Abstract
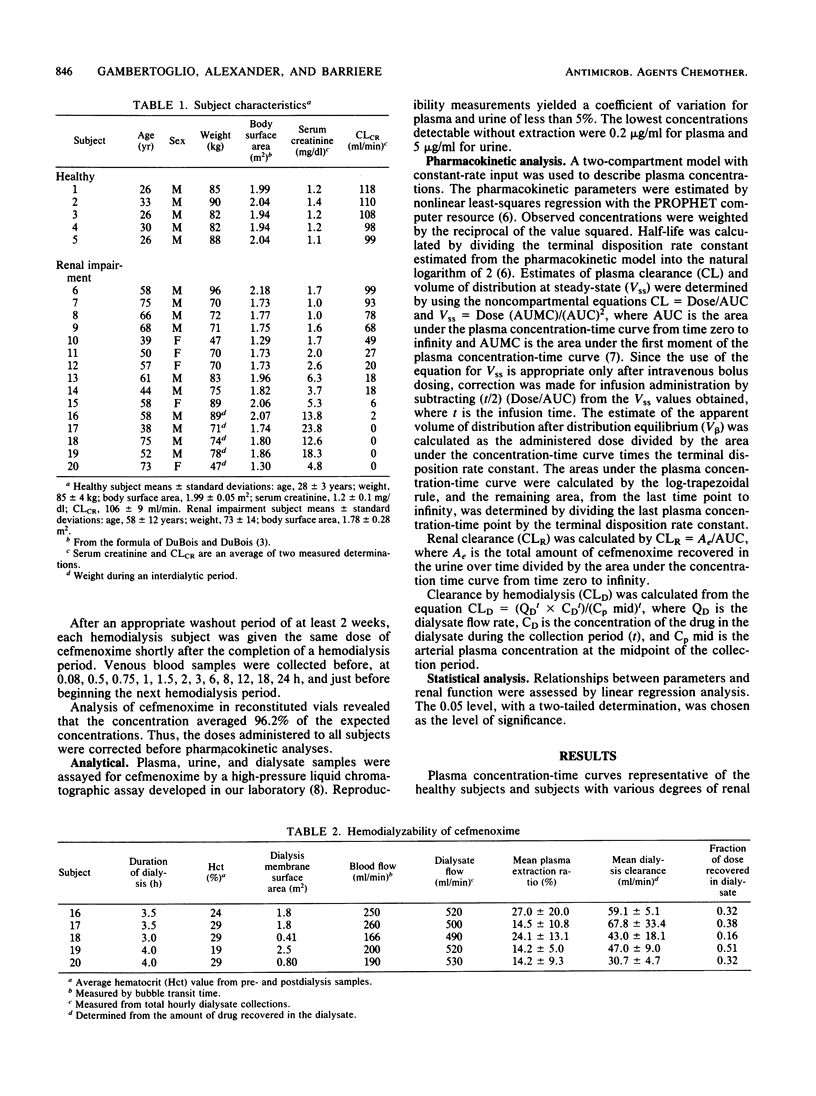
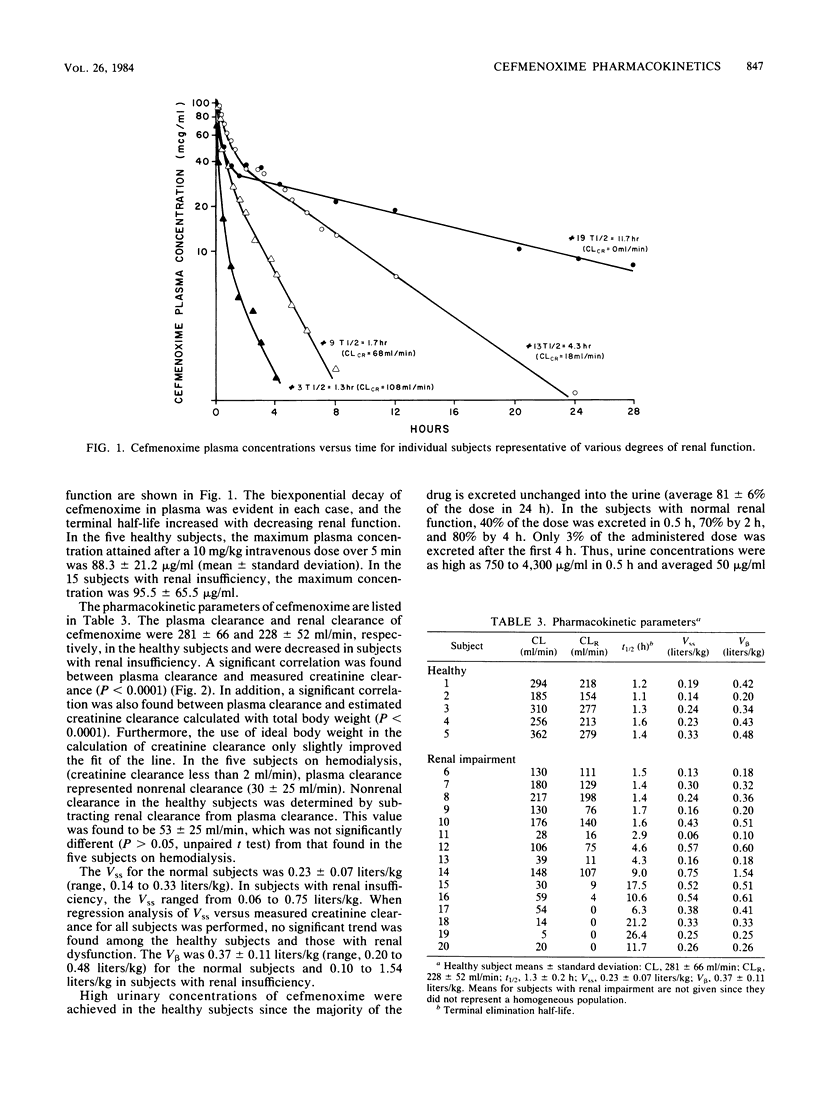
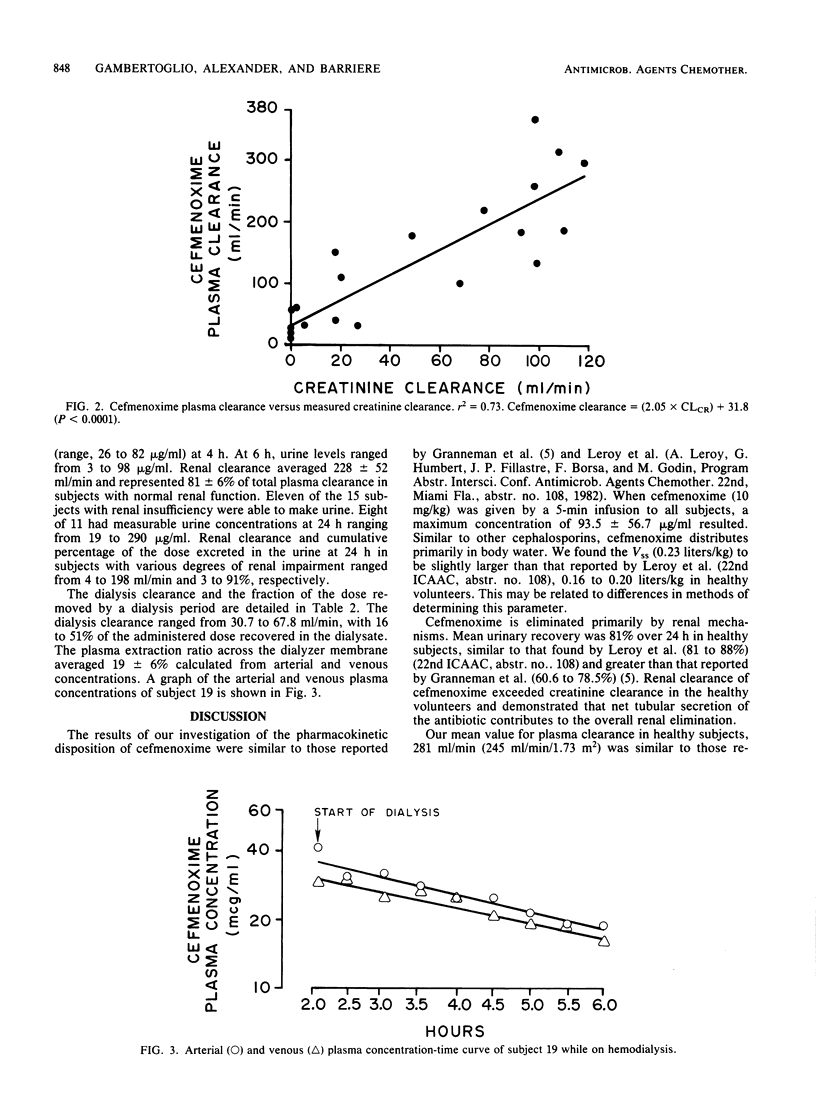
The pharmacokinetics of cefmenoxime were characterized in five healthy volunteers and in 15 subjects with various degrees of renal insufficiency after a single 10-mg/kg, 5-min intravenous infusion. Five of these subjects were studied both on hemodialysis and during an interdialytic period. Plasma, urine and dialysate were assayed for cefmenoxime by a specific high-pressure liquid chromatographic assay. Peak plasma concentrations of cefmenoxime were ca. 94 micrograms/ml after completion of the infusion. The mean plasma and renal clearances in the healthy volunteers were 281 +/- 66 and 228 +/- 52 ml/min, respectively. Plasma clearance declined in patients with renal insufficiency and correlated significantly with creatine clearance. The mean apparent volume of distribution at steady state in the healthy volunteers was 0.23 liters/kg and was not found to be significantly different in subjects with renal insufficiency. The mean cumulative 24-h urinary recovery of cefmenoxime in healthy volunteers was 81% of the administered dose and decreased with reduced renal function. Cefmenoxine dosage should be reduced in proportion to the decline in creatinine clearance. A simple nomogram for dose selection is provided.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Fuchs P. C., Jones R. N., Thornsberry C., Barry A. L., Gerlach E. H., Sommers H. M. Cefmenoxime (SCE-1365), a new cephalosporin: in vitro activity, comparison with other antimicrobial agents, beta-lactamase stability, and disk diffusion testing with tentative interpretive criteria. Antimicrob Agents Chemother. 1981 Dec;20(6):747–759. doi: 10.1128/aac.20.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman G. R., Sennello L. T., Steinberg F. J., Sonders R. C. Intramuscular and intravenous pharmacokinetics of cefmenoxime, a new broad-spectrum cephalosporin, in healthy subjects. Antimicrob Agents Chemother. 1982 Jan;21(1):141–145. doi: 10.1128/aac.21.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan I. A., Gambertoglio J. G., Barriere S. L., Conte J. E., Jr, Lin E. T. High-performance liquid chromatographic determination of cefmenoxime (AB-50912) in human plasma and urine. J Chromatogr. 1983 Apr 8;273(2):458–463. doi: 10.1016/s0378-4347(00)80971-8. [DOI] [PubMed] [Google Scholar]
- Stamm J. M., Girolami R. L., Shipkowitz N. L., Bower R. R. Antimicrobial activity of cefmenoxime (SCE-1365). Antimicrob Agents Chemother. 1981 Mar;19(3):454–460. doi: 10.1128/aac.19.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]


