Abstract
Few studies have investigated whether or not there is an interdependence between osmoregulation and vesicular trafficking. We previously showed that in Caenorhabditis elegans che-14 mutations affect osmoregulation, cuticle secretion, and sensory organ development. We report the identification of seven lethal mutations displaying che-14-like phenotypes, which define four new genes, rdy-1–rdy-4 (rod-like larval lethality and dye-filling defective). rdy-1, rdy-2, and rdy-4 mutations affect excretory canal function and cuticle formation. Moreover, rdy-1 and rdy-2 mutations reduce the amount of matrix material normally secreted by sheath cells in the amphid channel. In contrast, rdy-3 mutants have short cystic excretory canals, suggesting that it acts in a different process. rdy-1 encodes the vacuolar H+-ATPase a-subunit VHA-5, whereas rdy-2 encodes a new tetraspan protein. We suggest that RDY-1/VHA-5 acts upstream of RDY-2 and CHE-14 in some tissues, since it is required for their delivery to the epidermal, but not the amphid sheath, apical plasma membrane. Hence, the RDY-1/VHA-5 trafficking function appears essential in some cells and its proton pump function essential in others. Finally, we show that RDY-1/VHA-5 distribution changes prior to molting in parallel with that of actin microfilaments and propose a model for molting whereby actin provides a spatial cue for secretion.
THE ability to control solute and water balance during osmotic challenge is essential for cellular life (Yancey et al. 1982). Most cellular functions, in particular vesicle trafficking, depend on the specific balance of inorganic ions in the cytosol and the lumen. For instance, loss of the yeast endosomal Na+/H+ exchanger Nhx1 alters cytoplasmic and luminal pH, with profound consequences on the endocytic trafficking pathway (Brett et al. 2005). In Caenorhabditis elegans, disruption of the epidermal chloride channel gene clh-1 results in a significantly wider body and an abnormal structure of cuticular specializations called alae, which are secreted by the epidermis (Petalcorin et al. 1999). Conversely, many animals and plants respond to the need to modify their internal ion balance by regulating the trafficking of certain ion transporters, channels, and exchangers. For instance, vasopressin triggers the fusion of subapical vesicles containing the aquaporin-2 membrane water channel with the apical plasma membrane of kidney-collecting-duct principal cells to mediate water excretion (Nielsen et al. 1993, 1995). Although vesicle trafficking and osmoregulation seem interwoven, whether or not there is a genetic basis for their interdependence is largely unclear.
An obvious approach to addressing this problem is to find mutations that would affect both osmoregulation and trafficking. In contrast to Saccharomyces cerevisiae for which a wealth of information on the genetic control of osmoregulation (Hohmann 2002) or trafficking (Schekman and Novick 2004) is available, less is known about multicellular organisms. The nematode C. elegans provides many powerful experimental advantages for defining evolutionarily conserved genes, pathways, and mechanisms that give rise to diverse physiological processes (Jorgensen and Mango 2002). In particular, C. elegans has helped define genes that contribute to osmotic homeostasis (Kaitna et al. 2002; Solomon et al. 2004; Lamitina and Strange 2005) and trafficking (Nurrish 2002).
We previously found that the C. elegans protein CHE-14 is important for both osmoregulation and apical trafficking (Michaux et al. 2000). A proportion of che-14 larvae dies with an appearance of rods that are filled with fluid, which resemble larvae observed after laser ablation of the kidney-like excretory cell (Nelson and Riddle 1984). In addition, transmission electron microscopy (TEM) reveals that che-14 mutants accumulate large vesicles in support cells of the amphid sensory organs and dark material at the apical surface of the epidermis, while the cuticle normally secreted apically is much thinner than normal (Michaux et al. 2000). CHE-14 is homologous to Dispatched (Michaux et al. 2000), a protein required for the release of the Hedgehog morphogen in Drosophila (Burke et al. 1999). However, the C. elegans genome lacks a canonical Hedgehog homolog and several additional components of the Hedgehog-signaling pathway, in particular Smoothened and Costal2, indicating that CHE-14 does not act in a Hedgehog-patterning process.
To address how CHE-14 might affect trafficking and osmoregulation, we sought to identify new genes acting in the same process. Here we report the results of a screen to uncover mutations displaying che-14-like phenotypes, which led to the identification of seven new mutations. These mutations define four genes that we call rdy-1–4 (rod-like larval lethality and dye-filling defective), one of which corresponds to vha-5 and codes for one of the four C. elegans a-subunits of the vacuolar H+-ATPase (V-ATPase) proton pump. The V-ATPase is a multisubunit protein complex consisting of two distinct subcomplexes: the cytosolic V1 complex that catalyzes ATP hydrolysis and the transmembrane V0 complex, to which VHA-5 belongs, that is responsible for proton translocation (Nishi and Forgac 2002). While this work reports the actual molecular cloning of rdy-1 as vha-5, we recently used vha-5 mutations to show in a parallel study that the V0 sector of the V-ATPase is present at the limiting membrane of multivesicular bodies (MVBs) and at the epidermis apical membrane, where it allows the release of MVB internal vesicles (Liégeois et al. 2006). In particular, using hypomorphic mutations, we could genetically establish that VHA-5 has two distinct and separable functions—one involved in proton pumping within the entire V-ATPase complex and another involved in trafficking within the V0 complex alone (Liégeois et al. 2006). With this recent work as a background, here we molecularly characterize in parallel the genes rdy-1/vha-5 and rdy-2, investigate their function in sensory organs, and compare the cellular role of rdy-2 to that of rdy-1/vha-5 in the epidermis. We also test which protein among RDY-1, RDY-2, and CHE-14 acts upstream of the other in the epidermis and in support cells. Finally, to extend our previous conclusions about the role of the V0 sector in cuticle secretion (Liégeois et al. 2006), we examine whether VHA-5 expression changes during molting, as observed for many genes involved in cuticle formation.
MATERIALS AND METHODS
C. elegans strains and maintenance:
C. elegans strains were handled and maintained at 20° as described previously (Brenner 1974). The following strains were used: N2 (wild type); CB4856 (wild type); RW7000 (wild type); DH1206, rme-8(b1023) I; CB3823, eDf18/unc-24(e138) dpy-20(e1282) IV; KK627, itDf2 V/nT1[unc-?(n754) let-?] (IV;V); MT3751, dpy-5(e61) I; rol-6(e187) II; unc-32(e189)III; MT464, unc-5(e53) IV; dpy-11(e224) V; lon-2(e678) X; sqt-3(sc63) him-5(e1467) unc-76(e911); unc-24(e138) dpy-20(e1282); MT5813, nDf42/nT1[unc-(n754)] IV; +/nT1 V. CB4856 is an isolate from a Hawaiian island that shows a uniformly high density of polymorphisms compared with the reference Bristol N2 strain (http://genomeold.wustl.edu/projects/celegans/index.php?snp=1) (Wicks et al. 2001); RW7000 is a Bergerac strain with a high Tc1 copy number (Williams et al. 1992). Description of other strains, markers, and rearrangements can be obtained from WormBase (http://www.wormbase.org). Strains carrying genetic markers or deficiencies were obtained from the Caenorhabditis Genetics Center.
Mutagenesis and identification of rdy mutations:
We previously described a trimethylpsoralen/UV (TMP/UV) clonal screen (Michaux et al. 2000), which was based on two steps: (i) identification of F1 plates segregating rod-like larvae filled with fluid and (ii) staining of fluid-filled larvae that were still touch sensitive with the lipophilic dye 3, 3′-dioctadecyloxacarbocyanine (DiO) to retain plates in which rod-like larvae failed to take up DiO. DiO normally stains 12 amphid and phasmid sensory neurons, provided that their ciliated endings are normal and can access the environment through the channel formed by the surrounding socket and sheath cells (Perkins et al. 1986). Staining with DiO was performed as described before (Michaux et al. 2000). Of 13,000 haploid genomes, 19 F1 clones segregated dead rod larvae, among which 8 were DiO staining defective, including che-14(mc35) (Michaux et al. 2000); larvae with signs of necrosis under differential interference contrast (DIC) optics were discarded. These mutations, which defined four new complementation groups (rdy-1–rdy-4; see below), were outcrossed with N2 at least five times prior to further analyses. Their arrest stage was determined on the basis of the somatic gonad morphology, the migration/division of P cells, the division of seam and intestinal nuclei, and the appearance of postdeirid neurons.
Strong Ras pathway mutants (let-60, mpk-1, let-23, sem-5) also display a rod-like larval lethality (Yochem et al. 1997). We believe that rdy mutations do not affect the Ras pathway, as dying let-23(sy10), mpk-1(ku1), and sem-5(n2019) larvae could still take up DiO (data not shown).
Genetic mapping and complementation tests:
Details about the assignment of rdy mutations to four complementation groups corresponding to rdy-1(mc37) and rdy-1(mc38) (LGIV), rdy-2(mc39) and rdy-2(mc40) (LGV), rdy-3(mc41) (LGIII), rdy-4(mc42) and rdy-4(mc43) (LGII) and their mapping can be found in the supplemental material at http://www.genetics.org/supplemental/.
Transgenesis and complementation rescue:
DNA was injected into the syncytial gonads of hermaphrodites using injection mixes that generally contained 10 ng/μl construct or 100 ng/μl cosmid, 100 ng/μl pRF4 as a transformation marker (Mello et al. 1991), plus pBSKII plasmid to bring the total DNA concentration to 200 ng/μl. DNA mixes were injected into rdy-1(mc38)/unc-5(e53) or rdy-2(mc39)/sqt-3(sc63) him-5(e1467) unc-76(e911) animals; rescue was judged on the absence of uncoordinated (Unc) animals in the progeny. For rdy-1, we found that F35H10, one of the two cosmids in the interval where rdy-1 was predicted to map, could rescue the lethality of rdy-1(mc38). RNA interference (RNAi) (Fire et al. 1998) against vha-5/F35H10.4, which was known to be expressed in the excretory canal (Oka et al. 2001; Pujol et al. 2001), indicated that rdy-1 corresponds to vha-5. For rdy-2, we found that C50B8, F53F4, and a PCR fragment spanning F53F4.4 and F53F4.6 (10,785 bp; primers 5′-TGCTTCTGCCTTCTCTCATTC and 5′-CATTTCAGGACGAATACTTCC), but not PCR fragments spanning F53F4.1 and F53F4.2 (7773-bp fragment; primers 5′-GTCATAACAGCCTTAAACTAC and 5′-GCATTTGTAGTGATTTCAAGG) or F53F4.3 (4331-bp fragment; primers 5′-CGATGCTCCAATTAGTAAACC and 5′-CTTGCAACACTCGAAAGCTTG), could rescue the lethality of rdy-2(mc39). After digestion of the 10,785-bp PCR fragment with MscI or SalI and gel purification, the 6985-bp SalI-cut fragment containing only F53F4.6, but not the MscI-cut fragment containing F53F4.4, could rescue the lethality of rdy-2(mc39). We conclude that rdy-2 corresponds to the predicted gene F53F4.6.
RNA interference:
Hermaphrodites were injected with double-stranded RNA transcribed with the message machine T3 kit (Ambion, Austin, TX) from a PCR fragment obtained with primers (5′-aattaaccctcactaaaggGAGCTCTCTGAAGTCTTGTGG and 5′-aattaaccctcactaaaggCAGAACTCAAAAGAAAGAAGC; lowercase letters correspond to the T3 RNA Pol promoter) located in the 3′-end of vha-5. It induced a partial L2 larval lethality characterized by rod-like larvae filled with fluid.
DNA sequencing of vha-5 and rdy-2:
Using overlapping PCR reactions, we amplified the entire vha-5 and rdy-2 coding sequences and sequenced fragments bearing a deletion or all fragments for rdy-2(mc39). vha-5(mc37) corresponds to a 214-bp deletion, including the genomic positions 2498–2711 downstream of the first vha-5 coding nucleotide; vha-5(mc38) corresponds to a 124-bp deletion including the genomic positions 2298–2421 downstream of the first vha-5-coding nucleotide; rdy-2(mc39) corresponds to a T:A substitution at position 16,382 in the F53F4 cosmid sequence and is predicted to transform an AGA codon into an opal stop codon; rdy-2(mc40) corresponds to a 121-bp deletion, including the positions 15,506–15,626 in the F53F4 cosmid sequence.
RT–PCR in rdy-2:
To determine the 5′-end of rdy-2 transcripts, we used an RT–PCR strategy. Total RNA preparation and RT–PCR were carried out as described before (Bosher et al. 2003). Reverse transcription was initiated with either oligo(dT) or 5′-ACGTAGAGAAGTGCTCCAAGG, which bridges the final two exons. PCR was done using the primer 5′-TACTTCTTCCAAATCTCGACG or a primer corresponding to the sequence of the SL1 spliced leader and 5′-CAATGTGACACAGCTAACTCC (in the fourth exon) or the primer used for RT. RT–PCR products were then sequenced.
Fluorescent fusion proteins:
Constructs were generated using standard procedures and subsequently transformed into XL1-blue electro-competent bacteria. The rescuing vha-5∷gfp (pML670) and vha-5∷mrfp (pML698) constructs are described elsewhere (Liégeois et al. 2006). Substituting the GFP-coding sequence with that of cyan fluorescent protein (CFP) (taken from plasmid pPD136.61, a kind gift from A. Fire, http://www.ciwemb.edu/pub/FireLabInfo/) produced the vha-5∷cfp construct. pML680 (Ex[+ΔHyp]), carrying a 1542-bp deletion in the presumptive vha-5 promoter, was obtained by digesting pML670 with HindIII and AvrII followed by T4 DNA polymerase treatment. Other vha-5 promoter deletions were obtained by PCR using the primers 5′aaaacgcgtgAGAACTGTGAGAATTTCAATC and 5′-aaaacgcgtgAGGTGTTAAAGGCTAATCTGC (deletion 1493–2494; underlined sequence, MluI site) and 5′-aaaacgcgtgACAGTTCTCAATTCATATTGG and 5′-aaaacgcgtgATCTCTCTCCTTTGTTGCTC (deletion 2482–2652) starting from pML670. pML673, encoding a RDY-2∷GFP functional fusion protein, was obtained by cloning the promoter and the full-length coding sequence of F53F4.6 in frame with the GFP-coding sequence (primers 5′-cccgagctcGACAAGAAACGTGTTCGAGC, SacI underlined, and 5′-ggggtaccAACTTTTCACGAGATATGAAGATTA, KpnI underlined) in a modified version of pPD95.75 in which a SacI site had been engineered. Substituting the GFP-coding sequence with that of CFP produced the rdy-2∷cfp construct. A similar strategy was used to generate a che-14∷yfp construct starting from the previously described che-14∷gfp construct (Michaux et al. 2000). To examine the distribution of RDY-2 and CHE-14 mutants in weak rdy-1 mutants, we co-injected the selection marker pRF4 with the mutant vha-5(L786S)∷mrfp transgene (at 3 ng/μl) (Liégeois et al. 2006) with rdy-2∷cfp (at 5 ng/μl) and che-14∷yfp (at 20 ng/μl) constructs in vha-5(mc38)/unc-5(e53) animals and selected F2 transgenic animals that did not segregate Uncs. To generate the vha-8p∷vab-10ABD∷yfp construct, the vha-8 promoter (primers 5′-aaaagtggtaccAAAGTATTGTCCGCAAGGCAC and 5′-ttcccatggtaccAGTCGTTAGTGGTTTTCCCTG; KpnI underlined) was cloned upstream of a cDNA encoding the first 290 residues of the spectraplakin VAB-10 (Bosher et al. 2003) fused to the yellow fluorescent protein (YFP)-coding sequence in the pPD136.64 vector (kind gift from A. Fire, http://www.ciwemb.edu/pub/FireLabInfo/). This construct and the vha-5∷cfp plasmid (see above) were co-injected with or without a dlg-1∷rfp plasmid marking the C. elegans adherens junction (gift from Jeff Hardin); at least three animals for each larval stage from more than two independent transgenic lines were examined and gave identical results.
Mosaic analysis of vha-5:
Mosaic analysis of vha-5 was performed using two parallel strategies. In the first case, homozygous transgenic vha-5(mc38); Ex[pML670; pRF4] animals were allowed to lay eggs for 6–8 hr (pML670 is a rescuing vha-5∷gfp construct; see above). Rdy dead larvae were examined by DIC and GFP fluorescence 24 hr after egg laying; plates were also inspected over the next 2 days for rare dying larvae that could progress beyond the L2 stage. In the second case, we induced deletions in the vha-5 promoter by generating three plasmids: pML680 was obtained by digesting pML670 with HindIII and AvrII followed by T4 DNA polymerase treatment (1542-nt deletion); pML685 and pML689 were obtained by PCR to amplify the plasmid pML670 except the promoter region encompassing nucleotides 1494–2494 (primers 5′-aaaacgcgtgAGAACTGTGAGAATTTCAATC and 5′-aaaacgcgtg AGGTGTTAAAGGCTAATCTGC) or nucleotides 1494–2653 (primers 5′-aaaacgcgtgACAGTTCTCAATTCATATTGG and 5′-aaaacgcgtgATCTCTCTCCTTTGTTGCTC), respectively; the underlined MluI restriction site was used for religation.
Microscopy:
DIC, TEM, scanning electron microscopy (SEM), and confocal microscopy were performed as described elsewhere (Liégeois et al. 2006). TEM on rdy mutants was carried out by picking larvae as soon as signs of rigidity and translucence became apparent, which was <32 hr after egg laying. TEM and SEM on adults were done by picking L4 larvae 24 hr prior to fixation. For TEM, four vha-5(mc38) and three rdy-2(mc39) animals were observed. For both vha-5(mc38) and rdy-2(mc39) animals, a set of at least 20 adjacent ultrathin sections from the tip of the head and at least three sections taken in at least three different areas of the body were analyzed. For SEM, 15 animals were observed for the vha-5(mc38); Ex[pML680] strain, which all displayed alae defects.
RESULTS
A genetic screen for mutants displaying che-14-like phenotypes:
che-14 larvae display two phenotypes that are easy to score (Michaux et al. 2000). First, a proportion of che-14 larvae die looking as rods filled with fluid. Second, all che-14 L2 and older larvae display a dye-staining defect of amphid and phasmid chemosensory neurons after incubation with the lipophilic dye DiO. DiO normally stains neuronal cell bodies, provided that their ciliated endings are normal and have access to the environment through the channel formed by the surrounding socket and sheath cells (Perkins et al. 1986).
To identify essential as well as nonessential genes potentially acting in the same process as che-14, we used a two-step clonal strategy with the two criteria described above (Figure 1A). This screen led to the identification of che-14(mc35) (Michaux et al. 2000). We also recovered seven other mutations (not described at the time), named mc37–mc43. We completed this screen by a closer examination of the cuticle, as che-14 adults have stunted alae, which are cuticular specializations running along the lateral side of the animal (Figure 1B). We called the genes identified by these mutations rdy. Genetic analysis showed that they define four complementation groups, which mapped onto four chromosomes (see supplemental material at http://www.genetics.org/supplemental/). The gene rdy-3 is defined by a single allele, and the others by two alleles.
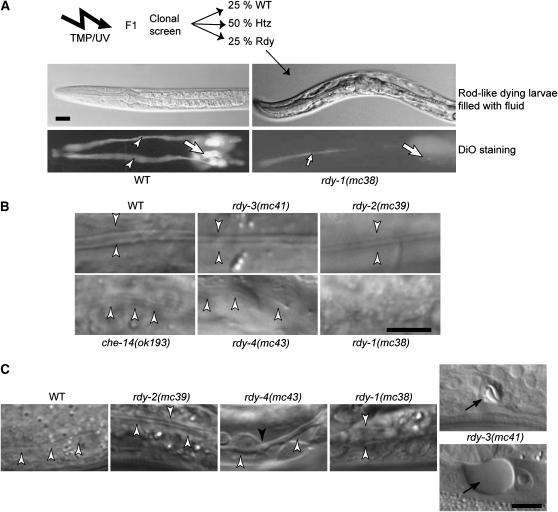
Figure 1.—
Mutagenesis and identification of rdy mutations. (A) Strategy for the identification of rdy mutations. After TMP/UV mutagenesis, the progeny of individual F1 animals were examined for the presence of rod-like larvae filled with fluid (top photos: DIC microscopy) and then for staining with the lipophilic dye DiO (bottom photos: fluorescence microscopy), which normally labels 12 neurons in the head (large arrows) and their dendrites (arrowheads). (Left) Wild-type (WT) larvae. (Right) rdy-1(mc38) mutants (the small arrow in the bottom photo points to the pharyngeal lumen). (B) L1 larva alae (arrowheads) of wild-type and rdy mutants viewed by DIC microscopy. Larvae are arranged in order of increased severity from WT = rdy-3 (normal alae) > rdy-2 > che-14 = rdy-4 > rdy-1 (no alae). (C) Excretory canal (open arrowheads) of L1 larvae viewed by DIC microscopy and arranged in order of increased severity: the canal was thicker in rdy-2 mutants; thicker and irregular (solid arrowhead) in rdy-4 mutants; extremely thick in rdy-1 mutants; essentially absent in rdy-3 mutants, in which vacuoles (arrow) that progressively grew in size (top—24 hr after egg laying; bottom—32 hr after egg laying) could be seen at the level of the excretory cell body. Bars, 5 μm.
Table 1 and Figure 1 present a more detailed account of the defects conferred by the rdy mutations. First, whereas che-14 mutants display a partial larval lethality, other rdy mutations induced a fully penetrant lethality during the L1 or L2 larval stages. Since all known che-14 mutations are strong or null alleles (Michaux et al. 2000), some essential functions carried by rdy genes must be che-14 independent. Second, they all displayed a penetrant dye-filling defect in amphids and phasmids, as do che-14 alleles. Third, DIC microscopy suggested that all rdy L1 larvae, except rdy-3(mc41), had abnormal alae (Figure 1B); the alae defect of che-14 larvae was intermediate between those of rdy-1 and rdy-2; that of rdy-4 larvae was slightly variable but generally like that of che-14 larvae. In wild-type animals, the excretory cell sends out long anterior and posterior processes, which run on both sides of the animal and are called the excretory canals. The posterior canals extend until the somatic gonad at the L1 stage and reach the rectum in L2 larvae. rdy-1, rdy-2, and rdy-4 mutants had an excretory canal of normal length, but it was abnormally wide and occasionally exhibited swelled areas (Figure 1C). In contrast, rdy-3(mc41) larvae had no excretory canal and developed a progressive vacuole at the level of the excretory cell body (Figure 1C; 21 of 29 had no canal; 6 had a canal extending up to the position of the H2 blast cell, and 2 until the position of the V1 blast cell). We thus suggest that rdy-3 may act in a different process than the other rdy genes.
TABLE 1.
Phenotypes of the rdy mutants
| Genotype | % Rdy (N)a | Stage of lethalityb | DiO(+) neuronsc | Alaed |
|---|---|---|---|---|
| Wild type | 0 | — | 10.5 ± 1 (n = 20) | +++ |
| che-14(mc35) | 49 (n = 305) | L1 to adult | 1.2 ± 1 (n = 20) | + |
| rdy-1(mc37) | 21 (n = 241) | Mid-L1 (n = 18) | 1.1 ± 2.2 (n = 24) | − |
| rdy-1(mc38) | 24 (n = 228) | Mid-L1 (n > 50) | 1.5 ± 2.7 (n = 13) | − |
| rdy-2(mc39) | 23 (n = 245) | L2 (n > 50) | 1.1 ± 2.1 (n = 10) | ++ |
| rdy-2(mc40) | 22 (n = 262) | L2 (n = 21) | 1.3 ± 2 (n = 21) | ++ |
| rdy-3(mc41)e | 21 (n = 571) | 90% L2/10% L3 (n = 38) | 0 (n = 20) | +++ |
| rdy-4(mc42) | 22 (n = 380) | Mid-L1 (n = 25) | 0 (n = 10) | + |
| rdy-4(mc43) | 23 (n = 248) | Mid-L1 (n > 50) | 1.2 ± 1 (n = 20) | + |
Percentage of rod-looking larvae filled with fluid in the progeny of heterozygous rdy mutant strains or in the progeny of homozygous che-14(mc35) animals. Although the proportion of Rdy larvae was always <25%, we believe that their lethality is fully penetrant, as their transparency makes them difficult to spot and as we never recovered viable adults laying only Rdy larvae.
Stage when larvae ceased to move, which was assessed at least 48 hr after egg laying.
No. of amphid chemosensory neurons that were stained by DiO, which can normally stain 12 neurons in wild-type animals.
Presence or absence of alae under DIC microscopy with an arbitrary score ranging from +++ to − (see Figure 1C).
rdy-3(mc41) larvae were frequently found to die off food, which is a further indication that they have defective chemosensory organs.
We decided to focus on rdy-1 and rdy-2, which had strong alae phenotypes. Our rdy-1 and rdy-2 alleles are strong loss-of-function or null alleles, since their phenotypes did not worsen in trans to deficiencies (see materials and methods). For simplicity, we will first describe their cloning and then their cellular phenotypes.
RDY-1 corresponds to the V-ATPase a-subunit VHA-5:
To determine the molecular identity of rdy-1, we used a classical strategy combining high-resolution genetic mapping, RNA interference, and transgene-mediated complementation rescue (Figure 2A) (see materials and methods). We found that rdy-1 corresponds to the previously described gene vha-5 (Oka et al. 2001; Pujol et al. 2001) (Figure 2A). In particular, we found that rdy-1(mc37) and rdy-1(mc38) are small deletions in vha-5, which induce premature stop codons (Figure 2B). This result is consistent with the nature of the mutagen used (trimethylpsoralen), the genetic tests showing that mc37 and mc38 are strong or null alleles (see supplemental material at http://www.genetics.org/supplemental/), and the absence of wild-type VHA-5 in Western blots of vha-5(mc38) animals (Liégeois et al. 2006). Below, we will refer to rdy-1 as vha-5. VHA-5 corresponds to one of the four C. elegans large transmembrane subunits of the V-ATPase, the so-called a-subunit. VHA-5 and the other a-subunits (UNC-32, VHA-6, and VHA-7) have specific tissue distributions (Oka et al. 2001; Pujol et al. 2001). The V-ATPase is involved in acidification of secretory and endocytic organelles and is essential for osmoregulation in animal excretory systems (Nishi and Forgac 2002; Sun-Wada et al. 2003). The transmembrane V0 sector, to which VHA-5 belongs, also plays a direct role in trafficking in yeast, Drosophila, and C. elegans (Peters et al. 2001; Bayer et al. 2003; Hiesinger et al. 2005; Liégeois et al. 2006).
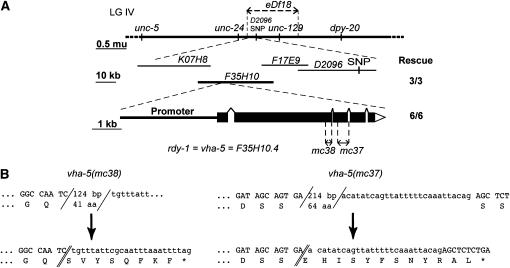
Figure 2.—
rdy-1 corresponds to vha-5 and codes for a V-ATPase a-subunit. (A, top). Genetic map of chromosome IV showing the positions of markers used to map rdy-1. rdy-1(mc37) and rdy-1(mc38) mutations were positioned between the left breakpoint of eDf18, which is defined by cosmid K07H8, and a SNP located in cosmid D2096. (Middle) Among cosmids in this region, F35H10 rescued the lethality of rdy-1(mc38) larvae (number of rescued lines indicated on the right). (Bottom) RNAi and further rescuing tests proved that rdy-1 corresponds to the gene F35H10.4/vha-5, whose exon–intron structure is shown along with the positions of mc37 and mc38. (B) More detailed description of the molecular lesions corresponding to vha-5(mc38) and vha-5(mc37). (Top) Wild-type sequence flanking the deletion breakpoints (slashes). (Bottom) Mutant sequence and distance to the nearest stop codon.
rdy-2 encodes a four-transmembrane protein of unknown function:
Using a similar strategy, we showed that the predicted gene F53F4.6 could rescue the lethality of rdy-2(mc39) larvae (see materials and methods and Figure 3A). In particular, we found that the mutation rdy-2(mc39) changes the Arg codon 71 into an opal stop codon, whereas rdy-2(mc40) is a deletion inducing a frameshift (Figure 3, B and C; supplemental Figure 1 at http://www.genetics.org/supplemental/), in agreement with the prediction that both are strong or null alleles (see supplemental material at http://www.genetics.org/supplemental/). RT–PCR experiments and sequence comparisons, which are further detailed in supplemental Figure 1 at http://www.genetics.org/supplemental/, suggest that there are two distinct rdy-2 transcripts with distinct 5′-ends. The long and short rdy-2 transcripts potentially encode two tetraspan membrane proteins (Figure 3D; supplemental Figure 1 at http://www.genetics.org/supplemental/) with predicted N- and C-terminal cytosolic tails. Many nematode species, including the distant parasitic nematodes Meloidogyne incognita and Strongyloides stercoralis (http://www.nematode.net/blast), encode homologs of the short RDY-2 isoform, but only the closely related C. briggsae and C. remanei species are predicted to encode homologs of the long RDY-2 isoform (supplemental Figure 1B at http://www.genetics.org/supplemental/). It suggests that the short RDY-2 isoform might carry the most important function. Outside of nematodes, there are no RDY-2 homologs. In particular, the sizes and sequences of RDY-2 extracellular and cytosolic loops differ from those of other tetraspan proteins, such as proteolipids, connexins, tetraspanins, and claudins. Nonetheless, we note that these well-characterized tetraspan proteins are involved in cell adhesion, membrane integrity, or possibly apical trafficking (Cheong et al. 1999).
Figure 3.—
rdy-2 encodes two putative tetraspan proteins. (A, top) Genetic map of chromosome V showing the positions of markers used to map rdy-2. rdy-2(mc39) and rdy-2(mc40) mutations were positioned between a SNP localized in the cosmid T10G3 and the left breakpoint of itDf2, which is defined by the cosmid T03D2. (Middle) Among cosmids in the area, only cosmids C50B8 and F53F4 (boldface type) could rescue the lethality of rdy-2(mc39) larvae (the number of rescuing lines tested for the presence of cosmid DNA by PCR is indicated on the right). (Bottom) Among PCR-generated subfragments of F53F4, only F53F4.6 could rescue the lethality of rdy-2(mc39) larvae. (B) Predicted exon–intron structure of F53F4.6 according to Wormbase version WS157 (top) and actual exon–intron structure of rdy-2 transcripts deduced from RT–PCR experiments using the primers symbolized between both structures (half arrows; S, sense; R, reverse; nucleotides are numbered relative to the first AUG according to WS157). The positions of conserved areas in the C. briggsae F53F4.6 homolog are symbolized by dashed lines. (C) A more detailed description of the molecular lesion corresponding to rdy-2(mc40) (see Figure 2B for symbols). (D) Topology of the long and short RDY-2 proteins as predicted by the software TMHMM version 2.0 (http://www.cbs.dtu.dk/services/TMHMM/); the N-term extension of the long protein is indicated shaded.
vha-5 and rdy-2 mutations affect osmoregulation and secretion:
As outlined above, rdy mutants share with che-14 mutants several phenotypes at the level of the dissecting microscope. To determine whether this could also be the case at the subcellular level, we examined vha-5(mc38) and rdy-2(mc39) larvae by transmission electron microscopy as they were becoming rods.
Excretory cell:
In wild-type animals, a system of beaded canaliculi feed into a central apical lumen of the excretory canal (Figure 4A), which is surrounded by a basal lamina that it shares with the neighboring epidermis. Typically, in che-14 mutants the excretory canal is poorly attached and frequently enlarged (Michaux et al. 2000). In agreement with the DIC analysis (Figure 1) and with the characterization of point mutations partially affecting the proton pump function of vha-5 (Liégeois et al. 2006), we found that vha-5(mc38) null larvae and, to a lesser extent, rdy-2 mutant larvae had an enlarged excretory canal (Figure 4A).
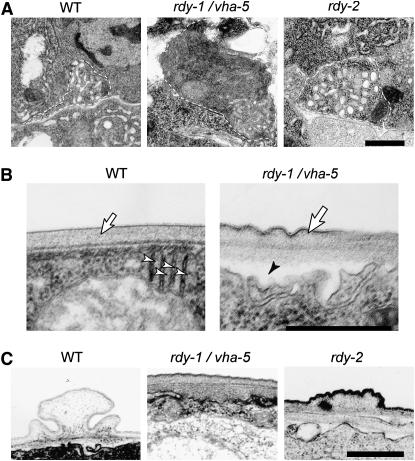
Figure 4.—
vha-5 and rdy-2 mutants have enlarged excretory canals and cuticle secretion defects. TEM images of wild-type (WT), vha-5(mc38), and rdy-2(mc39) L1 larvae. (A) Cross section through the excretory canal; its outline is marked by a dashed line. Note the enlarged section of the canal in rdy mutants and its dark appearance in the rdy-1/vha-5 larva. The average canal section was 0.22 ± 0.05 μm2 in wild-type L1 larvae, 0.69 ± 0.31 μm2 in vha-5(mc38) larvae (P < 0.0005), and 0.44 ± 0.11 μm2 in rdy-2(mc39) larvae (P < 0.0001). (B) Cross section through the epidermis. In vha-5(mc38) larvae, stacked sheets of membrane (arrowhead) originating from the apical plasma membrane are irregular, and the cuticle is irregular (arrow) and has partially detached from the epidermis (solid arrowhead). (C) The alae of the vha-5(mc38) larva are absent, and those of the rdy-2(mc39) larva have a flat structure, compared to control alae. Bars, 0.5 μm.
Epidermis:
The epidermis secretes the cuticle (Figure 4B), which in L1, dauer, and adult stages forms alae with contributions from seam cells and the hyp7 syncytial epidermis (Sapio et al. 2005). Typically, che-14 mutants have a thinner cuticle and alae. We could detect both epidermal and cuticular defects in vha-5 null larvae (Figure 4, B and C). Within the epidermis, we noted that the stacked sheets of membranes, which normally invaginate from the apical membrane, were either reduced in size or disorganized (Figure 4B, open arrowhead). Extracellularly, the cuticle had an abnormal structure (Figure 4, B and C). In some places, dense material accumulated under the cuticle (Figure 4C), while in others the cuticle was not closely apposed to the epidermal apical plasma membrane (Figure 4B, solid arrowhead). The cuticle's outer surface was wavy instead of flat, it had an irregular thickness, and alae were absent (Figure 4C) (consistent with the DIC phenotype). The apparent separation between the epidermis and the cuticle might reflect an osmoregulation defect; the structural cuticular defects are consistent with the observation that point mutations partially affecting the trafficking function of VHA-5, but not its proton pump function, had reduced alae and were dumpy (Liégeois et al. 2006). The cuticle of rdy-2 larvae was abnormal, too, but not as severely affected: its most distinctive feature was the irregular and generally flattened structure of its alae (Figure 4C).
Chemosensory organs:
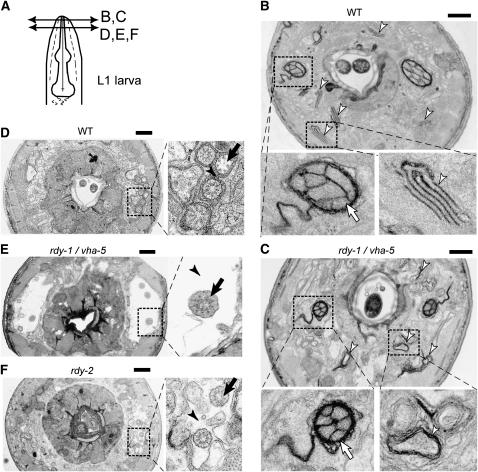
In wild-type animals, the bilateral amphid ciliated neurons go through two consecutive channels made by the amphid sheath and the amphid socket cells (Figure 5A), both of which are affected in che-14 mutants (Perkins et al. 1986; Michaux et al. 2000). The bilateral amphid sheath cells, and several other sensory neuron sheath cells, contain distinctive parallel membrane lamellae (Figure 5B, right enlargement), which are suspected to play a role in the formation of the large vesicles that are secreted within the amphid pocket and accumulate in che-14 mutants (Perkins et al. 1986). The content of these vesicles might correspond to the granular electron dense material surrounding the ciliated endings of chemosensory neurons (Perkins et al. 1986) (Figure 5D, enlargement). In vha-5 null larvae, these lamellae adopted a circular ring-like structure (Figure 5C, arrowheads). At a slightly posterior level, neurons were separated in an abnormally wide and electron-light lumen (Figure 5E), and we noted an absence of granular material around neurons in the amphid pocket (Figure 5E, arrowhead). Enlargement of the sheath pocket might also reflect an osmoregulation defect. Likewise, in rdy-2 mutant larvae, we could not find any granular material around neurons, which were also spread out in an abnormally wide electron-light lumen (Figure 5F, arrowhead). These defects are compatible with a direct or indirect (see discussion) role in secreting the granular material found in the amphid channel and in controlling osmoregulation in the amphid pocket.
Figure 5.—
VHA-5 and RDY-2 are essential for secretion of matrix material by sheath cells. (A) Schematic showing the approximate positions of the different TEM transverse sections in B–F. All images correspond to L1 larvae. (B) Wild-type (WT) and (C) vha-5(mc38) larvae, close to the tip of the nose. The left and right magnifications show the amphid opening at the level of the socket channel (arrow, terminal sensory dendrites) and a stack of membrane sheets (arrowhead), respectively; amphid neurons were normally enclosed by the socket channel in vha-5(mc38) mutants, but membrane sheets were disrupted (arrowhead). (D) Wild-type, (E) vha-5(mc38), and (F) rdy-2(mc39) larvae, further posteriorly, showing ciliated neurons surrounded by the amphid sheath. Note in the enlarged view (right), the dark material (arrowhead in D) in the extracellular space around ciliated dendrites (arrow), which is absent in the two rdy sections. Instead, there is empty space around the dendrites (arrowheads), particularly in the vha-5 larva. Bars, 1 μm.
vha-5 acts cell-autonomously:
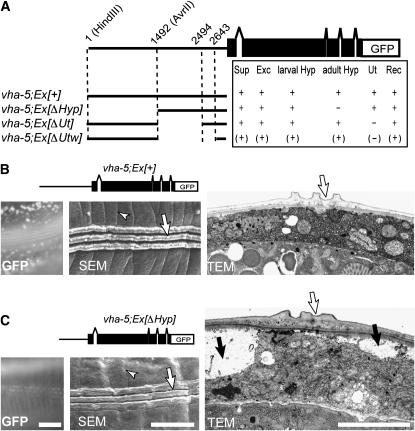
The early larval lethality and strong excretory cell defects of vha-5 mutants raise the prospect that the cuticle secretion defects might be indirect and result from lethality. To test this possibility, we performed a mosaic analysis of vha-5 using two parallel approaches that both rely on the use of a rescuing vha-5 transgene expressed in the excretory cell and the epidermis (Liégeois et al. 2006) (see also below). First, we analyzed the defects observed in dead larvae segregating from transgenic vha-5(mc38) animals carrying a rescuing vha-5∷gfp transgene. Second, we sought to rescue vha-5 function in the excretory canal but not in the epidermis. Using the first approach, we found that larval lethality is due mainly to loss of vha-5 expression in the excretory system, since all Rdy larvae had lost vha-5∷gfp expression in the excretory system (Table 2). However, larvae retaining expression in the epidermis (class I and II) could survive longer than Rdy larvae with no expression at all (class III), implying that the epidermis also requires VHA-5 function to maintain the animals' health (Table 2). To specifically rescue vha-5 function in the excretory cell, we used promoter deletions in the vha-5 promoter, as we could not find any well-characterized gene expressed in the excretory system but not in the epidermis (Figure 6A). One vha-5 promoter deletion (vha-5; Ex[Δhyp]) remained active in all cells expressing vha-5, except in the epidermis after the L4/adult switch, and rescued the larval lethality of vha-5(mc38). The corresponding adults displayed thinner alae than wild-type adults and their cuticle was not closely apposed to the epidermis everywhere when examined by electron microscopy (Figure 6, B and C), as also observed in vha-5 null mutants (Figure 4, B and C). Collectively, these data show that vha-5 acts cell-autonomously and that the cuticle defects do not result from the absence of VHA-5 function in the excretory system.
TABLE 2.
Mosaic analysis suggests that vha-5 is required for viability in the excretory system and, to a lesser extent, in the epidermis
| Presence of GFPa
|
||||
|---|---|---|---|---|
| Tissue | Non-Rdy larvae | Class I Rdy larvae | Class II Rdy larvae | Class III Rdy larvae |
| Excretory cell | + | − | − | − |
| Excretory duct cell | + | + | − | − |
| Epidermis | + | + | + | − |
| No. of larvae | >600b | 5 | 14 | 172 |
| Stage 26 hr post-egg laying | Mid-L2 | Late L1/young L2 | Late L1/young L2 | Mid-L1 |
Larvae segregating from vha-5(mc38); Ex[vha-5∷gfp] transgenic animals were examined for the presence of GFP in the excretory cell, the excretory duct cell, and the epidermis: +, GFP present; −, GFP absent.
All larvae expressing the GFP in the excretory system reached adulthood, except two that had very short excretory canals and filled with fluid in late larval development.
Figure 6.—
Cell-autonomous activity of vha-5 in the epidermis. (A) Dissection of the vha-5 promoter. Each line depicts the promoter sequence (thick line) present upstream from the VHA-5 and GFP coding regions. Expression (+), absence (−), or weak expression (parentheses) of the GFP in tissues where it is normally expressed were assayed: Sup, support cells; Exc, excretory cell; larval Hyp, larval epidermis; adult Hyp, adult epidermis; Ut, uterus; Rec, rectum. (B and C) GFP fluorescence (left), SEM (middle), and TEM (right) micrographs of a vha-5(mc38) animal rescued by the control vha-5∷gfp transgene (pML670, noted vha-5; Ex[+]) or by the promoter deletion construct that abrogates GFP expression in the adult epidermis (noted vha-5; Ex[ΔHyp]). Note in B the punctate GFP expression in the epidermis (left), the regular annulae (arrowhead in SEM), and the normal alae (arrow in SEM and TEM); conversely, note in C the absence of GFP expression in the epidermis (left), the disrupted annulae (arrowhead in SEM), the flat alae (arrow in TEM), and the large vacuoles causing an apparent detachment between the epidermis and the cuticule (solid arrows). Bars, 5 μm.
VHA-5 and RDY-2 are apical and have overlapping distributions:
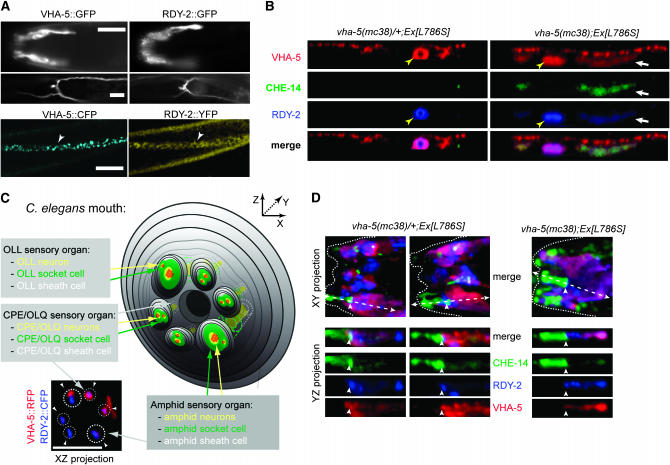
To further define the roles of rdy-1/vha-5 and rdy-2, we determined their expression patterns using functional translational fusions (see Figures 2 and 3). Using GFP constructs and antibodies, we previously showed that VHA-5 is expressed in the excretory canal and the epidermis (Liégeois et al. 2006). We found that RDY-2 has a distribution very similar to that of VHA-5 in these tissues (Figure 7A). Specifically, we saw expression in the lining of the excretory canal and excretory duct, but not around the excretory pore. As no expression was seen around the excretory cell body, we conclude that RDY-2 is localized at the apical side of the canal, as reported for VHA-5 (Liégeois et al. 2006). RDY-2 had a distribution in the epidermis similar to that of VHA-5 and was also excluded from seam cells (Figure 7A). In contrast to VHA-5, whose expression was observed throughout development, starting at midembryogenesis, RDY-2 expression became progressively fainter after the L1 stage. VHA-5 was also detected at the lumen of the vulva and rectum (data not shown). In addition, we found that VHA-5 as well as RDY-2 were expressed in the sheath cells associated with head and tail sensory organs (Figure 7A). Three-dimensional reconstructions showed that VHA-5 and RDY-2 formed a sixfold symmetrical pattern, which includes a larger spot that presumably corresponds to the amphid (Figure 7C). We thus suggest that RDY-2 and VHA-5 are present in CEP and/or OLQ support cells, in addition to amphid support cells. In the amphid sheath cell, VHA-5 and RDY-2 were found in the most distal part of the cell lining the sheath pocket, which can be equated to its apical side (Figure 7, C and D). Expression in the excretory system, in sheath cells, and in the epidermis matches well vha-5 and rdy-2 phenotypes, strengthening the notion that these genes act cell-autonomously.
Figure 7.—
CHE-14, VHA-5, and RDY-2 co-accumulate in the epidermis of vha-5 mutants affecting secretion but not in their sensory organs. (A) Fluorescence micrographs showing VHA-5∷GFP (left) and RDY-2∷GFP (right) fusion proteins in different adults (top), or VHA-5∷CFP and RDY-2∷YFP fusion proteins in the same L2 larva (bottom). Both proteins are expressed in the same tissues, i.e., the amphid sheath cell (top), the excretory cell and canal (middle), the epidermis (bottom, arrowheads). (Top) Lateral view. (Bottom) Ventral views. Bars, 10 μm. (B) Confocal Z-projections through the epidermis (apical, top of each panel) showing the distributions of CHE-14∷YFP and RDY-2∷CFP in heterozygous (left) or homozygous (right) vha-5(mc38) adults carrying a vha-5(L786S)∷mrfp mutant transgene (denoted Ex[L786S]). All three fluorescent proteins accumulate and colocalize (white arrrow) in homozygous vha-5(mc38) animals; the che-14 transgene was generally too weak to be detected in the excretory canal (yellow arrowhead). (C) Scheme of the C. elegans mouth (for the sake of simplicity, IL sensory organs are not represented), showing sensory organs where VHA-5 and RDY-2 are expressed. The XZ projection of confocal micrographs (bottom left) shows partial colocalization of VHA-5 and RDY-2 in the apical sheath cells of the amphids (larger dotted circles) and CEP (small dotted circles) and/or in the sheath cells of the OLQ sensory organs (arrowheads); two of the four CEP/OLQ organs seem to have no red signal because it was more difficult to detect them on the other side of the animal. (D) XY projections of confocal micrographs through the head of heterozygous (left) or homozygous (right) vha-5(mc38) adults carrying a vha-5(L786S)∷mrfp mutant transgene, and YZ section through an amphid organ (corresponding to the dotted arrow in the XY projection). CHE-14 was uniquely expressed in the socket cell (to the left of the arrowhead; the sheath cell is to the right of the arrowhead). Five transgenic animals were examined in detail by confocal microscopy. Bars, 5 μm.
vha-5 acts upstream of che-14 and rdy-2 in epidermal but not in sheath cells:
Our initial goal was to identify genes acting in the same process as che-14 in osmoregulation and trafficking. To test if this was the case for vha-5 and rdy-2, we reasoned that they should have, at a minimum, the same subcellular distributions as che-14 and possibly be mutually required for their localization. We generated a triple transgenic line expressing functional VHA-5∷mRFP, RDY-2∷CFP and CHE-14∷YFP constructs. Three-dimensional reconstructions through the head showed that CHE-14 was uniquely present in socket cells (green area in Figure 7D). This observation, along with the absence of RDY-2 and VHA-5 from seam cells, indicates that CHE-14 can work independently of VHA-5 and RDY-2 at least in some cells. We noted that RDY-2 was enriched in the anterior- and posterior-most areas of the amphid sheath cell surrounding the sheath pocket compared to CHE-14 or VHA-5 (YZ sections of Figure 7D); the significance of this enrichment is unclear. We next looked at the distribution of the three same transgenes in a weak vha-5 mutant background, which was obtained by rescuing the vha-5(mc38) null mutant with a vha-5 transgene carrying the point mutation L786S. This mutation affects the trafficking function of VHA-5, causing the fusion protein and some cargoes to accumulate within dark MVBs in the epidermis (Liégeois et al. 2006). We found that CHE-14 and RDY-2 colocalized with the mutant VHA-5 in the epidermis (Figure 7B), presumably within MVBs. We conclude that VHA-5 is required for a late trafficking step of CHE-14 and RDY-2 in the epidermis. In contrast, the distributions of the three proteins in sensory organs were overall quite similar to that observed in control heterozygous animals (Figure 7D). In particular, we did not detect their accumulation in areas distal to the sheath pocket. Moreover, vha-5(mc38) adults rescued by the trafficking mutant transgenes vha-5(L786S) or vha-5(E830Q) could normally take up DiO (data not shown). Together, these data suggest that the trafficking function of VHA-5 is not essential in amphid sheath cells.
VHA-5 and actin microfilaments are jointly reorganized during molting:
The finding that VHA-5 is required for cuticle formation prompted us to examine whether its expression changes during larval molts, as observed for many genes encoding cuticular proteins or proteins involved in their processing (Johnstone 2000; Hashmi et al. 2004; Frand et al. 2005; Hao et al. 2006). We did not see any major change in VHA-5 abundance during larval stages (not shown); however, we could see that its distribution changed from randomly organized spots at intermolts to aligned spots forming parallel circumferential bands at molts (Figure 8 and data not shown). These circumferential bands were strikingly reminiscent of the actin bundles, which disappear after molts and reform prior to molts (Costa et al. 1997). Interestingly, the V1 sector B- and C-subunits of the V-ATPase can bind actin (Lee et al. 1999).
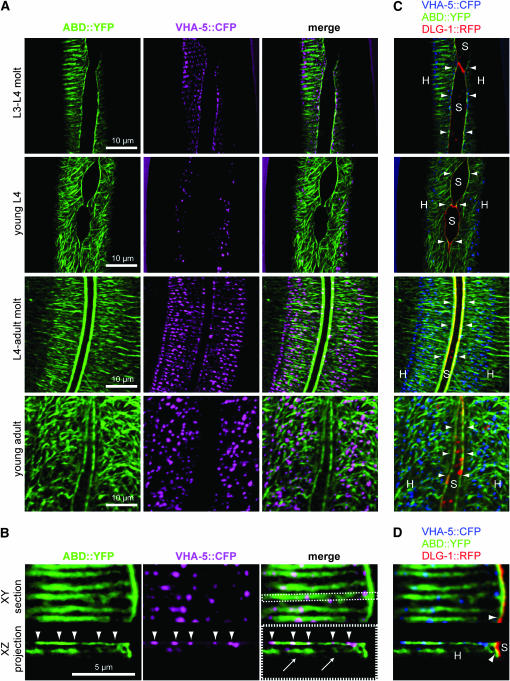
Figure 8.—
VHA-5 and actin microfilament distributions are jointly reorganized during molting. Confocal micrographs showing the distributions of actin, as revealed by the vha-8∷vab-10ABD∷yfp construct noted ABD∷YFP (it corresponds to the actin-binding domain of VAB-10 fused to YFP), VHA-5∷CFP, and DLG-1∷RFP transgenes at four different time points during larval development. (A) XY projections showing actin and VHA-5. Actin microfilaments form parallel bundles during molts (first and third rows), which become disorganized between molts (second and fourth rows). VHA-5 distribution (second column) follows the same pattern, forming aligned dots during molts and dispersed dots in intermolts. Both markers colocalized during molts (merge pictures). (B) Magnification of the apical side of the epidermis during the L4-adult molt when actin bundles are better organized. VHA-5∷CFP dots (arrowheads) colocalize with ABD∷YFP on the most apical and parallel actin bundles in both XY and XZ projections, rather than on the faint and less organized subapical actin filaments that we reproducibly observed in XZ projections (arrows). (C and D) ABD∷YFP bundles surrounding the seam cells (arrowheads) colocalize with DLG-1∷RFP at the apical junctions between the hyp7 syncytium (H) and seam cells (S). VHA-5∷CFP is shown in violet in A and B (to enhance the contrast) and in blue in C and D.
To test whether VHA-5 distribution might follow that of actin, we generated a probe to visualize actin in vivo. We fused the first 290 residues of the spectraplakin VAB-10 to YFP (Bosher et al. 2003), since the corresponding domain in human and Drosophila VAB-10 homologs is a well-defined actin-binding domain (Sun et al. 2001; Lee and Kolodziej 2002). This construct, which was driven by the vha-8 promoter, is described as ABD∷YFP in Figure 8 (it was validated as an actin-binding protein using an embryonic epidermal promoter; F. Landmann, C. Gally and M. Labouesse, unpublished results). We could thereby correlate changes in actin organization with that of a VHA-5∷CFP construct. Consistent with the findings reported by Costa et al. (1997), we observed that actin distribution was disorganized between molts and started to form parallel circumferential filaments slightly before molts (Figure 8). VHA-5 distribution did not correlate with actin between molts, although we assume that the randomly distributed puncta remain linked to the apical plasma membrane (Liégeois et al. 2006). Interestingly, however, at molting VHA-5 puncta became aligned along ABD∷YFP-decorated actin bundles and colocalized with them. We conclude that there is a close relationship between secretion of a new cuticle, actin remodeling, and VHA-5-containing V-ATPase distribution.
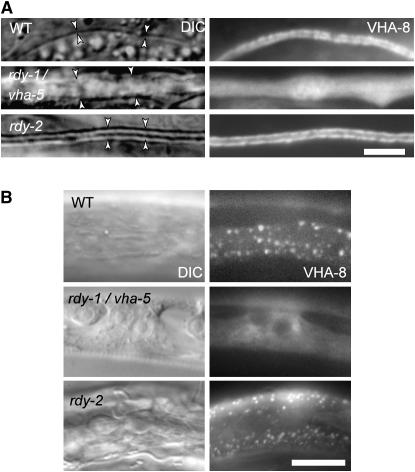
VHA-5 is required for V-ATPase assembly:
The V0 and V1 sectors constantly associate, in which case the V-ATPase proton pump is functional, and dissociate. In yeast, the a-subunit of the V-ATPase is necessary to assemble the V-ATPase complex (Nishi and Forgac 2002). To determine whether this might also be the case in C. elegans, we visualized the V-ATPase in rdy mutants by generating a YFP fusion construct for the V1 sector E-subunit called VHA-8 (Choi et al. 2003). VHA-8∷YFP distribution along the excretory canal of vha-5 null larvae was very diffuse and labeled a much larger area (Figure 9A). Likewise, it was very diffuse in the epidermis of vha-5 null mutants (Figure 9B) and failed to form the discrete puncta that normally colocalize with VHA-5 in the wild-type epidermis (Liégeois et al. 2006). These observations are consistent with our DIC and electron microscopy observations and with results in yeast. In contrast, in rdy-2 larvae, VHA-8 had a normal distribution along the excretory canal (Figure 9A) and a normal punctate distribution in the epidermis (Figure 9B), suggesting that rdy-2 is not required for V-ATPase complex formation and/or localization. Using the vha-8∷yfp reporter, we could also confirm that rdy-3 mutants had no excretory canal and that the excretory canal of rdy-4 larvae was not grossly abnormal except for its width (data not shown).
Figure 9.—
VHA-5 is required for normal assembly of the V-ATPase. DIC and GFP fluorescence views of transgenic larvae expressing a VHA-8∷GFP construct; VHA-8 is the V1 sector E-subunit. (A) Excretory canal (arrowheads); VHA-8 was very diffuse in vha-5 larvae. Bar, 2 μm. (B) Epidermis; VHA-8 was also very diffuse in the vha-5 larva, and the epidermis was vacuolated. Bar, 5 μm.
DISCUSSION
Our initial goal was to identify genes acting in the same process as the sterol-sensing domain protein CHE-14 during exocytosis to the apical membrane and/or osmoregulation (Michaux et al. 2000). The screen identified seven mutations defining four new genes, which share several features with che-14, including larval lethality; the failure to absorb lipid dyes such as DiO in chemosensory neurons; and, for six of them, an abnormal cuticle. While mutations displaying one of these phenotypes may affect osmoregulation or secretion in a very indirect manner, we reasoned that identifying mutations that combine all three phenotypes should identify genes that play a direct role in osmoregulation and/or secretion. For instance, strong alleles in the Ras pathway lead to rod-like larval lethality because the excretory duct cell adopts another fate (Yochem et al. 1997), but such dying larvae can still take up DiO (see materials and methods). Likewise, a search in WormBase (release WS160; http://www.wormbase.org) for genes giving dumpy (Dpy), molting (Mlt, indicative of cuticle defects), clear (Clr, indicative of an osmoregulation defect), and larval lethality phenotypes after RNAi identifies only two hits, one of which is vha-6. The latter encodes an intestinal V0 a-subunit >65% similar to vha-5 at the nucleotide level such that short vha-6 siRNA species might indirectly target vha-5. Consistent with the screen being specific, RDY-1/VHA-5 and RDY-2, which we studied further, are like CHE-14 transmembrane proteins found at the apical pole of the three cell types affected in mutants, namely the epidermis, the excretory cell, and support cells.
Despite their phenotypic similarities, there are some notable differences between che-14 and vha-5 or rdy-2, indicating that they may not act in the same pathway in all cells. First, che-14 mutations induce a partial lethality (Michaux et al. 2000), whereas the latter induce complete lethality. Second, large vesicles accumulate in chemosensory sheath cells and amorphous material in the seam cells of che-14 mutants (Perkins et al. 1986; Michaux et al. 2000), which we did not observe in vha-5 or rdy-2 mutants. Finally, CHE-14 is uniquely present in seam cells and socket cells (Michaux et al. 2000). The gene rdy-3 appears unique in our collection, since it was required for excretory canal formation but not for cuticle assembly. Future work should tell whether rdy-3 acts primarily to allow excretory lumen extension, as is the case for the chloride channel EXC-4 (Berry et al. 2003), or if it also controls osmoregulation.
VHA-5 exerts different functions in different tissues:
Our molecular identification of rdy-1 establishes that it corresponds to vha-5, which encodes an a-subunit of the V0 sector of the V-ATPase. The V-ATPase has long been known to represent the main enzymatic complex regulating the pH of internal organelles along the endocytic and secretory pathways (Nishi and Forgac 2002). It is also essential in maintaining osmoregulation in animal excretory systems (Sun-Wada et al. 2003), which is entirely consistent with the observation that vha-5 is expressed in the excretory cell and that its absence leads to dead larvae (see below). Our recent genetic analysis of vha-5 function in the epidermis (Liégeois et al. 2006) strongly suggests that VHA-5 also has a direct role in secretion. Likewise, biochemical and genetic analyses in yeast and Drosophila have suggested that the transmembrane V0 sector of the V-ATPase also has a role in membrane fusion, independently from the cytosolic V1 sector and proton pumping (Peters et al. 2001; Bayer et al. 2003; Hiesinger et al. 2005).
Three observations reported in this work further strengthen the notion that the V0 sector has a direct role in cuticle secretion. First, the null allele vha-5(mc38) prevents alae formation, which corroborates previous conclusions based on the analysis of weak alleles (Liégeois et al. 2006). Second, a promoter deletion in a vha-5 transgene, which rescued larval lethality but prevented expression in the adult epidermis, still induced severe cuticle defects, showing that the absence of alae in vha-5 null larvae is not an indirect consequence of larval lethality. Third, VHA-5 colocalized with actin microfilaments at the apical surface just prior to molting, suggesting that VHA-5 must form well-organized apical circumferential belts when the bulk of cuticle secretion is at its peak. Actin plays probably multiple, both inhibitory and activating, roles in exocytosis, including a role in positioning exocytotic vesicles (Eitzen 2003). In this framework, we suggest that the V-ATPase subunits B and C, which can bind actin (Lee et al. 1999), recruit the V0 sector when actin microfilaments get reorganized ahead of molting to position the V0 sector in circumferential stripes and can subsequently dissociate from the V0 sector, as observed during Manduca sexta molting (Sumner et al. 1995). In turn, we suggest that the V0 sector mediates the secretion of specific cuticle components and thereby contributes to cuticle assembly with its characteristic circumferential annulae. Actin, for instance, might play a role in positioning or allowing the fusion of MVBs, which we showed as playing a key role in cuticle formation (Liégeois et al. 2006). The observation that VHA-5 is necessary for targeting RDY-2 and CHE-14 to the epidermal plasma membrane (Figure 7B) predicts that they should act downstream of VHA-5 in cuticle formation, if they indeed act in the same process. It also suggests that CHE-14 and RDY-2 reach the apical epidermal plasma membrane through MVBs, as previously reported for WRT-2 and WRT-8 (Liégeois et al. 2006).
Two of the phenotypes observed by transmission electron microscopy in neuron-associated sheath cells are similar to those observed in epidermal cells of vha-5 null larvae. In both cell types, specialized membrane stacks were disorganized and secretion was affected: the granular material surrounding ciliated dendrites was strongly reduced or absent, much like alae were absent and the cuticle abnormal. A key question is whether the secretion defects in sheath cells are due to VHA-5 osmoregulation and pH pumping functions or whether they reflect its direct role in secretion, as in the epidermis. We would argue that it primarily reflects a proton-pumping function, since the vha-5 point mutation L786S, which affects secretion in the epidermis but not osmoregulation (Liégeois et al. 2006), could normally take up DiO and did not affect RDY-2 or CHE-14 distribution in sheath cells. If this interpretation is correct, it would indicate that the membrane stacks in sheath cells could have a role in osmoregulation rather than in a secretory process as once suspected (Perkins et al. 1986). They could increase the membrane surface for ion exchange as observed in the kidney.
As discussed above, our data thus suggest that VHA-5 trafficking function is central in the epidermis and its osmoregulation function is central in support cells. However, we do not exclude the possibility that VHA-5 also has a minor trafficking role in sheath cells and, conversely, that proton pumping across the epidermal apical membrane by the entire V-ATPase also contributes to epidermal homeostasis. As in support cells, membrane stacks, which are abnormal in vha-5 mutants (Figure 4B) and where the V-ATPase is present (Choi et al. 2003; Liégeois et al. 2006), could correspond to an osmoregulatory structure. Acidification of the extracellular matrix might facilitate the activity of collagen proconvertases. Interestingly, in many transporting epithelia and in vertebrate osteoclasts, whose main function is to resorb bone collagens, the V-ATPase is enriched at the level of mitochondria-rich membrane invaginations (for review, see Brown and Breton 1996; Jurdic et al. 2006).
The tetraspan protein RDY-2 has a minor role in secretion:
We have shown that RDY-2 is a novel tetraspan protein with no homology outside of nematodes. The main phenotype of rdy-2 mutants is an apparent osmoregulation defect, which is similar, albeit slightly less severe, to that of vha-5 mutants. In addition, we observed that alae were flattened and that the amount of granular material around sensory dendrites was reduced, indicating that its secretory function is also impaired. The RDY-2 protein appeared enriched at the apical membrane, which is compatible with a possible role in apical secretion. However, the absence of clear homology makes it difficult to suggest how it might act molecularly. Other well-characterized tetraspan proteins may provide some clues. Claudins within tight junctions and connexin 32 within gap junctions are involved in cell–cell adhesion, tetraspanins have multiple roles including cell adhesion and membrane integrity, and the small V0 sector c-subunits are proteolipid tetraspan proteins (Bronstein 2000; Nishi and Forgac 2002). The apical localization of RDY-2 makes it unlikely that it acts in cell adhesion and there was no apparent membrane integrity defect in rdy-2 mutants. More interestingly, several tetraspan proteins, such as synaptophysin, synaptoporin, synaptogyrin, and MAL/VIP17, have been implicated in vesicle formation or delivery (Cheong et al. 1999; Hubner et al. 2002).
Our preliminary characterization of rdy-4, which also has flattened alae, raises the possibility that it might act like rdy-2. Further characterization of these two genes should define their precise role in epithelial homeostasis and whether they have, as VHA-5, a direct role in secretion.
Genes involved in osmoregulation and their possible links with trafficking:
Our screening procedure identified several genes required for osmoregulation. The finding of a V-ATPase subunit among rdy- genes is not surprising, since mutations in the human genes encoding the V1 sector B-subunit and one of the V0 sector a-subunits result in a renal acidification defect, leading to metabolic acidosis (Karet et al. 1999; Smith et al. 2000; Wagner et al. 2004), while the excretory cell is considered as the kidney-like organ of the animal (Nelson and Riddle 1984). In addition, loss of this B-subunit also leads to sensorineural deafness (Karet et al. 1999; Smith et al. 2000), which is presumably linked to a role of the V-ATPase in maintaining the pH of the fluid that surrounds the mechanosensory hair cells (Karet et al. 1999). By analogy, it supports our proposal that the enlargement of the sheath pocket surrounding chemosensory neurons observed in vha-5 null mutants reflects a role of the V-ATPase in maintaining a pH balance in the sheath pocket. Defects in pH maintenance in turn might explain why chemosensory neurons fail to absorb the lipid dye DiO. In organisms that need to rapidly adapt to a changing environment, such as frogs, the V-ATPase plays a key role in maintaining the acid–base balance necessary for Na+ uptake in a process that involves hyperpolarization of the external plasma membrane (Ehrenfeld and Klein 1997). The free-living nematode C. elegans certainly falls in the category of organisms exposed to a rapidly changing environment, which could explain the prominent requirement for the V-ATPase in the excretory cell and epidermis. Our screening strategy and the fact that vha-5 and rdy-2 were subsequently found in the excretory system make it quite likely that rdy-4 also functions in the excretory cell to control osmoregulation.
The V-ATPase is an electrogenic H+ pumps that can energize membranes by the voltage component of the proton-motive force rather than by an ionic gradient (Wieczorek et al. 1999). As such, it influences osmoregulation in excretory systems and trafficking pathways, as the pH of both the endocytic and the secretory compartments is tightly controlled (Paroutis et al. 2004). A few other channels and transporters also contribute to osmoregulation and trafficking, such as chloride channels and sodium/proton antiporters (Marshansky et al. 2002). In C. elegans, for instance, the epidermal chloride channel gene clh-1 is required for the formation of alae (Petalcorin et al. 1999), and the intracellular chloride channel EXC-4 is required for extension of the excretory canal lumen by the fusion of smaller vesicles (Berry et al. 2003). The exc-4 phenotype and the finding that the V0 sector of the V-ATPase promotes membrane fusion in such different systems as yeast vacuoles, Drosophila synapses, and the C. elegans epidermis, raise the idea that the relationships between ion pump/transporters and membrane fusion are more promiscuous than could have been predicted.
Several rather indirect arguments favor the notion that the apparent link between osmoregulation and trafficking might reflect how the primitive cell acquired membranes and the need to exchange with its environment, rather than the result of evolution being parsimonious and using the same protein for multiple unrelated roles. The potential to secrete ions, waste, and peptides of different sizes might represent a continuum that gradually evolved over time, initially relying on proteins that could create an electrogenic gradient. Regarding the V-ATPase, in some Archaebacteria, genes encoding subunits of the V0 and V1 sectors are encoded by separate operons (Shibui et al. 1997), arguing that these two sectors evolved independently before becoming associated within a unique complex and perhaps had different functions in primitive cells. The reversible dissociation of V1 from the V0 sector in modern eukaryotes (Nishi and Forgac 2002) is consistent with this idea. Furthermore, the genomes of most mycetes have only one V0 a-subunit (Chavez et al. 2006), raising the possibility that this subunit originally had the osmoregulation and secretion functions. Hence, the secretion function of the V0 sector might be ancestral among eukaryotes.
Acknowledgments
We thank Anne Gansmuller [Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC) Imaging Facility] for help with electron microscopy and Marcel Boeglin and Didier Hentsch (IGBMC Imaging Facility) for help with confocal analysis. We thank Andy Fire, Yuji Kohara, Jeff Hardin, and the Caenorhabditis Genetic Center for reagents. S.L. and A.B. were supported by fellowships from the Ministère de la Recherche and from the Fondation Recherche Médicale. This work was supported by funds from the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, and by a grant from the Ministère de la Recherche (programme Action Concertée Incitative).
References
- Bayer, M. J., C. Reese, S. Buhler, C. Peters and A. Mayer, 2003. Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J. Cell Biol. 162: 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, K. L., H. E. Bulow, D. H. Hall and O. Hobert, 2003. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science 302: 2134–2137. [DOI] [PubMed] [Google Scholar]
- Bosher, J. M., B. S. Hahn, R. Legouis, S. Sookhareea, R. M. Weimer et al., 2003. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 161: 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett, C. L., D. N. Tukaye, S. Mukherjee and R. Rao, 2005. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell 16: 1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein, J. M., 2000. Function of tetraspan proteins in the myelin sheath. Curr. Opin. Neurobiol. 10: 552–557. [DOI] [PubMed] [Google Scholar]
- Brown, D., and S. Breton, 1996. Mitochondria-rich, proton-secreting epithelial cells. J. Exp. Biol. 199: 2345–2358. [DOI] [PubMed] [Google Scholar]
- Burke, R., D. Nellen, M. Bellotto, E. Hafen, K. A. Senti et al., 1999. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99: 803–815. [DOI] [PubMed] [Google Scholar]
- Chavez, C., E. J. Bowman, J. C. Reidling, K. H. Haw and B. J. Bowman, 2006. Analysis of strains with mutations in six genes encoding subunits of the V-ATPase: eukaryotes differ in the composition of the V0 sector of the enzyme. J. Biol. Chem. 281: 27052–27062. [DOI] [PubMed] [Google Scholar]
- Cheong, K. H., D. Zacchetti, E. E. Schneeberger and K. Simons, 1999. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proc. Natl. Acad. Sci. USA 96: 6241–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, K. Y., Y. J. Ji, B. K. Dhakal, J. R. Yu, C. Cho et al., 2003. Vacuolar-type H+-ATPase E subunit is required for embryogenesis and yolk transfer in Caenorhabditis elegans. Gene 311: 13–23. [DOI] [PubMed] [Google Scholar]
- Costa, M., B. W. Draper and J. R. Priess, 1997. The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev. Biol. 184: 373–384. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld, J., and U. Klein, 1997. The key role of the H+ V-ATPase in acid-base balance and Na+ transport processes in frog skin. J. Exp. Biol. 200: 247–256. [DOI] [PubMed] [Google Scholar]
- Eitzen, G., 2003. Actin remodeling to facilitate membrane fusion. Biochim. Biophys. Acta 1641: 175–181. [DOI] [PubMed] [Google Scholar]
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811. [DOI] [PubMed] [Google Scholar]
- Frand, A. R., S. Russel and G. Ruvkun, 2005. Functional genomic analysis of C. elegans molting. PLoS Biol. 3: e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, L., K. Mukherjee, S. Liegeois, D. Baillie, M. Labouesse et al., 2006. The hedgehog-related gene qua-1 is required for molting in Caenorhabditis elegans. Dev. Dyn. 235: 1469–1481. [DOI] [PubMed] [Google Scholar]
- Hashmi, S., J. Zhang, Y. Oksov and S. Lustigman, 2004. The Caenorhabditis elegans cathepsin Z-like cysteine protease, Ce-CPZ-1, has a multifunctional role during the worms' development. J. Biol. Chem. 279: 6035–6045. [DOI] [PubMed] [Google Scholar]
- Hiesinger, P. R., A. Fayyazuddin, S. Q. Mehta, T. Rosenmund, K. L. Schulze et al., 2005. The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann, S., 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66: 300–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner, K., R. Windoffer, H. Hutter and R. E. Leube, 2002. Tetraspan vesicle membrane proteins: synthesis, subcellular localization, and functional properties. Int. Rev. Cytol. 214: 103–159. [DOI] [PubMed] [Google Scholar]
- Johnstone, I. L., 2000. Cuticle collagen genes: expression in Caenorhabditis elegans. Trends Genet. 16: 21–27. [DOI] [PubMed] [Google Scholar]
- Jorgensen, E. M., and S. E. Mango, 2002. The art and design of genetic screens: Caenorhabditis elegans. Nat. Rev. Genet. 3: 356–369. [DOI] [PubMed] [Google Scholar]
- Jurdic, P., F. Saltel, A. Chabadel and O. Destaing, 2006. Podosome and sealing zone: specificity of the osteoclast model. Eur. J. Cell Biol. 85: 195–202. [DOI] [PubMed] [Google Scholar]
- Kaitna, S., H. Schnabel, R. Schnabel, A. A. Hyman and M. Glotzer, 2002. A ubiquitin C-terminal hydrolase is required to maintain osmotic balance and execute actin-dependent processes in the early C. elegans embryo. J. Cell Sci. 115: 2293–2302. [DOI] [PubMed] [Google Scholar]
- Karet, F. E., K. E. Finberg, R. D. Nelson, A. Nayir, H. Mocan et al., 1999. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat. Genet. 21: 84–90. [DOI] [PubMed] [Google Scholar]
- Lamitina, S. T., and K. Strange, 2005. Transcriptional targets of DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. Am. J. Physiol. Cell Physiol. 288: C467–C474. [DOI] [PubMed] [Google Scholar]
- Lee, B. S., S. L. Gluck and L. S. Holliday, 1999. Interaction between vacuolar H(+)-ATPase and microfilaments during osteoclast activation. J. Biol. Chem. 274: 29164–29171. [DOI] [PubMed] [Google Scholar]
- Lee, S., and P. A. Kolodziej, 2002. The plakin Short Stop and the RhoA GTPase are required for E-cadherin-dependent apical surface remodeling during tracheal tube fusion. Development 129: 1509–1520. [DOI] [PubMed] [Google Scholar]
- Liégeois, S., A. Benedetto, J. M. Garnier, Y. Schwab and M. Labouesse, 2006. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J. Cell Biol. 173: 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshansky, V., D. A. Ausiello and D. Brown, 2002. Physiological importance of endosomal acidification: potential role in proximal tubulopathies. Curr. Opin. Nephrol. Hypertens. 11: 527–537. [DOI] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux, G., A. Gansmuller, C. Hindelang and M. Labouesse, 2000. CHE-14, a protein with a sterol-sensing domain, is required for apical sorting in C. elegans ectodermal epithelial cells. Curr. Biol. 10: 1098–1107. [DOI] [PubMed] [Google Scholar]
- Nelson, F. K., and D. L. Riddle, 1984. Functional study of the Caenorhabditis elegans secretory-excretory system using laser microsurgery. J. Exp. Zool. 231: 45–56. [DOI] [PubMed] [Google Scholar]
- Nielsen, S., S. R. DiGiovanni, E. I. Christensen, M. A. Knepper and H. W. Harris, 1993. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc. Natl. Acad. Sci. USA 90: 11663–11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, S., C. L. Chou, D. Marples, E. I. Christensen, B. K. Kishore et al., 1995. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc. Natl. Acad. Sci. USA 92: 1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi, T., and M. Forgac, 2002. The vacuolar (H+)-ATPases: nature's most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 3: 94–103. [DOI] [PubMed] [Google Scholar]
- Nurrish, S. J., 2002. An overview of C. elegans trafficking mutants. Traffic 3: 2–10. [DOI] [PubMed] [Google Scholar]
- Oka, T., T. Toyomura, K. Honjo, Y. Wada and M. Futai, 2001. Four subunit a isoforms of Caenorhabditis elegans vacuolar H+-ATPase. Cell-specific expression during development. J. Biol. Chem. 276: 33079–33085. [DOI] [PubMed] [Google Scholar]
- Paroutis, P., N. Touret and S. Grinstein, 2004. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 19: 207–215. [DOI] [PubMed] [Google Scholar]
- Perkins, L. A., E. M. Hedgecock, J. N. Thomson and J. G. Culotti, 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117: 456–487. [DOI] [PubMed] [Google Scholar]
- Petalcorin, M. I., T. Oka, M. Koga, K. Ogura, Y. Wada et al., 1999. Disruption of clh-1, a chloride channel gene, results in a wider body of Caenorhabditis elegans. J. Mol. Biol. 294: 347–355. [DOI] [PubMed] [Google Scholar]
- Peters, C., M. J. Bayer, S. Buhler, J. S. Andersen, M. Mann et al., 2001. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409: 581–588. [DOI] [PubMed] [Google Scholar]
- Pujol, N., C. Bonnerot, J. J. Ewbank, Y. Kohara and D. Thierry-Mieg, 2001. The Caenorhabditis elegans unc-32 gene encodes alternative forms of a vacuolar ATPase a subunit. J. Biol. Chem. 276: 11913–11921. [DOI] [PubMed] [Google Scholar]
- Sapio, M. R., M. A. Hilliard, M. Cermola, R. Favre and P. Bazzicalupo, 2005. The zona pellucida domain containing proteins, CUT-1, CUT-3 and CUT-5, play essential roles in the development of the larval alae in Caenorhabditis elegans. Dev. Biol. 282: 231–245. [DOI] [PubMed] [Google Scholar]
- Schekman, R., and P. Novick, 2004. 23 genes, 23 years later. Cell 116: S13–S15. [DOI] [PubMed] [Google Scholar]
- Shibui, H., T. Hamamoto, M. Yohda and Y. Kagawa, 1997. The stabilizing residues and the functional domains in the hyperthermophilic V-ATPase of Desulfurococcus. Biochem. Biophys. Res. Commun. 234: 341–345. [DOI] [PubMed] [Google Scholar]
- Smith, A. N., J. Skaug, K. A. Choate, A. Nayir, A. Bakkaloglu et al., 2000. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat. Genet. 26: 71–75. [DOI] [PubMed] [Google Scholar]
- Solomon, A., S. Bandhakavi, S. Jabbar, R. Shah, G. J. Beitel et al., 2004. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 167: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner, J. P., J. A. Dow, F. G. Earley, U. Klein, D. Jager et al., 1995. Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J. Biol. Chem 270: 5649–5653. [DOI] [PubMed] [Google Scholar]
- Sun, D., C. L. Leung and R. K. Liem, 2001. Characterization of the microtubule binding domain of microtubule actin crosslinking factor (MACF): identification of a novel group of microtubule associated proteins. J. Cell Sci. 114: 161–172. [DOI] [PubMed] [Google Scholar]
- Sun-Wada, G. H., Y. Wada and M. Futai, 2003. Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct. Funct. 28: 455–463. [DOI] [PubMed] [Google Scholar]
- Wagner, C. A., K. E. Finberg, S. Breton, V. Marshansky, D. Brown et al., 2004. Renal vacuolar H+-ATPase. Physiol. Rev. 84: 1263–1314. [DOI] [PubMed] [Google Scholar]
- Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterston and R. H. Plasterk, 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164. [DOI] [PubMed] [Google Scholar]
- Wieczorek, H., D. Brown, S. Grinstein, J. Ehrenfeld and W. R. Harvey, 1999. Animal plasma membrane energization by proton-motive V-ATPases. BioEssays 21: 637–648. [DOI] [PubMed] [Google Scholar]
- Williams, B. D., B. Schrank, C. Huynh, R. Shownkeen and R. H. Waterston, 1992. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 131: 609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus and G. N. Somero, 1982. Living with water stress: evolution of osmolyte systems. Science 217: 1214–1222. [DOI] [PubMed] [Google Scholar]
- Yochem, J., M. Sundaram and M. Han, 1997. Ras is required for a limited number of cell fates and not for general proliferation in Caenorhabditis elegans. Mol. Cell. Biol. 17: 2716–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J., R. J. Hill, P. J. Heid, M. Fukuyama, A. Sugimoto et al., 1997. end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev. 11: 2883–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]