Abstract
The eukaryotic initiation factor (eIF) 4G family plays a central role during translation initiation, bridging between the 5′ and 3′ ends of the mRNA via its N-terminal third while recruiting other factors and ribosomes through its central and C-terminal third. The protein p97/NAT1/DAP5 is homologous to the central and C-terminal thirds of eIF4G. p97 has long been considered to be a translational repressor under normal cellular conditions. Further, caspase cleavage liberates a p86 fragment that is thought to mediate cap-independent translation in apoptotic cells. We report here that, surprisingly, human p97 is polysome associated in proliferating cells and moves to stress granules in stressed, nonapoptotic cells. Tethered-function studies in living cells show that human p97 and p86 both can activate translation; however, we were unable to detect polysome association of p86 in apoptotic cells. We further characterized the zebrafish orthologs of p97, and found both to be expressed throughout embryonic development. Their simultaneous knockdown by morpholino injection led to impaired mesoderm formation and early embryonic lethality, indicating conservation of embryonic p97 function from fish to mammals. These data indicate that full-length p97 is a translational activator with essential role(s) in unstressed cells, suggesting a reassessment of current models of p97 function.
Keywords: mRNA translation, eIF4G, apoptosis, stress granules, zebrafish development, morpholino
INTRODUCTION
p97 (also called DAP5 or NAT1) is a highly conserved and ubiquitously expressed eukaryotic protein (Imataka et al. 1997; Levi-Strumpf et al. 1997; Yamanaka et al. 1997) with an essential function during embryogenesis in the mouse (Yamanaka et al. 2000). p97 is structurally related to the eukaryotic initiation factor (eIF) 4G, a central player during the initiation phase of mRNA translation (Preiss and Hentze 2003; Sonenberg and Dever 2003). eIF4G can be roughly divided into three regions (Fig. 1A). The N-terminal third of eIF4G can bridge between the mRNA 5′ cap structure and 3′ poly(A) tail during early initiation. It does so by simultaneously interacting with the cap-binding protein eIF4E and the poly(A)-binding protein PAPB. eIF4G further coordinates the recruitment of the small (40S) ribosomal subunit and associated factors to the mRNA by binding to the 40S-associated eIF3 (through its central third) as well as to the RNA helicase eIF4A (through sites in its central and C-terminal thirds). Assembly of this preinitiation complex is then followed by scanning of the 5′ untranslated region (UTR), rearrangement of the complex upon recognition of the appropriate start codon on the mRNA, and large (60S) ribosomal subunit joining to commence polypeptide synthesis. p97 has homology with the central and C-terminal thirds of eIF4G and thus can interact with eIF3 and eIF4A (Imataka et al. 1997; Yamanaka et al. 1997), although only the more N-terminal eIF4A-binding region is functional (Imataka and Sonenberg 1997). It further interacts through its C terminus with the eIF4E-kinase Mnk1, another property it shares with eIF4G (Pyronnet et al. 1999). Importantly, p97 lacks regions found at the N terminus of eIF4G, which interact with eIF4E and PABP (Imataka et al. 1997; Marash and Kimchi 2005). Thus, p97 is thought to be incapable of supporting canonical cap-dependent mRNA translation.
FIGURE 1.
p97 is associated with polysomes. Apoptosis in Jurkat cells was induced by agonistic anti-Fas antibody treatment and extracts processed for linear sucrose density gradient analysis (17.5%–50%) as detailed in the Materials and Methods. (A) Schematic of eIF4GI, p97, and their caspase-cleavage products, for further details see Morley et al. (2005). (B) Density gradient profiles from mock-treated cells or cells treated with anti-Fas antibody for the time periods indicated (top of each panel). Each panel shows the 254-nm absorbance trace recorded during fractionation, with the position of various ribosomal complexes indicated below. Each gradient was divided into 12 fractions, which were processed for the Western blots displayed below each trace to indicate the sedimentation behavior of p97, its caspase-cleaved form p86, full-length eIF4GI, and its caspase-cleavage products. Probing was also carried out for RPS6 to locate ribosomal complexes and tubulin to demonstrate absence of nonspecific aggregation. Positions of all identified protein bands are indicated beside the panels; see Materials and Methods for details on the antibodies used. (C) Density gradient profiles from control cells, or cells treated with anti-Fas antibody for 8 h were done in duplicate as in B, and 10 fractions were taken. For each condition, one lysate aliquot was supplemented with 30 mM EDTA prior to formaldehyde cross-linking to disassemble polysomes (right).
Cells, however, employ cap-independent translation mechanisms by internal ribosome entry under conditions of blocked cap-dependent translation such as during mitosis in the cell cycle, environmental stress, or apoptosis (Holcik et al. 2000; Merrick 2004; Stoneley and Willis 2004; Holcik and Sonenberg 2005; Komar and Hatzoglou 2005). Large-scale surveys consistently suggest that ∼3% of eukaryotic mRNAs continue to be translated in such conditions (Johannes et al. 1999; Qin and Sarnow 2004; Bushell et al. 2006). Of note here is that both the C-terminal two-thirds and the central third of eIF4G can support translation initiation by internal ribosome entry in vivo (De Gregorio et al. 1999). The p97 protein was discovered in diverse contexts. It was reported as a protein with homology with eIF4G (Imataka et al. 1997; Shaughnessy et al. 1997). Its cDNA was cloned in a screen for targets of an RNA-editing enzyme and was named NAT1 (Yamanaka et al. 1997). Finally, a fragment of its cDNA was selected in a screen for interferon γ-induced apoptosis modulators, and the protein was termed DAP5 (Levi-Strumpf et al. 1997). The latter observation, and the subsequent finding that p97 (like several other translation factors) was processed by caspase-3 in apoptotic cells (Henis-Korenblit et al. 2000; Morley et al. 2005), led to a series of studies exploring its involvement in translation of apoptosis-related mRNAs by internal ribosome entry (Marash and Kimchi 2005). First, adding recombinant p97 or p86 to a cell-free translation system led to selectively increased translation of the second cistron in a bicistronic mRNA carrying the DAP5-IRES in the intercistronic space, p86 being more potent in this assay than p97 (Henis-Korenblit et al. 2000). Second, several studies have employed transient cotransfection of mammalian cell lines with p97 expression constructs and bicistronic reporters harboring different IRES sequences. One laboratory reported activation by p86 of c-myc, Apaf-1, DAP5, and XIAP IRESes (Henis-Korenblit et al. 2002), while another saw activation with Apaf-1, DAP5, and HIAP2 IRESes, but not with XIAP, EMCV, BIP, or c-Myc IRESes (Nevins et al. 2003; Warnakulasuriyarachchi et al. 2004). All these reports indicate preferential activation of second-cistron expression by p86, while full-length p97 was largely inactive. Further, it had been reported early on that overexpression of p97 in cultured mammalian cells led to approximately twofold repression of EMCV IRES- or cap-driven reporter expression and reduction of general protein synthesis by ∼20%–25% (Imataka et al. 1997). Contrasting with this result, p97 null mouse ES cells were reported to have normal levels of global translation, and to show no differences in expression from EMCV, c-myc, and DAP5 IRESes (Yamanaka et al. 2000). Despite the considerable inconsistencies in the literature, a model of p97 as a translation repressor under normal cellular conditions, which becomes activated by caspase cleavage during apoptosis to mediate critical translation of pro- as well as anti-apoptotic factors, was widely adopted.
Here, we have directly tested this model of p97 function in several ways. We analyzed the polysome association of p97 and its caspase cleavage product p86 in human Jurkat T cells. Surprisingly, we found p97 to sediment with polysomes from control cells, while p86 was not detectably polysomal in apoptotic cells. Tethered-function studies in living cells revealed that human p97 and p86 both could activate translation. Severe cellular stress led to accumulation of endogenous human p97 or eIF4GI in the repressive stress granules. These data suggest that, contrary to expectation, p97 is a translational activator in unstressed cells and does not require caspase cleavage for activation. Further, we detect expression of both orthologous genes in zebrafish, p97a&b, throughout the developing embryo. Their simultaneous knockdown by morpholino injection led to impaired mesoderm formation and early embryonic lethality. This indicates conservation of the embryonic p97 function from fish to mammals. Overall, our findings call for a reassessment of current models of the cellular role of p97.
RESULTS
Full-length p97 is associated with polysomes
Based on the model of p97 as a caspase-activated translation factor, one might expect the caspase-cleaved p86 fragment in apoptotic cells to be selectively mobilized into polysomes, while the full-length protein in unstressed cells should not be polysome associated. To test this, we treated Jurkat T cells with agonistic Fas antibodies for variable times and established efficient induction of apoptosis by detecting caspase cleavage of PARP by Western blotting (not shown). We then prepared cytoplasmic extracts of apoptotic and control cells in the presence of cycloheximide and separated these by ultracentrifugation through linear 17.5%–50% w/v sucrose gradients (see Materials and Methods for details). When fractions from control cell gradients were analyzed by Western blotting for eIF4GI, we found that all eIF4GI were in subpolysomal fractions, indicating that initiation factors were lost from polysomes during the procedure. This was analogous to observations made in Saccharomyces cerevisiae (Nielsen et al. 2004), where reversible formaldehyde cross-linking of cells had been shown to stabilize initiation factor association with polysomes. Thus, we modified our protocol by including a mild 1% formaldehyde cross-linking step of the cell lysates (Niranjanakumari et al. 2002). Following that modification, we could clearly detect eIF4GI sedimenting deep into the polysomal region of density gradients with extracts from control cells (Fig. 1B,C). Absorbance readings at 254 nm, and probing of the membrane for ribosomal protein rpS6, served to locate the different ribosomal complexes. The nonribosomal protein tubulin was found only in subribosomal fractions near the top of the gradient, suggesting that extract cross-linking did not cause formation of nonspecific protein aggregates.
Next, we probed the membranes with anti-p97 antisera and found that in control cells full-length p97 was clearly sedimenting into the polysomal fractions (Fig. 1B,C, top left panel). Following changes over the time course of agonistic Fas antibody treatment (2, 4, and 6 h), we noted caspase cleavage of both eIF4GI and p97, yielding the expected pattern of cleavage fragments. Two hours after induction of apoptosis, cleavage fragments of p97 and eIF4G (see Fig. 1A for a schematic of eIF4GI and its caspase cleavage products) were detectable (Fig. 1B, top right panel). At the 6-h time point, nearly all of the eIF4GI and most of p97 were cleaved. The OD254 nm traces indicate a drastic reduction in the amount of polysomal complexes, consistent with an expected overall decrease in translation rate (Morley et al. 2000; Bushell et al. 2001; Saelens et al. 2001). Notably, by 2 h of treatment, both full-length eIF4GI and p97 were largely withdrawn from the polysomal region of the gradient. We found that the eIF4GI fragments as well as p86 likewise accumulated mostly in the subpolysomal fractions (at 2, 4, or 6 h). Small amounts of p86, and the eIF4GI M-FAG and C-FAG fragments, were found in the lighter polysomal region of the gradient at 6 h of treatment (Fig. 1B, bottom right panel). As a further test for the specificity of the sedimentation patterns, we repeated the experiment for control cells and cells treated for 8 h with agonistic Fas antibodies, adding 30 mM EDTA to an aliquot of each cell extract to disassemble polysomes (Fig. 1C). This led to the loss of both eIF4GI and p97 from the heavy region of the gradient in control cells (Fig. 1C, top panels). Although EDTA treatment may also affect mRNP assemblies other than polysomes, this result is consistent with a bona fide association of these factors with polysomes. At 8 h of treatment (Fig. 1C, bottom panels), we again observed a slight tendency of p86 to sediment into the heavier regions of the gradient, which was, however, largely resistant to EDTA treatment. Co-sedimentation of the eIF4GI M-FAG and C-FAG fragments with polysomes could not be convincingly shown. Collectively, these data suggest that p97 and eIF4GI are both similarly associated with polysomes in actively growing cells. By contrast, we have been unable to convincingly demonstrate polysome association of either p86 or any of the eIF4GI caspase cleavage fragments. This indicates that full-length p97 is already active in translation in normally growing cells.
p97 and p86 both can activate translation in vivo
To address the issue of p97/86 as translational activators more directly, we decided to employ the dedicated initiation factor (DIF) assay (De Gregorio et al. 1999, 2001). In this assay, a fusion protein between an RNA-binding domain (the λ peptide) and the translation initiation factor under study is coexpressed in transfected cells with a bicistronic reporter construct harboring a specific binding site for the fusion protein (the boxB element) in the intercistronic space (Fig. 2A). The plasmid pSGλ-4G, expressing a λ fusion with the C-terminal two-thirds of eIF4GI was available from previous work (De Gregorio et al. 1999, 2001) and served as a positive control. Analogous constructs for p97 and p86 were made (pSGλ-p97 and -p86) and preliminary transfection experiments were conducted to detect and quantify expression of all λ fusion proteins by Western blotting using λ-specific antisera (not shown). Based on these results, cotransfection experiments of HeLa cells with cocktails of the λ-effector plasmids in variable amounts, a constant amount of the pSGBoxB reporter plasmid, and pSV-β Galactosidase as a transfection efficiency control were devised. The amount of λ-effector plasmids was adjusted to give comparable expression levels of all the fusion proteins (Fig. 2C). For each amount of λ-effector plasmid, a control transfection with “empty” pSGλ was further done. Expression of the first (firefly luciferase, LUC) and second (chloramphenicol acetyl transferase, CAT) cistron was measured and normalized for transfection efficiency and the relative increase due to the presence of the fusion proteins calculated (see Materials and Methods for details). The bar chart in Figure 2B illustrates a similar dose-dependent activation of the downstream cistron (about three- to fivefold) for λ-4G, -p97, or -p86, with a possible tendency of λ-p86 to have a weaker activity than λ-p97 or λ-4G. No significant change was detected in upstream LUC expression (Fig. 2B, numbers on the bottom of the bars). A minor amount of p86 cleavage product was detected in cells transfected with the pSGλ-p97, which may reflect a low level of apoptotic cells following transfection. Reference to the p86 expression levels in the pSGλ-p86 transfections indicates that such low levels cannot explain the robust levels of CAT activation seen with pSGλ-p97. We conclude that p97 as well as p86 can directly activate translation in intact cells to a similar degree as the homologous fragment of eIF4GI.
FIGURE 2.
Both p97 and p86 can activate translation in vivo. (A) Schematic representation of the experimental strategy to tether λ-initiation factor fusion proteins to a boxB element in the intercistronic space of a bicistronic mRNA, generating a dedicated initiation factor. (B) Effects of the fusion proteins λ4G, λp97, and λp86 on expression of the bicistronic reporter mRNA. Multiple HeLa cell aliquots were cotransfected with plasmid cocktails consisting of 2.88 μg of boxB reporter, and increasing amounts of λ-effectors: 0.02, 0.04, and 0.08 μg of pSGλ4G (lanes 1–3); 0.25, 0.5, and 1 μg of pSGλp97 (lanes 4–6); or pSGλp86 (lanes 7–9). The difference of 4 μg in each case was made up with pSV-β Gal plasmid, and for each λ-effector amount an equivalent control transfection was done with “empty” pSGλ. Cells were harvested 24 h after transfection. Reporter protein expression was measured in lysates, and relative stimulation of CAT or LUC expression due to λ-effector coexpression was calculated as detailed in the Materials and Methods. The bar chart shows averaged results for CAT with standard deviation from two to four independent repeat experiments for each effector concentration. Corresponding values for LUC expression are shown at the base of each bar. (C) Aliquots of cell extracts were used for Western blots with anti-λ antisera. Probing for tubulin was done as a loading control.
p97 is present in stress granules
During severe cellular stress, stalled translation initiation complexes including many initiation factors such as eIF4GI accumulate in cytoplasmic foci termed stress granules (SG) (Kedersha et al. 2005). We wished to explore a possible differential role of p97 in this phenomenon and thus treated HeLa cells with either 250 μM sodium arsenite for 30 min to induce oxidative stress or induced a heat shock by incubating the cells for 10 min at 44°C. Immunohistochemistry staining patterns in treated and control cells were observed by confocal microscopy (see Materials and Methods for details). As positive controls, we stained with antibodies against eIF4GI (Fig. 3) and the stress granule marker TIA-1 (Fig. 3, central images of all panels). In control cells (Fig. 3A), eIF4GI is diffusely distributed throughout the cytoplasm, while TIA-1 is predominantly nuclear. In arsenite-treated (Fig. 3B) or heat-shocked cells (Fig. 3C), both eIF4GI and TIA-1 become co-localized in the stress granules, as previously described (Kedersha et al. 2005). When staining for p97 (Fig. 3), we find that the protein is predominantly cytoplasmic in control cells (Fig. 3A), where it is diffusely localized. Both types of stress treatment lead to the accumulation of p97 in cytoplasmic foci (Fig. 3B,C; note that the focal plane in these images was chosen for best visualization of SG rather than showing a good cross section of the nucleus). The clear co-localization of p97 and TIA-1 indicates that these p97 foci are bona fide SG. As a negative control, we further stained cells for DNA methyl transferase 1 (DNMT1) and found no accumulation of signal in SG in stressed cells (M. Nousch and T. Preiss, unpubl.). In images with a cross section of both nucleus and cytoplasm (Fig. 3A), it can further be seen that the anti-p97 antibodies gave some nuclear staining. To investigate this further and to independently test the localization of p97 to SG, HeLa cells were transfected with a construct expressing p97 as a C-terminal fusion with eGFP (Fig. 3D, bottom panels), or a construct expressing eGFP alone as a control (Fig. 3D, top panels). eGFP and TIA-1 images were recorded of untreated cells (Fig. 3D) and sodium arsenite-treated cells (Fig. 3E). Closer inspection of the merged images of stressed and control cells (Fig. 3E, insets) reveals that the p97-eGFP fusion protein localizes to SG, while the negative control eGFP control does not. Since no nuclear staining was seen with the p97-eGFP fusion protein, we favor the overall interpretation that p97 is a predominately cytoplasmic protein. Finally, Western blots of material from stressed and control cells demonstrate that neither eIF4GI nor p97 are detectably cleaved under these conditions (Fig. 3F). Collectively, these results demonstrate that localization of both eIF4GI and p97 is diffusely cytoplasmic in untreated HeLa cells, and that importantly, in cells subjected to oxidative stress or heat shock both proteins move to SG to a similar extent.
FIGURE 3.
p97 accumulates in stress granules (SG). Stress granules were induced in HeLa cells by treatment with 250 μM arsenite for 30 min (B,E) or exposure of cells for 10 min to 44°C (C) as previously described (Kedersha et al. 2005). Cells were then immediately fixed and processed for confocal microscopy. (A,D) Untreated control cells are shown for comparison. (A–C) Cells were stained for endogenous eIF4GI or p97 protein (left, as indicated). (D,E) Cells transfected with peGFP-N1 vector or peGFP-p97 (left: GFP signal). In each case, cells were also stained for TIA-1 as an SG marker (center). (Right) Corresponding merged view. Enlarged details are shown (boxed areas) to highlight localization of proteins to SGs (TIA-1, eIF4GI, p97, eGFP-p97), or lack thereof (eGFP). Bars, 8 μm. (F) Aliquots of extracts from control (−) or stressed cells (HS, heat shock; Ars, sodium arsenite) were used for Western blots probed with p97- and eIF4G-specific antisera that recognize the full-length as well as the caspase-cleavage products of both proteins.
Zebrafish p97 is required for early embryonic differentiation
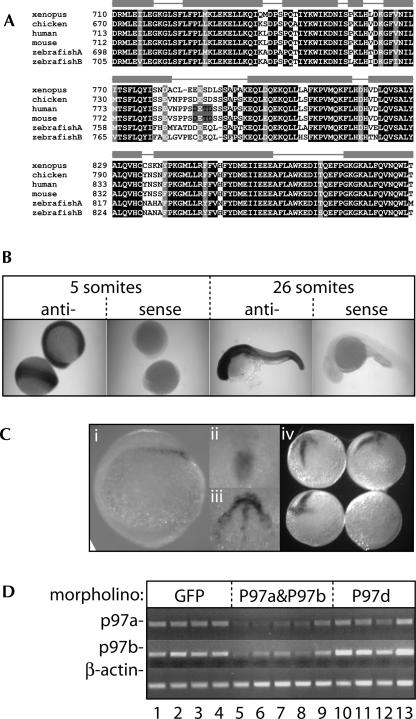
Given that the above experiments suggested an active role of human p97 in the absence of caspase cleavage, we aligned the amino acid sequences of p97 homologs from different species, focusing on the region around the caspase cleavage site in the mammalian protein (Fig. 4A, Henis-Korenblit et al. 2000) the DETD caspase cleavage site in the human and mouse sequence is highlighted by a gray box). To obtain the zebrafish p97 sequences, the human protein sequence of p97 (Accession: P78344) was used to search using BLAST against the available zebrafish genomic sequence at Ensembl and the Sanger Center. Two matching contigs were identified, and a predicted protein was obtained using Genewise (Birney et al. 2004) and the human protein sequence. These predicted sequences were used to search the NCBI EST database for matching ESTs. Matches were found to both p97a and p97b (Image 5410611 and Image 6961549). As this work was in progress, another study (Takahashi et al. 2005) reported the same sequences (eIF4G2a/NAT1a and eIF4G2b/NAT1b; referred to as p97a and p97b here for consistency), which we have further analyzed here. The two zebrafish isoforms have a high homology overall with their mammalian counterparts (p97a: 82.1%; and p97b: 82.7% identity to human p97), but the caspase-3 cleavage site identified in human p97 lies in a poorly conserved loop region between two well-conserved helical elements (Bellsolell et al. 2006) and is not conserved in the nonmammalian species. Thus, the zebrafish factor is very unlikely to be cleaved during apoptosis, at least not in the way the mammalian factor is, although we could not test this directly as our antibodies did not recognize zebrafish p97 on Western blots (data not shown). Instead, we made use of the opportunity to test the role of p97 in zebrafish embryonic development. Mammalian p97 is widely expressed in different embryonic and adult tissues (Imataka et al. 1997; Levi-Strumpf et al. 1997; Yamanaka et al. 1997). In mice, it has been shown that p97(−/−) embryos die during gastrulation and E7.5 embryos do not develop organized primary germ layers (Yamanaka et al. 2000). Using in situ hybridization, we showed that p97a (not shown) and p97b (Fig. 4B) were both expressed throughout the developing zebrafish embryo from early stages. We next designed morpholinos to knockdown expression of the two p97 isoforms either individually (p97aS, p97bS, against the “p97a-intron 2” splice donor site, and “p97b-intron 4” splice donor site, respectively) (Draper et al. 2001) or together (p97D, anti-sense to the translation start site, which is identical in sequence for both isoforms; see Materials and Methods for morpholino sequences). Only a joint morpholino knockdown of both isoforms (p97D, or p97aS and p97bS together) had consistent adverse effects on the developing embryos. Specifically, we noticed that injection of p97D led to poor survival of embryos compared with a control morpholino against GFP (Table 1). The defective p97(−/−) mouse embryos had been shown to develop trophectoderm and primitive endoderm, but their mesoderm did not emerge from the epiblast (Yamanaka et al. 2000). We looked at this in the zebrafish embryos by in situ hybridization for Goosecoid mRNA, a marker of mesoderm development (Stachel et al. 1993). When both p97 isoforms were targeted by knockdown with either p97D, or the splice site morpholinos in combination, we found that about one-fourth of embryos (five of 19 injected) failed to develop mesoderm (Fig. 4C). Semiquantitative RT-PCR reactions confirmed that the splice-site oligos specifically reduced p97 mRNA levels (Fig. 4D). As controls, we tested uninjected embryos or embryos injected separately with either GFP-, p97aS-, or p97bS morpholinos (six embryos each) and found normal Goosecoid expression in all cases. Taken together, these experiments indicate that loss of p97 function leads to defects in early zebrafish embryonic development, which mimic those observed in p97 knockout mice.
FIGURE 4.
p97 is required for zebrafish embryo development. (A) Partial alignment of known p97 amino acid sequences (accession numbers: Xenopus BAD83638, chicken BAD83637, human P78344, mouse NP_038535, zebrafish A BAD83639, -B BAD83640) indicating limited conservation of the caspase-3 cleavage site identified in mammalian p97 (DETD, gray box). (Gray bars) Predicted α-helical segments for mammalian and fly p97 (Bellsolell et al. 2006). (B) Zebrafish embryos at the 5- or 26-somite stage were used for in situ hybridization using a p97b RNA anti-sense probe (sense also shown as negative control). Equivalent results were obtained when probing for p97a mRNA expression (not shown). (C) In situ hybridization for goosecoid mRNA expression of ∼10-h embryos. (i–iii) Images of uninjected embryos highlight goosecoid expression during formation of the hypoblast, which will develop into the mesoderm. (iv) A group of four embryos injected with 0.25 mM of each p97aS and p97bS morpholino is shown illustrating loss of goosecoid expression in one in four injected embryos. (D) RNA isolated from individual embryos injected with morpholinos as indicated above the lanes was used for semiquantitative RT-PCR to confirm mRNA knockdown with splice-site morpholinos against p97a and p97b. PCR reactions were carried out for both isoforms of p97, as well as β-actin mRNA as a specificity control.
TABLE 1.
Embryo viability following morpholino-mediated knockdown of p97
DISCUSSION
This study yielded a number of surprising findings that suggest a reevaluation of longstanding models of p97 function. On a cellular level, we found that endogenous human p97 sediments with polysomes in actively growing cells (Fig. 1) and tethered p97 can act as translational activators in living cells (Fig. 2). Applying subapoptotic cellular stress elicited a movement of p97 protein to SG (Fig. 3). Although tethered p86 also activates translation in the DIF assay, we have been unable to demonstrate p86 association with polysomes in apoptotic cells. Thus, our data indicate that p97 is a translational activator in unstressed cells and does not require caspase cleavage for activation. This is partly at odds with conclusions drawn from the earlier studies (summarized in the Introduction) but receives support from recently emerging publications. We therefore discuss below several aspects of our data in the context of other available literature.
p97 as an activator of translation
Previous efforts to address this issue all manipulated the level of p97 protein in cells or cell extracts, by either adding exogenous protein or removing the endogenous factor through RNAi knockdown. Introducing additional p97 protein to cells by transient transfection, for instance, can lead to perturbations in the cellular translational machinery. The extent of p97 overexpression may then affect the experimental outcome, as recently proposed (Lee and McCormick 2006). These authors reported activation of cap-dependent reporter mRNA translation and global protein synthesis following p97 overexpression in cultured mammalian cells (Lee and McCormick 2006), contrary to earlier reports in which p97 overexpression led to repression of EMCV IRES- or cap-driven reporter expression and reduction of general protein synthesis (Imataka et al. 1997). The argument is that p97 as a specialized translation activator could still repress global cellular translation when overexpressed to a level at which it begins to out-titrate other initiation factors that normally interact with eIF4G in canonical translation initiation. RNAi knockdown of p97 avoids this complication and clearly led to reduction of global cellular protein synthesis as well as reporter mRNA translation (Lee and McCormick 2006). However, it also caused cell cycle arrest and a selective increase in translation of p27/Kip1 mRNA, encoding a cell cycle repressor (Lee and McCormick 2006), leaving at least residual uncertainty as to whether endogenous p97 promotes global protein synthesis directly, or through promotion of cell proliferation. There is also evidence in vitro that exogenous p97 can augment translation from the c-myc IRES or of uncapped mRNA (Hundsdoerfer et al. 2005; Mikami et al. 2006).
We employed experimental strategies here that were designed to avoid these complications. First, we used the association of endogenous p97 protein with polysomal complexes or SG as a surrogate measurement for its translational status and found that p97 behaved very much like the bona fide translation initiation factor eIF4GI, which we assayed as a reference. The focus on endogenous p97 ensures measurements within a native cellular context. Second, we used the DIF assay to generate direct evidence on the translation activation potential of p97/p86. Although the DIF assay is based on transient cell transfection, the initiation factor under study is expressed at low levels that do not perturb cellular translation, and factor activity depends strictly on the tethering interactions between the specific λ peptide fusion protein and the boxB element on the reporter mRNA (De Gregorio et al. 1999, 2001; Czaplinski et al. 2005). Using this direct assay, we found that p97 could activate translation in a manner similar to the C-terminal two-thirds of eIF4GI.
Caspase cleavage is not required for regulation of p97 function
It is well established that apoptosis triggers general polysome disassembly and caspase cleavage of p97 to p86. The model that cleavage of p97 during apoptosis serves to activate the factor is based predominantly on transient cotransfection studies with bicistronic cellular IRES reporters in mammalian cell lines (Henis-Korenblit et al. 2002; Nevins et al. 2003; Warnakulasuriyarachchi et al. 2004; Marash and Kimchi 2005). These reports indicate preferential activation of IRES-mediated translation by p86, while full-length p97 was largely inactive. Similar reasoning as outlined above would raise the concern that addition to the cellular environment of exogenous p97 or p86 protein can perturb the cellular balance between cap-dependent and cap-independent translation. This can make it difficult to distinguish between effects of a direct interaction of p97 or p86 with the IRES under study versus more indirect actions of the overexpressed proteins. Indeed, this type of problem was noted in one study, and alternative normalization strategies were employed to extrapolate from such indirect effects (Henis-Korenblit et al. 2002). Caspase cleavage removes the C terminus from the protein, leading to loss of interaction with the Mnk1 kinase. Recently, it has further been reported that the extreme C terminus of p97 interacts with eIF2β and that the p86 fragment has markedly reduced binding to eIF4A, while further deletion from the C terminus restored full eIF4A binding to its N-terminal interaction region on p97 (Lee and McCormick 2006). These new findings indicate additional roles of the p97 C terminus in mediating translation and possible allosteric consequences of caspase cleavage in other parts of the protein. From these interaction data, however, it is difficult to construct a coherent model for functional activation of the factor by caspase cleavage. Our own DIF assay results indicate that p97 and p86 can in broad terms be similarly potent translational activators, and we were unable to show selective polysome recruitment of p86 in apoptotic cells. This does not exclude a positive role for p86 in apoptotic cells, although the limited evolutionary conservation of the caspase cleavage site suggests that cleavage of the factor may only occur in the mammalian lineage. A plausible interpretation of our data is that the effect of caspase cleavage on p97 activity can be neutral, and that the interactions with Mnk1 and eIF2β are dispensable for translational activity, at least in the DIF assay context. Essentially all published work on p86 suffers from a lack of definitive functional data on this caspase-cleavage product in actual apoptotic cells, and thus further work will be necessary to clarify the physiological function of p86.
The question arises as to what, other than caspase cleavage, may regulate the activity of the factor. The literature offers some clues. First, the Web supplement of a large-scale proteomic study lists p97 as a phosphoprotein (phosphorylated at the highly conserved threonine 508; Beausoleil et al. 2004). Second, a study cited unpublished observations that the eIF4E-kinase Mnk1 can also phosphorylate p97 in vitro (Pyronnet et al. 1999). Third, p97 was shown to be recruited to 40S ribosomal subunits following growth stimulation (Lee and McCormick 2006). Thus, we speculate that p97 may be regulated by phosphorylation in response to growth factor signaling.
In search of a physiological role for p97
Given the evidence discussed above, it is clear that p97 can function as a translational activator in growing cells, shifting emphasis away from its cleavage by caspase and possible apoptotic function. Our data on SG accumulation of p97 further argue against a straightforward role as a major mediator of selective translation in typical cellular stress conditions. This highlights the necessity of identifying additional or alternative mRNA targets for this translation factor, a task that we have begun to address in current work. It is still most plausible that p97 somehow mediates cap-independent translation, and apart from situations of cellular stress this is known to be of importance, for instance, during the G2/M phases of the cell cycle (Cornelis et al. 2000; Pyronnet et al. 2000; Pyronnet and Sonenberg 2001). Finally, our observations in zebrafish demonstrate that the essential roles of p97 in early embryonic development and the formation of the primitive germ layers are conserved from fish to mammals. Again, this is most likely due to its role as a translation factor, although this still awaits a stringent test. Until then, even a role outside of translation, as previously suggested (Yamanaka et al. 2000), remains at least a formal possibility.
In summary, we have presented here direct evidence that p97 can be a translational activator in vivo that does not require caspase cleavage for activation. Together with the evolutionarily conserved role of p97 in embryonic differentiation, this presents the renewed challenge of identifying the major cellular and molecular functions of this translation factor.
MATERIALS AND METHODS
Plasmids and oligonucleotides
DIF assay
Plasmids pSGλ-4G and pSGBoxB were previously described (De Gregorio et al. 1999). To generate pSGλ, pSGλ-eIF4G was digested with BamHI to liberate the insert, and then religated. For pSGλ-p97 and -p86, a full-length human p97 clone (RZPD IMAGE #4299481) was used in PCR reactions to generate p97 and p86 DNA fragments, using primers GCGCGATATCGCGGCCGCGCGTGGAGAGTGCGATTGCAGAAGGG and GCGCGATATCGCGGCCGCGTCAGCTTCTTCCTCTGATTCTTC or primers GCGCGATATCGCGGCCGCGCGTGGAGAGTGCGATTGCAGAAGGG and GCGCGATATCGCGGCCGCATCTGTTTCATCGCTGGG, respectively. PCR products were digested with EcoRV and inserted into the blunted BamHI site of pSGλ. The pSV-β Galactosidase vector was from Promega.
SG induction
Human p97 DNA was amplified by PCR using primers CCGGAATTCTATGGAGAGTGCGATTGCAG and ACGCGTCGACTGGTCAGCTTCTTCCTCTGA. The PCR product was digested with SalI and EcoRI and inserted into the respective sites peGFP-N1 (Clontech).
Zebrafish development
Full-length zebrafish p97 cDNA clones were obtained from RZPD (IMAGE #5410611 for p97a/eIF4G2a/NAT1a, IMAGE #6961549 for p97b/eIF4G2b/NAT1b; as described in Takahashi et al. [2005]). Linearized p97a and goosecoid plasmids were used directly as templates for in vitro transcription; the p97b sequence was first subcloned into pBluescript II KS (+/−). Morpholinos antisense to the following sequences were purchased from Gene Tools: p97D: TTGTCAAGCCGCCAAAGTGGAGAGT, p97aS: atcatcttgctgGTGAGCGGGTTTT, p97bS: tcatcttgctgGTGAGGAATTTTAT. RT-PCR primers were as follows: forward for both p97–GGGGTGCATCTCAGCACTAT, reverse p97a–CCTTAAGTTGGACTCTTTC, reverse p97b–CTGGACCCTCTTCTTTTTCTCC, β actin forward–CATCAGCATGGCTTGTGCTCTGTATGG, β actin reverse–GACTTGTCAGTGTACAGAGACACCCTG. All plasmids were verified by sequencing.
Antibodies
Rabbit polyclonal antisera to p97 were raised against the synthetic peptide PARSTRRDDNSAAN (amino acids 52–65 of human p97). Rabbit polyclonal antisera against human eIF4GI were gifts from Simon Morley, Brighton, UK (anti-peptide antisera IAN [amino acids 43–62], Ann/antiserum E [amino acids 1179–1206], and Bloo2/antiserum H [amino acids 864–889]; Bushell et al. 2000). The rabbit polyclonal anti-λ peptide antibody was a gift from Matthias Hentze, Heidelberg, Germany, and has been previously described (De Gregorio et al. 2001). The following antibodies were obtained from commercial suppliers: mouse anti-tubulin, goat anti-TIA-1 (Santa Cruz), Mouse anti-NAT-1, mouse anti-eIF4G (BD Bioscience), and rabbit anti-rS6 (Cell Signaling). Secondary antibodies anti-rabbit, anti-mouse conjugated to HRP were from Amersham; anti-mouse, anti-rabbit, and anti-goat antibodies conjugated to TRITC, FITC, or Cy5 were from Jackson ImmunoResearch.
Cell culture
Jurkat T lymphocytes (ATCC CRL-2570) were grown in RPMI at low density (∼8×105/mL). For apoptosis induction, cells were washed twice in ice-cold PBS, resuspended into one-tenth of the original volume of medium without fetal bovine serum, and anti-fas antibody (Upstate clone CH11) was added at 800 ng/mL. Cells were left for 20 min on ice before addition of 10 volumes of warm medium containing fetal bovine serum and continued incubation.
HeLa S3 cells were maintained in DMEM supplemented with 10% fetal bovine serum, L-Glutamate, and penicillin/streptomycin.
Polysome analysis
Cells (2×107) were harvested by centrifugation and washed twice in ice-cold PBS containing 100 μg/mL cycloheximide. The cell pellet was then lysed by suspension in 450 μL of buffer A (20 mM HEPES pH 7.6, 125 mM KCl, 5 mM MgCl2, 2 mM DTT, 100 μg/mL cycloheximide, 1× complete protease inhibitor cocktail [Roche], 0.5 mM PMSF, and 1% Triton X-100) for 10 min on ice, followed by centrifugation for 10 min at 16,000g and 4°C to remove debris and nuclear components. The supernatant was cross-linked by adding formaldehyde to 1%, followed by incubation for 5 min on ice and then the addition of 100 mM glycine (Niranjanakumari et al. 2002). Lysates were layered immediately onto sucrose density gradients (10 mL, 17.5%–50% w/v in buffer A without protease inhibitors and Triton X-100, which were prepared in advance by a freeze–thaw method [Luthe 1983]). Gradients were centrifuged for 135 min at 35,000 rpm and 4°C in a Beckman L-80 ultracentrifuge using the SWT41 rotor. Fractionation was from the bottom of the gradient with concomitant recording of absorbance profiles at 254 nm. Fractions were concentrated by TCA precipitation; pellets were dissolved in 1× Laemmli sample buffer and resolved by 10% SDS-PAGE, followed by transfer to a PVDF membrane (Amersham), incubation with primary/secondary antibody combinations as indicated in the Figure 1 legend, and detection using the Western Lightning ECL kit (PerkinElmer) and exposure to hyperfilm (Amersham).
Dedicated initiation factor (DIF) assays
HeLa cell aliquots grown in six-well plates were cotransfected in duplicate with plasmid cocktails using lipofectamine 2000 (Invitrogen). Per well, a cocktail of 4 μg of plasmid DNA was applied, consisting of 2.88 μg of boxB reporter and increasing amounts of λ-effectors as detailed in the Figure 2 legend. The difference to 4 μg in each case was made up with pSV-β Gal plasmid, and for each λ-effector amount an equivalent control transfection was done with “empty” pSGλ. Cells were harvested 24 h after transfection and lysed with CAT Elisa lysis buffer. Reporter protein expression was measured with a BMG FLUOstar Optima plate reader using kit reagents for CAT Elisa, Firefly Luciferase activity, and β-Galactosidase chemiluminescence (all from Roche). CAT and LUC reporter activities were first normalized to β-Gal, then averaged for duplicates. Finally, λ-effector values were normalized against appropriate pSGλ controls to calculate the relative stimulation of CAT or LUC expression due to λ-effector coexpression. Aliquots of transfected cells were also processed for Western blot by boiling in 100 μL of 1× Laemmli sample buffer.
Stress granule (SG) induction
HeLa S3 cells were grown on cover slips until cell density reached 70%–90%. Cellular stress was induced by treating cells with 250 μM sodium arsenite (Sigma) for 30 min, or by incubating the cells for 10 min at 44°C as previously described (Kedersha et al. 2005). Cells were then fixed for 10 min in 4% paraformaldehyde and permeabilized for 10 min with ice-cold methanol at room temperature. Incubation with the primary and TRITC-, FITC-, or Cy5-coupled secondary antibodies was conducted for 1 h each at room temperature. For the GFP expression study, cells were transfected with 100 ng of plasmid DNA using lipofectamine 2000 (Invitrogen). Digital images were taken with a Leica TCS SP2 confocal microscope and analyzed with the associated software.
Zebrafish methods
Zebrafish (wild-type or golden mutant strains) maintenance, egg collection, and embryo culture were done as detailed elsewhere (Nusslein-Volhard and Dahm 2002). Digoxigenin-labeled p97a, p97b, and goosecoid RNA probes were prepared by in vitro transcription using appropriately linearized plasmids as templates. In situ hybridization to zebrafish embryos was done as previously described (Jowett 1999). For morpholino knockdown, ∼10 nL of morpholino solution (0.5 mM in water) was injected into the cytoplasm or nucleus of one- or two-cell stage zebrafish eggs, following previously described methods (Gilmour et al. 2002). To confirm the efficacy of morpholino injections, RNA was extracted from embryos after 30 h using the Trizol reagent (Invitrogen). Oligo(dT)-primed cDNA synthesis and PCR was done using standard procedures.
ACKNOWLEDGMENTS
We thank Simon Morley and Matthias Hentze for gifts of antisera. Alexandra Segref is acknowledged for her role in early exploratory experiments. This work was funded by grants from the National Health and Medical Research Council and the Sylvia & Charles Viertel Charitable Foundation to T.P.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.372307.
REFERENCES
- Beausoleil, S.A., Jedrychowski, M., Schwartz, D., Elias, J.E., Villen, J., Li, J., Cohn, M.A., Cantley, L.C., Gygi, S.P. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc. Natl. Acad. Sci. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellsolell, L., Cho-Park, P.F., Poulin, F., Sonenberg, N., Burley, S.K. Two structurally atypical HEAT domains in the C-terminal portion of human eIF4G support binding to eIF4A and Mnk1. Structure. 2006;14:913–923. doi: 10.1016/j.str.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Birney, E., Clamp, M., Durbin, R. GeneWise and Genomewise. Genome Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell, M., Poncet, D., Marissen, W.E., Flotow, H., Lloyd, R.E., Clemens, M.J., Morley, S.J. Cleavage of polypeptide chain initiation factor eIF4GI during apoptosis in lymphoma cells: Characterization of an internal fragment generated by caspase-3-mediated cleavage. Cell Death Differ. 2000;7:628–636. doi: 10.1038/sj.cdd.4400699. [DOI] [PubMed] [Google Scholar]
- Bushell, M., Wood, W., Carpenter, G., Pain, V.M., Morley, S.J., Clemens, M.J. Disruption of the interaction of mammalian protein synthesis eukaryotic initiation factor 4B with the poly(A)-binding protein by caspase- and viral protease-mediated cleavages. J. Biol. Chem. 2001;276:23922–23928. doi: 10.1074/jbc.M100384200. [DOI] [PubMed] [Google Scholar]
- Bushell, M., Stoneley, M., Kong, Y.W., Hamilton, T.L., Spriggs, K.A., Dobbyn, H.C., Qin, X., Sarnow, P., Willis, A.E. Polypyrimidine tract binding protein regulates IRES-mediated gene expression during apoptosis. Mol. Cell. 2006;23:401–412. doi: 10.1016/j.molcel.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Cornelis, S., Bruynooghe, Y., Denecker, G., Van Huffel, S., Tinton, S., Beyaert, R. Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol. Cell. 2000;5:597–605. doi: 10.1016/s1097-2765(00)80239-7. [DOI] [PubMed] [Google Scholar]
- Czaplinski, K., Kocher, T., Schelder, M., Segref, A., Wilm, M., Mattaj, I.W. Identification of 40LoVe, a Xenopus hnRNP D family protein involved in localizing a TGF-β-related mRNA during oogenesis. Dev. Cell. 2005;8:505–515. doi: 10.1016/j.devcel.2005.01.012. [DOI] [PubMed] [Google Scholar]
- De Gregorio, E., Preiss, T., Hentze, M.W. Translation driven by an eIF4G core domain in vivo. EMBO J. 1999;18:4865–4874. doi: 10.1093/emboj/18.17.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio, E., Baron, J., Preiss, T., Hentze, M.W. Tethered-function analysis reveals that eIF4E recruits ribosomes independent of its binding to the cap structure. RNA. 2001;7:106–113. doi: 10.1017/s1355838201000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper, B.W., Morcos, P.A., Kimmel, C.B. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: A quantifiable method for gene knockdown. Genesis. 2001;30:154–156. doi: 10.1002/gene.1053. [DOI] [PubMed] [Google Scholar]
- Gilmour, D.T., Jessen, J.R., Lin, S. Manipulating gene expression in the zebrafish. In: Nusslein-Volhard C., Dahm R., editors. Zebrafish: a practical approach. Oxford University Press; Oxford: 2002. pp. 121–143. [Google Scholar]
- Henis-Korenblit, S., Strumpf, N.L., Goldstaub, D., Kimchi, A. A novel form of DAP5 protein accumulates in apoptotic cells as a result of caspase cleavage and internal ribosome entry site-mediated translation. Mol. Cell. Biol. 2000;20:496–506. doi: 10.1128/mcb.20.2.496-506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis-Korenblit, S., Shani, G., Sines, T., Marash, L., Shohat, G., Kimchi, A. The caspase-cleaved DAP5 protein supports internal ribosome entry site-mediated translation of death proteins. Proc. Natl. Acad. Sci. 2002;99:5400–5405. doi: 10.1073/pnas.082102499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcik, M., Sonenberg, N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell. Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- Holcik, M., Sonenberg, N., Korneluk, R.G. Internal ribosome initiation of translation and the control of cell death. Trends Genet. 2000;16:469–473. doi: 10.1016/s0168-9525(00)02106-5. [DOI] [PubMed] [Google Scholar]
- Hundsdoerfer, P., Thoma, C., Hentze, M.W. Eukaryotic translation initiation factor 4GI and p97 promote cellular internal ribosome entry sequence-driven translation. Proc. Natl. Acad. Sci. 2005;102:13421–13426. doi: 10.1073/pnas.0506536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka, H., Sonenberg, N. Human eukaryotic translation initiation factor 4G (eIF4G) possesses two separate and independent binding sites for eIF4A. Mol. Cell. Biol. 1997;17:6940–6947. doi: 10.1128/mcb.17.12.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka, H., Olsen, H.S., Sonenberg, N. A new translational regulator with homology to eukaryotic translation initiation factor 4G. EMBO J. 1997;16:817–825. doi: 10.1093/emboj/16.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes, G., Carter, M.S., Eisen, M.B., Brown, P.O., Sarnow, P. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc. Natl. Acad. Sci. 1999;96:13118–13123. doi: 10.1073/pnas.96.23.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett, T. Analysis of protein and gene expression. Methods Cell Biol. 1999;59:63–85. doi: 10.1016/s0091-679x(08)61821-x. [DOI] [PubMed] [Google Scholar]
- Kedersha, N., Stoecklin, G., Ayodele, M., Yacono, P., Lykke-Andersen, J., Fitzler, M.J., Scheuner, D., Kaufman, R.J., Golan, D.E., Anderson, P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar, A.A., Hatzoglou, M. Internal ribosome entry sites in cellular mRNAs: Mystery of their existence. J. Biol. Chem. 2005;280:23425–23428. doi: 10.1074/jbc.R400041200. [DOI] [PubMed] [Google Scholar]
- Lee, S.H., McCormick, F. p97/DAP5 is a ribosome-associated factor that facilitates protein synthesis and cell proliferation by modulating the synthesis of cell cycle proteins. EMBO J. 2006;25:4008–4019. doi: 10.1038/sj.emboj.7601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Strumpf, N., Deiss, L.P., Berissi, H., Kimchi, A. DAP-5, a novel homolog of eukaryotic translation initiation factor 4G isolated as a putative modulator of γ interferone-induced programmed cell death. Mol. Cell. Biol. 1997;17:1615–1625. doi: 10.1128/mcb.17.3.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe, D.S. A simple technique for the preparation and storage of sucrose gradients. Anal. Biochem. 1983;135:230–232. doi: 10.1016/0003-2697(83)90755-8. [DOI] [PubMed] [Google Scholar]
- Marash, L., Kimchi, A. DAP5 and IRES-mediated translation during programmed cell death. Cell Death Differ. 2005;12:554–562. doi: 10.1038/sj.cdd.4401609. [DOI] [PubMed] [Google Scholar]
- Merrick, W.C. Cap-dependent and cap-independent translation in eukaryotic systems. Gene. 2004;332:1–11. doi: 10.1016/j.gene.2004.02.051. [DOI] [PubMed] [Google Scholar]
- Mikami, S., Masutani, M., Sonenberg, N., Yokoyama, S., Imataka, H. An efficient mammalian cell-free translation system supplemented with translation factors. Protein Expr. Purif. 2006;46:348–357. doi: 10.1016/j.pep.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Morley, S.J., Jeffrey, I., Bushell, M., Pain, V.M., Clemens, M.J. Differential requirements for caspase-8 activity in the mechanism of phosphorylation of eIF2α, cleavage of eIF4GI and signaling events associated with the inhibition of protein synthesis in apoptotic Jurkat T cells. FEBS Lett. 2000;477:229–236. doi: 10.1016/s0014-5793(00)01805-6. [DOI] [PubMed] [Google Scholar]
- Morley, S.J., Coldwell, M.J., Clemens, M.J. Initiation factor modifications in the preapoptotic phase. Cell Death Differ. 2005;12:571–584. doi: 10.1038/sj.cdd.4401591. [DOI] [PubMed] [Google Scholar]
- Nevins, T.A., Harder, Z.M., Korneluk, R.G., Holcik, M. Distinct regulation of internal ribosome entry site-mediated translation following cellular stress is mediated by apoptotic fragments of eIF4G translation initiation factor family members eIF4GI and p97/DAP5/NAT1. J. Biol. Chem. 2003;278:3572–3579. doi: 10.1074/jbc.M206781200. [DOI] [PubMed] [Google Scholar]
- Nielsen, K.H., Szamecz, B., Valasek, L., Jivotovskaya, A., Shin, B.S., Hinnebusch, A.G. Functions of eIF3 downstream of 48S assembly impact AUG recognition and GCN4 translational control. EMBO J. 2004;23:1166–1177. doi: 10.1038/sj.emboj.7600116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjanakumari, S., Lasda, E., Brazas, R., Garcia-Blanco, M.A. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods. 2002;26:182–190. doi: 10.1016/S1046-2023(02)00021-X. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C., Dahm R., editors. Zebrafish: A practical approach. Oxford University Press; Oxford: 2002. [Google Scholar]
- Preiss, T., Hentze, M.W. Starting the protein synthesis machine: Eukaryotic translation initiation. Bioessays. 2003;25:1201–1211. doi: 10.1002/bies.10362. [DOI] [PubMed] [Google Scholar]
- Pyronnet, S., Sonenberg, N. Cell-cycle-dependent translational control. Curr. Opin. Genet. Dev. 2001;11:13–18. doi: 10.1016/s0959-437x(00)00150-7. [DOI] [PubMed] [Google Scholar]
- Pyronnet, S., Imataka, H., Gingras, A.C., Fukunaga, R., Hunter, T., Sonenberg, N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits Mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyronnet, S., Pradayrol, L., Sonenberg, N. A cell cycle-dependent internal ribosome entry site. Mol. Cell. 2000;5:607–616. doi: 10.1016/s1097-2765(00)80240-3. [DOI] [PubMed] [Google Scholar]
- Qin, X., Sarnow, P. Preferential translation of internal ribosome entry site-containing mRNAs during the mitotic cycle in mammalian cells. J. Biol. Chem. 2004;279:13721–13728. doi: 10.1074/jbc.M312854200. [DOI] [PubMed] [Google Scholar]
- Saelens, X., Kalai, M., Vandenabeele, P. Translation inhibition in apoptosis: Caspase-dependent PKR activation and eIF2-α phosphorylation. J. Biol. Chem. 2001;276:41620–41628. doi: 10.1074/jbc.M103674200. [DOI] [PubMed] [Google Scholar]
- Shaughnessy J.D., Jr, Jenkins, N.A., Copeland, N.G. cDNA cloning, expression analysis, and chromosomal localization of a gene with high homology to wheat eIF-(iso)4F and mammalian eIF-4G. Genomics. 1997;39:192–197. doi: 10.1006/geno.1996.4502. [DOI] [PubMed] [Google Scholar]
- Sonenberg, N., Dever, T.E. Eukaryotic translation initiation factors and regulators. Curr. Opin. Struct. Biol. 2003;13:56–63. doi: 10.1016/s0959-440x(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Stachel, S.E., Grunwald, D.J., Myers, P.Z. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- Stoneley, M., Willis, A.E. Cellular internal ribosome entry segments: Structures, trans-acting factors and regulation of gene expression. Oncogene. 2004;23:3200–3207. doi: 10.1038/sj.onc.1207551. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Maruyama, M., Tokuzawa, Y., Murakami, M., Oda, Y., Yoshikane, N., Makabe, K.W., Ichisaka, T., Yamanaka, S. Evolutionarily conserved non-AUG translation initiation in NAT1/p97/DAP5 (EIF4G2) Genomics. 2005;85:360–371. doi: 10.1016/j.ygeno.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriyarachchi, D., Cerquozzi, S., Cheung, H.H., Holcik, M. Translational induction of the inhibitor of apoptosis protein HIAP2 during endoplasmic reticulum stress attenuates cell death and is mediated via an inducible internal ribosome entry site element. J. Biol. Chem. 2004;279:17148–17157. doi: 10.1074/jbc.M308737200. [DOI] [PubMed] [Google Scholar]
- Yamanaka, S., Poksay, K.S., Arnold, K.S., Innerarity, T.L. A novel translational repressor mRNA is edited extensively in livers containing tumors caused by the transgene expression of the apoB mRNA-editing enzyme. Genes & Dev. 1997;11:321–323. doi: 10.1101/gad.11.3.321. [DOI] [PubMed] [Google Scholar]
- Yamanaka, S., Zhang, X.Y., Maeda, M., Miura, K., Wang, S., Farese R.V., Jr, Iwao, H., Innerarity, T.L. Essential role of NAT1/p97/DAP5 in embryonic differentiation and the retinoic acid pathway. EMBO J. 2000;19:5533–5541. doi: 10.1093/emboj/19.20.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]