Summary
In the final stages of ovarian follicular development, the mouse oocyte remains arrested in the first meiotic prophase, and cAMP-stimulated PKA plays an essential role in this arrest. After the LH surge, a decrease in cAMP and PKA activity in the oocyte initiates an irreversible maturation process that culminates in a second arrest at metaphase II prior to fertilization [1]. A-kinase anchoring proteins (AKAPs) mediate the intracellular localization of PKA and control the specificity and kinetics of substrate phosphorylation [2]. Several AKAPs have been identified in oocytes including one at 140 kDa [3, 4] that we now identify as a product of the Akap1 gene. We show that PKA interaction with AKAPs is essential for two sequential steps in the maturation process: the initial maintenance of meiotic arrest and the subsequent irreversible progression to the polar body extruded stage. A peptide inhibitor (HT31) that disrupts AKAP/PKA interactions stimulates oocyte maturation in the continued presence of high cAMP. However, during the early minutes of maturation, type II PKA moves from cytoplasmic sites to the mitochondria, where it associates with AKAP1, and this is shown to be essential for maturation to continue irreversibly.
Results and Discussion
Generation of AKAP1 Knockout Mice
The central exon2 of Akap1 encodes the majority of the protein in all splice variants, and we replaced this exon with a neomycin resistance cassette to create a complete Akap1 knockout mouse (Figure 1). Western blot analysis indicated that all variants of the AKAP1 protein were absent in knockout tissues including the ovary (Figure 1C). Previous work has reported that the predominant AKAP in rodent oocytes migrates at 140 kDa on an RII overlay assay [3, 4]. By using a similar RII overlay assay, we showed that AKAP1 also migrated at 140 kDa and could be identified as the predominant AKAP in the oocyte since it was absent in the knockout (Figure 1D).
Figure 1. Generation of AKAP1 Knockout Mice.

(A) Targeting of the wild-type allele (top) by homologous recombination led to disruption of exon 2 of AKAP1 and insertion of the neomycin-resistant cassette (bottom). B, BamHI; BsrG, BsrGI; E, EcoRI; P, Pac.
(B) Genomic Southern blot of ES cells transfected with the mutant construct was conducted by digesting DNA with BamH1. The wild-type band migrates at 15 kb and the knockout band at 10 kb.
(C) Western blot analyses of protein samples of ovaries from AKAP1 wild-type and knockout mice indicated that the 140 kDa isoform is absent in knockout mice.
(D) RII overlay on oocytes indicated that AKAP1 is a predominant AKAP in the oocyte. Protein from 100 GV-stage oocytes from either wildtype or knockout females was separated on an SDS-PAGE gel. The protein was transferred to a nitrocellulose membrane and the membrane was incubated in RIIβ protein, followed by incubation with an RIIβ antibody. The red arrow indicates AKAP1.
(E) Confocal micrograph images showed immunostaining for αAKAP1 (green) in ovarian sections. Nuclei were stained with DAPI (blue). AKAP1 was predominantly expressed in the oocyte in different stages of follicular development. Preantral and preovulatory follicles are shown. The experiment was conducted three times and representative images are shown (250× magnification). gc, granulosa cells.
(F) Within the oocyte (GV stage), AKAP1 (green) showed a punctate distribution that colocalized to the mitochondria (red). Nuclei are stained with ToPro3 (blue). AKAP1 was excluded from cumulus granulosa cells (gc), as shown in the middle panels.
AKAP1 Is Highly Expressed in the Oocyte and Associates with Mitochondria
Breeding studies revealed that AKAP1 knockout females had greatly reduced fertility, so we examined the expression and localization of AKAP1 in the ovary. AKAP1 was found primarily in the oocyte in various stages of follicular development, including preantral and antral follicles (Figure 1E). Immunocytochemical studies of wild-type oocytes showed mitochondrial localization of AKAP1 and absence of AKAP1 in the cumulus granulosa cells (Figure 1F). Furthermore, real-time RT-PCR indicated that N0, the mitochondrial splice variant of AKAP1, is the predominant splice variant in the oocyte, whereas the N1 endoplasmic reticulum splice variant is absent (data not shown).
AKAP1 Knockout Females Are Subfertile
Although AKAP1 knockout males were fertile, AKAP1 knockout females were subfertile or infertile, whereas the AKAP1 heterozygous females had normal fertility. When AKAP1 heterozygous females were mated to C57BL/6J male mice, the expected number of litters per female per month were produced (0.86), with 7.7 ± 1.2 pups/litter. In contrast, AKAP1 knockout females gave birth at a rate of 0.26 litters/female/month and a litter size of 2.2 ± 0.8 pups/litter. This reduced fertility was present regardless of the background strain of the females (129X1/SvJ versus C57BL6/J) and was independent of the AKAP1 genotype of the males to which they were mated (see Figure S1 in the Supplemental Data available with this article online).
Ovarian histology was normal in prepubertal supero-vulated (3- to 4-week-old) and mature nonsuperovulated (12-week-old) knockout females (data not shown), and serum LH and FSH levels were also unchanged, indicating that the effect of AKAP1 deficiency on female fertility was not hormonally related. Superovulation resulted in a similar number of eggs ovulated in both knockout and control females (Figure S1), although many of the eggs in knockout females appeared to be immature or degenerated (Figure 2A).
Figure 2. Embryos from AKAP1 Knockout Females Failed to Mature and Were More Sensitive to cAMP.
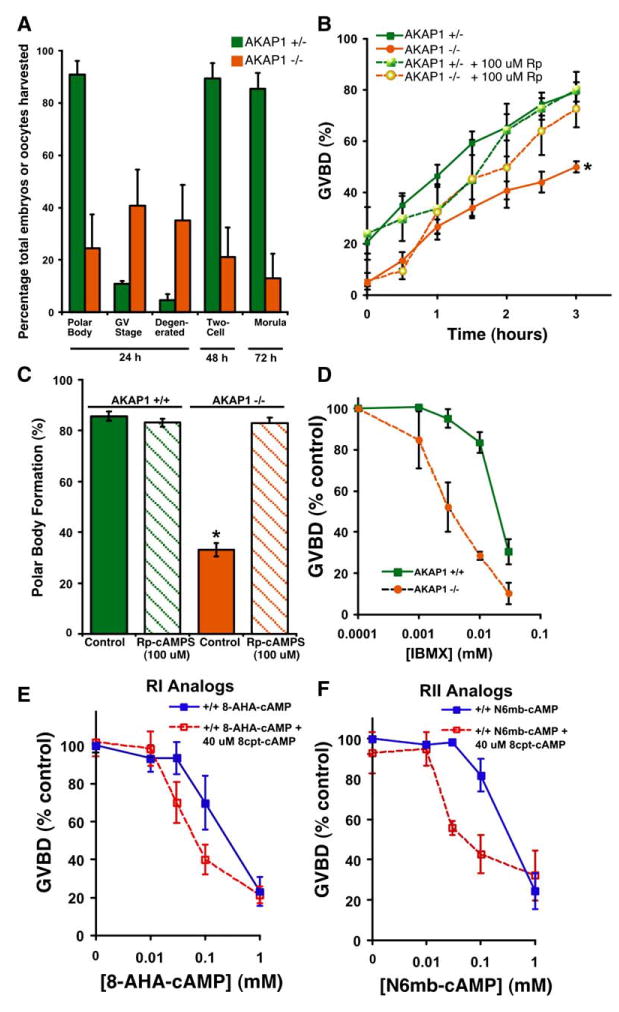
(A) Female AKAP1 knockout or heterozygous mice (22- to 26-days-old) were injected with PMSG, followed by hCG 48 hr later. Immediately after hCG injection, females were housed individually with wild-type males. Embryos were harvested from the oviduct of the females 24 hr later. The embryos were placed in HTF (Irvine Scientific) media supplemented with 0.25 mM EDTA for 72 hr. Embryonic stage was monitored during this time. n = 135 embryos from heterozygous females, n = 97 embryos from knockout females.
(B) In vitro GVBD was decreased in oocytes from knockout females. Denuded oocytes from heterozygous and knockout females were collected from ovaries 48 hr after PMSG injection. These oocytes were then assessed for GVBD (meiotic resumption) 3 hr later. Oocytes with a clear nuclear membrane (GV) and nucleoli were classified as being at the GV stage, while those without a visible nuclear structure were classified as GVBD stage. An inhibitor of PKA, Rp-cAMPS (100 μM), reversed the defect in GVBD in knockout oocytes but had no effect in oocytes from heterozygous females. Results are expressed as mean ± SEM for seven experiments (no treatment) and four experiments (Rp-cAMPS). *p < 0.05 by a repeated measures ANOVA with a Newman Kuels post test.
(C) In vitro polar body formation was restored to wild-type levels in AKAP1 knockout oocytes treated with 100 μM Rp-cAMPS. Denuded oocytes from wild-type and knockout females were collected from ovaries 48 hr after PMSG injection and incubated at 37ºC with or without Rp-cAMPS (100 μM). After 2 hr of incubation, the Rp-cAMPS was washed out of the media, the oocytes were returned to the incubator, and polar body formation was assessed 18 hr later. Results are expressed as mean ± SEM for six experiments (knockout) and three experiments (wild-type). *p < 0.001 by a one-way ANOVA with a Newman Kuels post test.
(D) Oocytes from AKAP1 knockout females were more sensitive to the inhibitory effects of IBMX on GVBD. Oocytes incubated with increasing concentrations of the IBMX were scored for GVBD 3 hr after treatment. Values are expressed as mean ±SEM for four experiments.
(E) RI-specific cAMP analogs synergistically inhibit GVBD in oocytes from AKAP1 wild-type mice. Denuded oocytes were incubated with 40 μM of 8cpt-cAMP and increasing concentrations of 8-AHA-cAMP to stimulate RI. Oocytes were scored for GVBD 3 hr after treatment. Values are expressed as mean ± SEM for three experiments.
(F) RII-specific cAMP analogs synergistically inhibit GVBD in oocytes from AKAP1 wild-type mice. Denuded oocytes were incubated with 40 μM of 8cpt-cAMP and increasing concentrations of N6mb-cAMP to stimulate RII. Oocytes were scored for GVBD 3 hr after treatment. Values are expressed as mean ± SEM for three experiments.
Ovulated Oocytes from AKAP1 Knockout Females Failed to Resume Meiosis
After superovulation with gonadotropins and in vivo mating, we found that most oocytes from knockout females were either degenerated or arrested in GV stage and had not extruded a polar body, an indication that the oocytes had not progressed to metaphase II and fertilization competence. However, when these embryos were cultured in vitro, those that had extruded a polar body were able to become two-cell embryos and subsequently morula (Figure 2A).
AKAP1 Knockout Oocytes Harvested from the Ovary Are Defective in Maturation In Vitro
Oocytes were harvested from the ovaries of PMSG-primed females, and cumulus granulosa cells were removed. After 3 hr in culture, 79% of germinal vesicle (GV)-stage oocytes from heterozygous females resumed meiosis and underwent maturation, as indicated by germinal vesicle breakdown (GVBD). However, only 50% of oocytes from knockout females underwent GVBD (Figure 2B). Oocytes from knockout females that matured in vitro showed activation of maturation promoting factor (MPF) and MAP kinase (MAPK), as did wild-type (data not shown). Interestingly, the maturation of knockout oocytes could be rescued by the addition of 100 μM Rp-adenosine 3,5-cyclic monophos-phorothioate (Rp-cAMPS), an inhibitor of PKA (Figure 2B). Even more strikingly, 83% of knockout oocytes incubated in 100 μM Rp-cAMPS for 2 hr and then washed were rescued to polar body stage 20 hr later (Figure 2C). In contrast, only 33% of oocytes from knockout females extruded polar bodies in the absence of Rp-cAMPS treatment. Based on this result, we hypothesized that PKA is inappropriately active in the knockout oocytes. The defect seen in oocyte maturation when triggered in vitro in granulosa-free oocytes is less dramatic than that observed in vivo when oocytes mature in the presence of the cumulus granulosa cells. This is consistent with the possibility that knockout oocytes have become more sensitive to cAMP-activated arrest because of a change in PKA localization and that granulosa cells are still providing signals to the oocyte that partially maintain levels of cAMP even during LH-induced ovulation.
AKAP1 Knockout Oocytes Are More Sensitive to cAMP
Either the total activity or subcellular localization of PKA might be affected in the oocyte as a result of deletion of AKAP1. Total cAMP levels were unchanged in knockout GV-stage oocytes (data not shown), and the levels of PKA subunits and the activity of PKA was also normal (Figure S2). However, knockout oocytes were more sensitive to inhibition of GVBD by the PDE inhibitor, 3-isobu-tyl-1-methylxanthine (IBMX) (Figure 2D). To confirm that AKAP1 knockout oocytes are more sensitive to cAMP and not merely PDE inhibition, GVBD experiments were conducted with oocytes incubated in increasing concentrations of the cAMP analogs N6,2-O-Dibutyryla-denosine cAMP (db-cAMP) and 6-Dichloro-1-b- D-ribo-furanosylbenzimidazole 3′,5′-cyclic monophosphorothioate, Sp-isomer (Sp-5,6-DCl-cBIMPS). Oocytes from AKAP1 knockout females were more sensitive to the inhibitory effects of db-cAMP and Sp-5,6-DCl-cBIMPS on GVBD than oocytes from wild-type females (data not shown).
The RIIα Subtype of PKA Is a Major Regulator of Meiotic Arrest in Wild-Type Oocytes
Although it was previously thought that RIIα was not expressed in rodent oocytes, more recent work has documented the presence of both RIα and RIIα [3, 4], although it has been suggested that RIα-PKA is the major subtype responsible for maintaining meiotic arrest [5, 6]. To reexamine the relative contribution of RIα and RIIα containing PKA to meiotic arrest, we measured GVBD in response to stimulation of oocytes with cAMP analogs that preferentially activate holoenzymes containing either RIα or RIIα. We used cAMP analogs that differ in their relative affinities for the A and B cAMP binding sites on RIα and RIIα, choosing analog combinations that act synergistically on only one of the two isoforms, while activation of the other isoform is additive [7, 8].
GV-stage oocytes were obtained from PMSG-treated wild-type ovaries, denuded, and incubated with increasing concentrations of the subtype-specific analogs. In AKAP1 wild-type oocytes, activation of RIIα (Figure 2F) gave a more synergistic inhibition of GVBD than seen with RIα (Figure 2E), suggesting that RIIα PKA plays an important role in maintaining meiotic arrest.
PKA Localization Is Unchanged in AKAP1 Knockout GV-Stage Oocytes
We hypothesized that the increased sensitivity to cAMP in knockout oocytes results from changes in PKA localization. Subcellular fractionation of mitochondria from wild-type GV-stage oocytes indicated that AKAP1 localized completely to the mitochondrial compartment as expected from immunocytochemistry (ICC). However, RIIα was not associated with the mitochondrial compartment and was found in the 17,000 × g supernatant (Figure S4). Localization of the RIα, RIIα, and Cα subunits of PKA was further examined in GV-stage oocytes by ICC. RIIα localization in knockout oocytes was indistinguishable from that of wild-type oocytes (Figure 3A), and in agreement with fractionation studies did not co-localize with mitochondria. The other PKA subunits expressed in the oocyte (RIα, Cα) also did not colocalize with mitochondria by ICC or direct fractionation studies (data not shown). Unexpectedly, these data indicate that PKA is unable to interact with AKAP1 at the mitochondrial membrane in GV-stage oocytes.
Figure 3. RIIα and AKAP1 Localization during Oocyte Maturation.

(A) ICC was conducted on GV-stage oocytes with primary antibodies to PKA RIIα (green), Mitotracker (red), and ToPro (blue).
(B) ICC was conducted in MII-stage oocytes.
(C) ICC was conducted in wild-type oocytes collected from ovarian follicles and incubated for 1.5 or 6 hr, respectively. Primary antibodies to RIIα (green) and Mitotracker (red) were used. Oocytes were counterstained with ToPro (blue). This experiment was conducted three times, and representative images are shown.
(D) ICC was conducted in MII-stage wild-type oocytes with primary antibodies to AKAP1 (green) or AKAP7γ (green) and Mitotracker (red) or RIIα (red). Oocytes were counter-stained with ToPro (blue). These experiments were conducted three times, and representative images are shown.
Dynamic Relocalization of RIIα -PKA to Mitochondria Occurs prior to GVBD and Is Dependent on AKAP1
Other groups have demonstrated that PKA localization changes as the oocyte resumes meiosis [3, 4]. We asked whether any of these changes in PKA localization were dependent on the presence of AKAP1. Metaphase II (MII) stage oocytes were collected from the oviducts of superovulated females. Although most knockout oocytes do not mature to the MII stage, sufficient numbers of MII knockout oocytes could be obtained for the ICC experiments. Although RIIα did not associate with the mitochondria in wild-type GV-stage oocytes, it colocalized with mitochondria in wild-type MII oocytes but not in knockout MII oocytes (Figure 3B). We also directly demonstrated that RIIα colocalized with AKAP1 in wild-type MII oocytes (Figure 3D). RIIα did not colocalize with mitochondria in AKAP1 knockout oocytes that reached the MII stage, but instead localized at the cortical region of the oocyte (Figure 3B) in a pattern that was similar to the localization of another AKAP, AKAP7γ (Figure 3D). The intracellular localization of mitochondria and the movement of mitochondria during maturation were the same in both wild-type and knockout oocytes (Figure S3).
We examined whether the relocalization of RIIα to the mitochondria occurred early or late in the maturation process. GV-stage oocytes were isolated from the ovaries of wild-type mice, incubated for 1.5 to 6 hr, and assessed for meiotic stage and type II PKA localization. As shown in Figure 3C and Figure S4, RIIα begins to localize to the mitochondria within 1.5 hr after maturation is initiated and prior to the GVBD stage and remains localized throughout the MI stage after GVBD (6 hr). After a 2 hr maturation, subcellular fractionation of oocyte extracts indicated that about 50% of the RIIα was now associated with the mitochondrial pellet and AKAP1 (Figure S4).
Localization Plays a Dual Role in the Process of Oocyte Maturation
Our results suggest that anchoring of RIIα-PKA to AKAP1 on mitochondria effectively prevents that holoenzyme from interfering with the maturation process once it is initiated. Since other AKAPs are also present in the oocyte [3, 4, 9–11], we asked whether this was the only role for PKA anchoring in oocyte maturation. A peptide inhibitor of AKAP interactions with R subunits, HT31, was injected into wild-type GV-stage oocytes that were incubated in the presence of IBMX to maintain them in an arrested state. Surprisingly, HT31 stimulated oocyte maturation as measured by GVBD, overcoming the IBMX-induced arrest; as a control, the inactive pro-line-substituted HT31-P had no effect. HT31 was just as effective at promoting maturation as PKI (data not shown), a well-studied stimulator of frog and mouse oocyte maturation [1, 12]. These data indicate that PKA localization to an AKAP other than AKAP1 is essential at an early stage in order for cAMP to maintain the arrest of oocytes in meiosis I prophase.
Previous work has shown that once the process of oocyte maturation is initiated, it proceeds to completion in an irreversible manner [13]. This implies that there is some type of molecular ‘‘switch’’ that initiates meiosis and cannot be turned off easily once this process has begun. We suggest that this switch might involve the relocalization of PKA within the oocyte during the early stages of GVBD. To determine whether changes in PKA localization can affect the reversibility of meiotic maturation, we treated wild-type and knockout GV-stage oocytes with the PKA inhibitor Rp-cAMPS for 1 hr. We then removed the Rp-cAMPS and added IBMX to increase cAMP levels. Wild-type oocytes had activated their ‘‘meiotic switch’’ after 1 hr, and GVBD could not be inhibited by the addition of IBMX. However, the rescue in GVBD that was observed in knockout oocytes with Rp-cAMPS was partially reversed just by washing out the Rp-cAMPS at 1 hr and completely reversed when IBMX was added to elevate cAMP (Figure 4B).
Figure 4. Correct PKA Localization Is Required for Meiotic Resumption.

(A) Oocytes microinjected with 0.1 mM HT31 or 0.1 mM HT31-P were incubated with or without 0.2 mM IBMX and scored for GVBD 3 hr after treatment. Values are expressed as mean ± SEM for three experiments.
(B) Wildtype and knockout GV-stage oocytes were treated with Rp-cAMPS (100 μM) for 1 hr followed by a wash out. IBMX (0.2 mM) was then added, and GVBD was observed 3 hr later. Values are expressed as mean ± SEM for three experiments.
(C) Model of AKAP function in the oocyte. In the wild-type GV oocyte, the RIIα subunit of PKA does not interact with AKAP1, but we hypothesize that it is associated with an unidentified AKAP, AKAPx. As the wild-type oocyte begins to mature, RIIα translocates and binds AKAP1 at the mitochondria. MPF, maturation promoting factor, a complex of CDK1 and cyclin B; cdc25b, a dual specificity phosphatase shown to act on CDK1 to activate it; wee1, a tyrosine kinase that phosphorylates and inactivates CDK1. AKAP1 is shown associated with mitochondria at all times.
Studies in vitro demonstrate that PKA activation inhibits GVBD [14–16], and inhibitors of either cAMP production or PKA activity promote maturation [1, 17–19]. The maturation pathway in oocytes is controlled by MPF, a complex of CDK1 and cyclin B. The CDK1 kinase is inhibited by phosphorylation at tyrosine 15 by Wee1, and this site as well as threonine 14 is dephosphorylated by the dual specificity phosphatase, Cdc25b. Xenopus Cdc25 has been shown to be phosphorylated by PKA, and this phosphorylation may lead to association of the phosphatase with 14-3-3 and inhibition of its activity [20]. Disruption of the mouse Cdc25b gene demonstrates that this phosphatase is an important activator of maturation, and in its absence, oocytes remain arrested at prophase with low MPF activity [21].
Our work reveals that the localization of PKA is also an essential element of the decision of oocytes to commit to the maturation program. In GV-stage oocytes, the RIIα PKA holoenzyme localizes in a widely distributed punctate pattern throughout the cytoplasm. As shown in the model depicted in Figure 4C, we speculate that this holoenzyme is binding to an as yet unidentified AKAP that positions PKA close to its targets (e.g., Cdc25b phosphatase and the recently identified Wee1B kinase [22]), maintaining the inactive state of Cdc25b and the active state of Wee1B by phosphorylation. In support of this, we find that GV-stage oocytes injected with the PKA anchoring disruptor HT31 initiate maturation even when the oocytes are maintained in IBMX to keep cAMP and PKA activity high. Our experiments with analogs demonstrate greater synergistic inhibition of GVBD with RII analogs compared with RI analogs, suggesting that the type II kinase is more closely linked to the arrest of maturation in wild-type oocytes.
When cAMP levels in the oocyte decline, PKA activity declines and no longer maintains meiotic arrest. CDK1 is dephosphorylated and becomes active, and maturation is initiated. We suggest that activation of CDK1 sets in motion the relocalization of RIIα-PKA to the mitochondria, which occurs prior to GVBD. The molecular mechanisms that regulate this relocalization are unknown but may involve posttranslational modifications of either RIIα or AKAP1 that increase their interaction with each other. In the AKAP1 knockout, RIIα does not associate with mitochondria, and this loss of RIIα localization prevents the ‘‘meiotic switch’’ from being thrown irreversibly. AKAPs are therefore playing a dual role in oocyte maturation: they are required to maintain the PKA-dependent arrest of oocytes at meiosis I prophase prior to the LH surge, and, after the initiation of maturation, AKAP1 plays an alternative and equally important role in sequestering PKA away from its targets in order for oocyte maturation to proceed normally and irreversibly. Jackson et al. [23] suggested a sequestration role for Drosophila AKAP200 similar to that proposed here for AKAP1 based on studies of egg chamber and actinenriched ring canal development.
The increase in IVF procedures in the human population has focused attention on oocyte maturation as a determinant of successful fertilization, and several reports have documented oocyte maturation defects as a cause of IVF failure [24, 25]. Our results demonstrate the importance of temporal and spatial control of PKA signaling during mammalian oocyte maturation.
Supplemental Data
Supplemental Data include four figures and Supplemental Experimental Procedures and can be found with this article online athttp://www.current-biology.com/cgi/content/full/16/3/321/DC1/.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development, of the National Institutes of Health through a cooperative agreement (U54-HD12629) as part of the Specialized Cooperative Centers Program in Reproduction Research. K.J.N. is a recipient of training grant support from National Institutes of Health (T32 GM07270). Confocal imaging (Leica TCA SP/MP) was conducted at the Keck Imaging Center of the University of Washington. Serum hormone levels were measured at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (NICHD [SCCPRR] Grant U54-HD28934), and gene array analysis was done at the SCCPRR Gene Array Center, University of Washington. We would like to thank Charlie S. Rubin for AKAP1 genomic clones. Susan S. Taylor kindly supplied AKAP1 antibody, and AKAP7γ antibody was a gift from William A. Catterall. The authors also thank Donner Babcock and Kimberly Burton for critical reading of the manuscript.
References
- 1.Bornslaeger EA, Mattei P, Schultz RM. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev Biol. 1986;114:453–462. doi: 10.1016/0012-1606(86)90209-5. [DOI] [PubMed] [Google Scholar]
- 2.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 3.Kovo M, Schillace RV, Galiani D, Josefsberg LB, Carr DW, Dekel N. Expression and modification of PKA and AKAPs during meiosis in rat oocytes. Mol Cell Endocrinol. 2002;192:105–113. doi: 10.1016/s0303-7207(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 4.Brown RL, Ord T, Moss SB, Williams CJ. A-kinase anchor proteins as potential regulators of protein kinase A function in oocytes. Biol Reprod. 2002;67:981–987. doi: 10.1095/biolreprod.101.003046. [DOI] [PubMed] [Google Scholar]
- 5.Downs SM, Hunzicker-Dunn M. Differential regulation of oocyte maturation and cumulus expansion in the mouse oocyte-cumulus cell complex by site-selective analogs of cyclic adenosine monophosphate. Dev Biol. 1995;172:72–85. doi: 10.1006/dbio.1995.0006. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez KF, Petters RM, Crosier AE, Farin CE. Roles of gene transcription and PKA subtype activation in maturation of murine oocytes. Reproduction. 2002;123:799–806. doi: 10.1530/rep.0.1230799. [DOI] [PubMed] [Google Scholar]
- 7.Otten AD, Parenteau LA, Doskeland S, McKnight GS. Hormonal activation of gene transcription in ras-transformed NIH3T3 cells overexpressing RII alpha and RII beta subunits of the cAMP-dependent protein kinase. J Biol Chem. 1991;266:23074–23082. [PubMed] [Google Scholar]
- 8.Ogreid D, Ekanger R, Suva RH, Miller JP, Doskeland SO. Comparison of the two classes of binding sites (A and B) of type I and type II cyclic-AMP-dependent protein kinases by using cyclic nucleotide analogs. Eur J Biochem. 1989;181:19–31. doi: 10.1111/j.1432-1033.1989.tb14689.x. [DOI] [PubMed] [Google Scholar]
- 9.Brown RL, August SL, Williams CJ, Moss SB. AKAP7gamma is a nuclear RI-binding AKAP. Biochem Biophys Res Commun. 2003;306:394–401. doi: 10.1016/s0006-291x(03)00982-3. [DOI] [PubMed] [Google Scholar]
- 10.Louvet S, Aghion J, Santa-Maria A, Mangeat P, Maro B. Ezrin becomes restricted to outer cells following asymmetrical division in the preimplantation mouse embryo. Dev Biol. 1996;177:568–579. doi: 10.1006/dbio.1996.0186. [DOI] [PubMed] [Google Scholar]
- 11.Rawe VY, Payne C, Navara C, Schatten G. WAVE1 intranuclear trafficking is essential for genomic and cytoskeletal dynamics during fertilization: cell-cycle-dependent shuttling between M-phase and interphase nuclei. Dev Biol. 2004;276:253–267. doi: 10.1016/j.ydbio.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 12.Eyers PA, Liu J, Hayashi NR, Lewellyn AL, Gautier J, Maller JL. Regulation of the G(2)/M transition in Xenopus oocytes by the cAMP-dependent protein kinase. J Biol Chem. 2005;280:24339–24346. doi: 10.1074/jbc.M412442200. [DOI] [PubMed] [Google Scholar]
- 13.Ferrell JE, Xiong W. Bistability in cell signaling: how to make continuous processes discontinuous, and reversible processes irreversible. Chaos. 2001;11:227–236. doi: 10.1063/1.1349894. [DOI] [PubMed] [Google Scholar]
- 14.Maller JL, Krebs EG. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′ :5′ -monophosphate-dependent protein kinase. J Biol Chem. 1977;252:1712–1718. [PubMed] [Google Scholar]
- 15.Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 16.Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 17.Vivarelli E, Conti M, De Felici M, Siracusa G. Meiotic resumption and intracellular cAMP levels in mouse oocytes treated with compounds which act on cAMP metabolism. Cell Differ. 1983;12:271–276. doi: 10.1016/0045-6039(83)90023-4. [DOI] [PubMed] [Google Scholar]
- 18.Wiersma A, Hirsch B, Tsafriri A, Hanssen RG, Van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJ. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest. 1998;102:532–537. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duckworth BC, Weaver JS, Ruderman JV. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci USA. 2002;99:16794–16799. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, Donovan PJ. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet. 2002;30:446–449. doi: 10.1038/ng856. [DOI] [PubMed] [Google Scholar]
- 22.Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15:1670–1676. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 23.Jackson SM, Berg CA. An A-kinase anchoring protein is required for protein kinase A regulatory subunit localization and morphology of actin structures during oogenesis in Drosophila. Development. 2002;129:4423–4433. doi: 10.1242/dev.129.19.4423. [DOI] [PubMed] [Google Scholar]
- 24.Levran D, Farhi J, Nahum H, Glezerman M, Weissman A. Maturation arrest of human oocytes as a cause of infertility: case report. Hum Reprod. 2002;17:1604–1609. doi: 10.1093/humrep/17.6.1604. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Ami S, Zlotkin E, Brandes JM, Itskovitz-Eldor J. Failure of meiotic competence in human oocytes. Biol Reprod. 1994;50:1100–1107. doi: 10.1095/biolreprod50.5.1100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


