Abstract
Our earlier report suggested that androst-5-ene-3β,7β-diol (Δ5-androstenediol or Adiol) is a natural hormone with androgenic activity and that two potent antiandrogens, hydroxyflutamide (Eulexin) and bicalutamide (Casodex), fail to block completely the Adiol-induced androgen receptor (AR) transactivation in prostate cancer cells. Here, we report the development of a reporter assay to screen several selective steroids with anti-Adiol activity. Among 22 derivatives/metabolites of dehydroepiandrosterone, we found 4 steroids [no. 4, 1,3,5(10)-estratriene-17α-ethynyl-3,17β-diol; no. 6, 17α-ethynyl-androstene-diol; no. 8, 3β,17β-dihydroxy-androst-5-ene-16-one; and no. 10, 3β-methylcarbonate-androst-5-ene-7,17-dione] that have no androgenic activity and could also block the Adiol-induced AR transactivation in prostate cancer PC-3 cells. Interestingly, these compounds, in combination with hydroxyflutamide, further suppressed the Adiol-induced AR transactivation. Reporter assays further showed that these four anti-Adiol steroids have relatively lower glucocorticoid, progesterone, and estrogenic activity. Together, these data suggest some selective steroids might have anti-Adiol activity, which may have potential clinical application in the battle against the androgen-dependent prostate cancer growth.
Prostate cancer represents the most commonly diagnosed noncutaneous malignancy in aging males and is the second leading cause of cancer-related death in North American men (1). Androgen ablation has been the cornerstone of treatment for advanced forms of this disease, and a combination therapy of surgical or medical castration with an antiandrogen, such as hydroxyflutamide (HF; Eulexin) or bicalutamide (Casodex), is now widely used to reduce the level of endogenous androgens coming from, for example, adrenal sources (2).
Limiting the availability of androgens to regional or metastatic prostate cancers usually induces remission, but after some time, the cancer may become refractory to treatment. It has been suggested that genetic changes of the androgen receptor (AR) gene may contribute to a short response to hormone therapy (3). However, the mechanisms responsible for androgen independence remain uncharacterized. The reason for this poor response is enigmatic, but the recent findings (4) that androst-5-ene-3β,7β-diol (Adiol) can activate AR target genes and that two potent antiandrogens, HF and bicalutamide, fail to block completely the androgenic activity of Adiol in human prostate cancer cells may offer one of the possible explanations.
Adiol, derived from dehydroepiandrosterone (DHEA) and convertible to testosterone, is classified as belonging to the “adrenal androgen” group. It has also been known that Adiol can bind directly to the estrogen receptor (ER) and act as an estrogen at physiological concentrations (5). The in vitro androgenic activity of Adiol, like that of testosterone, is relatively weak but greatly augmented by some selective AR coactivators, such as ARA70 (6–12). Orchiectomy decreases the blood concentration of Adiol by approximately 50% (13). Adiol is produced in very small amounts by the adrenal glands (14) but in greater amounts from DHEA conversion in several tissues (15). Its concentration in blood plasma is directly proportional to the higher intracellular concentration of DHEA and DHEA-sulfate (16, 17). Another important piece of evidence, as demonstrated by Labrie et al. (18), is that total androgen blockage caused only a 41% reduction in the serum Adiol level. Thus, the serum levels of Adiol in patients undergoing total androgen blockage treatment remain relatively high and may still be able to activate AR target genes. Therefore, blocking the androgenic activity of the remaining Adiol may be worth considering. However, our previous study suggested that treatment with HF or bicalutamide may be insufficient to block Adiol action in AR-positive prostate cancer and may provide a possible explanation for the well documented disappointing clinical findings (4).
This evidence suggested that there could be a potential benefit in blocking androgenic activity of Adiol in patients with prostate cancer. To accomplish this blocking, we have tested 22 DHEA derivatives/metabolites (Fig. 1) as potential antiandrogenic compounds to see whether these compounds can compete with Adiol and block its action on AR transcriptional activity in human prostate cancer cells. We found that four of them can inhibit Adiol-induced AR transcriptional activity in PC-3 cells.
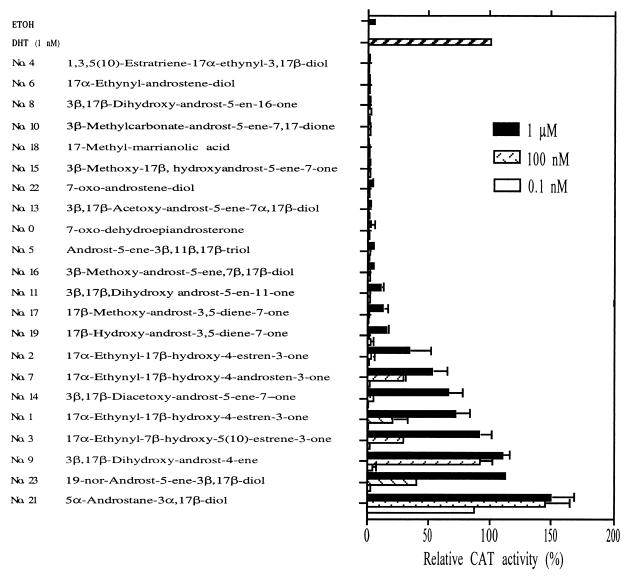
Figure 1.
The effects of various DHEA metabolites on the transcriptional activity of AR. PC-3 cells were transiently transfected with 1.5 μg of wild-type AR (WtAR) and 4.5 μg of mouse mammalian tumor virus (MMTV)-chloramphenicol acetyltransferase (CAT). After a 24-h transfection, cells were cultured without hormones (mock) or with 1 nM 5α-dihydrotestosterone (DHT) or with 1,000, 10, or 0.1 nM of various DHEA metabolites. The CAT activity was determined, and the ethanol (ETOH) treatment was set as 1-fold. Values are the means ± SD of at least three determinations. The numbers of various DHEA metabolites and derivatives were assigned arbitrarily. On the y axis, these compounds were arranged in order of the strength of induced AR transcriptional activity by the compounds at the concentration of 1 μM.
MATERIALS AND METHODS
Chemicals and Plasmids.
Adiol, DHT, 17β-estradiol, and progesterone were purchased from Sigma; ethynyl-derivatized steroids were from Steraloids (Wilton, NH); HF (Eulexin) was provided by G. Wilding (University of Wisconsin, Madison, WI); pSG5-WtAR and MMTV-CAT were constructed as described (6). Other steroid compounds, derivatives of DHEA, were synthesized; some have been described (19, 20).
Cell Culture, Transfection, and Reporter Gene Expression Assays.
The human prostate cancer cell line PC-3 and human breast cancer cell line MCF-7 were maintained in DMEM containing 10% (vol/vol) FCS. Transfection and CAT assays were performed as described (4, 6, 7). Briefly, 4 × 105 cells were plated on 60-mm dishes 24 h before transfection, and the medium was changed to phenol-red-free DMEM with 10% (vol/vol) charcoal-stripped FCS 1 h before transfection. The cells were transfected by using the calcium phosphate precipitation method. The total amount of DNA was adjusted to 8.5 μg with pSG5 in each transfection assay. After a 24-h transfection, the medium was changed again, and the cells were treated with hormones for another 24 h. The cells were then harvested, and whole-cell extracts were used for CAT assay. Transfection efficiency was normalized by β-galactosidase activity. The CAT activity was quantitated with a PhosphorImager (Molecular Dynamics).
RESULTS
Induction of AR Transcriptional Activity by DHEA Derivatives.
For the screening of these 22 DHEA derivatives, we first investigated their ability to induce AR transcriptional activity in the AR-negative PC-3 cell line. The results of the CAT assay were obtained by transient cotransfection of AR plasmid and androgen response element-reporter plasmid (MMTV-CAT). After transfection, the cells were treated with various DHEA derivatives at 1,000, 10, and 0.1 nM. As shown in Fig. 1, 11 compounds (nos. 0, 4, 5, 6, 8, 10, 13, 15, 16, 18, and 22) had very little androgenic activity and induced only small AR transactivation.
Further Screening of Anti-Adiol Activity of DHEA Derivatives with Low Androgenic Effects.
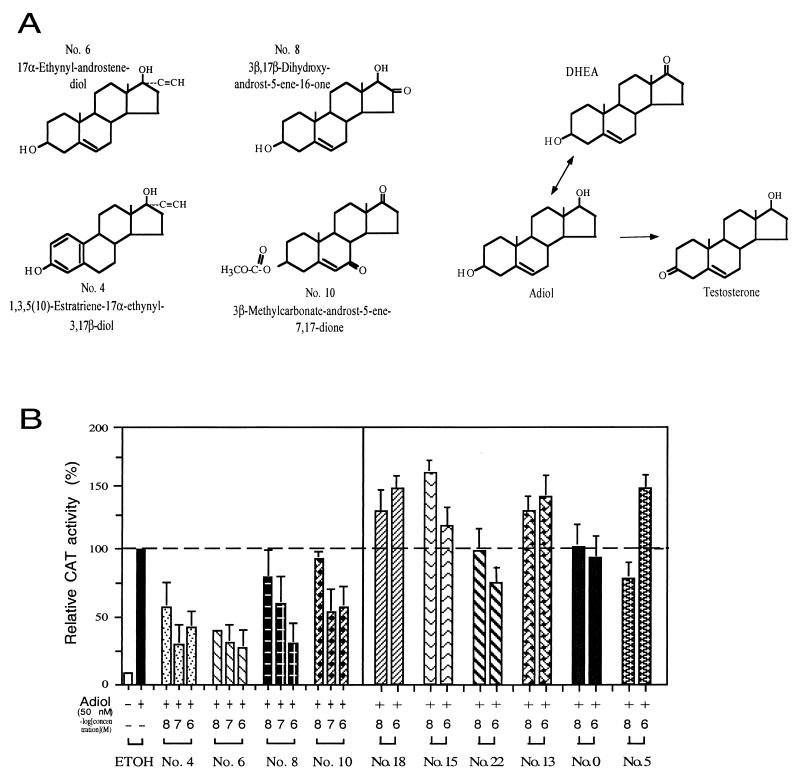
DHEA derivatives (n = 10) were screened further for their anti-Adiol activity on AR transactivation in PC-3 cells. Cells were cotransfected with AR plasmid and MMTV-CAT reporter in the presence of 50 nM Adiol and these 10 DHEA derivatives at 10, 100, or 1,000 nM. As shown in Fig. 2B, compounds nos. 4, 6, 8, and 10 have better suppression effects on Adiol-induced AR transcriptional activity. At the concentration of 0.1–1 μM, compounds nos. 4 and 6 can suppress the Adiol-induced AR transactivation to less than 30%. The chemical structures of compounds nos. 4, 6, 8, and 10 are shown in Fig. 2A. The six other DHEA derivatives (nos. 0, 5, 13, 15, 18, and 22) show either activation or no suppression effect on the Adiol-mediated AR transcriptional activity (Fig. 2B).
Figure 2.
The structures of DHEA derivatives and effects on the Adiol-induced AR transcriptional activity. (A) The structures of compounds nos. 4, 6, 8, and 10, DHEA, Adiol, and testosterone. (B) CAT activity was determined in PC-3 cells transiently cotransfected with 1.5 μg of WtAR and 4.5 μg of MMTV-CAT. After a 24-h transfection, cells were cultured in the presence of 50 nM Adiol and simultaneously treated with increasing concentrations of various DHEA derivatives for an additional 24 h. The second bar from the left shows the activity of Adiol alone (set as 100%). Values represent the means ± SD of at least three determinations. Suppression effects are seen with nos. 4, 6, 8, and 10. No suppression effects are seen with nos. 18, 15, 22, 13, 0, and 5.
Anti-DHT Effect of DHEA Derivatives (Nos. 4, 6, 8, and 10).
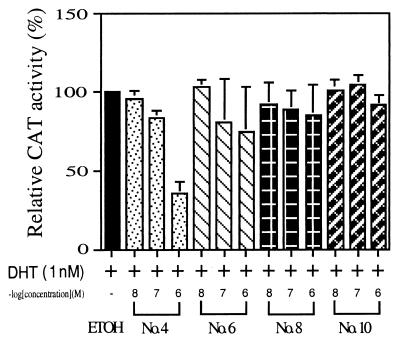
We then investigated whether these four anti-Adiol compounds have the ability to repress DHT-induced AR transactivation. PC-3 cells were cotransfected with AR plasmid and MMTV-CAT reporter in the presence of 1 nM DHT and these compounds at 10, 100, or 1,000 nM. As shown in Fig. 3, compound no. 4 turned out to be the best suppressor and can repress the DHT-induced AR transactivation to less than 40% at 1 μM.
Figure 3.
The suppression effects of nos. 4, 6, 8, and 10 DHEA derivatives on the DHT-induced AR transcriptional activity. CAT activity was determined in PC-3 cells transiently cotransfected with 1.5 μg of WtAR and 4.5 μg of MMTV-CAT. After a 24-h transfection, cells were cultured for an additional 24 h in the presence of 1 nM DHT and simultaneously treated with increasing concentrations of various DHEA derivatives. The leftmost bar shows the activity of DHT alone (set as 100%). Values represent the means ± SD of at least three determinations.
DHEA Derivatives (Nos. 4, 6, 8, and 10) Can Further Suppress the Adiol-Induced AR Transcriptional Activity in the Presence of HF.
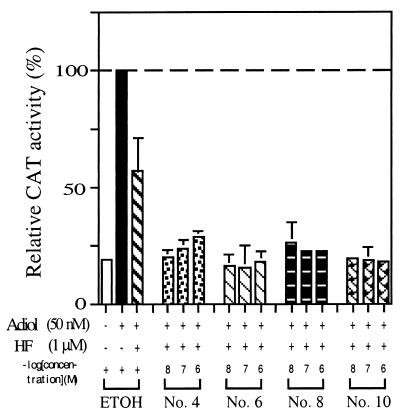
To mimic the in vivo condition of those patients with prostate cancer undergoing total androgen blockage (treated with castration plus antiandrogen), we further tested the ability of these four compounds to block Adiol-induced AR transactivation in combination with HF. In the presence of 1 μM HF, 50 nM Adiol, and 10–1,000 nM of these four compounds, PC-3 cells were transiently transfected with AR plasmid and MMTV-CAT reporter. As shown in Fig. 4, HF could suppress the Adiol-mediated AR transcription activity to 60%. After adding these four compounds, transcription activity was decreased to less than 25%.
Figure 4.
The effects of nos. 4, 6, 8, and 10 DHEA derivatives on the Adiol-induced and HF-blocked AR transcriptional activity. CAT activity was determined in PC-3 cells transiently cotransfected with 1.5 μg of WtAR and 4.5 μg of MMTV-CAT. After a 24-h transfection, 50 nM Adiol and 1 μM HF were added, and 30 min later, DHEA derivatives were added into the culture medium. The second bar to the left shows the activity of Adiol alone (set as 100%). Values represent the means ± SD of at least three determinations.
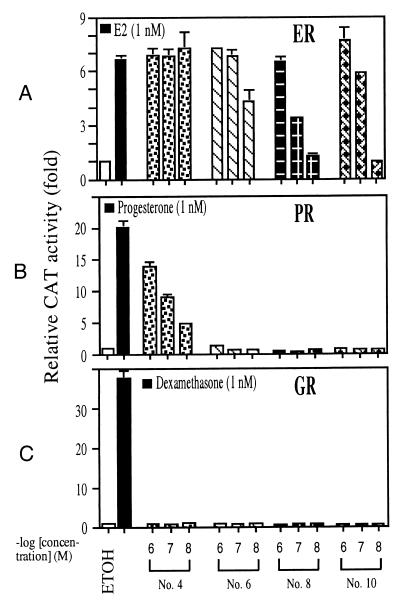
Steroid Hormone Specificity of DHEA Metabolites (Nos. 4, 6, 8, and 10).
The ER-positive MCF-7 cells were transfected with ERE-CAT reporter, and PC-3 cells were transfected with MMTV-CAT reporter and progesterone receptor (PR) or glucocorticoid receptor (GR) to test the steroid hormone specificity of these four compounds. As shown in Fig. 5, all four compounds have some estrogenic activity, and only compound no. 4, which has a 17α-ethynyl group, shows some weak PR activity. This result agreed with previous findings that steroids with an ethynyl group may have some progesterone activity. None of these four steroid compounds show any GR activity.
Figure 5.
The effects of nos. 4, 6, 8, and 10 DHEA derivatives on the ER, PR, or GR transcriptional activity. PC-3 cells were transfected with 1.5 μg of PR and 4.5 μg of MMTV-CAT or 1.5 μg of GR and 4.5 μg MMTV-CAT. MCF-7 cells were transfected with ERE-CAT. After a 24-h transfection, cells were incubated for an additional 24 h without hormone (ETOH) or with 1 nM 17β-estradiol (E2; A), 1 nM progesterone (B), or 1 nM dexamethasone (C) as positive controls as compared with 1, 0.1, or 0.01 μM of different DHEA metabolites. CAT activity was determined, and the ethanol treatment was set as 1-fold. Values are the means ± SD of at least three determinations.
DISCUSSION
We have screened many steroids derived from DHEA for their ability to block the Adiol-induced AR transactivation and found four steroid derivatives that can inhibit Adiol-induced AR transcriptional activity in prostate cancer cells. Because this screening system, which uses transient transfection and reporter gene assays, mimics the condition in patients with prostate cancer undergoing androgen ablation therapy, some compounds with anti-DHT and/or anti-Adiol effects identified from the screening may have potential values in prostate cancer treatment.
Androgen ablation has been the cornerstone of treatment for advanced prostate cancer, but most of the androgen-dependent prostate cancers progress into androgen independence. In addition, the phenomenon that antiandrogens act as agonists, the so-called antiandrogen-withdrawal syndrome, has been reported in many patients with prostate cancer (21). However, the detailed molecular mechanisms for the progression and withdrawal syndrome remain unclear. It has been suggested that mutations in the AR gene may contribute to androgen independence and the agonist effect of antiandrogens (22, 23). Other reports showed that HF could also activate the transcriptional activity of WtAR (24, 25). Our previous reports also showed that, under some conditions, certain selective AR coactivators could enhance the agonist effect of antiandrogens, including HF (8, 9, 26, 27). Moreover, our early studies showed that Adiol itself is an androgenic hormone and its androgenic activity also can be enhanced significantly by some selective AR coactivators in DU145 prostate cancer cells (4). All these data may provide some possible explanations for androgen independence and withdrawal response.
Based on the above explanations, we have been interested in identifying some compounds that have the ability to block Adiol-mediated AR transcriptional activity in prostate cancer cells. Our results have shown four DHEA derivatives that have no intrinsic androgenic activity (Fig. 1) and also block Adiol-induced AR transactivation with (Fig. 3) or without (Fig. 2) addition of HF. Because compounds that can repress AR transactivation may become potential therapeutic drugs for prostate cancer, these four compounds screened from this study may have some chance of becoming therapeutic drugs. Furthermore, as these four DHEA metabolites were shown to have no agonist effect, they may have a lower risk of causing withdrawal syndrome.
We have developed a reliable method to screen compounds that can block Adiol-mediated AR transactivation and have found four DHEA derivatives as potential antiandrogenic drugs to block the Adiol-induced AR transactivation in prostate cancer. These compounds have no intrinsic androgenic activity as well as the capacity to significantly repress the Adiol-induced AR transactivation with or without other antiandrogens in prostate cancer cells. Further modification of these potentially useful compounds may allow us to develop new and better antiandrogenic drugs for the treatment of prostate cancer.
Acknowledgments
This work was supported by National Institutes of Health Grants CA55639 and DK47258.
ABBREVIATIONS
- Adiol
androst-5-ene-3β,17β-diol or Δ5-androstenediol
- HF
hydroxyflutamide
- AR
androgen receptor
- DHEA
dehydroepiandrosterone
- ER
estrogen receptor
- DHT
5α-dihydrotestosterone
- WtAR
wild-type AR
- MMTV
mouse mammary tumor virus
- CAT
chloramphenicol acetyltransferase
- ETOH
ethanol
- PR
progesterone receptor
- GR
glucocorticoid receptor
References
- 1.Landis S H, Murray T, Bolden S, Wingo P A. Ca Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Garnick M B. Urology. 1997;49:5–15. doi: 10.1016/s0090-4295(97)00163-5. [DOI] [PubMed] [Google Scholar]
- 3.Gaddipati J P, McLeod D G, Heidenberg H B, Sesterhenn I A, Finger M J, Moul J W, Srivastava S. Cancer Res. 1994;54:2861–2864. [PubMed] [Google Scholar]
- 4.Miyamoto H, Yeh S, Lardy H, Messing E, Chang C. Proc Natl Acad Sci USA. 1998;95:11083–11088. doi: 10.1073/pnas.95.19.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams J B. Mol Cell Endocrinol. 1985;41:1–17. doi: 10.1016/0303-7207(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 6.Yeh S, Chang C. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh S, Miyamoto H, Shima H, Chang C. Proc Natl Acad Sci USA. 1998;95:5527–5532. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang H-Y, Yeh S, Fujimoto N, Chang C. J Biol Chem. 1999;274:8570–8576. doi: 10.1074/jbc.274.13.8570. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto N, Yeh S, Kang H-Y, Inui S, Chang H-C, Mizokami A, Chang C. J Biol Chem. 1999;274:8316–8321. doi: 10.1074/jbc.274.12.8316. [DOI] [PubMed] [Google Scholar]
- 10.Yeh S, Miyamoto H, Nishimura K, Kang H, Ludlow J, Hsiao P, Wang C, Su C, Chang C. Biochem Biophys Res Commun. 1998;248:361–367. doi: 10.1006/bbrc.1998.8974. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao P-W, Lin D-L, Nakao R, Chang C. J Biol Chem. 1999;274:20229–20234. doi: 10.1074/jbc.274.29.20229. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao P-W, Chang C. J Biol Chem. 1999;274:22373–22379. doi: 10.1074/jbc.274.32.22373. [DOI] [PubMed] [Google Scholar]
- 13.Belanger A, Brochu M, Cliche J. J Clin Endocrinol Metab. 1986;62:812–815. doi: 10.1210/jcem-62-5-812. [DOI] [PubMed] [Google Scholar]
- 14.Hirschmann H, de Courcy C, Levy R P, Miller K L. J Biol Chem. 1960;235:PC48–PC49. [PubMed] [Google Scholar]
- 15.Kirschner M A, Sinhamahapatra S, Zucker I R, Loriaux L, Nieschiag E. J Clin Endocrinol Metab. 1973;37:183–189. doi: 10.1210/jcem-37-2-183. [DOI] [PubMed] [Google Scholar]
- 16.Bonney R C, Scanlon M J, Jones D L, Beranek P A, Reed M J, James V H. J Steroid Biochem. 1984;20:1353–1355. doi: 10.1016/0022-4731(84)90168-7. [DOI] [PubMed] [Google Scholar]
- 17.Mills I H. Proc R Soc Med. 1967;60:905–906. [PMC free article] [PubMed] [Google Scholar]
- 18.Labrie F, Dupont A, Giguere M, Borsanyi J P, Lacourciere Y, Monfette G, Emond J, Bergeron N. Br J Urol. 1988;61:341–346. doi: 10.1111/j.1464-410x.1988.tb13971.x. [DOI] [PubMed] [Google Scholar]
- 19.Lardy H, Kneer N, Wei Y, Partridge B, Marwah P. Steroids. 1998;63:158–165. doi: 10.1016/s0039-128x(97)00159-1. [DOI] [PubMed] [Google Scholar]
- 20.Reich I, Lardy H, Wei Y, Marwah P, Kneer N, Powell D, Reich H J. Steroids. 1998;63:542–553. doi: 10.1016/s0039-128x(98)00066-x. [DOI] [PubMed] [Google Scholar]
- 21.Kelly W K, Slovin S, Scher H I. Urol Clin North Am. 1997;24:421–431. doi: 10.1016/s0094-0143(05)70389-x. [DOI] [PubMed] [Google Scholar]
- 22.Veldscholte J, Ris-Stalpers C, Kuiper G G J M, Jenster G, Berrevoets C, Claassen E, van Rooij H C, Trapman J, Brinkmann A O, Mulder E. Biochem Biophys Res Commun. 1990;173:534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Akakura K, Komiya A, Aida S, Akimoto S, Shimazaki J. Prostate. 1996;29:153–158. doi: 10.1002/1097-0045(199609)29:3<153::aid-pros2990290303>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Kuil C W, Berrevoets C A, Mulder E. J Biol Chem. 1995;270:27569–27576. doi: 10.1074/jbc.270.46.27569. [DOI] [PubMed] [Google Scholar]
- 25.Wong C, Kelce W R, Sar M, Wilson E M. J Biol Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- 26.Yeh S, Miyamoto H, Chang C. Lancet. 1997;349:852–853. doi: 10.1016/S0140-6736(05)61756-4. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto H, Yeh S, Wilding G, Chang C. Proc Natl Acad Sci USA. 1998;95:7379–7984. doi: 10.1073/pnas.95.13.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]