, K. Liolios, T. Harrison, C. Lopez-Estrano, and K. Haldar. 2004. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306:1934-1937. [DOI] [PubMed] [Google Scholar]
- 18.Hooper, N. M. 2001. Determination of glycosyl-phosphatidylinositol membrane protein anchorage. Proteomics 1:748-755. [DOI] [PubMed] [Google Scholar]
- 19.Hua, L., P. S. Hefty, Y. J. Lee, Y. M. Lee, R. S. Stephens, and C. W. Price. 2006. Core of the partner switching signalling mechanism is conserved in the obligate intracellular pathogen Chlamydia trachomatis. Mol. Microbiol. 59:623-636. [DOI] [PubMed] [Google Scholar]
- 20.Jackson, M. D., C. C. Fjeld, and J. M. Denu. 2003. Probing the function of conserved residues in the serine/threonine phosphatase PP2Cα. Biochemistry 42:8513-8521. [DOI] [PubMed] [Google Scholar]
- 21.Karasov, A. O., J. C. Boothroyd, and G. Arrizabalaga. 2005. Identification and disruption of a rhoptry-localized homologue of sodium hydrogen exchangers in Toxoplasma gondii. Int. J. Parasitol. 35:285-291. [DOI] [PubMed] [Google Scholar]
- 22.Keeley, A., and D. Soldati. 2004. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 14:528-532. [DOI] [PubMed] [Google Scholar]
- 23.Kim, K., M. S. Eaton, W. Schubert, S. Wu, and J. Tang. 2001. Optimized expression of green fluorescent protein in Toxoplasma gondii using thermostable green fluorescent protein mutants. Mol. Biochem. Parasitol. 113:309-313. [DOI] [PubMed] [Google Scholar]
- 24.Komaki, K., K. Katsura, M. Ohnishi, M. Guang Li, M. Sasaki, M. Watanabe, T. Kobayashi, and S. Tamura. 2003. Molecular cloning of PP2Cη, a novel member of the protein phosphatase 2C family. Biochim. Biophys. Acta 1630:130-137. [DOI] [PubMed] [Google Scholar]
- 25.Luan, S. 2003. Protein phosphatases in plants. Annu. Rev. Plant Biol. 54:63-92. [DOI] [PubMed] [Google Scholar]
- 26.Macara, I. G. 2001. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65:570-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marti, M., R. T. Good, M. Rug, E. Knuepfer, and A. F. Cowman. 2004. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306:1930-1933. [DOI] [PubMed] [Google Scholar]
- 28.McGowan, C. H., and P. Cohen. 1988. Protein phosphatase-2C from rabbit skeletal muscle and liver: an Mg2+-dependent enzyme. Methods Enzymol. 159:416-426. [DOI] [PubMed] [Google Scholar]
- 29.Mordue, D. G., S. Hakansson, I. Niesman, and L. D. Sibley. 1999. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp. Parasitol. 92:87-99. [DOI] [PubMed] [Google Scholar]
- 30.Mota, L. J., and G. R. Cornelis. 2005. The bacterial injection kit: type III secretion systems. Ann. Med. 37:234-249. [DOI] [PubMed] [Google Scholar]
- 31.Nagel, S. D., and J. C. Boothroyd. 1989. The major surface antigen, P30, of Toxoplasma gondii is anchored by a glycolipid. J. Biol. Chem. 264:5569-5574. [PubMed] [Google Scholar]
- 32.Ngo, H. M., M. Yang, and K. A. Joiner. 2004. Are rhoptries in Apicomplexan parasites secretory granules or secretory lysosomal granules? Mol. Microbiol. 52:1531-1541. [DOI] [PubMed] [Google Scholar]
- 33.Saeij, J. P., S. Coller, J. P. Boyle, M. Jerome, M. White, and J. C. Boothroyd. Toxoplasma injects a polymorphic kinase homologue that targets the host nucleus and co-opts transcription. Nature, in press. [DOI] [PMC free article] [PubMed]
- 34.Seeber, F., J. F. Dubremetz, and J. C. Boothroyd. 1998. Analysis of Toxoplasma gondii stably transfected with a transmembrane variant of its major surface protein, SAG1. J. Cell Sci. 111:23-29. [DOI] [PubMed] [Google Scholar]
- 35.Shaw, M. K. 2003. Cell invasion by Theileria sporozoites. Trends Parasitol. 19:2-6. [DOI] [PubMed] [Google Scholar]
- 36.Shiels, B., G. Langsley, W. Weir, A. Pain, S. McKellar, and D. Dobbelaere. 2006. Alteration of host cell phenotype by Theileria annulata and Theileria parva: mining for manipulators in the parasite genomes. Int. J. Parasitol. 36:9-21. [DOI] [PubMed] [Google Scholar]
- 37.Sibley, L. D., E. Weidner, and J. L. Krahenbuhl. 1985. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature 315:416-419. [DOI] [PubMed] [Google Scholar]
- 38.Sinai, A. P., and K. A. Joiner. 2001. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J. Cell Biol. 154:95-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinai, A. P., T. M. Payne, J. C. Carmen, L. Hardi, S. J. Watson, and R. E. Molestina. 2004. Mechanisms underlying the manipulation of host apoptotic pathways by Toxoplasma gondii. Int. J. Parasitol. 34:381-391. [DOI] [PubMed] [Google Scholar]
- 40.Suss-Toby, E., J. Zimmerberg, and G. E. Ward. 1996. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. USA 93:8413-8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swan, D. G., K. Phillips, A. Tait, and B. R. Shiels. 1999. Evidence for localisation of a Theileria parasite AT hook DNA-binding protein to the nucleus of immortalised bovine host cells. Mol. Biochem. Parasitol. 101:117-129. [DOI] [PubMed] [Google Scholar]
- 42.Swan, D. G., L. Stadler, E. Okan, M. Hoffs, F. Katzer, J. Kinnaird, S. McKellar, and B. R. Shiels. 2003. TashHN, a Theileria annulata encoded protein transported to the host nucleus displays an association with attenuation of parasite differentiation. Cell. Microbiol. 5:947-956. [DOI] [PubMed] [Google Scholar]
- 43.Tachibana, T., M. Hieda, and Y. Yoneda. 1999. Up-regulation of nuclear protein import by nuclear localization signal sequences in living cells. FEBS Lett. 442:235-240. [DOI] [PubMed] [Google Scholar]
- 44.Topolska, A. E., A. Lidgett, D. Truman, H. Fujioka, and R. L. Coppel. 2004. Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J. Biol. Chem. 279:4648-4656. [DOI] [PubMed] [Google Scholar]
- 45.Villard, O., E. Candolfi, D. J. Ferguson, L. Marcellin, and T. Kien. 1997. Loss of oral infectivity of tissue cysts of Toxoplasma gondii RH strain to outbred Swiss Webster mice. Int. J. Parasitol. 27:1555-1559. [DOI] [PubMed] [Google Scholar]
- 46.Wera, S., and B. A. Hemmings. 1995. Serine/threonine protein phosphatases. Biochem. J. 311:17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu, L. P., A. K. Miller, and S. E. Clark. 2003. POLTERGEIST encodes a protein phosphatase 2C that regulates CLAVATA pathways controlling stem cell identity at Arabidopsis shoot and flower meristems. Curr. Biol. 13:179-188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.