Abstract
Cryptococci survive and replicate within macrophages and can use exogenous arachidonic acid for the production of eicosanoids. Phospholipase B1 (PLB1) has a putative, but uninvestigated, role in these processes. We have shown that uptake and esterification of radiolabeled arachidonic, palmitic, and oleic acids by the Cryptococcus neoformans var. grubii H99 wild-type strain and its PLB1 deletion mutant strain (the Δplb1 strain) are independent of PLB1, except under hyperosmolar stress. Similarly, PLB1 was required for metabolism of 1-palmitoyl lysophosphatidylcholine (LysoPC), which is toxic to eukaryotic cell membranes, under hyperosmolar conditions. During both logarithmic and stationary phases of growth, the physiologically relevant phospholipids, dipalmitoyl phosphatidylcholine (DPPC) and dioleoyl phosphatidylcholine, were taken up and metabolized via PLB1. Exogenous DPPC did not enhance growth in the presence of glucose as a carbon source but could support it for at least 24 h in glucose-free medium. Detoxification of LysoPC by reacylation occurred in both the H99 wild-type and the Δplb1 strains in the presence of glucose, but PLB1 was required when LysoPC was the sole carbon source. This indicates that both energy-independent (via PLB1) and energy-dependent transacylation pathways are active in cryptococci. Phospholipase A1 activity was identified by PLB1-independent degradation of 1-palmitoyl-2-arachidonoyl phosphatidylcholine, but the arachidonoyl LysoPC formed was not detoxified by reacylation. Using the human macrophage-like cell line THP-1, we demonstrated the PLB1-dependent incorporation of macrophage-derived arachidonic acid into cryptococcal lipids during cryptococcus-phagocyte interaction. This pool of arachidonate can be sequestered for eicosanoid production by the fungus and/or suppression of host phagocytic activity, thus diminishing the immune response.
Secreted phospholipase B1 (PLB1), a multifunctional enzyme exhibiting three activities (PLB, lysophospholipase [LPL], and lysophospholipase transacylase [LPTA]), is a proven virulence determinant for the pathogenic fungus Cryptococcus neoformans. The functions of this enzyme include facilitation of adhesion of cryptococci to a human lung epithelial cell line (14), initiation of interstitial pulmonary infection, and dissemination from the lung via the lymphatics and blood (24). PLB1 is also involved in the survival and replication of cryptococci in macrophages (22). It has been postulated to enhance intracellular survival by down-regulating macrophage fungistatic activity through production of eicosanoids, such as prostaglandins and leukotrienes (22, 23). PLB1 may promote escape from the phagolysosome by breaking down the surrounding membrane bilayer, since partial dissolution of this membrane has been observed in cryptococcus-laden macrophages (12, 28).
The biochemical basis of PLB1-dependent virulence is poorly understood. It has been shown previously that an energy source is not required for PLB1 activity and that preferred substrates of cryptococcal PLB1 include dipalmitoyl phosphatidylcholine (DPPC) and phosphatidylglycerol, which are abundant in lung surfactant (the fluid lining pulmonary alveoli) and cell membrane phospholipids (PL), such as dioleoyl phosphatidylcholine (DOPC) (5, 25). 1-Oleoyl and 1-palmitoyl lysophosphatidylcholine (LysoPC) can be generated from mammalian membrane lipids by other phospholipases and are the best substrates for the LPL and LPTA activities of PLB1 (5). There is indirect evidence from rat models of pulmonary and cerebral cryptococcosis that DPPC is a substrate for cryptococcal PLB in vivo since much larger amounts of glycerophosphorylcholine, the product of PLB hydrolysis, are found in lung, which is rich in DPPC, than in brain, which is not (16, 17). Hence, PLB1 activity may promote lung penetration by cryptococci.
The function of cryptococcal PLB1 in degradation and metabolism of lipids in macrophages has not been investigated. The fatty acid arachidonic acid (AA) is an important precursor for eicosanoid production, but the origin of the arachidonic acid incorporated into cryptococcal eicosanoids during infection is unknown. Possibilities include the degradation of phagocyte membrane phospholipids by host phospholipase A2 (PLA2) activity, hydrolysis of host lipids by secreted fungal phospholipase activity, and the degradation of fungal lipids by fungal or host enzymes. Arachidonic acid is not known to be a component of cryptococcal cell membranes (18) but is taken up by cryptococci when supplied exogenously, suggesting that it could be derived from mammalian cells (23). PLB1 could also liberate host fatty acids other than arachidonic acid, providing an energy source for optimal intracellular growth, since glucose is believed to be deficient in the phagosome. In support of this hypothesis, the H99 wild-type strain and the PLB1 deletion mutant (Δplb1) and PLB1 reconstituted Δplb1rec strains can all grow intracellularly, but the growth of the Δplb1 strain is deficient (9, 22, 24).
To determine the role of cryptococcal PLB1 in the hydrolysis and metabolism of lipids, we investigated the uptake and metabolism of exogenous palmitic acid (PA), oleic acid (OA), and arachidonic acid and phospholipids containing these fatty acid species into lipids from the H99 and Δplb1 cryptococcal strains under conditions of minimal nutrition, such as might be present within host phagocytes, and during hyperosmotic stress, conditions for which we have previously shown increases in both PLB secretion and adhesion to a lung epithelial cell line (14). We then sought and found evidence of PLB1-dependent transcellular metabolism of host cell-derived arachidonic acid during cryptococcal infection of a macrophage-like cell line, THP-1.
MATERIALS AND METHODS
Reagents.
Isotopically labeled lipids were supplied by Amersham Pharmacia and included [9,10(n)-3H]palmitic acid, [9,10(n)-3H]oleic acid, [5,6,8,9,11,12,14,15-3H]arachidonic acid, l-α-1-palmitoyl-2-arachidonoyl [arachidonoyl-1-14C]phosphatidylcholine, 1,2-di[1-14C]palmitoyl-phosphatidylcholine, 1,2-di[1-14C]oleoyl-phosphatidylcholine, and 1-[1-14C]palmitoyl-2-lysophosphatidylcholine. Carrier lipids were from Sigma Chemical Co. (St. Louis, MO) and included 1-palmitoyl-sn-glycero-3-phosphocholine, DPPC, DOPC, 1-palmitoyl-2-arachidonoyl phosphatidylcholine (PAPC), AA, PA, and OA. Solvents used were of analytical or nanograde quality (Crown Scientific or Mallinckrodt Chemicals USA). High-performance thin-layer chromatography (TLC) plates were from Merck (Germany).
Cryptococcal strains.
Cryptococcus grubii serotype A strain H99 (the wild type) and the Δplb1 (PLB1 deletion mutant strain HCM5) and Δplb1rec (reconstituted strain HCM15) isogenic strains were generated and kindly supplied by Gary Cox (9). Working cultures of these strains were prepared from the lyophilized cells in the culture collection and maintained by growth on Sabouraud's dextrose agar plates from Difco Laboratories (Detroit, MI) for 48 h at 30°C. These were stored at 4°C and subcultured every 2 to 3 weeks. New working cultures were prepared every 3 months. The presence of PLB1 activity in the H99 and Δplb1rec strains and the absence of PLB1 activity in the Δplb1 strain were confirmed by radiometric assays at regular intervals (5).
Culture and activation of THP-1 cells.
The human macrophage-like cell line THP-1 was maintained in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum at 37°C and 5% CO2. THP-1 cells were grown to confluence in 75-cm2 tissue culture flasks and then treated with 0.32 μM phorbol-12-myristate 13-acetate and incubated for a further 48 to 72 h to allow cell differentiation (determined microscopically, by formation of an adherent monolayer).
Radiolabeling, opsonization, and phagocytosis of cryptococci.
Radiolabeled [9,10(n)-3H]oleic acid was added to cryptococci in phosphate-buffered saline without calcium and magnesium [PBS(−)], and the cells were incubated at 35°C in a rotary shaker at 150 rpm for 24 to 36 h. The cells were washed to remove unincorporated radioactivity, and a sample was counted with a scintillation counter using NCS tissue solubilizer (Amersham Pharmacia) and Ultima Gold scintillant (Canberra-Packard, Groningen, The Netherlands). To prepare for phagocytosis, the oleic acid-labeled cryptococci were then opsonized in PBS(−) containing 10% human AB serum for 30 min, washed in PBS(−), and added to differentiated THP-1 monolayers in 24-well plates at a 10:1 ratio of cryptococci to THP-1 cells. In control experiments, radiolabeled cryptococci were autoclaved for 15 min at 121°C (killed cells) before opsonization. The nonadherent cryptococci were gently aspirated, and the monolayers containing ingested/strongly adherent cryptococci were washed twice with PBS(−), dislodged from the wells by scraping, and counted in a scintillation counter with the addition of NCS tissue solubilizer and Ultima Gold. Percent phagocytosis/tight adhesion was calculated and found to be maximal after 4 to 5 h of incubation at 37°C/5% CO2.
Preparation of cryptococci for fatty acid analysis.
THP-1 cells in 75-cm2 tissue culture flasks were infected with Cryptococcus neoformans (live or autoclaved) at a ratio of 10 cryptococci to 1 phagocyte and incubated for 5 h at 37°C and 5% CO2. Control THP-1 cells were incubated with a corresponding amount (1 ml) of PBS(−). Nonadherent cryptococci were gently washed off the THP-1 monolayers with prewarmed PBS(−). The THP-1 cells containing strongly adherent/phagocytosed cryptococci were scraped from the flasks with a rubber policeman (both cryptococcal and THP-1 viabilities were unaffected), layered on top of Ficoll-Paque, and centrifuged at 500 × g for 30 min. The interface layer was substantially enriched in THP-1 cells containing ingested cryptococci, as reported previously by Chretien et al. (7). This layer was removed and washed twice with PBS(−). The cryptococci were released from the THP-1 cells by freeze-thawing and exposure to ice-cold water for 10 to 15 min. Contaminating THP-1 debris was then removed by incubation with a combination of trypsin (50 μl of 10× trypsin), DNase (2.8 μg; Sigma), and Zymolyase 20T (50 μg; Seikagaku America, MA) for 1 h at 37°C. The cryptococci were then washed three times with PBS(−) and stored frozen for analysis by gas chromatography.
Fatty acid analysis.
Opsonized cryptococci, obtained before or after phagocytosis, or differentiated THP-1 cells incubated for 4 to 5 h in PBS(−) without cryptococci were saponified, methylated, and extracted as in the MIDI Sherlock version 4.0 microbial identification system (Newark, DE). Briefly, each sample was suspended in 1 ml methanolic base (15g NaOH-50 ml water plus 50 ml methanol) and boiled at 100°C for 30 min to achieve saponification. Released fatty acids were then methylated with 2 ml methylation reagent (230 ml methanol in 270 ml 6 N HCl) at 80°C for 10 min. After being cooled, the samples were extracted with isohexane-methyl tetra-butyl ether (1:1, vol/vol) and the lipids extracted in the organic phase were base washed with 3 ml of 2% (wt/vol) NaOH. The organic phase was removed and stored at −20°C for gas chromatographic analysis. For this, fatty acid methyl esters were separated on an HP Series II 5890 gas chromatograph with an Agilent Ultra-2 capillary column (19091B-102). Peak identification was achieved using either the CLIN 50 or the Yeast 28 library. Results are expressed as percentages of the sum of the areas of all peaks identified.
Uptake and metabolism of exogenous lipids by cryptococci.
Cryptococci were cultured for 2 to 3 days on Sabouraud's dextrose agar at 30°C to form a lawn, harvested by scraping, and then washed twice with saline and once with one of the following media: (i) buffer at pH 5.5 (close to the pH optimum for cryptococcal PLB1) containing 10 mM imidazole, 2 mM CaCl2, 2 mM MgCl2, and 56 mM d-glucose in 0.9% NaCl; (ii) yeast nitrogen broth (YNB) at pH 7.3 (close to the physiological pH) containing 0.5% glucose; and (iii) hypertonic medium (providing an osmotic stress) consisting of 10× YNB (pH 7.3) and 5% glucose. The pelleted cells (0.5 ml) were resuspended in 0.5 ml of medium in an Eppendorf tube and incubated with 50,000 to 100,000 disintegrations per minute (dpm) of radiolabeled lipid and 1 μM carrier lipid for 24 h at 37°C. The cryptococci were then centrifuged, and the pH of the supernatant was determined. The pellets were washed twice with PBS(−) containing 0.1% fatty acid-free bovine serum albumin and then once with PBS(−) only. Lipids were extracted with 4 volumes of chloroform-methanol (2:1) and then partitioned against water as in the Bligh-Dyer method (2), and an aliquot of the organic phase was counted for radioactivity to obtain the total lipid uptake level before separation of the component lipids by TLC. The radioactivity in the component lipids was expressed as a percentage of the total lipids recovered from the plate. The solvent systems used were petroleum ether (bp, 60 to 80°C)-diethyl ether-acetic acid (90:15:1, vol/vol/vol) for neutral lipid and chloroform-methanol-water (65:25:4, vol/vol/vol) for polar lipid separation. Spots were revealed with iodine vapor and identified by comparison of their retardation factor values with those of authentic standards. Spots were scraped from the plates and their radioactivity levels determined by scintillation counting (Instagel; Canberra-Packard).
Growth and metabolism of cryptococci in the presence of lipids.
The H99, Δplb1, and Δplb1rec cryptococcal strains were each inoculated into 50 ml of YNB containing 0.5% or 1% (wt/vol) glucose and cultured for 24 h at 35°C with gentle agitation. Aliquots of 1 ml (containing about 106 cells) were transferred subsequently to 50 ml YNB containing radiolabeled lipids and their corresponding carrier lipids or YNB alone at pH 5.5. Lipids included 100,000 dpm 1,2-di[1-14C]palmitoyl-phosphatidylcholine or 1-[1-14C]palmitoyl-2-lysophosphatidylcholine with 1 μM DPPC or LysoPC carrier lipid, respectively. Cultures were then incubated at 35°C with gentle agitation for up to 48 h. Plate counts were performed at zero time and at various time points during growth and the CFU/ml determined.
When lipids were substituted for glucose as the source of carbon, the procedure was similar except that the YNB was prepared without glucose, carrier DPPC was used at 2 mM, and LysoPC was used at 1 mM and 2.5 mM. Bovine lung surfactant (25 mg/ml phospholipid content; Survanta; Abbott Laboratories), containing the equivalent of 2 mM DPPC, was also tested. l-α-1-Palmitoyl-2-arachidonoyl [arachidonoyl-1-14C]phosphatidylcholine was added at 100,000 dpm, together with carrier PAPC, at 869 μM. In all growth experiments, YNB containing 0.5% glucose (PAPC) or 1% glucose (DPPC and LysoPC) was used as the control, and the concentrations of the exogenous lipids were chosen to give similar numbers of carbon atoms to these controls.
To determine lipid uptake and metabolism during growth, aliquots of the cryptococcal suspensions were pelleted by centrifugation and washed with PBS(−) containing 0.1% fatty acid-free bovine serum albumin and then PBS(−) alone. Lipids were extracted and separated by TLC as described above, using chloroform-methanol-water (65:25:4, vol/vol/vol) as a solvent system. The radioactivity in the separate lipid classes was estimated. Using this solvent system, triacylglycerol (TAG) and diacylglycerol (DAG) could not be adequately separated and are reported as TAG/DAG.
RESULTS
Effect of PLB1 on cryptococcal uptake and metabolism of exogenous lipids. (i) Fatty acid uptake.
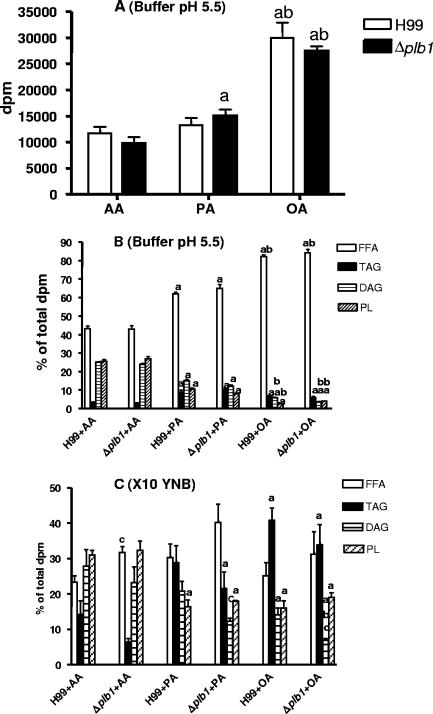
The uptake and incorporation of exogenous radiolabeled AA, PA, and OA into cryptococcal lipids from packed cell suspensions (stationary phase) were compared in three different media (see Materials and Methods). These were (i) imidazole buffer (pH 5.5) containing 1% glucose, close to the optimal pH for PLB1 activity and also close to pH values found in cryptococcomas and inside macrophages (21, 29); (ii) YNB containing 0.5% glucose at pH 7.3, close to the physiological pH; and (iii) 10× YNB at pH 7.3, providing an osmotic stress analogous to that provided by cryptococcal polyols at sites of infection (16, 17), predominantly by the addition of 5% glucose. Previously, we showed that this medium markedly increased the secretion of active PLB from cryptococci (H99) and their adhesion to lung epithelial cells (14). Radiolabeled AA, PA, and OA were each taken up into lipids extracted from packed cell suspensions of the H99 and Δplb1 strains to the same extent in the three different media after a 24-h incubation period. Oleic acid was taken up most avidly, followed by palmitic acid and arachidonic acid (shown for incubation in buffer-glucose at pH 5.5 in Fig. 1A).
FIG. 1.
Uptake and metabolism of exogenous [9,10(n)-3H]PA, [9,10(n)-3H]OA, and [5,6,8,9,11,12,14,15-3H]AA by cryptococci. Uptake was performed in the presence of 1 μM carrier fatty acid. Total incorporation of radioactivity into lipids of 0.5 ml of cryptococci (H99 and Δplb1 strains) at stationary phase after 24 h of incubation in imidazole buffer containing glucose (pH 5.5) is shown in panel A, with the distributions of radioactivity in the individual lipid classes (calculated as percentages of the total lipids recovered from the TLC plate; see Materials and Methods) shown in panel B. In panel C, the distributions of radioactivity into these lipid classes after uptake under hypertonic conditions (10× YNB) are shown. Values presented are the means and standard errors of the means for four experiments, and statistical significance was determined by the unpaired two-tailed t test and analysis of variance. a, different from AA values; b, different from PA values; c, different from H99 values (P < 0.05).
(ii) Fatty acid metabolism.
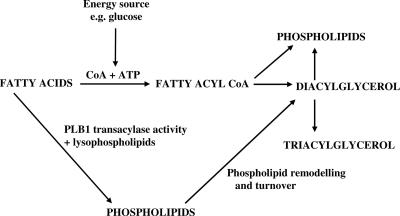
The distribution of the total radioactivity incorporated into the cryptococcal lipids was assessed by separating the individual lipid species in the total lipid extract by TLC, thus enabling the extent and direction of the metabolism of the fatty acids to be determined. After 24 h of incubation, radioactivity in cryptococci was almost exclusively found in lipid fractions containing free fatty acids (FFA), TAG, DAG, and PL. In the two iso-osmolar media (pH 5.5 and pH 7.3), the utilization levels for exogenous fatty acids were identical and there was no difference between levels for cryptococcal strain H99 and its PLB1 deletion mutant. The utilization levels for the fatty acids were inversely proportional to the uptake levels, with both strains incorporating around 55% of radioactive arachidonic acid predominantly into PL and DAG (Fig. 1B). About 35% and 15% of palmitic and oleic acids, respectively, were esterified into TAG, DAG, and PL. Notably, after 24 h, the pH in YNB media, initially at pH 7.3, was 4.5 to 5.0 for both the H99 and the Δplb1 strains, due to acetic acid production (3). This pH range is optimal for PLB1 activity (5), but clearly, PLB1 activity does not affect either the uptake or the esterification of fatty acids under these conditions. Putative pathways for cryptococcal fatty acid metabolism are shown in Fig. 2.
FIG. 2.
Proposed pathways for incorporation of fatty acids into cryptococcal lipids. The fatty acids can be added exogenously or derived from phospholipids by the PLB activity of PLB1 or phospholipase A1 or A2. The energy-dependent pathway involves the activation of fatty acids by coenzyme A (CoA), whereas the energy-independent pathway involves the transacylase activity of PLB1. The exact mechanism of this transacylase is unknown.
Under conditions of osmotic stress, both the H99 and the Δplb1 strains esterified more of all three fatty acids (but especially oleic acid) into TAG, a storage lipid (Fig. 1C). Arachidonic acid was converted more to DAG and PL than palmitic or oleic acids. Less esterification of all three fatty acids (significant for arachidonic) occurred in the Δplb1 strain than in H99, indicating that this mutant may be less efficient at removing potentially lytic free fatty acids in cryptococci under hypertonic (or possibly other stressful) conditions.
(iii) Phospholipids.
The uptake and metabolism of radiolabeled phospholipids were then tested in the same three media, and under the same conditions, used for the fatty acid studies. These phospholipids were DPPC and DOPC, which are favored substrates for PLB1 (5), and LysoPC, which could form via hydrolysis of DPPC by host or cryptococcal phospholipase A2 activity and which also serves as a substrate for the lysophospholipase and transacylase activities of the PLB1 enzyme. Lysophospholipids are not detected as intermediates in cryptococcal PLB activity.
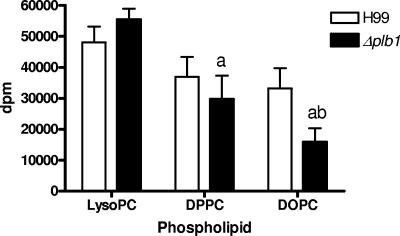
In all media, the H99 wild-type and Δplb1 strains incorporated radioactivity from LysoPC and DPPC to similar extents (results for incubation in buffer at pH 5.5 are shown in Fig. 3), although there was a trend, which was not statistically significant, for less incorporation of DPPC into the Δplb1 strain. Compared with what was found for H99, there was significantly less uptake of DOPC into the Δplb1 strain under all conditions (i.e., buffer, YNB, and 10× YNB). Less DPPC and DOPC were taken up by the Δplb1 strain than LysoPC (Fig. 3).
FIG. 3.
Incorporation of radioactive label from the fatty acids of exogenous phospholipids into lipids of cryptococci. Uptakes of 1,2-di[1-14C]palmitoyl phosphatidylcholine, 1,2-di[1-14C]oleoyl-phosphatidylcholine, and 1-[1-14C]palmitoyl-2-lysophosphatidylcholine were performed using 1 μM carrier lipid with 0.5 ml of H99 and Δplb1 strain cells at stationary phase after 24 h of incubation in imidazole buffer containing glucose at pH 5.5. Results are the means and standard errors of the means for four experiments, and significance was determined by the unpaired two-tailed t test and analysis of variance. a, different from LysoPC values; b, different from H99 values (P < 0.05).
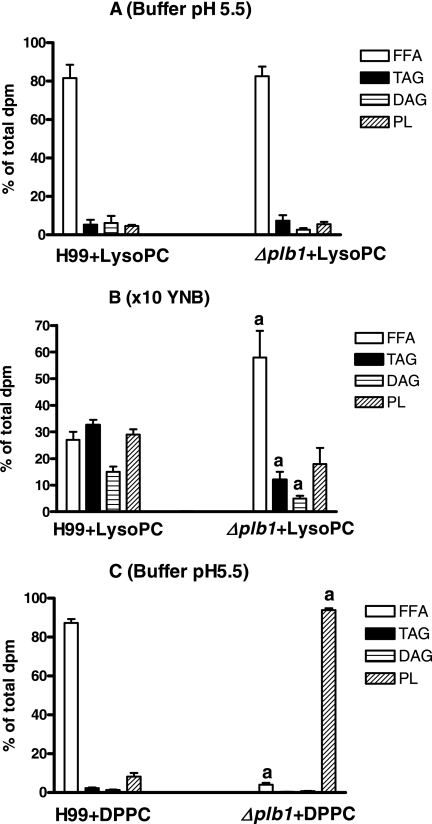
The extent and direction of metabolism of the radiolabeled phospholipids were determined by separating the individual lipid species present in the total lipid extract by TLC, as described above for the fatty acids. Most of the LysoPC was deacylated and remained as free fatty acid (around 80%) in pH 5.5 buffer (Fig. 4A) or YNB (not shown), with no differences between levels for wild-type and mutant strains, indicating that deacylation was not PLB1 dependent. In contrast, under hypertonic conditions (10× YNB), more LysoPC was metabolized by H99 into TAG, DAG, and PL, with only around 25% remaining as free fatty acid compared with 60% for the Δplb1 strain, again indicating a possible role for PLB1 in the removal of potentially lytic free fatty acids under hypertonic conditions (Fig. 4B). It was confirmed by use of TLC with a polar solvent system that 75 to 78% of the PL (Fig. 4A and B) was composed of phosphatidylcholine (PC) rather than LysoPC.
FIG. 4.
Metabolism of radioactive label from the fatty acids of exogenous phospholipids into separate lipid classes of cryptococci. Uptakes of 1,2-di[1-14C]palmitoyl phosphatidylcholine and 1-[1-14C]palmitoyl-2-lysophosphatidylcholine were performed with H99 and Δplb1 strain cells at stationary phase using 1 μM carrier lipids after 24 h of incubation in imidazole buffer containing glucose at pH 5.5 (A and C) or under hypertonic conditions (B, 10× YNB). Separation into lipid classes was performed by TLC, as described in Materials and Methods. Results are means and standard errors of the means for four experiments, with significance calculated by the unpaired two-tailed t test. a, different from H99 values (P < 0.05).
As expected, more than 85 to 95% of exogenous DPPC or DOPC was taken up but not metabolized by the Δplb1 strain, whether incubated in buffer, YNB, or 10× YNB (results for DPPC in pH 5.5 buffer are shown in Fig. 4C), whereas 80 to 90% of DPPC or DOPC was recovered as free fatty acid in H99. Only trace amounts of LysoPC were detected in any of the PL fractions isolated by TLC, confirming that breakdown of phospholipid was occurring by PLB1 activity rather than PLA.
Role of PLB1 in the metabolism of phospholipids during growth. (i) LysoPC.
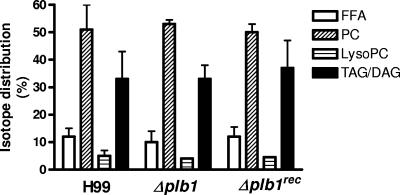
We have shown above that in media supplemented with glucose as an energy source, stationary-phase H99 and Δplb1 strain cells degrade LysoPC, which is normally toxic to cells (13), by disrupting their membranes. We next tested whether these strains and the reconstituted Δplb1rec strain could grow in the presence of LysoPC and utilize it as a substrate for growth. No differences were observed in the growth of all three strains over 24 h in YNB containing 0.5% glucose and 1 μM LysoPC at pH 5.5 (not shown). The levels for total uptake of radioactivity from 1-[1-14C]palmitoyl-2-lysophosphatidylcholine in the presence of 1 μM LysoPC into extracted lipids by all strains were similar, and separation of lipid species by TLC showed that all strains degraded LysoPC and reesterified the released fatty acids into PC and TAG/DAG (Fig. 5). This indicates that PLB1 is not required to detoxify LysoPC in the presence of glucose as an energy source.
FIG. 5.
Distribution of radioactive fatty acid label from 1-[1-14C]palmitoyl-2-lysophosphatidylcholine in cryptococcal lipid fractions after 24 h of growth in YNB plus glucose. Total lipids were extracted and fractionated into separate classes by TLC, as described in Materials and Methods. Lipid fractions are FFA, PC, LysoPC, and TAG/DAG. Cryptococci (H99, Δplb1, and Δplb1rec strains) were grown in YNB with 0.5% glucose plus 1 μM carrier LysoPC for 24 h at pH 5.5. Data represent the means ± ranges of isotope distribution as percentages of the overall uptake levels for two separate experiments.
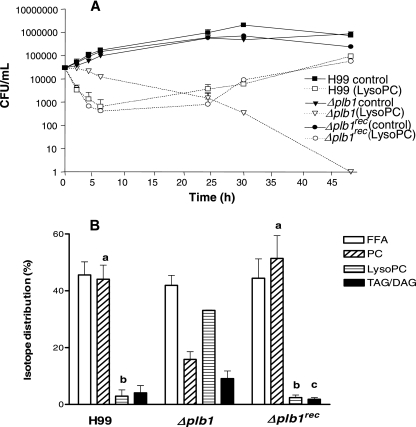
To test whether LysoPC could be used as an energy source, this experiment was repeated in the absence of glucose in YNB medium containing LysoPC (2.5 mM) as the sole source of carbon. In contrast to what was found for cryptococci grown in the presence of glucose, growth was now reduced in all three strains over the first 24 h (Fig. 6A). Although some LysoPC was degraded, with radiolabel detected in free fatty acids, significantly less was metabolized to PC in the Δplb1 strain than in H99 or the Δplb1rec strain (Fig. 6B).
FIG. 6.
Effects of substituting 2.5 mM 1-palmitoyl lysophosphatidylcholine for glucose on cryptococcal growth (A) and radiolabeled fatty acid distribution from 1-[1-14C]palmitoyl-2-lysophosphatidylcholine (B) into FFA, PC, LysoPC, and TAG/DAG. Cryptococci were grown for 24 h at pH 5.5 in YNB. Data represent the means ± standard deviations of CFU/ml (A) or isotope distributions (B) as percentages of the overall uptake levels for three separate experiments. Significantly reduced growth was observed for all strains of cryptococci grown in LysoPC at 24 h compared with what was found for corresponding controls grown in YNB containing 0.5% glucose (P < 0.05). The Δplb1 strain had significantly higher CFU/ml at 2 to 5 h than the H99 and Δplb1rec strains (P < 0.001). Radiolabel in PC (a) (P < 0.01), LysoPC (b) (P < 0.001), and TAG/DAG (c) (P < 0.05) was significantly different from that in corresponding Δplb1 strain samples; data were analyzed using analysis of variance.
The conversion of LysoPC to PC was also observed in the H99 culture supernatant, which became quite flocculent due to the insolubility of PC in aqueous media, whereas that from the Δplb1 strain remained clear (not shown). Thin-layer chromatography of the extracted supernatant lipids confirmed these observations, indicating a high level of secreted PLB1 activity from the H99 and Δplb1rec strains. Growth of the H99 and Δplb1rec strains was observed between 24 to 48 h, in contrast to that of the Δplb1 strain, which had not recovered by 30 h in this experiment or by 30 and 48 h in a separate experiment using both 1 mM and 2.5 mM LysoPC (data not shown). Because the fatty acid levels are similar in wild-type, mutant, and recombinant strains (Fig. 6B), we can conclude that the Δplb1 strain cells are dying due to LysoPC toxicity rather than a lack of a carbon source (fatty acid) for growth. Together, these data indicate a role for PLB1 in detoxifying LysoPC in the absence of an energy supply such as glucose. This is not unexpected, since the transacylase activity of PLB1 does not require the addition of ATP to activate the fatty acids involved (Fig. 2, pathways).
(ii) DPPC.
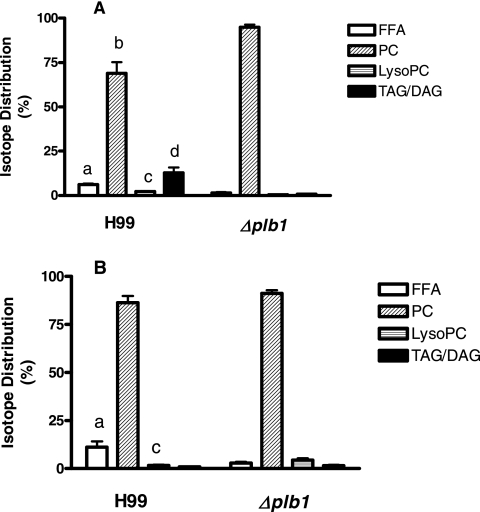
When the H99 and Δplb1 strains were cultured for 24 h in YNB containing 1% glucose at pH 5.5, there was a trend, which was not statistically significant, for increased growth of H99 in the presence of 1 μM DPPC (data not shown). Similarly, radiolabeling experiments and lipid analyses indicated that a small but significant amount of DPPC was metabolized under these conditions by H99 (none by the Δplb1 strain), mainly to DAG/TAG (Fig. 7A). The Δplb1 strain incorporated 2.3 times more total radiolabeled DPPC than H99 (P < 0.05, paired two-tailed t test), indicating that metabolism of the DPPC substrate by the PLB1-competent strain had occurred in the medium as well as intracellularly. Both exogenous and endogenous metabolism of DPPC occurred only under acidic conditions (pH 5.5), which would favor PLB1 activities, and were not evident when the experiments were carried out at pH 7.0 (data not shown).
FIG. 7.
Comparison of the distributions of radioactive fatty acid from 1,2-di[1-14C]palmitoyl phosphatidylcholine in cryptococci grown in the presence of glucose (A) or as a substitute for glucose (B). In the experiment for panel A, the cryptococci were grown for 24 h in YNB at pH 5.5 containing 1% glucose plus 1 μM carrier DPPC, whereas in the experiment for panel B, 2 mM DPPC was used instead of glucose. Isotope distributions into FFA, PC, LysoPC, and TAG/DAG are represented as the means ± standard deviations of the total isotope uptake levels, with four separate experiments for panel A and seven separate experiments for panel B. Data were compared using the unpaired two-tailed t test and analysis of variance. In the experiment for panel A, FFA (a) (P < 0.01), PC (b) (P = 0.02), LysoPC (c) (P < 0.01), and TAG/DAG (d) (P = 0.02) were significantly different from those observed for the Δplb1 strain. In the experiment for panel B, radiolabel in FFA (a) (P < 0.01) and LysoPC (c) (P < 0.01) was significantly different from that observed in corresponding Δplb1 strain samples.
When 2 mM DPPC was substituted for glucose, there were no significant differences between the growth rates of the H99 and Δplb1 strains at 24 h and those of their glucose-containing controls (data not shown), indicating that DPPC can support growth in the absence of glucose, and it does not require PLB1. Strain-dependent differences were observed, however, in the distribution of radiolabeled DPPC into the various lipid classes, mainly involving conversion by H99 to free fatty acid, presumably for energy production (Fig. 7B). In contrast to total uptake of radiolabeled DPPC in the presence of glucose, uptake in its absence was significantly higher in H99 than in the Δplb1 strain (2.5-fold, P < 0.05, paired two-tailed t test). Thus, it appears that PLB1 is metabolizing DPPC intracellularly in the PLB1-competent strain, but it does not appear to be essential for growth, at least over 24 h.
It is of interest that DPPC (equivalent to 2 mM) supplied as lung surfactant produced the same effects as those described above for pure DPPC (data not shown).
(iii) PAPC.
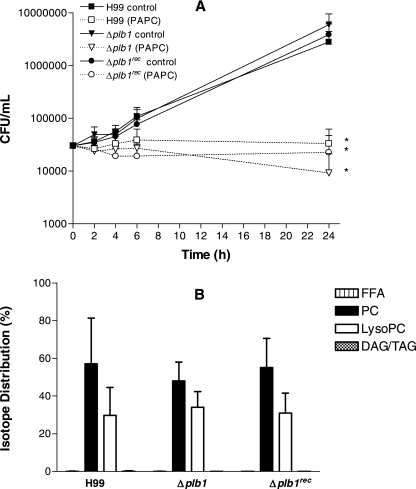
Strain H99 has been shown (22) to produce eicosanoids from exogenous diarachidonoyl phosphatidylcholine (DAPC), which contains arachidonic acid in both the sn-1 and the sn-2 positions. However, in macrophages, the lipid species commonly contain arachidonic acid in the sn-2 position and a saturated fatty acid, such as palmitic or stearic acid, or an ether-linked fatty acid in the sn-1 position (27, 32). We therefore tested the effects of PAPC, radiolabeled in the arachidonic acid chain, together with 869 μM carrier PAPC, on growth and metabolism in the presence or absence of glucose at pH 5.5. In the presence of glucose, all three strains grew to similar extents over 24 h, and as with DPPC (Fig. 7), both the H99 and the Δplb1rec strains, but not the Δplb1 strain, metabolized significant amounts of PAPC, predominantly into storage TAG (data not shown).
The levels of total uptake of radiolabel derived from PAPC were similar in all three strains, but there was no growth over 24 h when PAPC was used as the sole source of carbon (Fig. 8A). Surprisingly, the arachidonic acid label derived from the sn-2 position of PAPC was found in all strains only in LysoPC and not in free fatty acid, indicating removal of the unlabeled fatty acid from the sn-1 position and suggesting that a PLA1 rather than PLB1 was responsible for the hydrolysis (Fig. 8B).
FIG. 8.
Effect of substituting 869 μM PAPC for glucose on cryptococcal growth in YNB (A) and distribution of radioactivity from l-α-1-palmitoyl-2-arachidonoyl [arachidonoyl-1-14C]phosphatidylcholine into cryptococcal lipid classes (B). Data represent the means ± standard deviations of CFU/ml (A) and isotope distributions (B) as percentages of the overall uptake. Radiolabeled PAPC (PC) and LysoPC were detected in lipid fractions after 24 h. FFA and DAG/TAG levels are not visible above the baseline. *, significant difference in growth after 24 h was observed with all strains grown in PAPC compared with what was found for corresponding controls grown in YNB containing 0.5% glucose (P < 0.01), using the paired two-tailed t test, in three separate experiments. No significant differences were observed in lipid distributions between strains.
The role of PLB1 in the phagocytosis/strong adhesion of cryptococci by the macrophage-like cell line THP-1.
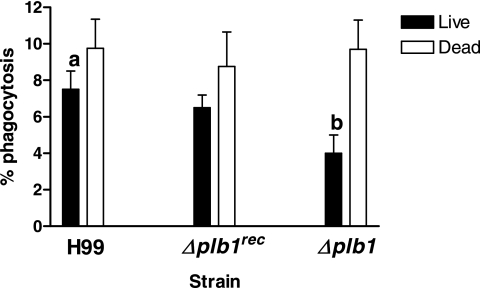
Cox et al. (9) reported that the budding of intracellular Δplb1 strain after ingestion by the macrophage-like cell line J774 was reduced compared with that of H99 but that phagocytosis was not affected. We sought to determine the effect of active cryptococcal PLB1, which is heat labile, by measuring the phagocytosis of live and heat-killed (autoclaved) H99, Δplb1, and Δplb1rec strain cells (Fig. 9). Under our conditions, the phagocytosis of live H99 cells (7.3% ± 1.2%) was significantly greater than that for Δplb1 strain cells (4.3% ± 0.8%). The uptake levels of killed cryptococci for all three strains were similar and higher than those for the live organisms, though the difference was significant only for the Δplb1 strain (Fig. 9). This is an interesting observation, as the level of adherence of dead Δplb1 strain cells to the lung epithelial cell line A549 is similarly higher than that of live cells (14).
FIG. 9.
Phagocytosis of the H99, Δplb1, and Δplb1rec C. neoformans strains by THP-1 cells. Live or autoclaved cryptococci, radiolabeled with oleic acid, were opsonized with 10% human AB serum and then incubated with THP-1 cells for 4 to 5 h. a, P < 0.05, significant relative to what was found for Δplb1 strain cells; b, P < 0.05, significant relative to what was found for dead Δplb1 strain cells by the paired two-tailed t test (three separate experiments, done in triplicate).
Effect of phagocytosis on fatty acid composition of C. neoformans.
In Fig. 1, it was shown that, in vitro, exogenous arachidonic, oleic, and palmitic acids can be taken up and metabolized to DAG, TAG, and phospholipids by both wild-type and PLB1 deletion mutant cryptococci (eicosanoid production was not measured). Evidence of transcellular metabolism of arachidonic acid by cryptococci was sought by allowing live and killed (autoclaved) cryptococci to be phagocytosed by THP-1 cells and analyzing the fatty acid compositions of THP-1 cell lipids and of cryptococcal lipids before and after phagocytosis. The autoclaved cryptococci served as a control for nonenzymatic activity and for the presence of residual THP-1 membranes or nonincorporated fatty acids that remained adherent to the cryptococci.
The fatty acid composition of lipids extracted from THP-1 cells was markedly different from that of cryptococcal strain H99 as revealed by gas chromatographic analysis. The fatty acids of lipids in the phagocytic cells tended to have more of the saturated fatty acids 14:0, 16:0, and 18:0 and the monounsaturated fatty acid 16:1 than both live and dead cryptococci, with the major component being palmitic acid (29%) (Table 1). Cryptococci contained much more oleic acid ( 1ω9c) and linoleic acid (18:2ω6,9c), with the compositions being comparable with those of other clinical strains of C. neoformans (18). The absence of arachidonic acid and 18:1ω7 in cryptococci is noteworthy (18 (Table 1).
TABLE 1.
Fatty acid compositions of live and killed cryptococcal strain H99 cells
| Fatty acid | Value (% [SEM])a for indicated cell group
|
||||
|---|---|---|---|---|---|
| THP-1 | Live
|
Killed
|
|||
| Before ϕϕ | After ϕϕ | Before ϕϕ | After ϕϕ | ||
| 14:0 | 3.50 (0.97) | 1.53b (0.24) | 3.58 (1.02) | 0.72c (0.29) | 3.08 (1.08) |
| 14:0 aldehyde | 1.05 (0.46) | 0.24b (0.14) | 1.46d(0.38) | 0 (0) | 1.11 (0.31) |
| 16:0 | 28.94 (1.17) | 19.36b (1.1) | 25.39 (1.32) | 18.14 (0.17) | 25.19 (1.19) |
| 16:1 cis 9 (ω7c) | 14.10 (1.59) | 0.96b (0.16) | 11.82 (1.23) | 0.98 (0.45) | 11.13 (1.45) |
| 18:0 | 9.27 (1.18) | 3.86b (0.22) | 7.22 (0.68) | 4.29 (0.42) | 8.30 (0.96) |
| 18:1ω7ce | 10.69 (0.49) | 0b (0) | 8.42d(0.40) | 0 (0) | 7.91 (0.51) |
| 18:1ω9c | 21.83 (0.76) | 40.32b (1.25) | 25.25 (3.33) | 42.46 (0.85) | 26.47 (1.47) |
| 18:2ω6,9cf | 4.30 (0.35) | 33.04b (0.42) | 9.44d(2.02) | 31.93 (1.63) | 12.58 (1.88) |
| 20:4ω6,9,12,15 arachidonic acid | 6.31 (0.53) | 0b (0) | 7.42d(1.38) | 0 (0) | 4.20 (1.21) |
Composition was determined by gas chromatography using the CLIN 50 library before and after phagocytosis by THP-1 macrophages. Enzyme-mediated changes to lipid composition during cryptococcal-phagocyte interaction were analyzed by pair-wise comparison of each lipid class in live and killed cryptococci after phagocytosis (values in bold) using the two-tailed t test. Values are expressed as percentages of the sum of the areas of all peaks identified and are the means (with standard errors of the means in parentheses) for five separate experiments. ϕϕ symbolizes phagocytosis.
P < 0.05, relative to value for THP-1.
P < 0.05, relative to value for live H99, before phagocytosis.
P < 0.05, relative to value for killed H99, after phagocytosis.
May contain traces of 18:1 cis 11/trans 9/trans 6.
May contain traces of 18:1ω6,9c and 18:0 ANTEISO.
Prior to phagocytosis, both live and autoclaved H99 cells showed no significant differences in fatty acid composition (P > 0.05) except for myristic acid (14:0), which was reduced by 53% in autoclaved cryptococci (Table 1). After phagocytosis, the fatty acid compositions of both live and autoclaved cryptococci showed selective changes superimposed on a general profile more like that of the THP-1 cells. The latter observation indicates that despite extensive hypotonic lysis and washing of cryptococci to free them from macrophages, residual macrophage-derived lipids remained attached to the cryptococcal cells. Live and autoclaved cryptococci were therefore compared after phagocytosis to determine the extent to which the observed fatty acid changes were due to PLB1 enzyme activity versus adhesion of residual THP-1 lipids. Uptake of arachidonic acid from THP-1 cells was 77% higher in live than in dead cryptococci, consistent with an enzyme-mediated effect. The fatty acids 14:0 aldehyde and 18:1ω7c were also significantly enriched in live cryptococci, whereas 18:2ω6,9c was decreased (Table 1).
Prior to phagocytosis, the fatty acid compositions of the Δplb1 and Δplb1rec strains showed no significant differences from those of the H99 wild-type strain when analyzed using the CLIN 50 library (data not shown). Slight differences were noted using the Yeast 28 library database (e.g., in minor components 14:0, 14:0 aldehyde, and 16:1) when the Δplb1 strain was studied in a separate set of experiments (Table 2). Arachidonic acid and 18:1ω7c were again absent.
TABLE 2.
Fatty acid compositions of live and killed Δplb1 cryptococcal strain cells
| Fatty acid | Value (% [SEM])a for indicated cell group
|
||||
|---|---|---|---|---|---|
| THP-1 | Live
|
Killed
|
|||
| Before ϕϕ | After ϕϕ | Before ϕϕ | After ϕϕ | ||
| 14:0 | 5.39 (0.49) | 0b (0) | 0 (0) | 0 (0) | 0 (0) |
| 14:0 aldehyde | 3.39 (0.94) | 0b (0) | 0 (0) | 0 (0) | 0 (0) |
| 16:0 | 27.03 (0.54) | 19.79b (0.66) | 31.01d(2.82) | 21.73c (1.01) | 26.51 (1.36) |
| 16:1 cis 9 (ω7c) | 14.47 (1.36) | 0b (0) | 0 (0) | 0 (0) | 0 (0) |
| 18:0 | 9.08 (0.86) | 4.70b (0.41) | 8.35d(1.25) | 6.52 (0.86) | 7.05 (1.07) |
| 18:1ω7ce | 10.08 (0.27) | 0b (0) | 0 (0) | 0 (0) | 0 (0) |
| 18:1ω9c | 19.99 (0.55) | 37.42b (0.64) | 34.86 (2.46) | 35.94c (0.33) | 34.96 (2.59) |
| 18:2ω6,9cf | 5.68 (0.83) | 37.69b (1.21) | 25.78d(1.88) | 35.61 (1.88) | 31.47 (2.92) |
| 20:4ω6,9,12,15 arachidonic acid | 3.29 (0.32) | 0b (0) | 0 (0) | 0 (0) | 0 (0) |
Composition was determined by gas chromatography using the Yeast 28 library before and after phagocytosis by THP-1 cells. Enzyme-mediated changes to lipid composition during cryptococcal-phagocyte interaction were analyzed by pair-wise comparison of each lipid class in live and killed cryptococci after phagocytosis (values in bold) using the two-tailed t test. Values are expressed as percentages of the sum of the areas of all peaks identified and are the means (with standard errors of the means in parentheses) for three separate experiments. ϕϕ symbolizes phagocytosis.
P < 0.05, relative to value for THP-1.
P < 0.05, relative to value for live Δplb1, before phagocytosis.
P < 0.05, relative to value for killed Δplb1, after phagocytosis.
May contain traces of 18:1 cis 11/trans 9/trans 6.
May contain traces of 18:1ω6,9c and 18:0 ANTEISO.
Following phagocytosis, the pattern of THP1-derived fatty acids incorporated into the Δplb1 strain differed from that observed for H99 (Table 2). Two saturated fatty acids, palmitic acid (16:0) and stearic acid (18:0), were preferentially incorporated into live Δplb1 strain cells compared with the results for killed Δplb1 strain cells, whereas unlike with H99, neither arachidonic acid nor 18:1ω7c was present in the Δplb1 strain. As with H99, the 18:2ω6,9c level was lower in live than in dead cells after phagocytosis, consistent with transfer of this fatty acid from cryptococci to the THP-1 cells.
DISCUSSION
In this study, we have proven the hypothesis that cryptococci phagocytosed by mammalian macrophages take up and metabolize fatty acids of macrophage origin and that incorporation of arachidonic acid, a precursor of cryptococcal eicosanoid production, is specifically PLB1 dependent. Cryptococci can thereby act as a “sink” for macrophage-derived arachidonate, reducing its availability for phagocytic activation.
In addition, we have shown for the first time that cryptococci utilize PLB1 to metabolize phospholipids present at sites of human infection, specifically, its preferred substrates DPPC (5), found in host surfactant, and DOPC (5), a component of mammalian cell membranes. In the absence of glucose as an energy source, PLB1 is required for reacylation of a toxic product of DPPC hydrolysis, LysoPC, which could be formed by phospholipase A2 activity.
The finding of reduced phagocytosis of live cryptococci in the absence of a functional PLB1 gene is at variance with published data (9). This may be explained by our use of a different phagocytic cell line (THP-1 rather than J774) and different opsonization conditions but is most likely due to the fact that Cox et al. (9) measured phagocytosis in the presence of 10% fetal calf serum. We have shown previously that fetal calf serum inhibits PLB activity (30). Furthermore, our results are consistent with previous data implicating PLB1 in the adhesion of cryptococci (a prerequisite for phagocytosis) to a human lung epithelial cell line (14).
PLB1 and lipid metabolism in cryptococci.
Exogenous fatty acids were taken up readily by cryptococci independently of PLB1. The mechanisms of fatty acid uptake in cryptococci are unknown, but those in mammalian cells involve protein-mediated transport and/or simple diffusion (1, 19). Surprisingly, much free fatty acid, which is potentially toxic to cell membranes, was taken up by the fungus but not incorporated into lipids, suggesting that it is stored, possibly in the capsule or cell wall to avoid lytic membrane damage. There was also some esterification of fatty acids into the signaling lipid, DAG, the storage lipid, TAG, and membrane phospholipids by a mechanism that was independent of PLB1, except under hypertonic conditions where esterification of arachidonic acid, in particular, was reduced in the Δplb1 strain. Arachidonic acid, in contrast to palmitic and oleic acids, is not normally found in cryptococci (18) (Tables 1 and 2). The high rate of esterification for arachidonic acid compared with those for palmitic and oleic acids may be a protective mechanism, since in mammalian systems, intracellular unesterified arachidonic acid can signal apoptosis (4).
In addition, PLB1 was required to esterify the free fatty acid produced by hydrolysis of LysoPC under hypertonic conditions. Since the transacylase activity of PLB1 is energy independent (5), this would provide an energy-saving alternative transacylation pathway that is active under stress conditions (Fig. 2). Usually, fatty acids are activated by ATP formed in the presence of an energy source, such as glucose, to coenzyme A derivatives before metabolic utilization (6, 11) (Fig. 2). The ability of the Δplb1 strain to acylate LysoPC to form PC only in the presence of glucose indicates a switch to a PLB1-independent transacylase pathway which is energy dependent. Together, these data suggest that in cryptococci, as in mammalian cells (6, 32), there are energy-dependent and -independent pathways of fatty acid esterification. It is likely that PLB1 activity is essential during stress, when energy needs to be conserved. A role for PLB1 in cryptococcal membrane integrity and/or remodeling during stress is consistent with our previous findings of enhanced PLB1 secretion at a physiological temperature (30), during osmotic stress (14), and demonstration of PLB1 activity in experimental cryptococcomas (16).
PLB1 was also essential for maintaining viability of cryptococci when cultured in medium containing LysoPC as a sole carbon source, since this condition was lethal for the Δplb1 strain. Although growth of PLB1-competent strains was also reduced upon initial transfer into glucose-free medium, growth resumed within 24 h, most likely due to the initiation of the reacylation of both cell-associated and extracellular LysoPC to form PC. In contrast, in this medium, LysoPC was minimally degraded or esterified by the Δplb1 strain.
During growth in the presence of glucose, LysoPC was detoxified by conversion to PC for new membranes and TAG for storage and deacylated, producing free fatty acid, by a PLB1-independent pathway, indicating that a lysophospholipase other than PLB1 was responsible. Two additional lysophospholipases have been observed in cryptococci (8, 31).
Uptake of the physiologically important phospholipids, DPPC and DOPC, was not dependent on PLB activity since both were present in stationary-phase Δplb1 strain cells. Subsequent hydrolysis was achieved by PLB1, with almost complete release of fatty acids from both substrates observed in H99 and no hydrolysis observed in the Δplb1 strain. Metabolism of DPPC was greatly reduced during logarithmic growth of H99 in the presence of glucose, with some conversion to free fatty acid and storage as TAG in H99, whereas in the absence of glucose, the degraded DPPC remained as free fatty acid, presumably for energy production.
Evidence for PLA1 activity in Cryptococcus.
Metabolism of exogenous DAPC by cryptococci has been shown to be PLB1 dependent and to result in the generation of anti-inflammatory eicosanoids from DAPC-derived arachidonic acid (22). In this study, we tested the hypothesis that arachidonic acid is released by cryptococcal PLB1 from the sn-2 position of the physiologically more common PAPC. In the absence of glucose, PAPC did not support cryptococcal growth but was taken up equally by all cryptococcal strains. However, PAPC was hydrolyzed to 2-arachidonoyl LysoPC, consistent with PLA1 rather than PLB1 activity and suggesting that 2-arachidonoyl LysoPC cannot be metabolized and detoxified by cryptococcal LPL/LPTA in the absence of glucose. Recently, cell-associated PLA activity has been observed in membrane fractions of C. neoformans (15).
Transcellular metabolism of arachidonic acid from phagocytes by cryptococci.
PLB1-dependent generation of prostaglandins from exogenously supplied arachidonic acid was observed by Noverr et al. (23). These workers postulated that generation of prostaglandins by cryptococci in vivo explains their survival within host macrophages, since macrophage fungistatic activity is suppressed by prostaglandins. The source of the arachidonic acid was not determined.
We confirmed in this study that there is no naturally occurring arachidonic acid in cryptococci (18). Furthermore, we showed that arachidonic acid is selectively enriched in live H99 wild-type cryptococci compared with that in heat-killed H99 wild-type cryptococci after ingestion by and/or strong adhesion to the macrophage-like cell line THP-1. This difference was significant despite a greater index of adhesion/ingestion for heat-killed than for live organisms under these conditions (Fig. 9) and confirms that the arachidonic acid found in cryptococci after phagocytosis is of macrophage origin. The observation that some arachidonate was extracted from killed H99 cells after phagocytosis is presumably due to residual host-derived fatty acid that is present despite extensive enzyme treatment and washing. This interpretation is supported by a background increase in other THP-associated lipids in both live and killed cryptococci after phagocytosis and by our previous observation of differences in capsule structure after heat killing, detected by infrared spectroscopy (14), leading to differences in “stickiness” toward mammalian cells.
Arachidonic acid is almost exclusively found in the sn-2 position of the glycerol backbone of phospholipids in mammalian cell membranes and could be released by the activity of mammalian PLA2 (secondary to cell membrane perturbation during adhesion or phagocytosis) (6, 26, 32) or via the activity of secreted or cell surface-associated cryptococcal PLB1 (10, 15). THP-1 cells are rich in arachidonic acid and demonstrate almost equal distributions of arachidonate in phosphatidylethanolamine, PC, and phosphatidylinositol species. In addition, proliferating THP-1 cells remodel arachidonate at extremely high rates (27). As no arachidonate was present in Δplb1 strain cells after phagocytosis, it can be concluded that arachidonic acid present in wild-type cryptococci was hydrolyzed from macrophage phospholipids by the action of cryptococcal PLB1. Arachidonic acid taken up by cryptococci is then esterified into membrane phospholipids or DAG/TAG for storage or signal transduction (Fig. 1) or converted into prostaglandins (22, 23).
In a preliminary in vivo experiment, cryptococci were isolated by bronchoalveolar lavage from mice inoculated intranasally with either H99 or the Δplb1 strain. Fatty acid analyses indicated that arachidonic acid constituted 6.6% of H99 fatty acids but only 4.7% of Δplb1 strain fatty acids, despite the presence of large amounts of contaminating host cellular debris in the latter (J. Djordjevic, unpublished data).
Incorporation of host fatty acids other than arachidonic.
In addition to incorporation of arachidonic acid into H99 cells, there was significant incorporation of other fatty acids, e.g., 14:0 aldehyde and 18:1ω7c in H99 and 16:0 and 18:0 in the Δplb1 strain, after passage via THP-1 cells. In both cell lines, 18:2ω6,9c was decreased. The function of these fatty acids in cryptococcal metabolism is unknown. Overall, Δplb1 strain cells acquire more saturated fatty acids than H99 cells after passage via THP-1 cells, making membranes potentially more rigid and less flexible. Membrane remodeling via fatty acid biosynthesis and saturation-desaturation are important regulatory mechanisms for overcoming stress responses in C. neoformans (20). Cryptococci may acquire host fatty acids for this function, as the LPTA activity of PLB1 requires no input of energy. We postulate that the relative increase in unsaturated fatty acids in H99 under the osmotic and other stresses present in the phagocytic vacuole results in increased membrane fluidity, which promotes replication and intracellular survival of H99 compared with those of the Δplb1 strain.
We conclude that C. neoformans can acquire and utilize lipid components from its environment, including the mammalian host. We propose that cryptococcal PLB1, which we have demonstrated in experimental cryptococcosis in vivo and have shown to be essential for establishment of pulmonary infection and dissemination to the brain and other organs, also promotes survival and replication of cryptococci within macrophages, where the intraphagosomal pH is known to be similar to the pH optimum for cryptococcal PLB1 (5.5) (21). In particular, transcellular metabolism of macrophage-derived arachidonic acid is a potentially novel means of diverting arachidonate from mammalian cell pathways of phagocyte activation to generate more flexible cryptococcal membranes enriched in unsaturated fatty acids and to enhance the production of cryptococcal eicosanoids that inhibit macrophage fungistatic activity, with consequent suppression of the host immune response.
Acknowledgments
This work received the support of National Health and Medical Research Council grants no. 211040 and no. 352354 as well as a Harry Windsor Postgraduate Research Scholarship.
We also thank Christabel Wilson and Gary Martinic for technical assistance and Leanne Hicks for performing the fatty acid analyses.
Footnotes
Published ahead of print on 10 November 2006.
REFERENCES
- 1.Abumrad, N. C., C. Harmon, and A. Ibrahimi. 1998. Membrane transport of long chain fatty acids: evidence for a facilitated process. J. Lipid Res. 39:2309-2318. [PubMed] [Google Scholar]
- 2.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 3.Bubb, W. A., L. C. Wright, M. Cagney, R. T. Santangelo, T. C. Sorrell, and P. W. Kuchel. 1999. Heteronuclear NMR studies of metabolites produced by Cryptococcus neoformans in culture media: identification of possible virulence factors. Magn. Reson. Med. 42:442-453. [DOI] [PubMed] [Google Scholar]
- 4.Cao, Y., A. T. Pearman, G. A. Zimmerman, T. M. McIntyre, and S. M. Prescott. 2000. Intracellular unesterified arachidonic acid signals apoptosis. Proc. Natl. Acad. Sci. USA 97:11280-11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, S. C., L. C. Wright, J. C. Golding, and T. C. Sorrell. 2000. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 347:431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chilton, F. H., A. N. Fonteh, M. E. Surette, M. Triggiani, and J. D. Winkler. 1996. Control of arachidonate levels within inflammatory cells. Biochim. Biophys. Acta 1299:1-15. [DOI] [PubMed] [Google Scholar]
- 7.Chretien, F., O. Lortholary, I. Kanau, S. Neuville, F. Gray, and F. Dromer. 2002. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J. Infect. Dis. 186:522-530. [DOI] [PubMed] [Google Scholar]
- 8.Coe, J. G. S., C. F. Wilson, T. C. Sorrell, N. G. Latouche, and L. C. Wright. 2003. Cloning of CNLYSO1, a novel extracellular lysophospholipase of the pathogenic fungus Cryptococcus neoformans. Gene 316:67-78. [DOI] [PubMed] [Google Scholar]
- 9.Cox, G. M., H. C. McDade, S. C. A. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Casadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166-175. [DOI] [PubMed] [Google Scholar]
- 10.Djordjevic, J. T., M. Del Poeta, T. C. Sorrell, K. M. Turner, and L. C. Wright. 2005. Secretion of cryptococcal phospholipase B1 (PLB1) is regulated by a glycosylphosphatidylinositol (GPI) anchor. Biochem. J. 389:803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faergeman, N. J., P. N. Black, X. D. Zhao, J. Knudsen, and C. C. DiRusso. 2001. The acyl-CoA synthetases encoded within FAA1 and FAA4 in Saccharomyces cerevisiae function as components of the fatty acid transport system linking import, activation, and intracellular utilization. J. Biol. Chem. 276:37051-37059. [DOI] [PubMed] [Google Scholar]
- 12.Feldmesser, M. S., S. Tucker, and A. Casadevall. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9:273-278. [DOI] [PubMed] [Google Scholar]
- 13.Fyrst, H., B. Oskouian, F. A. Kuypers, and J. D. Saba. 1999. The PLB2 gene of Saccharomyces cerevisiae confers resistance to lysophosphatidylcholine and encodes a phospholipase B/lysophospholipase. Biochemistry 38:5864-5871. [DOI] [PubMed] [Google Scholar]
- 14.Ganendren, R., E. Carter, T. Sorrell, F. Widmer, and L. Wright. 2006. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 8:1006-1015. [DOI] [PubMed] [Google Scholar]
- 15.Ganendren, R., A. Widmer, V. Singhal, C. Wilson, T. Sorrell, and L. Wright. 2004. In vitro antifungal activities of inhibitors of phospholipases from the fungal pathogen Cryptococcus neoformans. Antimicrob. Agents Chemother. 48:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himmelreich, U., C. Allen, S. Dowd, R. Malik, P. B. Shehan, P. Russell, C. E. Mountford, and T. C. Sorrell. 2003. Identification of metabolites of importance in the pathogenesis of pulmonary cryptococcoma using nuclear magnetic resonance spectroscopy. Microbes Infect. 5:285-290. [DOI] [PubMed] [Google Scholar]
- 17.Himmelreich, U., T. E. Dzendrowskyj, C. Allen, S. Dowd, R. Malik, P. B. Shehan, P. Russell, C. E. Mountford, and T. C. Sorrell. 2001. Cryptococcomas distinguished from gliomas with MR spectroscopy: an experimental rat and cell culture study. Radiology 220:122-128. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim, A. S., H. Sanati, and M. A. Ghannoum. 1996. Lipids of Cryptococcus neoformans, p. 160. In R. Prasad and M. A. Ghannoum (ed.), Lipids of pathogenic fungi. CRC Press Inc., Boca Raton, FL.
- 19.Kleinfeld, A. 2000. Lipid phase fatty acid flip-flop. Is it enough for cellular transport? J. Membr. Biol. 175:79-86. [DOI] [PubMed] [Google Scholar]
- 20.Kraus, P. R., M. J. Bolly, S. S. Giles, J. E. Stajich, A. Allen, G. M. Cox, F. S. Dietrich, J. R. Perfect, and J. Heitman. 2004. Identification of Cryptococcus neoformans temperature-regulated genes with a genomic-DNA microarray. Eukaryot. Cell 3:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levitz, S. M., S. H. Nong, K. F. Seetoo, T. S. Harrison, R. A. Speizer, and E. R. Simons. 1999. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect. Immun. 67:885-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noverr, M. C., G. M. Cox, J. R. Perfect, and G. B. Huffnagle. 2003. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 71:1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noverr, M. C., S. M. Phare, G. B. Toews, M. J. Coffey, and G. B. Huffnagle. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santangelo, R., H. Zoellner, T. Sorrell, C. Wilson, C. Donald, J. Djordjevic, Y. Shounan, and L. Wright. 2004. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 72:2229-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santangelo, R. T., M. H. Nouri-Sorkhabi, T. C. Sorrell, M. Cagney, S. C. Chen, P. W. Kuchel, and L. C. Wright. 1999. Biochemical and functional characterization of secreted phospholipase activities from Cryptococcus neoformans in their naturally occurring state. J. Med. Microbiol. 48:731-740. [DOI] [PubMed] [Google Scholar]
- 26.Scott, K. F., G. G. Graham, and K. J. Bryant. 2003. Secreted phospholipase A2 enzymes as therapeutic targets. Expert Opin. Ther. Targets 7:427-440. [DOI] [PubMed] [Google Scholar]
- 27.Surette, M. E., and F. H. Chilton. 1998. The distribution and metabolism of arachidonate-containing phospholipids in cellular nuclei. Biochem. J. 330:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker, S. C., and A. Casadevall. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 99:3165-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright, L. C., W. Bubb, J. Davidson, R. Santangelo, M. Krockenberger, U. Himmelreich, and T. C. Sorrell. 2002. Metabolites released by Cryptococcus neoformans var. neoformans and var. gattii differentially affect human neutrophil function. Microbes Infect. 4:1427-1438. [DOI] [PubMed] [Google Scholar]
- 30.Wright, L. C., S. C. A. Chen, C. F. Wilson, M. F. Simpanya, R. Blackstock, G. M. Cox, J. W. Murphy, and T. C. Sorrell. 2002. Strain-dependent effects of environmental signals on the production of extracellular phospholipases by Cryptococcus neoformans. FEMS Microbiol. Lett. 209:175-181. [DOI] [PubMed] [Google Scholar]
- 31.Wright, L. C., J. Payne, R. T. Santangelo, M. F. Simpanya, S. C. A. Chen, F. Widmer, and T. C. Sorrell. 2004. Cryptococcal phospholipases: a novel lysophospholipase discovered in the pathogenic fungus Cryptococcus gattii. Biochem. J. 384:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita, A., T. Sugiura, and K. Waku. 1997. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J. Biochem. 122:1-16. [DOI] [PubMed] [Google Scholar]