Abstract
The colony of a filamentous ascomycete fungus typically grows as a multinucleate syncytium. While this syncytial organization has developmental advantages, it bears the risk of extensive damage caused by local injury of hyphae. Loss of cytoplasm in injured hyphae is restricted by the fast and efficient sealing of the central pores of hyphal crosswalls, or septa, by a peroxisome-derived organelle called the Woronin body. The formation of septal plugs is also associated with development and leads to separation of certain parts of the colony. Septal plugs associated with developmental processes or aging hyphae typically occur by the accumulation of sealing material. Here we report that in Neurospora crassa, a protein necessary for hyphal fusion and proper colony development called SO (SOFT) localizes to septal plugs. In response to injury, SO accumulates at the septal plug in a Woronin body-independent manner. However, the presence of the Woronin body affects the speed of accumulation of SO at the septal pore. We determined that SO contributes to, but is not essential for, septal plugging. SO accumulation was also observed at septal plugs formed during hyphal aging and during programmed cell death mediated by genetic differences at heterokaryon incompatibility (het) loci.
Filamentous ascomycete fungi typically form mycelial colonies consisting of a network of interconnected multinucleate hyphae. Colonies grow by hyphal tip extension, branching, and fusion (4, 10). In filamentous ascomycete species, hyphal crosswalls or septa are incomplete and contain a single central pore. Septal pores allow cytoplasm and organelles, including nuclei, to move between hyphal compartments, thus making the fungal colony a syncytium. The syncytial, interconnected organization of a fungal colony enables translocation of cellular contents, such as organelles, metabolites, nutrients, or signaling compounds throughout the colony, presumably facilitating growth and reproduction. However, cytoplasmic continuity bears the risk of catastrophic loss of cellular contents as a result of hyphal injury. To prevent the loss of cytoplasm, septal pores become rapidly plugged in response to hyphal damage. In filamentous ascomycete species, plugging of septal pores is executed by a specialized organelle called the Woronin body (4, 5, 19, 30, 33). Woronin bodies are a specialized class of peroxisomal vesicles containing a crystalline proteinaceous core (34). In Neurospora crassa, the main component of the Woronin body core is the HEX-1 protein. Deletion of the hex-1 gene results in strains that lack Woronin bodies and results in the catastrophic loss of cytoplasm from hyphae after injury (13, 29). Occlusion of septal pores in the hex-1 mutant eventually occurs but is significantly delayed. In a wild-type strain, the plugging of septal pores by the Woronin body initiates subsequent processes termed consolidation (6) in which the hyphal plug becomes permanently sealed by the deposition of additional material. Studies with N. crassa showed that electron-dense material often associates with Woronin bodies in plugged septal pores. However, the molecular nature of this material remains unknown. Consolidation is often followed by reinitiation of growth (4, 18). One or more hyphal tips are formed at the plugged septum, resulting in the invasion of a growing hypha into the dead compartment. These newly formed hyphae can fuse with each other or an adjoining undamaged compartment, resulting in the reconnection of hyphal compartments separated by injury.
Within the fungal colony, plugging of septal pores has also been observed independently of hyphal injuries (18). Hyphae within the interior of a colony often show a high frequency of septal plugging. Studies with N. crassa revealed that plug formation in intact hyphae is fundamentally different from septal plugging in response to injury (18, 29). In undisturbed hyphae, septal plugs are formed not by Woronin bodies but by the accumulation of electron-dense material within and around the septal pore. The origin and molecular identity of this material are unknown. The relative number of plugged septal pores increases toward the interior of the colony, suggesting that plug formation is related to hyphal aging. Septal plugging is also associated with nonself recognition and programmed cell death in filamentous ascomycete species (11), a phenomenon termed “heterokaryon incompatibility.” Hyphal fusion between different fungal individuals results in the formation of a heterokaryon in which genetically different nuclei coexist in a common cytoplasm. Fusion between genetically incompatible isolates generally results in rapid septal plugging and death of the fusion cell (2, 9, 12), a process that is also apparently Woronin body independent.
Deletion of the so gene (NCU02794.3) in N. crassa results in mutants showing a pleiotropic phenotype, including lack of anastomosis, reduced aerial hyphae, an altered conidiation pattern, and female sterility (7). The molecular function of the SO protein is unknown, although it contains a WW domain predicted to mediate protein-protein interactions. The so gene is conserved in the genomes of filamentous ascomycete species; homologs are not found in Saccharomyces cerevisiae, Schizosaccharomyces pombe, or basidiomycete species. Here we show that SO-green fluorescent protein (GFP) fusions accumulate at septal pores in response to injury, in aging hyphae, and during heterokaryon incompatibility. Although so mutants showed plugged hyphae via the Woronin body in response to hyphal injury, the time required for cytoplasmic bleeding to cease via septal plugging was increased in so mutants. SO-GFP localized to septal plugs in the absence of a functional Woronin body, although with a delayed response and reduced efficiency.
MATERIALS AND METHODS
N. crassa strains and growth media.
The strains used in this study are listed in Table 1. Strains were grown on Vogel's minimal medium (MM) (31). For strains carrying auxotrophic markers, required supplements were added to the medium. Crosses were performed on Westergaard's medium (32). If strains carrying auxotrophic markers were used as females, 1/10 of the normally required concentration of the supplement was added to the mating medium. Alternatively, a heterokaryon between the helper strain FGSC 4564 (Fungal Genetic Stock Center) (Table 1) and the auxotrophic strain was used as a female in crosses (23). To test the mating types of strains, they were crossed with mating type tester strains FGSC 4347 (fl a) and FGSC 4317 (fl A) (Table 1).
TABLE 1.
Origins and genotypes of the strains used in this study
| Strain | Genotype | Origin or reference |
|---|---|---|
| FGSC 2489 (74-OR23-1VA) | A | FGSCa |
| FGSC 508 | so A | FGSC |
| FGSC 542 | so a | FGSC |
| FGSC 11292 | Δso a | FGSC |
| FGSC 11293 | Δso A | FGSC |
| FGSC 6103 | his-3 A | FGSC |
| AF-H4 | his-3 so A | 7 |
| AF-T8 | his-3+::Pccg1-so+-gfp+A | This study |
| AF-S1 | his-3+::Pso-so+-gfp+A | This study |
| AF-MF1 | his-3+::Pccg1-gfp+A | This study |
| R9-08 | hex-1 A | 13 |
| AF-T8.11 | his-3+::Pccg1-so+-gfp+a | This study |
| AF-SoT8 | his-3+::Pccg1-so+-gfp+so A | This study |
| AF-SoS1 | his-3+::Pso-so+-gfp+so A | This study |
| AF-SoMF | his-3+::Pccg1-gfp+so A | This study |
| HSG8.2.5 | his-3+::Pccg1-so+-gfp+hex-1 A | This study |
| AF-SH1 | hex-1 so A | This study |
| AF-SG1 | his-3+::Pccg1-so+-gfp+so; het-c1 pyr-4 A | This study |
| C9-15 | cyh-1; thr-2 het-c2 A | 25, 26 |
| AF-S567 | his-3+::Pccg1-soW567G-gfp+A | This study |
| AF-S590 | his-3+::Pccg1-soW590G-gfp+A | This study |
| AF-SoS567 | his-3+::Pccg1-soW567G-gfp+so A | This study |
| AF-SoS590 | his-3+::Pccg1-soW590G-gfp+so A | This study |
| FGSC 4564 | ad-3B cyh-1 am1 | FGSC |
| FGSC 4317 | fl A | FGSC |
| FGSC 4347 | fl a | FGSC |
| R13-23 | mak-2 A | 22 |
FGSC, Fungal Genetic Stock Center.
Plasmid construction.
Genomic template DNA extraction was performed as described in reference 15. Wild-type DNA was extracted from FGSC 2489. Proofreading PfuTurbo (Stratagene) or Phusion DNA polymerase (Finnzymes; distributed by New England Biolabs) was used to amplify expression fragments. For sequencing and further amplification, all PCR products were cloned into pCR-BluntII-TOPO (Invitrogen). Fragments were cut out of the TOPO plasmid and cloned into pMF272 (8).
To fuse the so gene to gfp, so was amplified by PCR with primers SO-GFP-F (5′ TCTAGAATGTCTCGATTCCGCGGTGTTCCT 3′) and SO-GFP-R (5′ TTAATTAAATGCCCATACTCCAAATGCGGCAC 3′). The fragment was cloned into pMF272 (8) at the XbaI and PacI restriction sites, resulting in plasmid pSO8. To express so-gfp under the control of the so promoter, so and its promoter were amplified with primers SO-GFP-FII (5′ GCGGCCGCATCCACAGCTCATCTCCCC 3′) and SO-GFP-R. The fragment was cloned into pMF272 (8) at the NotI and PacI restriction sites, leading to replacement of the ccg-1 promoter and resulting in plasmid pSOSO1. Point mutations in the codons encoding the tryptophan residues of the WW domain were introduced by fusion PCR. To mutate the first codon, fragments were amplified by primer pairs WWA-WW1R (5′ CCCCCACAGCAGAATAGCTTCAAC 3′, 5′ CGAGATGGGCAATCCAGCCCTCAGGCAAC 3′) and WW1F-WWB (5′ GTTGCCTGAGGGCTGGATTGCCCATCTCG 3′, 5′ TCGAAGTGGCTTGTGTAGTAGTGG 3′). The fragments were then fused with primers WWA and WWB. The second codon was mutated in the same way with primer pairs WWA-WW2R (5′ GCCCTTGGGAAACTCCCACTGCGTGGCTTGG 3′) and WW2F-WWB (5′ CCAAGCCACGCAGTGGGAGTTTCCCAAGGGC3′). The mutations were confirmed by sequencing a 1,351-bp SphI fragment, which was used to replace the respective SphI fragment in pSO8, resulting in plasmids pSO8W567G and pSO8W590G. The presence of the point mutations in the completed constructs was again confirmed by DNA sequence analysis.
Western analysis.
Assayed strains were grown in liquid culture at 30°C for 16 h. The mycelium was harvested by filtering through one layer of Miracloth (Calbiochem) and frozen in liquid nitrogen. Protein extraction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblot analysis were performed as previously described (22). Membranes were subsequently incubated with anti-GFP mouse immunoglobulin G monoclonal antibody (clones 7.1 and 13.1; dilution, 1:1,000; Roche) and anti-mouse immunoglobulin G (heavy and light chains) peroxidase (dilution, 1:4,000; Roche). To assess protein loading, blots were stripped and probed with anti-β-tubulin monoclonal antibody (clone TU27; dilution, 1:1,000; BabCo).
Light and fluorescence microscopy.
For light or fluorescence microscopy, hyphae were observed on agar blocks containing either Vogel's MM or 2% water agar. To stain cell walls and septa, calcofluor (100 μg/ml) was added before coverslips were placed upon the sample. Mycelia were examined by bright-field or differential interference contrast (DIC) optics with a Zeiss Axioskop II microscope. Fluorescence microscopy was performed with a Zeiss Axioskop II and the Chroma Standard Filter Set 31019 (Chroma Technology Corp.) filter block for GFP and a standard 4′,6′-diamidino-2-phenylindole (DAPI) filter set for calcofluor staining. Photographs were taken with a Hamamatsu digital charge-coupled device camera (Hamamatsu). Files were processed in Openlab 4.0.3 and Adobe Photoshop 7.0. Deconvolution microscopy was performed with a Deltavision Spectris DV4 deconvolution microscope (Applied Precision Instruments). Image processing and visualization were done with Bitplane Imaris. For time courses of hyphal injury, hyphae were cut with a UV pulse laser with a laser microdissection microscope (Leica AS LMD).
Neurospora transformation.
Conidia of N. crassa were transformed by electroporation as described in reference 17, with 1.5 kV and 1-mm gap cells (R. L. Metzenberg and K. Black, personal communication).
Quantification of cytoplasmic bleeding.
To quantify cytoplasmic bleeding, the method described in reference 13 was adapted. Strains were inoculated on a central spot on 20 ml MM agar in 125-ml Erlenmeyer flasks. When the developing colony reached the flask walls (wild type and hex-1 after around 16 h, so after around 24 h), mycelia were covered with 2 ml double-distilled H2O and incubated for 2 min. After brief vortexing, the water was recovered and briefly centrifuged to remove particulates. A 100-μl aliquot of the supernatant was used to determine the protein content by Bradford analysis.
Heterokaryon formation.
To form a het-c/pin-c incompatible heterokaryon (14), conidial suspensions of AF-SG1 (his-3+::Pccg1-so+-gfp+ so; pyr-4 het-c1 pin-c1) and C9-15 (thr-2 het-c2 pin-c2) were mixed and spotted onto MM agar plates. After 48 h of incubation at room temperature, a piece measuring 5 by 5 mm was cut out of the developed colony and transferred to MM agar containing 0.003% methylene blue to assess dead hyphal compartments (14, 27). Cultures were incubated for another 48 h at room temperature and analyzed by light and fluorescence microscopy.
RESULTS
SO-GFP fusions are functional and are cytoplasmically localized.
In an earlier study, we showed that mutations in the so gene of N. crassa resulted in a pleiotropic phenotype, including lack of hyphal anastomoses, shortened aerial hyphae, and female sterility (7). In order to gain a better understanding of the molecular function of the SO protein, we sought to determine its subcellular localization by fusing it to GFP. Two different expression cassettes were assembled. One construct contained 988 bp of the so promoter, followed by the so open reading frame fused to the gfp encoding sequence (pSOSO1). The other construct (pSO8) contained the so-gfp fusion construct under the control of the ccg-1 promoter, which has been used for studies of both GFP localization and overexpression in N. crassa (8). Both fusion cassettes were constructed in plasmid pMF272, which allows targeting to the his-3 locus after transformation (8). To test if the SO-GFP fusions were functional, the pSOSO1 and pSO8 plasmids were transformed into so strain AF-H4. Transformants containing pSOSO1 (AF-SoS1) or pSO8 (AF-SoT8) showed a phenotype comparable to that of a wild-type strain (Fig. 1A), including restoration of aerial hyphae, conidiation pattern, and hyphal fusion. By contrast, so strains obtained from a control transformation with plasmid pMF272 (AF-SoMF1) showed the so phenotype of the recipient strain. These data indicate that the SO-GFP fusions are functional and complement the loss of the native SO protein.
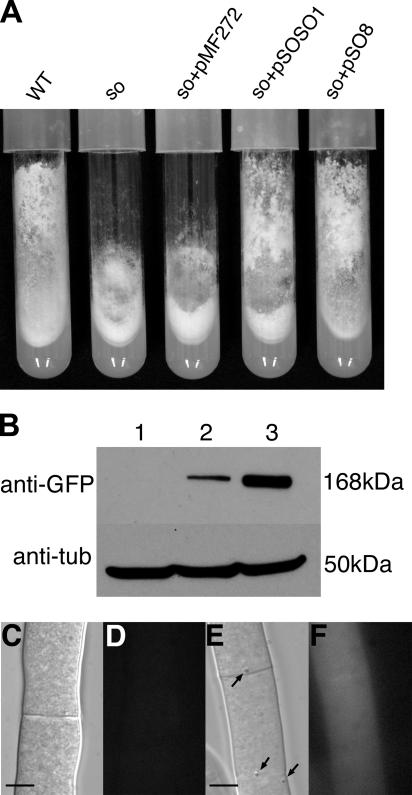
FIG. 1.
The SO-GFP fusion protein complements the so phenotype. (A) Strains were grown in tubes containing Vogel's MM. Cultures were grown for 7 days at 30°C. The so mutant shows reduced aerial hyphae and an altered conidiation pattern. Expression of so-gfp under control of the so or the ccg-1 promoter restores the wild-type phenotype. Shown, from left to right, are strains FGSC 2489 (wild type [WT]), FGSC 508 (so), AF-SoMF (his-3+::Pccg1-gfp+ so), AF-SoS1 (his-3+::Pso-so+-gfp+ so), and AF-SoT8 (his-3+::Pccg1-so+-gfp+ so). (B) Western analysis of strains expressing so-gfp with anti-GFP antibody. Expression of so-gfp under the control of the ccg1 promoter results in a higher protein concentration than expression under the control of the so promoter. Protein extraction and Western blot analysis were done as described in Materials and Methods. Lanes: 1, FGSC 2489 (wild type); 2, AF-S1 (his-3+::Pso-so+-gfp+); 3, AF-T8 (his-3+::Pccg1-so+-gfp+). Loading of comparable amounts of protein per lane was tested by hybridization with an anti-tubulin (anti-tub) antibody. (C and D) Hyphae of wild type (FGSC 2489). (C) DIC image. (D) Fluorescent image with GFP filter. (E and F) Hyphae of AF-T8 expressing SO-GFP. (E) DIC image; Woronin bodies in close proximity to the septa are shown (arrows). (F) Fluorescent image with GFP filter. Bars represent 5 μm.
The so-gfp plasmids were also transformed into wild-type strain FGSC 6103, resulting in transformants AF-S1 (carrying pSOSO1) and AF-T8 (carrying pSO8) (Table 1). To determine the subcellular localization of SO-GFP, the AF-SoS1, AF-SoT8, AF-S1, and AF-T8 strains were inoculated onto MM agar plates and incubated for 20 h at 30°C. Fluorescence microscopy showed that the SO-GFP signal was evenly distributed throughout the cytoplasm in all of the strains (Fig. 1C to F and data not shown). No association with subcellular structures or organelles was apparent. To test if AF-S1 and AF-T8 expressed full-length SO-GFP fusions, they were subjected to Western blot analysis; only a single band of ∼170 kDa was observed, which is the predicted size of the SO-GFP fusion protein in both AF-S1 and AF-T8 (Fig. 1B). These data indicated that proteolytic products of the SO-GFP fusion were not the cause of the cytoplasmic GFP fluorescence. An approximately fivefold increase in the protein level of the SO-GFP fusion products was apparent in AF-T8 (Fig. 1B), in which so-gfp is regulated by the ccg-1 promoter. This fact was also apparent by fluorescence microscopy, where the GFP signal in AF-T8 was much brighter than in AF-S1 (data not shown). These data indicate that SO-GFP is primarily a cytoplasmically localized protein, although minor association of SO-GFP with organelles within hyphae cannot be excluded.
SO-GFP localizes to septal plugs in damaged hyphae.
During the course of the evaluation of subcellular localization of SO-GFP, we observed a strong GFP signal at the edges of the sample, where the hyphae were cut with a razor blade during sample preparation. We therefore tested whether SO-GFP accumulation was induced by hyphal injury. Hyphae of strains AF-T8 and AF-SoT8, which express so-gfp under the control of the ccg-1 promoter, were purposely injured by cutting with a razor blade. The injured hyphae were subsequently analyzed by light and fluorescence microscopy. In the majority of cases, the septal pores at the end of the injured hyphal compartment were plugged by Woronin bodies (Fig. 2A and E), which were apparent as diffractive particles under light microscopy (see Fig. 1E). Fluorescence microscopy showed that SO-GFP also localized to these plugs in both AF-T8 and AF-SoT8 (Fig. 2B to D and data not shown). In control strains, which expressed cytoplasmic GFP (AF-MF1, AF-SoMF), a similar accumulation of GFP signal at septal plugs was never observed (Fig. 2E to H and data not shown). Accumulation of SO-GFP at septal plugs in injured hyphae was not dependent upon the regulation of so by the ccg-1 promoter, because accumulation of SO-GFP at septa in injured hyphae was observed in strain AF-S1, in which so-gfp is under the regulation of the native so promoter (data not shown). The localization and intensity of the GFP signal were also comparable in strains with or without a functional untagged copy of so (AF-T8 compared to AF-SoT8 or AF-S1 compared to AF-SoS1). However, the GFP signal at plugged septa was weaker in AF-S1 and AF-SoS1 compared to the overexpressing strains AF-T8 and AF-SoT8, consistent with the results of the Western blot analysis. Together, these data indicate that the localization of SO-GFP to septal plugs is specific for the SO protein.
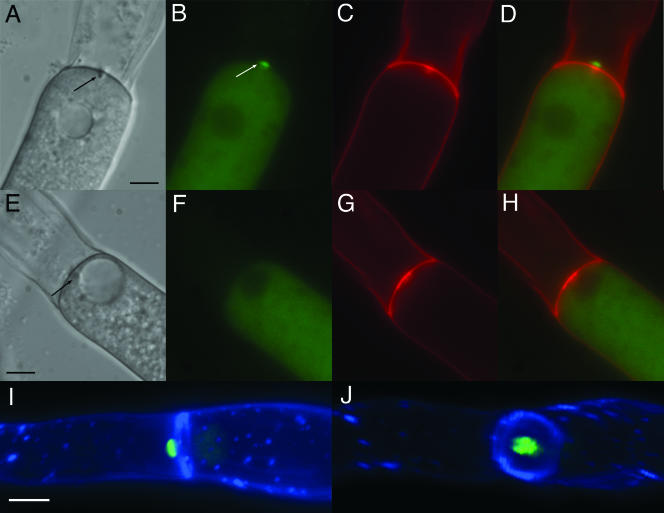
FIG. 2.
SO-GFP localizes to septal plugs in injured hyphae. Strains were grown for 18 h at 30°C on Vogel's MM agar plates. Hyphae were cut with a razor blade and immediately analyzed by light and fluorescence microscopy. (A to D) AF-T8 (his-3+::Pccg1-so+-gfp+). (E and F) Control strain AF-MF1 (his-3+::Pccg1-gfp+). The septal pores are plugged by the Woronin body (black arrows in panels A and E), SO-GFP localizes to the septal plug (white arrow in panel B). (A and E) DIC images. (B and F) Fluorescent image with GFP filter. (C and G) Calcofluor staining of the cell wall. (D and H) Merged images from panels B and C or F and G (GFP and Calcofluor). (I and J) Hyphae of AF-T8 were cut with a razor blade, stained with calcofluor, and immediately analyzed by deconvolution microscopy. (I) The left hyphal compartment was injured. SO-GFP forms a cap-like structure at the septal plug facing the side of the injury. (J) View of the septal plane. SO-GFP covers the septal pore in the center of the septum (see also movie S1 in the supplemental material). All size bars represent 5 μm.
The two-dimensional images showed that SO-GFP accumulated on the side of the septum facing the injury (Fig. 2D). To analyze the localization in more detail and to gain a better understanding of the three-dimensional structure of the SO-GFP aggregate, hyphae of AF-T8 were cut with a razor blade and imaged by deconvolution microscopy. The data obtained were used for three-dimensional reconstruction of the injured hyphae. These three-dimensional images showed that SO-GFP was localized to the center of the septa, where the septal pore is located (Fig. 2I and J). While Woronin bodies are known to plug the septal pore from the side of the intact compartment (30), SO-GFP accumulates on the side of the injury, forming a cap-like structure opposite from the position of the Woronin body (three-dimensional reconstruction; see also in movie S1 in the supplemental material).
SO-GFP accumulates at hyphal plugs in response to injury and remains there after consolidation and reinitiation of growth.
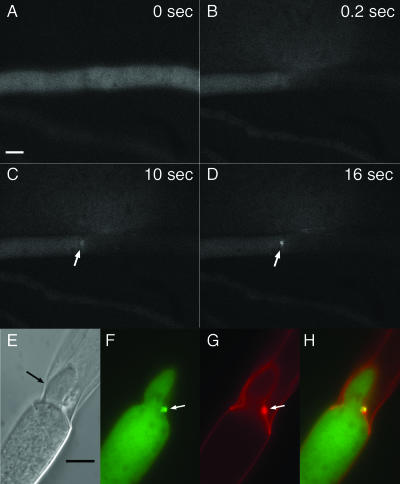
Since the SO-GFP signal was evenly distributed throughout the cytoplasm, we hypothesized that the fusion protein accumulated at the septal pore over time in response to injury. To test this hypothesis, we followed SO-GFP localization to the septal plug after hyphal injury by time-lapse microscopy. A laser microdissection microscope was used to cut through hyphal compartments of strain AF-T8. Subsequent plugging and SO-GFP localization events were followed by bright-field and fluorescence microscopy. Upon injury, cytoplasmic streaming through the septal pore toward the injury site was observed immediately after cutting with the laser (see movie S2 in the supplemental material). The injured hyphal compartments immediately lost their entire cytoplasmic contents, although cytoplasmic flow slowed quickly and eventually ceased because of septal plugging. Most of the time, neighboring uninjured hyphal compartments stayed intact. Fluorescence microscopy showed that SO-GFP accumulated at the septal plug (Fig. 3A to D; see movie S3 in the supplemental material); the time period between injury via the laser cut and the first visible accumulation of SO-GFP ranged from only 3 s to 30 s.
FIG. 3.
In AF-T8, SO-GFP accumulates sequentially at the septal plug and remains there after consolidation and reinitiation of tip growth. (A to D) Between 0 and 0.2 s, the hypha was cut with a UV pulse laser. After 10 s, SO-GFP accumulation becomes visible at the septal plug and intensifies during the next 6 s (white arrows) (see also movies S2 and S3 in the supplemental material). (E to H) Hyphae were cut with a razor blade and incubated for 30 min at room temperature prior to their analysis by light and fluorescence microscopy. A hypha from the adjoining compartment had formed a new tip, which grew into the dead compartment (black arrow). The new hypha is growing around the septal plug (white arrows). SO-GFP remains at the plug. (E) DIC image. (F) Fluorescent image with GFP filter. (G) Fluorescent image of calcofluor-stained hyphae. (H) Panels F and G merged. Bars represent 5 μm.
Septal plugging by the Woronin body following injury is followed by consolidation and reinitiation of growth (4, 13, 18). One or more hyphal tips are formed at the plugged septum, resulting in invasion of a growing hypha into the dead compartment. Our time-lapse fluorescence microscopy of the events following hyphal injury was only possible for up to around 1 min because of photobleaching of the GFP signal. Therefore, to evaluate SO-GFP localization during consolidation and regrowth, we assessed SO-GFP localization over a longer period of time. Hyphae of AF-T8 were cut with a razor blade, incubated for 30 min at room temperature, and subsequently evaluated. In many cases, one or two hyphal tips formed at the site of plugged septa, resulting in a new hypha invading the dead compartment (Fig. 3E). Often the septal plug was still visible but was moved to the side by the growing tips. Fluorescence microscopy showed that SO-GFP was still present, indicating that SO-GFP remains stable at the plug structure (Fig. 3F to H). Calcofluor staining revealed the presence of cell wall material at these plugs, consistent with earlier reports describing the deposition of cell wall material during consolidation of septal plugs (6).
To test if localization of SO to septal plugs is essential for subsequent reinitiation of growth at the plugged septum, recovery after injury was compared between the wild type and the so mutant. Wild-type strain FGSC 2489 and so mutant FGSC 508 (Table 1) were grown for 16 h on MM. Between 8 and 10 mm from the colony edge, hyphae were cut with a razor blade and incubated for 30 min at room temperature. One hundred injured and plugged hyphae were observed in three replicate experiments. In the wild-type strain (FGSC 2489), 65 ± 4 plugged septa showed newly formed growing tips at the site of injury. In the so mutant (FGSC 508), 64 ± 3 plugged septa showed reinitiation of growth. These data indicate that the SO protein is dispensable for growth recovery following injury via the formation of new hyphal tips at plugged septa.
SO contributes to sealing of the septal pore.
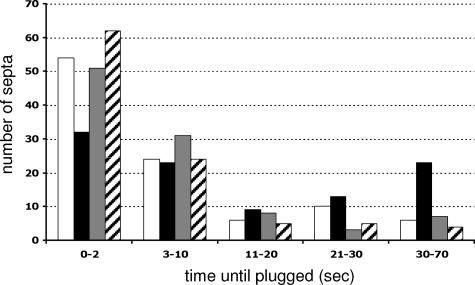
The accumulation of SO at the septal plug suggested that SO may contribute to the process of septal pore plugging in response to injury. To test this hypothesis, hyphae of the so deletion strain (FGSC 11293) were cut with a razor blade and analyzed by light microscopy. Similar to the situation in wild-type strains, the septal pores in the so mutant were plugged; extensive loss of cytoplasm was not observed (data not shown). Also, macroscopically, so mutants show no cytoplasmic bleeding (see Fig. 6A). These data indicated that septal plugging in response to injury occurs in the absence of so. To evaluate whether SO contributes to the efficiency of septal plugging, we compared the times between injury and complete sealing of the septal pore in a wild-type strain versus the so mutant. One hundred hyphae of the wild type (FGSC 2489) or the so mutant (FGSC 11293) were cut with the UV pulse laser, and the time between injury and complete sealing, which was defined as the point at which cytoplasmic flow through the septal pore ceased, was measured. These measurements were made independently of the presence or absence of Woronin bodies at the septal pore, because we frequently observed some cytoplasmic leakage through pores that had Woronin bodies associated with them, presumably because they were not positioned correctly in the septal pore or consolidation had not taken place. Only healthy hyphae approximately 5 to 12 μm in diameter within 1 cm of the growing margin of the developing colony were cut; hyphae showing high vacuolization or already plugged septa were omitted from this analysis. Both hyphal tip proximal and distal septa were observed. While more than 50% of the wild-type septal pores were immediately sealed, in the so mutant this value was reduced to 30% (Fig. 4). In addition, more than 20% of the so hyphae showed cytoplasmic leakage through the septal pore after more than 30 s, while only 6% of the wild-type septa were still bleeding at these later time points. These data suggest that SO is not essential for septal plugging but contributes to effective and fast sealing of septal pores in response to injury. We also tested if the overexpression of so-gfp in AF-T8 would lead to faster sealing of septal pores but found no significant difference compared to the wild-type strain (Fig. 4).
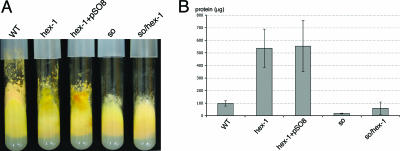
FIG. 6.
Overexpression of so does not complement the hex-1 phenotype. (A) Macroscopic phenotype of wild-type (WT) and mutant strains. Strains were grown in MM agar tubes for 5 days. The hex-1 mutant (R9-08) and the hex-1-overexpressing so mutant (HSG8.2.5) show extensive cytoplasmic bleeding. The so hex-1 double mutant (AF-SH1) looks similar to the so mutant and shows no macroscopic cytoplasmic bleeding. (B) Quantification of cytoplasmic loss in the wild-type (FGSC 2489) and mutant (R9-08, HSG8.2.5, FGSC 11293, and AF-SH1) strains shown in panel A. The experiment was conducted as described in Materials and Methods. The error bars represent the 95% confidence interval for the standard deviation.
FIG. 4.
The so mutant shows delayed septal pore plugging. Hyphae of the wild type (FGSC 2489), the so mutant (FGSC 11293), a so-gfp-overexpressing strain (AF-T8), and the fusion mutant mak-2 (R13-23) were cut with a UV pulse laser. The time between injury and sealing of the septal pore was determined in seconds. One hundred septa per strain were observed. White, wild type; black, so; gray, AF-T8 (his-3+::Pccg1-so+-gfp+); hatched, mak-2 mutant.
The so mutant is defective in hyphal fusion and is therefore unable to form an interconnected hyphal network. Thus, the delay of pore sealing observed in the so mutant could be a secondary effect of altered colony architecture. To test this possibility, we measured the time between injury and pore sealing in another fusion mutant, which contains a deletion of a predicted mitogen-activated protein kinase, mak-2 (R13-23). MAK-2 is also essential for germling-hyphal fusion (22) and, similar to so mutants, shows an altered mycelial organization and conidiation pattern. However, in the mak-2 mutant (R13-23), sealing of septal pores in response to hyphal injury was even slightly more efficient than in the wild-type strain, FGSC 2489 (Fig. 4). These data indicate that the plugging defect in the so mutant is not a secondary consequence of the hyphal fusion defect and altered colony architecture but that accumulation of SO at the septal pore in response to injury facilitates complete occlusion of the pore.
SO-GFP localization to septal plugs is Woronin body independent.
The Woronin body is the essential and major component of septal plugging in injured hyphae in N. crassa and other filamentous ascomycete species (5, 13, 19, 20, 28). The HEX-1 protein forms the core of Woronin bodies; mutants missing the hex-1 gene do not form these organelles. The septal pores of N. crassa hex-1 hyphae are not effectively sealed after injury and show extensive loss of cytoplasm (13, 28). To test if SO-GFP localization to plugged septal pores requires the presence of a functional Woronin body, we expressed so-gfp in the hex-1 mutant. Strain AF-T8 was crossed with hex-1 mutant strain R9-08 (Table 1). Progeny HSG8.2.5 (his-3+::Pccg1-so+ -gfp+ hex-1 A) showed the macroscopic bleeding phenotype of the hex-1 mutant (see Fig. 6A). To study SO-GFP localization in HSG8.2.5 during injury, hyphae were cut with a razor blade and analyzed by light and fluorescence microscopy. Injured hyphae of HSG8.3.5 showed extensive cytoplasmic bleeding (Fig. 5B), which eventually ceased when septal pores became occluded. Fluorescence microscopy showed that SO-GFP accumulated at these late-forming plugs (Fig. 5C to F). Note the accumulation of cell wall material in the septal plug area, a phenomenon previously reported for N. crassa (30). These data indicated that SO-GFP localization to septal plugs in response to injury is Woronin body independent.
FIG. 5.
SO-GFP localization at septal plugs is Woronin body independent. Strains were grown on Vogel's MM agar plates for 18 h at 30°C. Hyphae were cut with a razor blade and immediately analyzed by light and fluorescence microscopy. (A) Wild-type strain FGSC 2498. Septal pores are plugged in response to the injury (*). Hyphal compartments adjacent to the cut compartment remain intact. (B) HSG8.2.5 (his-3+::Pccg1-so+-gfp+ hex-1). Injured hyphae are not plugged by the Woronin body and lose extensive amounts of cytoplasm. (C to F) Septal pores of HSG8.2.5 hyphae are eventually plugged in a Woronin body-independent manner (black arrow in panel C). SO-GFP localizes to these late-forming plugs (white arrow in panel E). (C) DIC image. (D) Fluorescent image of calcofluor-stained hypha. (E) Fluorescent image with GFP filter. (F) Panels D and E merged. Bars in panels A and B represent 10 μm; the bar in panel C represents 5 μm.
To study the accumulation of SO-GFP at the septal pores in a hex-1 mutant, hyphae of HSG8.2.5 were cut with a laser and analyzed over time. In some HSG8.2.5 injured hyphae, the initial bleeding slowed down quickly and was restricted, as indicated by a stuttering cytoplasmic flow through the septal pore. In these cases, the loss of cytoplasm ceased completely within a few minutes and the septal pores appeared to be plugged. In other hyphae, the cytoplasm streamed through the septal pore with undiminished speed for several minutes; in some cases, cytoplasmic bleeding could be observed for up to 20 min postinjury. Fluorescence microscopy revealed a strong correlation between the time of the first visible accumulation of SO-GFP at the septal pore and the slowing down of the cytoplasmic bleeding. However, in almost every case, significant loss of cytoplasm was still observed, even though SO-GFP was present at the septal pore. Thus, the accumulation of SO-GFP at septal pores was correlated with restriction of cytoplasmic flow in hex-1 mutants in response to injury but was not sufficient for the efficient sealing of septal pores.
Overexpression of so-gfp does not suppress the cytoplasmic bleeding phenotype of hex-1, and a so hex-1 double mutant does not show more severe bleeding than a hex-1 mutant.
Strain HSG8.2.5 expresses so-gfp under the regulation of the ccg-1 promoter, resulting in higher (about fivefold) SO protein levels (Fig. 1B). We therefore tested if overexpression of so-gfp could decrease cytoplasmic loss after injury compared to the hex-1 mutant itself. Cytoplasmic leakage was measured and compared in the wild-type, hex-1, and HSG8.2.5 strains as described in reference 13. As shown in Fig. 6B, a significant difference in cytoplasmic leakage was not detected between the hex-1 strain (R9-08) and HSG8.2.5, indicating that the overexpression of so-gfp does not significantly complement the hex-1 bleeding phenotype.
Our results indicated that SO contributes to consolidation of the septal plug after hyphal injury. We therefore hypothesized that a hex-1 so double mutant might show a more severe bleeding phenotype than a hex-1 mutant. hex-1 mutant strain R9-08 was crossed with so mutant strain R8-02, and hex-1 so progeny were recovered. Interestingly, hex-1 so progeny (AF-SH1) did not show the macroscopic bleeding phenotype characteristic of hex-1 mutants (Fig. 6A). Measurement of leakage showed that the amount of cytoplasm lost in the hex-1 so double mutant (AF-SH1) was strongly reduced compared to that of the hex-1 mutant (Fig. 6B). These data suggested that mutations in so actually suppressed the hex-1 bleeding phenotype. However, analysis by light microscopy showed that cut hyphae in AF-SH1 (hex-1 so) were unable to plug septal pores (see below). These data suggest that the reduction in cytoplasmic bleeding in a fungal colony appears to be a secondary effect reflecting the altered colony architecture due to lack of hyphal fusion in the so mutant compared to a wild-type strain (for more details, see Discussion).
Since the role of SO in septal plugging in the hex-1 mutant could not be evaluated by studies on a colony-wide scale, we performed time-lapse studies on injured hyphae to determine if loss of so in a hex-1 background affects septal plugging. Hyphae of R9-08 (hex-1), HSG8.2.5 (hex-1 overexpressing so-gfp), and AF-SH1 (hex-1 so) were cut with the UV pulse laser and analyzed by light microscopy. In each experiment, 50 hyphae were observed over a 1-min time scale. In all cases, injured hyphae showed extensive cytoplasmic bleeding. Since the accumulation of SO-GFP was correlated with a visible decrease in the cytoplasmic flow rate, we determined the number of hyphae that were able to restrict cytoplasmic bleeding within 30 s. In hex-1 (R9-08), 25 ± 3 hyphae showed a quick restriction of bleeding. In the hex-1 so double mutant (AF-SH1), 26 ± 1 hyphae and in the hex-1 strain overexpressing so (HSG8.2.5), 25 ± 4 hyphae also showed quick restriction of cytoplasmic loss. These data indicated that the presence and the amount of SO protein do not significantly influence the restriction of hyphal bleeding in strains lacking functional Woronin bodies.
The WW domain is dispensable for SO-GFP localization to septal plugs.
The SO protein contains a WW domain, as well as a proline-rich domain. Since WW domains have been shown to mediate protein-protein interactions by binding proline-rich ligands, we hypothesized that SO proteins might interact with each other, leading to accumulation at the septal pore. To test this hypothesis, we individually mutated the two conserved tryptophan residues at positions 567 and 590 to glycine; these two tryptophan residues have been shown to be essential for the structure of the WW domain (16). The SO-GFP constructs pSO-GFPW567G and pSO-GFPW590G were introduced into FGSC 6103 (so+) (AF-S567 and AF-S590, respectively). Hyphae of AF-S567 and AF-S590 were cut with a razor blade, and localization of SO-GFP to septal plugs was quantified compared to that in AF-T8. In each experiment, 100 septal plugs formed in response to injury were observed. In AF-S567 and AF-S590, 93 ± 1 and 96 ± 2 of the septal plugs, respectively, showed the SO-GFP signal, which was not significantly different from that of control strain AF-T8 (95 ± 2).
Since AF-S567 and AF-S590 contain a functional wild-type copy of the so gene, we speculated that the SO mutated proteins might still interact with functional wild-type SO protein, which might lead to localization of SO-GFP at septal plugs. To test this hypothesis, AF-S567 and AF-S590 were crossed with FGSC 11292 (Δso) to obtain strains expressing the mutated so-gfp constructs in a Δso background. From the AF-S567 cross, we evaluated AF-SoS567, and from the AF-S590 cross, we evaluated AF-SoS590. Both strains showed only partial complementation of the so mutant phenotype. Quantification of SO-GFP localization in AF-SoS567 in response to injury showed that 95 ± 1 out of 100 plugs showed the GFP signal. In AF-SoS590, 95 ± 4 out of 100 injured septa showed the SO-GFP signal. These data indicate that the conserved tryptophan residues at positions 567 and 590 of the WW domain are dispensable for localization of SO to septal plugs.
SO-GFP localizes to septal plugs of aging and dying hyphae.
Septal pores in filamentous ascomycete fungi are plugged not only as a result of injury but also at different developmental stages such as aging (18, 29) or during heterokaryon incompatibility (9, 12). Previous studies have shown that these plugging events are Woronin body independent (29; N. L. Glass, unpublished data). Since SO-GFP does not depend on the presence of Woronin bodies, we determined if SO-GFP localizes to these different types of septal plugs.
To analyze SO-GFP localization, strain AF-T8 was inoculated onto 2% water agar. Under these growth conditions, only a sparse hyphal network with few aerial hyphae develops, allowing microscopy of the inner parts of the colony. In the interior of the colony, hyphae were highly vacuolized (Fig. 7A); no cytoplasmic flow was observed. While it was often difficult to clearly determine if septal pores were plugged, fluorescence microscopy revealed that SO-GFP frequently accumulated at these septal pores (Fig. 7A to D). The fact that in most cases the adjoining hyphal compartments were intact and contained cytoplasm and organelles indicated that the accumulation of SO-GFP was not triggered by hyphal injury. However, other plugged septal pores were detected that showed no SO-GFP signal. These findings suggest that SO localizes to septal pores during hyphal aging but is not essential for plugging.
FIG. 7.
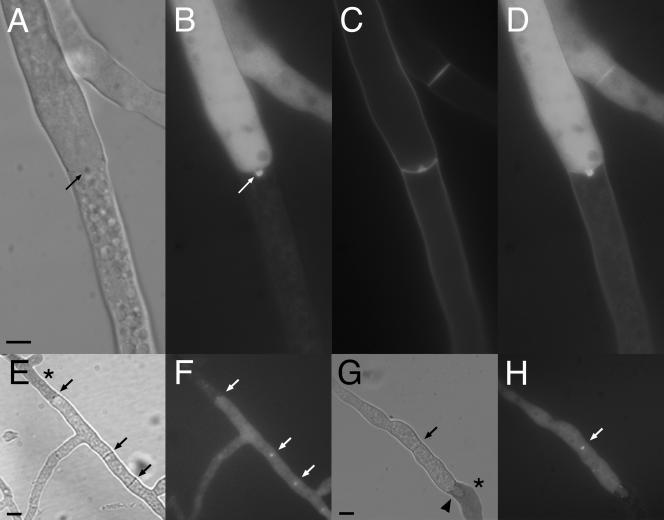
SO-GFP localizes to plugs in aging and incompatible hyphae. (A to D) Strain AF-T8 was grown on 2% water agar for 24 h. Hyphae in the inner part of the colony were analyzed by light and fluorescence microscopy. Septa of aging hyphal compartments were plugged (A, arrow). SO-GFP localizes to these plugs (B to D). (A, DIC image; B, GFP; C, calcofluor; D, panels B and C merged). (E to H) Incompatible heterokaryon (strains AF-SG1 and C9-15; Table 1). These two strains contain different het-c haplotypes, resulting in heterokaryon incompatibility. Hyphae show compartmentalization and cell death (dark compartments [*]). Septa are plugged (black arrows), and SO-GFP localizes to these plugs (white arrows). A newly formed hyphal tip has grown into a dead compartment (black triangle in panel G). (E and G) DIC images; hyphae stained with methylene blue. (F and H) GFP filter. All size bars represent 5 μm.
In addition to aging hyphae, septal plugs are associated with dead hyphal compartments that occur during heterokaryon incompatibility (9, 12). In N. crassa, hyphal fusion between individuals that differ in allelic specificity of at least 1 of 11 heterokaryon incompatibility (het) loci results in plugging of the septal pore and programmed cell death. To test if SO-GFP localizes to septal plugs formed during programmed cell death, a heterokaryon between AF-SG1 (his-3+::Pccg1 so+-gfp+ so; pyr-4 het-c1 pin-c1) and C9-15 (thr-2 het-c2 pin-c2) was forced with auxotrophic markers. The resulting colony showed typical signs of heterokaryon incompatibility, such as slow and sparse growth, no conidiation, and frequent cell death; septal pores of dying compartments were plugged (Fig. 7E). In addition, pores of septa in the proximity of dying compartments were also occluded. By fluorescence microscopy, SO-GFP accumulation at septal plugs at and around dying hyphal compartments was observed (Fig. 7E to H); a mean of 37 ± 3/50 plugged septa showed SO-GFP accumulation. These data indicate that SO-GFP localizes not only to septal plugs after injury but also to occlusions in septal pores that are formed during colony development and are not dependent upon the Woronin body.
DISCUSSION
The results of this study demonstrate that the SO protein accumulates at septal plugs in N. crassa. To our knowledge, this is the first time that a protein showing this type of localization has been identified in a filamentous fungus. SO-GFP localizes to septal pores plugged by Woronin bodies and was also found at plugs of aging and incompatible hyphae. In young, uninjured hyphae, SO-GFP was cytoplasmically localized. However, it is possible that an undetectable portion of SO-GFP is associated with cellular organelles in uninjured hyphae and with the preformed Woronin body in particular. While the phenotype of the hex-1 mutant clearly demonstrates that the Woronin body is the key component in septal plugging in response to injury, our data indicate that the damage response involves more than just the positioning of the Woronin body in the septal pore. The fact that the so mutant showed a slight delay in cessation of cytoplasmic flow as a result of injury suggests that SO might, in fact, contribute to sealing of the septal pore. This aspect of septal plugging has been described previously as “consolidation”.
Consolidation of septal occlusions formed via the Woronin body has been described in both N. crassa and Penicillium chrysogenum (6, 30); deposition of material over the plugged pores occurred over a 3-h time period, resulting in consolidation of the septal seal (6). Septal plugs remain stable after the reinitiation of growth at plugged septa and are pushed to the side by the new forming tips (4, 18). Our finding that SO-GFP is still detected at septal plugs after the reinitiation of growth indicates that the SO protein is a structural part of these plugs.
In healthy hyphae, Woronin bodies are often positioned close to the septa but do not obstruct cytoplasmic flow through the pore (18, 19, 30). When catastrophic injury occurs, the Woronin body moves from its normal position to that of the septal pore. Most plugged septal pores only contain a single Woronin body; other Woronin bodies in both the injured hypha and adjoining hyphae maintain their position (19). These observations suggest an active mechanism for transport of the Woronin body to the septal plug upon hyphal injury; the nature of this mechanism is unknown.
Our observation that SO-GFP accumulates at the side of the septum facing the injury raises the question of the mechanism of SO-GFP localization and accumulation at the septal pore. Since the UV laser immediately destroyed the injured compartment, which resulted in the complete loss of cytoplasm, it is likely that SO is derived from the hyphal compartment adjoining the injured one. SO-GFP transported by cytoplasmic streaming could accumulate at the septal pore. However, the polarized nature of the SO localization suggests a mechanism for anchoring SO to the outer section of the septal plug. We hypothesize that the presence of some signal or landmark at septal pores after injury triggers SO accumulation and results in consolidation of the septal pore.
Our data showing that SO-GFP accumulates at septal plugs in the hex-1 mutant indicate that this localization does not require physical interaction with the Woronin body. However, in a wild-type strain, localization of SO-GFP to septa plugged by the Woronin body is very rapid. The SO-GFP aggregate and the Woronin body are part of the stable plug structure and therefore must have either direct or indirect physical interaction with each other. Cell fractions of purified Woronin bodies (presumably septal plugs were included in these fractions) contained five abundant polypeptides (13). It would be of interest to determine if SO is one of these polypeptides that copurified with Woronin bodies.
Although the so mutant showed a delay in complete pore sealing in response to injury, overexpression or inactivation of so in the hex-1 mutant did not result in detectable differences in the plugging capacity of injured hyphae. However, the hex-1 so double mutant showed significantly less cytoplasmic bleeding than a hex-1 mutant. This surprising result might be caused by a combination of factors. so mutants are hyphal fusion mutants (7). Compared to those of wild-type strains, hyphae within a so colony are significantly less interconnected. Injury of a hypha in the hex-1 so mutant results in cytoplasmic loss from only a single filament and its branches. In the hex-1 mutant, which still undergoes hyphal fusion and therefore forms an interconnected, syncytial mycelium, damage of a single hypha leads to cytoplasmic draining from all of the hyphae connected to it via hyphal fusion bridges. In addition, the macroscopic cytoplasmic bleeding phenotype in the hex-1 mutant occurs mostly at aerial hyphae, indicating that these hyphae might be prone to injury. Since the so mutant has reduced production of aerial hyphae, injury, and thus cytoplasmic bleeding, might be less frequent in the hex-1 so mutant.
In filamentous ascomycete species, preformed Woronin bodies are the main plugging structures found in injured hyphae. However, plug formation by de novo deposition of material is a much more common theme in syncytial species. These types of septal plugs are found in asco-, basidio-, and zygomycete fungal species (1, 18), as well as in syncytial red and green algae (3, 21, 24). It is clear from SO-GFP localization studies that SO is often associated with plugged septa in aging hyphae and in hyphae compartmentalized during heterokaryon incompatibility. While the function of deposition plugs in different species has been postulated to be involved in similar processes, they almost certainly differ in origin and composition. However, the presence of proteins in these structures is common (3, 18, 21). The SO protein, whose gene is only present in the genomes of filamentous ascomycete species, is associated with de novo plug formation in this group of fungi. Deposition plugs have been observed in occluded septa that do not contain Woronin bodies, although normal-looking Woronin bodies were present in these hyphae (18, 19). In fact, in a study of N. crassa, Woronin bodies were never found to be associated with these deposition plugs (29). Until our studies, no component of these deposition plugs had been identified.
Mutants lacking functional SO show reduced aerial hyphal length, female sterility, and a slower growth rate and lack hyphal anastomoses (7). An obvious question is how these phenotypes relate to SO localization to septa. Preliminary data indicate that SO-GFP fusions with mutations in the WW domain do not fully complement the phenotypic defects of the so mutant, including aerial hypha formation and hyphal fusion. However, these mutated SO proteins still localize to septal plugs with an efficiency similar to that of wild-type SO protein. Together, these data suggest multiple independent functions of SO. While protein-protein interactions mediated through the WW domain might be essential for the proper regulation of colony development, accumulation of SO at septal pores could be a separate function that leads to stabilization and consolidation of the septal plug. Future experiments will illuminate the relationship of the different so mutant phenotypes and whether they are caused by an underlying common or different mechanism of SO function.
Supplementary Material
Acknowledgments
We thank Steven Ruzin and Denise Schichnes (CNR Biological Imaging Facility, University of California, Berkeley) for help and support with the deconvolution microscopy, three-dimensional reconstruction, and laser microdissection and Robert Moody (JH Technologies, Inc.) for help with imaging during the microdissection experiments. We thank Greg Jedd for providing the hex-1 strain.
This study was funded by a grant from the National Science Foundation (MCB-0517660) to N.L.G.
Footnotes
Published ahead of print on 10 November 2006.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Aylmore, R. C., G. E. Wakley, and N. K. Todd. 1984. Septal sealing in the basidiomycete Coriolus versicolor. J. Gen. Microbiol. 130:2975-2982. [Google Scholar]
- 2.Biella, S., M. L. Smith, J. R. Aist, P. Cortesi, and M. G. Milgroom. 2002. Programmed cell death correlates with virus transmission in a filamentous fungus. Proc. R. Soc. Lond. Ser. B Biol. Sci. 269:2269-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brawley, S. H., and J. R. Sears. 1982. Septal plugs in a green alga. Am. J. Bot. 69:455-463. [Google Scholar]
- 4.Buller, A. H. R. 1933. Researches on fungi, vol V. Longmans, Green & Co. London, United Kingdom.
- 5.Collinge, A. J., and P. Markham. 1985. Woronin bodies rapidly plug septal pores of severed Penicillium chrysogenum hyphae. Exp. Mycol. 9:80-85. [Google Scholar]
- 6.Collinge, A. J., and P. Markham. 1987. Response of severed Penicillium chrysogenum hyphae following rapid Woronin body plugging of septal pores. FEMS Microbiol. Lett. 40:165-168. [Google Scholar]
- 7.Fleissner, A., S. Sarkar, D. J. Jacobson, M. G. Roca, N. D. Read, and N. L. Glass. 2005. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot. Cell 4:920-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freitag, M., P. C. Hickey, N. B. Raju, E. U. Selker, and N. D. Read. 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41:897-910. [DOI] [PubMed] [Google Scholar]
- 9.Garnjobst, L., and J. F. Wilson. 1956. Heterokaryosis and protoplasmic incompatibility in Neurospora crassa. Proc. Natl. Acad. Sci. USA 42:613-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass, N. L., and A. Fleissner. 2006. Re-wiring the network: understanding the mechanism and function of anastomosis in filamentous ascomycete fungi, p. 123-139. In U. Kües and R. Fischer (ed.), The Mycota. I. Growth, differentiation and sexuality. Springer-Verlag, Berlin, Germany.
- 11.Glass, N. L., and I. Kaneko. 2003. Fatal attraction: non-self recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot. Cell 2:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson, D. J., K. Beurkens, and K. L. Klomparens. 1998. Microscopic and ultrastructural examination of vegetative incompatibility in partial diploids heterozygous at het loci in Neurospora crassa. Fungal Genet. Biol. 23:45-56. [DOI] [PubMed] [Google Scholar]
- 13.Jedd, G., and N. H. Chua. 2000. A new self-assembled peroxisomal vesicle required for efficient resealing of the plasma membrane. Nat. Cell Biol. 4:226-231. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko, I., K. Dementhon, Q. Xiang, and N. L. Glass. 2006. Nonallelic interactions between het-c and a polymorphic locus, pin-c, are essential for nonself recognition and programmed cell death in Neurospora crassa. Genetics 172:1545-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, S. B., and J. T. Taylor. 1990. Isolation of DNA from fungal mycelia and single spores, p. 282-287. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols, a guide to methods and applications. Academic Press, Inc., New York, N.Y.
- 16.Macias, M. J., V. Gervais, C. Civera, and H. Oschkinat. 2000. Structural analysis of WW domains and design of a WW prototype. Nat. Struct. Biol. 7:375-379. [DOI] [PubMed] [Google Scholar]
- 17.Margolin, B. S., M. Freitag, and E. U. Selker. 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Newsl. 44:34-36. [Google Scholar]
- 18.Markham, P. 1994. Occlusion of septal pores in filamentous fungi. Mycol. Res. 98:1089-1106. [Google Scholar]
- 19.Markham, P., and A. J. Collinge. 1987. Woronin bodies of filamentous fungi. FEMS Microbiol. Rev. 46:1-11. [Google Scholar]
- 20.Maruyama J. I., R. R. Juvvadi, K. Ishi, and K. Kitamoto. 2005. Three-dimensional image analysis of plugging at the septal pore by Woronin body during hypotonic shock inducing hyphal tip bursting in the filamentous fungus Aspergillus oryzae. Biochem. Biophys. Res. Commun. 331:1081-1088. [DOI] [PubMed] [Google Scholar]
- 21.Menzel, D. 1988. How do giant plant cells cope with injury? The wound response in siphonous green algae. Protoplasma 144:73-91. [Google Scholar]
- 22.Pandey, A., M. G. Roca, N. D. Read, and N. L. Glass. 2004. Role of mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot. Cell 3:348-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkins, D. D. 1984. Advantages of using the inactive-mating-type am1 strain as a helper component in heterokaryons. Fungal Genet. Newsl. 31:41-42. [Google Scholar]
- 24.Pueschel, C. M., and K. M. Cole. 1982. Rhodophycean pit plugs: an ultrastructural survey with taxonomic implications. Am. J. Bot. 69:703-720. [Google Scholar]
- 25.Smith, M. L., O. C. Micali, S. P. Hubbard, N. Mir-Rashed, D. J. Jacobson, and N. L. Glass. 2000. Vegetative incompatibility in the het-6 region of Neurospora crassa is mediated by two linked genes. Genetics 155:1095-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, M. L., C. J. Yang, R. L. Metzenberg, and N. L. Glass. 1996. Escape from het-6 incompatibility in Neurospora crassa partial diploids involves preferential deletion within the ectopic segment. Genetics 144:523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki, C., M. Kawano, T. Kashiwagi, Y. Arata, T. Kawasumi, and Y. Kashiwagi. 2000. Lethal effect of the expression of a killer gene SMK1 in Saccharomyces cerevisiae. Protein Eng. 13:73-76. [DOI] [PubMed] [Google Scholar]
- 28.Tenney, K., I. Hunt, J. Sweigard, J. I. Pounder, C. McClain, E. J. Bowman, and B. J. Bowman. 2000. Hex-1, a gene unique to filamentous fungi, encodes the major protein of the Woronin body and functions as a plug for septal pores. Fungal Genet. Biol. 3:205-217. [DOI] [PubMed] [Google Scholar]
- 29.Trinci, A. P., and A. J. Collinge. 1973. Structure and plugging of septa of wild type and spreading colonial mutants of Neurospora crassa. Arch. Biol. 91:355-364. [DOI] [PubMed] [Google Scholar]
- 30.Trinci, A. P., and A. J. Collinge. 1974. Occlusion of the septal pores of damaged hyphae of Neurospora crassa by hexagonal crystals. Protoplasma 80:57-67. [DOI] [PubMed] [Google Scholar]
- 31.Vogel, H. J. 1956. A convenient growth medium. Microb. Genet. Bull. 13:42-46. [Google Scholar]
- 32.Westergaard, M., and H. K. Mitchell. 1947. Neurospora. V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34:573-577. [Google Scholar]
- 33.Woronin, M. 1864. Zur Entwicklungsgeschichte der Ascobolus pulcherrimus Cr. und einiger Pezizen. Abh. Senkenb. Naturforsch. 5:333-344. [Google Scholar]
- 34.Yuan, P., G. Jedd, D. Kumaran, S. Swaminathan, H. Shio, D. Hewitt, N. H. Chua, and K. Swaminathan. 2003. A HEX-1 crystal lattice required for Woronin body function in Neurospora crassa. Nat. Struct. Biol. 10:264-270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.