Abstract
The methylation of the ribose 2′-OH of RNA occurs widely in nature and in all stable RNAs and occurs at five positions in yeast tRNA. 2′-O-methylation of tRNA at position 4 is interesting because it occurs in the acceptor stem (which is normally undermodified), it is the only 2′-O-methylation that occurs in the middle of a duplex region in tRNA, the modification is conserved in eukaryotes, and the features of the tRNA necessary for substrate recognition are poorly defined. We show here that Saccharomyces cerevisiae ORF YOL125w (TRM13) is necessary and sufficient for 2′-O-methylation at position 4 of yeast tRNA. Biochemical analysis of the S. cerevisiae proteome shows that Trm13 copurifies with 2′-O-methylation activity, using tRNAGly(GCC) as a substrate, and extracts made from a trm13-Δ strain have undetectable levels of this activity. Trm13 is necessary for activity in vivo because tRNAs isolated from a trm13-Δ strain lack the corresponding 2′-O-methylated residue for each of the three known tRNAs with this modification. Trm13 is sufficient for 2′-O-methylation at position 4 in vitro since yeast Trm13 protein purified after expression in Escherichia coli has the same activity as that produced in yeast. Trm13 protein binds substrates tRNAHis and tRNAGly(GCC) with KD values of 85 ± 8 and 100 ± 14 nM, respectively, and has a KM for tRNAHis of 10 nM, but binds nonsubstrate tRNAs very poorly (KD > 1 μM). Trm13 is conserved in eukaryotes, but there is no sequence similarity between Trm13 and other known methyltransferases.
Keywords: tRNA methyltransferase, Trm13, 2′-O-methylation, S. cerevisiae, tRNA processing, tRNA modification
INTRODUCTION
tRNA is the most abundant class of RNA found in the cell, accounting for nearly 90% of the RNA molecules that are produced in a typical yeast cell. tRNA molecules are extensively modified relative to other classes of RNA. Indeed, 101 of 120 known RNA modifications are found in tRNA (Limbach et al. 1994; Dunin-Horkawicz et al. 2006), and mature nonorganeller tRNA contains an average of 9.3 modified residues (Sprinzl et al. 1998). In the yeast Saccharomyces cerevisiae, 25 modifications have been found within the 34 sequenced cytoplasmic tRNAs, and the average tRNA bears ∼13 modifications (Limbach et al. 1994; Sprinzl et al. 1998).
One modification of particular interest is 2′-O-methylation of ribose. This modification is found in all three major phylogenetic domains and is one of the most common RNA modifications, comprising ∼8% of all tRNA modifications (366/4781) (Grosjean et al. 1995; Sprinzl et al. 1998), as well as a large fraction of the modifications found in rRNA and snRNA (Decatur and Fournier 2002, 2003). Formation of 2′-O-methylated residues is effected by either of two methods. The vast majority of 2′-O-methylation of rRNA, and some 2′-O-methylation of snRNA, is catalyzed by a common snoRNP complex comprised of Nop1p, Nop58p, Nop56p, and Snu13p subunits (Schimmang et al. 1989; Tyc and Steitz 1989; Wu et al. 1998; Lafontaine and Tollervey 1999, 2000; Watkins et al. 2000), which derives its specificity from Box C/D-dependent small nucleolar RNAs (snoRNAs) that guide modification to the appropriate base (Balakin et al. 1996; Kiss-Laszlo et al. 1996; Yu et al. 2005). Alternatively, the formation of 2′-O-methylated bases at all sites in eukaryotic and bacterial tRNAs, as well as at some sites in archaeal tRNAs (Clouet-d'Orval et al. 2005; Renalier et al. 2005), requires specific methyltransferases that act independently of exogenous guide RNAs.
2′-O-methylation is found at 13 positions in tRNA, but most commonly at 7 positions in eukaryotes and bacteria (4, 18, 32, 34, 39, 44, and 54), five of which (4, 18, 32, 34, and 44) are found in S. cerevisiae (Sprinzl et al. 1998). The S. cerevisiae genes responsible for three of these modifications are known. Trm7 methylates the ribose at positions 32 and 34 of three tRNAs, and trm7 mutants have an important role in translational fidelity (Pintard et al. 2002b). Trm3 modifies G18 on 10 of the 34 sequenced tRNAs (Cavaille et al. 1999), and, like many other modification proteins that act at remote sites from the anticodon (Hopper and Phizicky 2003), disruption of the gene has little obvious effect on growth or translation (Cavaille et al. 1999).
We are interested in methylation of ribose 2′-OH at position 4 of tRNA. This modification is interesting to study for four reasons. First, this modification is one of only a few that occurs in the amino acid acceptor stem of tRNA. The acceptor stem accounts for only 1.7% of all tRNA modifications, although it comprises 18% of the nucleotides of tRNA; moreover, 2′-O-methylation is one of only four modifications that are found in the acceptor stem. Second, this modification is one of very few 2′-O-methylations found in the middle of a duplex region. Of the 422 known occurrences of 2′-O-methylation found among characterized tRNAs (Sprinzl et al. 1998), 18 occur at position 4 in the middle of the acceptor stem, and only two other 2′-O-methylated residues are found in the middle of stems (at positions 29 and 64, within the anticodon stem and the T stem, respectively). Third, although 2′-O-methylation at position 4 is not common, this modification is widely conserved in eukaryotes, including humans. Fourth, there is no apparent common feature among tRNAs that are 2′-O-methylated at this position, although there must be high specificity, since only three of 34 sequenced tRNAs in S. cerevisiae bear this modification: tRNAHis, tRNAPro, and tRNAGly(GCC) (Fig. 1).
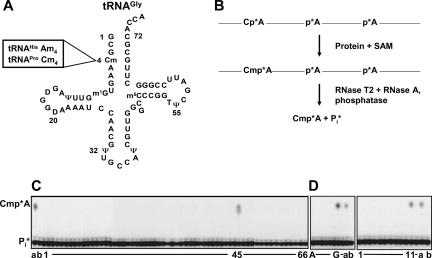
FIGURE 1.
Identification of the yeast ORF associated with 2′-O-methylation of tRNAGly(GCC) (A) Schematic of the two-dimensional structure of the tRNAGly(GCC) from S. cerevisiae. The box highlights the 2′-O-methylated base at position four of tRNAHis and tRNAPro. (B) Assay scheme to detect 2′-O-methylation of [α-32P] labeled tRNAGly. After incubation with protein and S-adenosylmethionine, tRNA is treated with RNase T2/RNase A and phosphatase and the products are resolved by thin-layer chromatography. This assay yields Cmp*A if the substrate is modified or Pi* (inorganic phosphate) if the substrate remains unmodified. (C) Assay of genomic collection of purified MORF fusion proteins for 2′-O-methylation activity. Labeled tRNAGly was incubated at 30°C for 18 h in 10 μL reaction mixtures containing S-adenosylmethionine and 2 μL (∼0.7 μg) protein from 59 pools of purified fusion proteins from the MORF collection (numbered 1–53, 55, 56, 58, 59, 64, and 66), as indicated, and analyzed as described in B and Materials and Methods. (a) S. cerevisiae crude extract, 30 μg; (b) buffer control. (D) Assay of subpools from pool 45 for tRNA 2′-O-methylation activity. (First panel) labeled tRNAGly was incubated with 2 μL of pools of purified MORF fusion proteins derived from the strains in rows A–H of plate 45, as indicated. (a) crude extract; (b) buffer. (Second panel) substrate was incubated with MORF fusion proteins from columns 1–12 from plate 45. (a) crude extract; (b) buffer.
We report here that the S. cerevisiae protein encoded by ORF YOL125w (now called TRM13) is responsible for 2′-O-methylation of tRNA at position 4 in vitro and in vivo and that one feature important for Trm13 selectivity appears to be the binding of tRNA substrates. Trm13 exhibits no obvious similarity to other known methyltransferases and may therefore be a member of a previously undescribed methyltransferase family.
RESULTS
Identification of a yeast ORF associated with 2′-O-methylation of position 4 of tRNAGly(GCC)
To identify the protein responsible for 2′-O-methylation at position 4 of tRNA, we biochemically screened the yeast proteome with tRNAGly(GCC) from S. cerevisiae (Fig. 1A), which is known to bear a 2′-O-methylcytidine (Cm) at position 4 (Yoshida 1973). To detect 2′-O-methylation, we transcribed a tRNAGly gene construct with [α-32P]ATP; incubated the labeled tRNA with a protein source and S-adenosyl-methionine (SAM); treated the RNA with RNase A, RNase T2, and phosphatase, to generate CmA arising from methylation of the 2′-OH between C4 and A5, and Pi from unreacted substrate and other labeled residues; and separated the CmA and Pi by thin layer chromatography (Fig. 1B). We then screened the S. cerevisiae proteome with this assay, using proteins purified from the movable open reading frame (MORF) library of yeast strains, each of which expresses a yeast ORF fused at its C terminus to a tri-partite affinity tag (Gelperin et al. 2005). To do this, we used the biochemical genomics approach described previously for another genomic library (Xing et al. 2002; Gu et al. 2003; Jackman et al. 2003; Alexandrov et al. 2005), employing pools of purified proteins. We first assayed pools of purified ORF-fusion proteins, each derived from 96 strains expressing ORFs, and found that pool 45 has the appropriate 2′-O-methyltransferase activity (Fig. 1C). We then assayed subpools of purified ORF-fusion proteins derived from the strains in microtiter plate 45 and found activity in Row G and column 11, indicating that ORF YOL125w in the strain at this position is associated with the activity (Fig. 1D). To confirm this assignment, we showed that the purified protein from this MORF strain had activity (data not shown) and sequenced the plasmid DNA from the MORF strain to confirm its identity. We assigned the name TRM13 to ORF YOL125w because, as shown below, this ORF encodes the 2′-O-methyltransferase that modifies residue 4 of tRNA in vitro and in vivo.
Trm13 (YOL125w) protein is necessary for 2′-O-methylation of position 4 of tRNAGly in vitro and in vivo
To determine if Trm13 is required for the 2′-O-methylation of tRNA, we prepared crude extracts from a MATα trm13-Δ strain, an otherwise isogenic control deletion strain (MATα ynr069c-Δ) and a related wild-type TRM13+/TRM13+ control strain, and compared their activities. As measured by protein titrations with the tRNAGly substrate, 30 μg extract from the MATα trm13-Δ strain has undetectable levels of 2′-O-methyltransferase activity, whereas activity is easily detected from both control strains containing Trm13p, using as little as 0.3 μg extract (Fig. 2A). Thus, Trm13 is necessary for 2′-O-methylation of tRNAGly in vitro. We show below that this modification occurs at position 4.
FIGURE 2.
Trm13 is required in vitro and in vivo for 2′-O-methylation of tRNA. (A) Extract from a trm13-Δ strain has no detectable 2′-O-methyltransferase activity. A MATα trm13-Δ strain, a control MATα strain (ynr069c-Δ), and a control diploid (TRM13 +/TRM13 +) strain were grown to mid-log phase in YP media containing 2% glucose, and extracts were assayed for 2′-O-methyltransferase activity for 2 h at 30°C with [α-32P] labeled tRNAGly as described in Figure 1, with decreasing amounts of crude extract (by factors of 10, beginning with ∼30 μg protein). (B–D) A trm13-Δ strain lacks 2′-O-methylated residues expected at position 4 of tRNA. tRNAPro (B), tRNAHis (C), and tRNAGly (D) were purified from wild-type and trm13-Δ strains, and their nucleosides were resolved by HPLC as described in Materials and Methods. tRNAs isolated from wild type contain all expected modifications as annotated in the literature (Yoshida 1973; Keith et al. 1983), whereas tRNAs isolated from trm13-Δ strains contain all expected modifications except the 2′-O-methylated residue. Traces shown in the left panel for each tRNA describe a sample modification whose quantification is unaffected by the deletion of TRM13. Traces shown in the right panel correspond to the 2′-O-methylated nucleosides found in each tRNA.
To determine if Trm13 is responsible for the 2′-O-methylation at position 4 of tRNA in vivo, we purified individual tRNAs from both the trm13-Δ strain and the wild-type control strain and analyzed their nucleoside composition using HPLC. As shown in Figure 2B–D, each of the three individual tRNAs known to have a 2′-O-methylated residue at position 4 has the expected 2′-O-methylated nucleoside (Cm for tRNAPro and tRNAGly and Am for tRNAHis), as measured by appearance of a peak in the expected location (Gehrke and Kuo 1989) and by the UV absorption spectrum of the corresponding peak. By contrast, each of the corresponding tRNAs from the trm13-Δ strain lack the 2′-O-methylated residues (Fig. 2B–D). Moreover, quantification of the nucleoside composition of these tRNAs revealed that the trm13-Δ strain had <1% of the corresponding 2′-O-methylated nucleosides (Table 1), whereas other nucleosides were present at the expected molar levels. Since each of these tRNAs has only a single 2′-O-methylated residue and this residue is known to be at position 4 (Yoshida 1973; Keith et al. 1983), we conclude that Trm13 is responsible for the production of 2′-O-methylated nucleosides at position 4 in vivo.
TABLE 1.
Quantification of nucleoside composition of tRNA purified from trm13-Δ and control strain by HPLC analysis
To confirm the position of the 2′-O-methylated residue that is lost in a trm13-Δ strain, we used primer extension to assay 2′-O-methylation of tRNAHis. As shown in Figure 3, there is a clear pause at position 5 in the primer extension of tRNAHis from wild-type cells, but no corresponding signal in the primer extension of tRNAHis from the trm13-Δ strain. Since, in addition, the band is only observed at low NTP concentrations (data not shown), we conclude that the band is a pause, as expected of 2′-O-methylation (Maden et al. 1995).
FIGURE 3.
tRNAHis from a trm13-Δ strain lacks 2′-O-methylation at position 4. (A) Clover leaf structure of tRNAHis. The position of the primer used for primer extension analysis is indicated by the arrow. The horizontal arrow indicates the base expected to be 2′-O-methylated. (B) Primer extension to assess 2′-O-methylation of tRNAHis at position 4. Low-molecular-weight RNA derived from wild-type or trm13-Δ strains, as indicated, was analyzed by primer extension using the primer shown in panel A. Lanes A, C, G, T contain the indicated dideoxynucleotide species to generate a sequencing ladder for tRNAHis, with the expected sequence as indicated. The arrow indicates the position of the expected primer extension stop due to the presence of the 2′-O-methylated nucleotide.
Trm13 is sufficient for 2′-O-methylation at position 4 on tRNAGly in vitro
To determine if Trm13 is sufficient for activity, we expressed and purified the protein from Escherichia coli and compared its activity to that produced from yeast, using a tRNAGly substrate uniquely labeled at the phosphate between C4 and A5 to enhance the sensitivity of the activity assay (Fig. 4A). To this end, we cloned TRM13 into a pET14b-based vector and purified the His6-Trm13 protein expressed in E. coli, resulting in a preparation that was at least 60% pure as measured by SDS-PAGE (data not shown). A corresponding purification of the yeast Trm13-MORF protein resulted in protein that was ∼50% pure and comprised of a doublet polypeptide as visualized by SDS-PAGE (data not shown).
FIGURE 4.
Purified His6-Trm13 protein catalyzes formation of 1 mol of 2′-O-methylcytidine at position 4 of tRNAGly in vitro. (A) Schematic of substrate tRNAGly bearing a single labeled 32P between C4 and A5 (C4*-tRNAGly). (B) His6-Trm13 protein purified from E. coli catalyzes 2′-O-methylation of residue 4 of tRNAGly. Reaction mixtures containing a labeled C4*-tRNAGly substrate and decreasing concentrations of purified His6-Trm13 protein (by factors of 10, beginning with 0.68 mg/mL) were incubated for 1.5 h at 30°C, and then RNA was analyzed as described in Materials and Methods. (C) His6-Trm13 protein catalyzes formation of 1 mol of 2′-O-methylcytidine per mole of tRNAGly. His6-Trm13 protein (3.2 μM) or buffer was incubated with 10 μM (2 nmol) unlabeled transcribed tRNAGly for 2 h, and RNA was treated with P1 nuclease and phosphatase to form nucleosides, which were resolved by HPLC. The extra product formed with Trm13 protein matches the accepted retention time and UV spectrum for 2′-O-methylcytidine (Gehrke and Kuo 1989). The expanded area shows a high-resolution view of the region of the HPLC trace corresponding to 2′-O-methylcytidine.
Comparison of the activity in crude extracts (CE) and purified proteins leads to two conclusions: First, the activity of His6-Trm13 protein purified from E. coli is comparable to that of Trm13 protein prepared from yeast, based on approximate specific activity measurements (Fig. 4B; Table 2). Thus, Trm13 is sufficient for activity on its own without additional S. cerevisiae proteins or RNA. Second, the yield of activity for both purifications is reasonable (20% for the yeast Trm13-MORF protein and 30% for the His6-YOL125w protein from E. coli), suggesting that we are not losing necessary cofactors during the purification.
TABLE 2.
Analysis of the specific activity of Trm13 protein purified from yeast or E. coli
To show that the modification catalyzed by Trm13 protein is indeed 2′-O-methylation and only occurs at a single position, we incubated the His6-Trm13 protein purified from E. coli with unlabeled tRNAGly and analyzed products by HPLC after digestion to form nucleosides. After incubation of 3.2 μM protein and 10 μM tRNAGly for 2 h, we observed the formation of 0.93 mol 2′-O-methylcytidine per mole tRNA, as determined by observation of a nucleoside with the mobility and the spectrum of Cm, and quantification of the yield (Fig. 4C). Since Trm13 can modify position 4 (Fig. 4B), and ∼1 mol of the 2′-O-methylcytidine product is produced per mole of tRNA substrate, we conclude that Trm13 protein catalyzes 2′-O-methylation of residue 4 and most likely of no other residue. Based on this analysis of catalytic activity of Trm13 and the in vivo analysis of trm13-Δ strains described above, we conclude that Trm13 is both necessary and sufficient for 2′-O-methylation at position 4 of substrate tRNAs.
Trm13 recognizes its tRNA substrates by binding
One important question about Trm13 tRNA Nm4 2′-O-methyltransferase is the nature of the common features of its tRNA substrates. Trm13 protein recognizes and modifies residue 4 of tRNAGly(GCC), tRNAHis, and tRNAPro, but these tRNAs have no obvious features in common that are not also shared with some of the 31 other yeast tRNAs that are not 2′-O-methylated at residue 4.
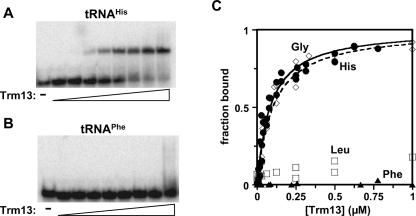
To begin to address this question, we investigated the binding of tRNA to Trm13. Trm13 binds its substrate tRNAHis efficiently, with a KD value of 85±8 nM, measured using an electrophoretic mobility shift assay (EMSA), with Trm13 protein in excess over tRNA (Fig. 5A,C). Using the same assay, we demonstrated that Trm13 also binds its substrate tRNAGly efficiently, with a KD = 100 ± 14 nM (Fig. 5C). The observed KD values for these tRNA species were not dependent on the amount of tRNA in the binding experiment and therefore reflect an apparent binding constant, not simply a titration artifact.
FIGURE 5.
Trm13 protein binds tRNA substrates with higher affinity than nonsubstrate tRNAs. (A,B) Analysis of binding by EMSA. tRNAHis (0.3 nM) and tRNAPhe (0.3 nM) were incubated with varying amounts of His6-Trm13 protein in the presence of 10 μg/mL poly(A) RNA and electrophoresed on a 5% acrylamide gel as described in Materials and Methods. His6-Trm13 protein varied from 2 μM to 0.9 nM (by factors of 3). The first lane in each panel contains no added Trm13 protein. (C) Determination of binding constants. The fraction of total RNA bound in each EMSA assay as shown in panel A was quantified by a PhosphorImager and plotted as a function of Trm13 protein concentration (-◊-, tRNAGly; -•-, tRNAHis; -□-, tRNALeu; and -▴-, tRNAPhe). For tRNAHis and tRNAGly, the resulting data were well fit by a single binding isotherm, and apparent binding constants were determined to be 85 ± 8 nM for tRNAHis and 100 ± 14 nM for tRNAGly. Since little or no apparent binding is observed for tRNALeu and tRNAPhe at even the highest protein concentrations tested, we can estimate lower limits to the KD for these tRNA species of >1 μM and >3 μM, respectively.
Comparison of binding to nonsubstrate tRNAs demonstrates that Trm13 binds preferentially to substrate tRNAs. As shown in Figure 5, tRNAPhe (Fig. 5B,C) and tRNALeu (Fig. 5C) bind very poorly (KD values estimated at >1000 nM and >3000 nM, respectively). Similar binding differences between substrate and nonsubstrate tRNAs are observed whether or not Mg++ is present in the gel buffer. In addition, binding to nonsubstrate tRNAs is as weak as binding to an equivalent amount of poly(A) (data not shown). Thus, it appears that Trm13 protein efficiently discriminates substrates from nonsubstrates by binding.
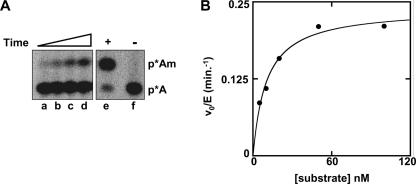
The steady-state kinetic parameters for Trm13 activity with tRNAHis demonstrate that Trm13 efficiently catalyzes 2′-O-methylation on this substrate. To obtain these parameters, we assayed 2′-O-methylation of a transcribed tRNAHis substrate singly labeled between C3 and A4, followed by P1 nuclease treatment and thin-layer chromatography to separate pAm from pA and quantification by a PhosphorImager to measure activity (Fig. 6A). Although this synthetic tRNAHis substrate lacks the G−1 residue that is normally added by tRNAHis guanylyltransferase (Thg1) to tRNAHis in vivo, we note that tRNAHis isolated from Thg1-depleted cells lacks G−1 but is fully modified at position 4 in vivo (Gu et al. 2005). A plot of the linear initial rates measured at each substrate concentration follows Michaelis–Menten kinetics (Fig. 6B), and a fit of these data yields a KM of 10 nM and kcat of 0.24 min−1, which combine to yield a kcat/KM of 4.0×105 M−1sec−1.
FIGURE 6.
Kinetic parameters for His6-Trm13 2′-O-methyltransferase activity with tRNAHis. His6-Trm13 protein (0.5 nM) was incubated with C3*-tRNAHis at different concentrations (5 nM, 10 nM, 20 nM, 50 nM, and 100 nM) for 2.5–20 min at 30°C and analyzed for 2′-O-methylation by TLC as described in Materials and Methods. (A) Representative time course of 2′-O-methyltransfease activity of His6-Trm13 protein with tRNAHis. 2′-O-methyltransferase activity was assayed with 0.5 nM Trm13 and 5 nM tRNAHis at (lane a) 2.5 min, (lane b) 5 min, (lane c) 10 min, (lane d) 20 min; (lane e) the results of reaction with 100 nM tRNAHis and 920 nM His6-Trm13 protein for 20 min, showing that all the substrate can react; (lane f) 100 nM tRNAHis with no Trm13 protein for 20 min. (B) Determination of steady-state kinetic parameters for Trm13 2′-O-methyltransferase activity with tRNAHis. Trm13-catalyzed 2′-O-methylation of tRNAHis occurs with a steady-state kcat = 0.24 min−1, KM = 10 nM, and kcat/KM = 4.0 × 105 M−1sec−1, as determined by fit of the data to the Michealis–Menten steady-state rate equation (Materials and Methods).
Trm13 protein is highly conserved within eukaryotes and is not similar to other methyltransferases
Analysis of the Trm13 protein sequence by BLAST reveals a number of predicted eukaryotic proteins with homology with Trm13, but no proteins with homology in organisms from other domains. Homologs are found widely in fungi (including Candida glabrata [E value e−72, 41% identity, 57% similarity]; Kluyveromyces lactis, e−65; Candida albicans, e−39; and Schizosaccharomyces pombe, e−15), vertebrates (such as Homo sapiens, e−26; Mus musculus, e−26; Xenopus laevis, e−29; Canis familiaris, e−27; and Danio rerio, e−26), invertebrates (such as Drosophila melanogaster, e−30 Anopheles gambiae, e−26; and Caenorhabditis elegans, e−15), plants (such as Arabidopsis thaliana, e−24; and Oryza sativa, e−12), and protists (such as Trypanosoma cruzi, e−14, 22% identity, 39% similarity). Although the overall similarity between yeast Trm13 and its protein homologs from other organisms is not extremely high, clusters of completely conserved amino acids are observed over the entire sequence (Fig. 7), perhaps indicating a common active site for 2′-O-methylation in the context of a varied structural environment between different members of this protein family. The widespread distribution of putative Trm13 homologs in eukaryotes contrasts with its absence in archaea and bacteria and is consistent with the known distribution of 2′-O-methylation at position 4 in eukaryotic tRNA, but not in tRNA from other domains.
FIGURE 7.
Alignment of Trm13 homologs. Homologs of Trm13 were identified by BLAST search (Altschul et al. 1997) and sequences from several representative species, as indicated next to each sequence line, were aligned using Multalin (Corpet 1988) with the following parameters: gap weight = 6 and gap length weight = 2. Dark shading indicates >80% consensus; light shading indicates >50% consensus at each position. For clarity, several regions with very low homology were omitted from this alignment; these breaks in the sequence are indicated with double hash marks. The numbering shown above each block of sequence represents the numbering of the relevant S. cerevisiae protein residue.
The Trm13 protein family appears to represent a new family of methyltransferases. There is no obvious homology with other methyltransferases, based on BLAST search. Moreover, there is little, if any, similarity between Trm13 protein and other tRNA 2′-O-methyltransferases identified in yeast (Cavaille et al. 1999; Pintard et al. 2002a,b; Lapeyre and Purushothaman 2004), as determined by pairwise BLAST, or with the RrmJ/fibrillarin superfamily of 2′-O-methyltransferases (Feder et al. 2003). Since Trm13 protein is described in the PFAM database as a domain of unknown function (DUF 715) and all of the putative Trm13 protein homologs are annotated as novel or hypothetical proteins, the Trm13 family might represent an additional family of methyltransferase proteins, although the low degree of similarity among methyltransferases within the family precludes a definitive statement in this regard (Schubert et al. 2003).
We note that trm13-Δ strains do not have an obvious growth defect. No growth difference was observed between trm13-Δ/trm13-Δ strains and its otherwise isogenic TRM13 +/TRM13 + parent when grown on rich media (YP) containing different fermentable sugars (raffinose, galactose, and glucose) or nonfermentable carbon sources (ethanol and glycerol), or in synthetic complete media containing glucose, at a variety of temperatures including 18°C, 25°C, 30°C, 33°C, 35°C, and 37°C (data not shown).
DISCUSSION
We have described several lines of evidence demonstrating that the protein encoded by S. cerevisiae ORF YOL125w (now called Trm13 protein) is responsible for 2′-O-methylation of tRNAGly(GCC), tRNAHis, and tRNAPro at residue 4. Trm13 protein copurifies with 2′-O-methyltransferase activity during screening of a genomic collection of purified S. cerevisiae fusion proteins from the MORF genomic library (Gelperin et al. 2005) when assayed with labeled tRNAGly(GCC) and S-adenosylmethionine as methyl donor (Fig. 1). Trm13 protein is necessary for 2′-O-methylation at position 4 in vitro since a crude extract of a trm13-Δ strain has no detectable 2′-O-methyltransferase activity, <1% of that observed in extracts of wild-type cells (Fig. 2). Trm13 is required in vivo since each of three tRNAs that are known to have 2′-O-methylated residues at position 4 lack the corresponding modified residues when isolated from a trm13-Δ strain (Figs. 2,3; Table 1). Trm13 protein is sufficient for activity since yeast His6-Trm13 protein purified from E. coli is as active as Trm13-fusion protein purified from yeast (Table 2), 2′-O-methylates position 4 of a specifically labeled tRNAGly substrate, and methylates the same residue of an unlabeled tRNAGly substrate with molar efficiency (Fig. 4). These data demonstrate that Trm13 is both necessary and sufficient for 2′-O-methylation of substrate tRNAs at position 4 in vitro and in vivo.
Our analysis of Trm13 protein conservation suggests that Trm13 may be part of a new family of methyltransferases found within eukaryotes (Fig. 7), consistent with the known eukaryotic distribution of 2′-O-methylated residues at the 4 position of tRNA (Sprinzl et al. 1998), although the large variations among members of this family and other methyltransferase families precludes a definitive conclusion in this regard (Schubert et al. 2003). The lack of similarity between the Trm13 family and other known methyltransferases and 2′-O-methyltransferases suggests a novel mechanism for catalysis and/or substrate recognition for this protein family or, alternatively, that subtle mechanistic or structural similarities exist between Trm13 and other known methyltransferases. Moreover, the variations within the Trm13 family may imply that some proteins in the family have slightly altered activities or substrate recognition properties. Presumably the differences in substrate modification for the Trm13 family are found in the structure of the tRNA substrates themselves, since there is no obvious pattern among the identity of tRNAs containing a 2′-O-methylated residue at position 4 or the base that is modified. tRNAGly is the most common species with this modification, with seven occurrences in yeast, plants, and humans; followed by five tRNAPro species found in fungi and mouse; two tRNAVal and two tRNASer found exclusively in Drosophila melanogaster; and two tRNAHis in S. cerevisiae. Furthermore, all four bases are found to be 2′-O-methylated at position 4 in the sequenced tRNAs, including two instances of Gm, seven of Um, seven of Cm, and two of Am. It is interesting that the two occurrences of Am at position 4 are the two isoforms of tRNAHis in S. cerevisiae, which are the only tRNAs of 561 sequenced tRNAs that contain a 2′-O-methylated adenosine residue at any location. By contrast, there are 65 tRNAs containing Um, 141 tRNAs that contain Cm, and 162 that have Gm (Sprinzl et al. 1998).
Analysis of Trm13 protein activity reveals that tRNA binding may be an important factor in determining how Trm13 2′-O methylates its three substrates. Our data demonstrate that Trm13 binds to its tRNA substrates with high affinity (KD, tRNAGly = 100 ± 14 nM and KD, tRNAHis = 85 ± 8 nM) and efficiently catalyzes 2′-O-methylation of tRNAHis, with a kcat/KM of 4.0 × 105 M−1sec−1. Yet, Trm13 exhibits little, if any, detectable binding (Fig. 5) or activity (data not shown) with yeast tRNA species such as tRNALeu and tRNAPhe, which are not known to contain the 2′-O-methyl modification at position 4 in vivo. Thus, we suggest that the binding of Trm13 protein to substrate tRNAs is an important factor in determination of tRNAs that are methylated at position 4. However, it is unclear which features are shared by the three Trm13 substrate tRNAs and/or by the 31 nonsubstrate yeast tRNAs that could account for the differences in modification or perhaps binding. Further experiments and analysis will be required to delineate the specific determinants for tRNA recognition by Trm13 that are responsible for the selective modification of substrate tRNAs.
The role of 2′-O-methylation of tRNA at position 4 remains to be determined. Structurally, 2′-O-methylation stabilizes the 3′-endo ribose conformation commonly found in A-form RNA (Kawai et al. 1992), although a role such as this in the middle of the acceptor stem might seem unnecessary. Mechanistically, the translocation step of E. coli ribosomes is inhibited by 2′-O-methylation of the acceptor stem at positions 66, 70, or 71 (as well as at 76), or by 2′-deoxy substitution at positions 71 or 76 (Feinberg and Joseph 2001), suggesting different causes of the inhibition. Thus, it is possible that 2′-O-methylation at position 4 of certain tRNAs could be important for one of the steps of translation in yeast. An earlier investigation of phenotypes arising from disruption of seven ORFs in chromosome XV of S. cerevisiae showed that there was no phenotype of trm13-Δ (yol125w) mutants in a variety of conditions, although slower than normal growth of a trm13-Δ mutant was reported (Hajji et al. 1999), which we do not observe. Despite the lack of phenotype of trm13 mutants, the widespread occurrence in eukaryotes of 2′-O-methylated residues at position 4 of certain tRNAs underscores the importance of this modification on evolutionary timescales. Like mutation of a number of other genes encoding modifications remote from the anticodon, a role for this modification may emerge from study of the appropriate combinations of mutations (Alexandrov et al. 2006) or the appropriate biochemical step.
MATERIALS AND METHODS
Growth of strains from the MORF library and purification of proteins
Strains from the MORF library were grown and purified using IgG Sepharose affinity chromatography as described previously (Gelperin et al. 2005). After cleavage of the bound protein by E. coli-purified GST-3C protease (Alexandrov et al. 2001; Gelperin et al. 2005), the resulting eluted MORF proteins were dialyzed overnight into storage buffer (20 mM HEPES at pH 7.5, 200 mM NaCl, 2 mM DTT, 50% glycerol) and stored at −20°C.
MORF-Trm13 was purified by immobilized metal ion affinity chromatography (IMAC) on Talon resin (Clontech) (Gelperin et al. 2005). Crude extracts prepared in CE2 buffer (20 mM HEPES at pH 7.5, 1 M NaCl, 5% glycerol, 2 mM β-mercaptoethanol) containing 2.5 μg/mL pepstatin, 2.5 μg/mL leupeptin, and 1 mM Pefabloc were diluted 1:1 with no salt CE2 buffer, mixed with prewashed Talon resin (4 mL per 500 mL cells), and mixed for 1 h at 4°C. Unbound proteins were removed by low-speed centrifugation, followed by two washes with 10 mL buffer containing 0.5 M NaCl and two washes with buffer containing 0.5 M NaCl and 10 mM imidazole (pH 7.7), and then bound protein was eluted with 1 mL buffer containing 0.5 M NaCl and 250 mM imidazole (pH 7.7), followed by dialysis into storage buffer and storage at −20°C.
2′-O-methyltransferase activity assay
2′-O-methyltransferase activity was assayed with [α-32P] labeled tRNA in 10 μL reaction mixtures containing 50 mM Tris HCl (pH 8.0), 2.5 mM MgCl2, 1 mM dithiothreitol (DTT), 50 mM ammonium acetate, 0.05 mM ethylenediaminetetraacetic acid (EDTA), 1 mM spermidine, 0.5 mM S-adenosyl-methionine (SAM), ∼200,000 cpm (6.3×1017 cpm/mol) [α-32P] labeled tRNAGly, and a protein source (either crude extract or purified protein). Reactions were initiated by addition of protein, incubated for 5 min to overnight at 30°C and then stopped by adding 90 μL of buffer containing 0.5 M Tris HCl (pH 8.0) and 10–20 μg of carrier RNA, followed by phenol extraction and ethanol precipitation. Then, RNA was treated with either RNase T2 or nuclease P1 as indicated. To produce nucleotide 3′-monophosphate species, the RNA was resuspended in a 4 μL mixture containing 20 mM sodium acetate (pH 5.2), 1 mM EDTA, 1 U of RNase T2 (Invitrogen), and 10 μg RNase A, incubated at 50°C for 1 h, and then treated with 1 U alkaline phosphatase (Roche) for 30 min. To produce nucleotide 5′-monophosphate species, the RNA was resuspended in a 5 μL mixture containing 25 mM sodium acetate (pH 5.2), 0.2 mM ZnCl2 and 2.5 μg P1 nuclease (MP Biomedicals), and incubated at 50°C for 1 h. The digested RNA was then applied to cellulose TLC plates (EM Scientific) and resolved in buffer containing isobutyric acid:NH4OH:H2O (66:1:33).
Preparation of crude extracts and tRNA from yeast strains lacking Trm13
The MATα and MATa haploid trm13-Δ strains, the homozygous diploid deletion strain (trm13-Δ/trm13-Δ), and its isogenic diploid parent (BY4743) were obtained from Open Biosystems. Preparation of crude extracts by disruption of the yeast cells in the presence of zirconium beads, isolation of low-molecular-weight RNA by hot phenol extraction, and purification of individual tRNA species from the resulting low-molecular-weight RNA using biotinylated oligonucleotide probes (Integrated DNA Technologies) complementary to the tRNA of interest were all performed as previously described (Xing et al. 2004). Probes were:
BioHis for tRNAHis (5′/Biotin/GCCATCTCCTAGAATCGAACC)
BioGly for tRNAGly(GCC) (5′/Biotin/TGGTGCGCAAGCCCGGAATCAACC)
BioPro for tRNAPro (5′/Biotin/GGAATTGAACCCAGGGCCTCTCGCA)
HPLC analysis of nucleosides
To analyze the individual nucleoside content of each tRNA species, purified tRNAGly(GCC), tRNAPro, or tRNAHis isolated from yeast (1 μg) or in vitro transcribed tRNAGly(GCC) (50 μg) incubated with Trm13 protein were treated with 5–10 μg of P1 nuclease at 37°C for 16 h, and then with 8 U of calf intestinal alkaline phosphatase at 37°C for 3 h. Nucleosides were resolved by HPLC (Waters Alliance Model 2690, equipped with Waters 996 photodiode array detector) on a reverse phase C18 column as described (Gehrke and Kuo 1989), and modified nucleosides were quantified as described (Jackman et al. 2003; Xing et al. 2004), using either known extinction coefficients for the modified nucleosides where available (Ψ, m5C, m1G, DHU, and T) or using the corresponding extinction coefficients for the unmodified major nucleosides for the species for which this information is not available (Cm, Am).
Primer extension assays
Primer extension assays were performed essentially as described by Jackman et al. (2003), with 5′-end-labeled primers, using 0.1–0.4 mM of each dNTP (G, A, T, and C) and 0.7 U of AMV-reverse transcriptase (Promega, high concentration) in 1× AMV-RT reaction buffer (Promega). Reactions were resolved on 10% polyacrylamide, 4 M urea gels, and visualized using the PhosphorImager (Molecular Dynamics).
Preparation and assay of specifically labeled tRNA substrates
tRNAGly(GCC) was specifically labeled at the 3′ phosphate of C4, essentially as previously described (Yu 1999; Jackman et al. 2003). Briefly, in vitro transcribed tRNAGly(GCC) was used as the substrate for RNase H digestion (RNase H generously provided by Y.-T. Yu, University of Rochester Medical Center) between residues 4 and 5, as directed by a chimeric oligonucleotide (5′-GmCmUmUmGCGCUmCmCmAmUmCmCmAmUmCm-3′, HHMI/Keck Synthesis facility, Yale University). After dephosphorylation and rephosphorylation of the 3′ fragment using [γ-32P]ATP (ICN, 7000 Ci/mmol), a synthetic RNA oligonucleotide comprising the first 4 nt of tRNAGly (5′-GCGC-3′; Dharmacon Research) was ligated to the 5′ end of the labeled RNA using T4 DNA ligase (USB) and a bridging DNA oligonucleotide, yielding full-length, specifically labeled tRNAGly (C4*Gly) after PAGE purification.
tRNAHis specifically labeled at the phosphate following C3 was prepared in a similar method, by RNase H digestion with a different chimeric oligonucleotide (5′-AmAmGmAmUmGGCTCmCmAmGmUmGmCmAmCm-3′). However, since ligation of the 3-nucleotide (nt) 5′ fragment from tRNAHis does not readily occur, the labeled 3′-half-molecule was ligated to a synthetic 18-mer RNA comprised of a 15-residue leader and the first 3 nt of tRNAHis (5′-CCCUGUGCAAGCAACGCC-3′). This 5′-extended tRNAHis molecule was cleaved in a second RNase H reaction (Gu et al. 2003) designed to produce a full-length, specifically labeled, wild-type tRNAHis (C3*His), which was subsequently purified by PAGE.
Reactions with specifically labeled tRNA species were carried out using the same 2′-O-methyltransferase activity assay as described above, except that the RNase T2 digestion products from C4*Gly were resolved on PEI-cellulose TLC plates in 0.5 M LiCl. The P1 nuclease products from C3*His were resolved by cellulose TLC in isobutyric acid:NH4OH:H2O (66:1:33), as was used for the assay of uniformly labeled tRNAGly described above.
Purification of His6-Trm13 from E. coli
To express Trm13 as an N-terminal His6-ORF fusion protein in E. coli, TRM13 DNA was amplified from yeast genomic DNA (derived from strain BY4700, ATCC # 200,866) with Pfu ultra-high-fidelity DNA polymerase (Stratagene), and cloned by ligation-independent cloning (LIC) into LIC vector AVA421 derived from pET14b (Alexandrov et al. 2004). The resulting plasmid (pMLW40-1) contained a silent polymorphism at codon 96 of TRM13, which was found to be AAC but is annotated as AAT in the Saccharomyces Genome Database, both of which encode asparagine. After transformation of pMLW40-1 into E. coli BL21(DE3) pLysS cells (Novagen), an individual transformant was grown at 30°C in LB with 100 μg/mL ampicillin to OD600 0.5 and induced overnight at 18°C with 1 mM isopropyl β-D-thiogalactopyranoside. His6-Trm13 was purified by IMAC as previously described (Jackman et al. 2003), and the protein was dialyzed into storage buffer containing 20 mM HEPES (pH 7.5), 200 mM NaCl, 50% glycerol, and 2 mM β-mercaptoethanol for storage at −20°C. Trm13 protein (3.4 mg/mL) was judged to be ∼60% pure by SDS-PAGE and Coomassie staining.
Electrophoretic mobility shift assays (EMSA) to measure tRNA binding
Binding of Trm13 protein to tRNA was performed as described (Apostol and Greer 1991) in buffer containing 28 mM HEPES (pH 7.5), 40 mM NaCl, 5 mM MgCl2, 0.5 mM DTT, 2.5 mM spermidine, 50 μg/mL BSA, and 10 μg/mL poly(A) RNA. Binding reactions were incubated for 20 min on ice, and samples were loaded on a prerun 5% polyacrylamide gel (49:1 acrylamide:bis) prepared in buffer containing 50 mM Tris borate (pH 8.3), 1 mM EDTA, 3 mM MgCl2, and 5% glycerol, and electrophoresed at 4°C in buffer containing 50 mM Tris-Borate (pH 8.3), 3 mM MgCl2, and 1 mM EDTA. Thermodynamic binding data were fit using KaleidaGraph (Synergy software) to a standard binding isotherm (Equation 1), in which the fraction of substrate bound ([E·L]/[Ltotal]), normalized to the endpoint, was plotted against the concentration of enzyme ([E]).
ACKNOWLEDGMENTS
We are grateful to L. Kotelawala for valuable help and discussions and Y. Kon for technical assistance. This research was supported by NIH grant GM52347 to E.M.P.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.399607.
REFERENCES
- Alexandrov, A., Dutta, K., Pascal, S.M. MBP fusion protein with a viral protease cleavage site: One-step cleavage/purification of insoluble proteins. Biotechniques. 2001;30:1194–1198. doi: 10.2144/01306bm01. [DOI] [PubMed] [Google Scholar]
- Alexandrov, A., Vignali, M., LaCount, D.J., Quartley, E., de Vries, C., De Rosa, D., Babulski, J., Mitchell, S.F., Schoenfeld, L.W., Fields, S., et al. A facile method for high-throughput co-expression of protein pairs. Mol. Cell. Proteomics. 2004;3:934–938. doi: 10.1074/mcp.T400008-MCP200. [DOI] [PubMed] [Google Scholar]
- Alexandrov, A., Grayhack, E.J., Phizicky, E.M. tRNA m7G methyltransferase Trm8p/Trm82p: Evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA. 2005;11:821–830. doi: 10.1261/rna.2030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov, A., Chernyakov, I., Gu, W., Hiley, S.L., Hughes, T.R., Grayhack, E.J., Phizicky, E.M. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol, B.L., Greer, C.L. Preferential binding of yeast tRNA ligase to pre-tRNA substrates. Nucleic Acids Res. 1991;19:1853–1860. doi: 10.1093/nar/19.8.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakin, A.G., Smith, L., Fournier, M.J. The RNA world of the nucleolus: Two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–834. doi: 10.1016/s0092-8674(00)80156-7. [DOI] [PubMed] [Google Scholar]
- Cavaille, J., Chetouani, F., Bachellerie, J.P. The yeast Saccharomyces cerevisiae YDL112w ORF encodes the putative 2′- O-ribose methyltransferase catalyzing the formation of Gm18 in tRNAs. RNA. 1999;5:66–81. doi: 10.1017/s1355838299981475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouet-d'Orval, B., Gaspin, C., Mougin, A. Two different mechanisms for tRNA ribose methylation in Archaea: A short survey. Biochimie. 2005;87:889–895. doi: 10.1016/j.biochi.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decatur, W.A., Fournier, M.J. rRNA modifications and ribosome function. Trends Biochem. Sci. 2002;27:344–351. doi: 10.1016/s0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- Decatur, W.A., Fournier, M.J. RNA-guided nucleotide modification of ribosomal and other RNAs. J. Biol. Chem. 2003;278:695–698. doi: 10.1074/jbc.R200023200. [DOI] [PubMed] [Google Scholar]
- Dunin-Horkawicz, S., Czerwoniec, A., Gajda, M.J., Feder, M., Grosjean, H., Bujnicki, J.M. MODOMICS: A database of RNA modification pathways. Nucleic Acids Res. 2006;34:D145–D149. doi: 10.1093/nar/gkj084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder, M., Pas, J., Wyrwicz, L.S., Bujnicki, J.M. Molecular phylogenetics of the RrmJ/fibrillarin superfamily of ribose 2′-O-methyltransferases. Gene. 2003;302:129–138. doi: 10.1016/s0378-1119(02)01097-1. [DOI] [PubMed] [Google Scholar]
- Feinberg, J.S., Joseph, S. Identification of molecular interactions between P-site tRNA and the ribosome essential for translocation. Proc. Natl. Acad. Sci. 2001;98:11120–11125. doi: 10.1073/pnas.211184098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke, C.W., Kuo, K.C. Ribonucleoside analysis by reversed-phase high-performance liquid chromatography. J. Chromatogr. 1989;471:3–36. doi: 10.1016/s0021-9673(00)94152-9. [DOI] [PubMed] [Google Scholar]
- Gelperin, D.M., White, M.A., Wilkinson, M.L., Kon, Y., Kung, L.A., Wise, K.J., Lopez-Hoyo, N., Jiang, L., Piccirillo, S., Yu, H., et al. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes & Dev. 2005;19:2816–2826. doi: 10.1101/gad.1362105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean, H., Sprinzl, M., Steinberg, S. Post-transcriptionally modified nucleosides in transfer RNA: Their locations and frequencies. Biochimie. 1995;77:139–141. doi: 10.1016/0300-9084(96)88117-x. [DOI] [PubMed] [Google Scholar]
- Gu, W., Jackman, J.E., Lohan, A.J., Gray, M.W., Phizicky, E.M. tRNAHis maturation: An essential yeast protein catalyzes addition of a guanine nucleotide to the 5′ end of tRNAHis. Genes & Dev. 2003;17:2889–2901. doi: 10.1101/gad.1148603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, W., Hurto, R.L., Hopper, A.K., Grayhack, E.J., Phizicky, E.M. Depletion of Saccharomyces cerevisiae tRNA(His) guanylyltransferase Thg1p leads to uncharged tRNAHis with additional m(5)C. Mol. Cell. Biol. 2005;25:8191–8201. doi: 10.1128/MCB.25.18.8191-8201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajji, K., Clotet, J., Arino, J. Disruption and phenotypic analysis of seven ORFs from the left arm of chromosome XV of Saccharomyces cerevisiae . Yeast. 1999;15:435–441. doi: 10.1002/(SICI)1097-0061(19990330)15:5<435::AID-YEA367>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hopper, A.K., Phizicky, E.M. tRNA transfers to the limelight. Genes & Dev. 2003;17:162–180. doi: 10.1101/gad.1049103. [DOI] [PubMed] [Google Scholar]
- Jackman, J.E., Montange, R.K., Malik, H.S., Phizicky, E.M. Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA. 2003;9:574–585. doi: 10.1261/rna.5070303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, G., Yamamoto, Y., Kamimura, T., Masegi, T., Sekine, M., Hata, T., Iimori, T., Watanabe, T., Miyazawa, T., Yokoyama, S. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2′-hydroxyl group. Biochemistry. 1992;31:1040–1046. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- Keith, G., Pixa, G., Fix, C., Dirheimer, G. Primary structure of three tRNAs from brewer's yeast: tRNAPro2, tRNAHis1 and tRNAHis2. Biochimie. 1983;65:661–672. doi: 10.1016/s0300-9084(84)80030-9. [DOI] [PubMed] [Google Scholar]
- Kiss-Laszlo, Z., Henry, Y., Bachellerie, J.P., Caizergues-Ferrer, M., Kiss, T. Site-specific ribose methylation of preribosomal RNA: A novel function for small nucleolar RNAs. Cell. 1996;85:1077–1088. doi: 10.1016/s0092-8674(00)81308-2. [DOI] [PubMed] [Google Scholar]
- Lafontaine, D.L., Tollervey, D. Nop58p is a common component of the box C+D snoRNPs that is required for snoRNA stability. RNA. 1999;5:455–467. doi: 10.1017/s135583829998192x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine, D.L., Tollervey, D. Synthesis and assembly of the box C+D small nucleolar RNPs. Mol. Cell. Biol. 2000;20:2650–2659. doi: 10.1128/mcb.20.8.2650-2659.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyre, B., Purushothaman, S.K. Spb1p-directed formation of Gm2922 in the ribosome catalytic center occurs at a late processing stage. Mol. Cell. 2004;16:663–669. doi: 10.1016/j.molcel.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Limbach, P.A., Crain, P.F., McCloskey, J.A. Summary: The modified nucleosides of RNA. Nucleic Acids Res. 1994;22:2183–2196. doi: 10.1093/nar/22.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden, B.E., Corbett, M.E., Heeney, P.A., Pugh, K., Ajuh, P.M. Classical and novel approaches to the detection and localization of the numerous modified nucleotides in eukaryotic ribosomal RNA. Biochimie. 1995;77:22–29. doi: 10.1016/0300-9084(96)88100-4. [DOI] [PubMed] [Google Scholar]
- Pintard, L., Bujnicki, J.M., Lapeyre, B., Bonnerot, C. MRM2 encodes a novel yeast mitochondrial 21S rRNA methyltransferase. EMBO J. 2002a;21:1139–1147. doi: 10.1093/emboj/21.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintard, L., Lecointe, F., Bujnicki, J.M., Bonnerot, C., Grosjean, H., Lapeyre, B. Trm7p catalyses the formation of two 2′-O-methylriboses in yeast tRNA anticodon loop. EMBO J. 2002b;21:1811–1820. doi: 10.1093/emboj/21.7.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renalier, M.H., Joseph, N., Gaspin, C., Thebault, P., Mougin, A. The Cm56 tRNA modification in archaea is catalyzed either by a specific 2′-O-methylase, or a C/D sRNP. RNA. 2005;11:1051–1063. doi: 10.1261/rna.2110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang, T., Tollervey, D., Kern, H., Frank, R., Hurt, E.C. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989;8:4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, H.L., Blumenthal, R.M., Cheng, X. Many paths to methyltransfer: A chronicle of convergence. Trends Biochem. Sci. 2003;28:329–335. doi: 10.1016/S0968-0004(03)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl, M., Horn, C., Brown, M., Ioudovitch, A., Steinberg, S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc, K., Steitz, J.A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989;8:3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, N.J., Segault, V., Charpentier, B., Nottrott, S., Fabrizio, P., Bachi, A., Wilm, M., Rosbash, M., Branlant, C., Luhrmann, R. A common core RNP structure shared between the small nucleoar box C/D RNPs and the spliceosomal U4 snRNP. Cell. 2000;103:457–466. doi: 10.1016/s0092-8674(00)00137-9. [DOI] [PubMed] [Google Scholar]
- Wu, P., Brockenbrough, J.S., Metcalfe, A.C., Chen, S., Aris, J.P. Nop5p is a small nucleolar ribonucleoprotein component required for pre-18 S rRNA processing in yeast. J. Biol. Chem. 1998;273:16453–16463. doi: 10.1074/jbc.273.26.16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, F., Martzen, M.R., Phizicky, E.M. A conserved family of Saccharomyces cerevisiae synthases effects dihydrouridine modification of tRNA. RNA. 2002;8:370–381. doi: 10.1017/s1355838202029825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, F., Hiley, S.L., Hughes, T.R., Phizicky, E.M. The specificities of four yeast dihydrouridine synthases for cytoplasmic tRNAs. J. Biol. Chem. 2004;279:17850–17860. doi: 10.1074/jbc.M401221200. [DOI] [PubMed] [Google Scholar]
- Yoshida, M. The nucleotide sequency of tRNA Gly from yeast. Biochem. Biophys. Res. Commun. 1973;50:779–784. doi: 10.1016/0006-291x(73)91312-0. [DOI] [PubMed] [Google Scholar]
- Yu, Y.-T. Construction of 4-thiouridine site-specifically substituted RNAs for cross-linking studies. Methods. 1999;18:13–21. doi: 10.1006/meth.1999.0752. [DOI] [PubMed] [Google Scholar]
- Yu, Y.-T., Terns, R.M., Terns, M.P. Mechanisms and functions of RNA-guided RNA modifications. In: Grosjean H., editor. Fine-tuning of RNA functions by modification and editing, Topics in Current Genetics series. vol. 12. Springer-Verlag Press; New York: 2005. pp. 223–262. [Google Scholar]