Abstract
Cell competition is a homeostatic mechanism that regulates the size attained by growing tissues. We performed an unbiased genetic screen for mutations that permit the survival of cells being competed due to haplo-insufficiency for RpL36. Mutations that protect RpL36 heterozygous clones include the tumor suppressors expanded, hippo, salvador, mats, and warts, which are members of the Warts pathway, the tumor suppressor fat, and a novel tumor-suppressor mutation. Other hyperplastic or neoplastic mutations did not rescue RpL36 heterozygous clones. Most mutations that rescue cell competition elevated Dpp-signaling activity, and the Dsmurf mutation that elevates Dpp signaling was also hyperplastic and rescued. Two nonlethal, nonhyperplastic mutations prevent the apoptosis of Minute heterozygous cells and suggest an apoptosis pathway for cell competition . In addition to rescuing RpL36 heterozygous cells, mutations in Warts pathway genes were supercompetitors that could eliminate wild-type cells nearby. The findings show that differences in Warts pathway activity can lead to competition and implicate the Warts pathway, certain other tumor suppressors, and novel cell death components in cell competition, in addition to the Dpp pathway implicated by previous studies. We suggest that cell competition might occur during tumor development in mammals.
ADULT Drosophila grow to a consistent size and proportion. One way in which tissue size is regulated is through the “cell competition” that can occur when growth is perturbed. In the imaginal discs, which give rise to much of the external tissues of the adult fly, cell competition coordinates growth and apoptosis and is required for consistent size regulation (de la Cova et al. 2004).
Cell competition was first described by Morata and Ripoll (1975) while studying the growth parameters of Minute mutations (M). Many Minutes are now known to correspond to mutations in ribosomal protein genes (Lambertsson 1998). Homozygosity for M mutations is cell lethal. Heterozygous M/+ cells have a reduced rate of cell division (Morata and Ripoll 1975), and while M/+ flies grow to a size and shape similar to wild-type flies, they take longer to do so (Lindsley and Zimm 1992).
In mosaic compartments containing both M/+ and wild-type cells, the M/+ cells are disproportionately eliminated from the developing tissue and may not contribute to the adult animal, even though a wholly M/+ animal would be viable (Morata and Ripoll 1975). At the same time, growth of the wild-type cells is correspondingly enhanced, sometimes leading the entire compartment to be constructed from just these cells (Simpson 1979; Simpson and Morata 1981). These reciprocal growth effects in mosaic compartments define cell competition and indicate that the growth rates of cells are moderated in response to that of their neighbors. Cell competition has also been described in mesodermal compartments as well as in imaginal discs, and between cells differing in myc gene expression as well as in ribosome complement (Lawrence 1982; de la Cova et al. 2004; Moreno and Basler 2004). Recent evidence suggests that cell competition occurs during repopulation of rat liver (Oertel et al. 2006).
It has been proposed that, in the Drosophila wing primordium, cells compete for the extracellular signaling molecule Dpp (Moreno et al. 2002). Dpp signaling is proposed to repress the expression of the transcription factor Brinker and thereby prevent Jun N-terminal kinase (JNK)-mediated apoptosis. This model is based on the findings that cell competition correlates with and can be corrected by Dpp signaling (Moreno et al. 2002; Moreno and Basler 2004). When M/+ cells are introduced into wing discs by mitotic recombination, these cells and their descendants exhibit reduced Dpp signaling, elevate JNK activity, and are lost by apoptosis. Such competed M/+ clones can be protected by elevated Dpp signaling [achieved by mutating brinker (brk)] or reduced JNK signaling (achieved by mutating the JunKK hemipterous) (Moreno et al. 2002). Similarly, in the case of competition between cells with differing doses of myc gene expression, overexpression of activated Dpp receptors can rescue the cells with a lower myc dose (Moreno and Basler 2004).
This model was based on studies of the X-linked genes brk and hemipterous, exploiting a translocation T(1,2)scS2 to obtain circumstances where FLP-mediated mitotic recombination of the X chromosome uncovered heterozygosity for the second chromosome M(2)60E locus in one class of somatically recombinant cells (Moreno et al. 2002). Because it focused on candidate genes located on the X chromosome, it is uncertain how many other genes may be required for cell competition and whether novel pathways might also be involved. In addition, a complete version of the model would explain how reduced translational capacity interferes with competition for Dpp, how reduced Dpp signaling activates JNK, and how JNK activity promotes cell death. Furthermore, Dpp availability is expected to differ even among wild-type cells, depending on their distance from the Dpp source, so the survival response to Dpp signaling must be calibrated in some way to explain why differing Dpp levels do not induce cell death during normal development. Thus it is likely that other genes and pathways that have not yet been identified by the candidate gene approach are involved in cell competition.
Another interesting observation is that stimulating cell growth by overexpression of Myc turns such cells into “supercompetitors” that can eliminate nearby wild-type cells. Other methods of activating cellular growth, such as overexpression of the phosophoinositide 3-kinase Dp110 or of cyclin D/Cdk4, do not cause supercompetition (de la Cova et al. 2004; Moreno and Basler 2004). It remains to be determined how many types of growth perturbation induce cell competition and what distinguishes them from growth pathways that do not affect competition.
Both to test the existing model and to identify other genes and pathways involved in cell competition, we performed a genetic screen for autosomal mutations that protect M/+ cells from cell competition. One would predict that mutations in autosomally located, negative regulators of the Dpp pathway, or in positive components of the JNK pathway, would protect M/+ cells from cell competition. The results indicate a complex relationship among cell competition, Dpp signaling, and JNK activity. In addition, we identify a hyperplastic tumor-suppressor pathway and novel cell death genes that are related to cell competition. Not all hyperplastic mutations rescued M/+ clones, confirming that cell competition reflects specific growth perturbations and identifying some of the components.
MATERIALS AND METHODS
p[RpL36+ w+] tranformants:
A 4-kb BamHI fragment from cytogenetic region 1D, spanning the RpL36 locus (Chen et al. 1996), was inserted into the pCasPer P-element vector marked with w+. Flies were transformed with this construct, and insertions that rescued the Minute bristle phenotype of the deficiency Df(1)R194, which removes the RpL36 gene, were recovered on each chromosome arm.
Drosophila strains and husbandry:
The following mutations were employed: ftGrv (Bryant et al. 1988); dsUA071 (Adler et al. 1998); exe1 (Boedigheimer and Laughon 1993); aosΔ7 (Freeman et al. 1992); fzR52 (Adler et al. 1994); jun2 (Adler et al. 1994); RhoABH, bsk2 (Tearle and Nusslein-Volhard 1987); bsk170B (Sluss et al. 1996); msn102 (Treisman et al. 1997); dap4 (Lane et al. 1996); sav1, sav2, sav3, wtsMGH1 (Tapon et al. 2002); ago3 (Moberg et al. 2001); hpoMGH4, gap1E21F.2, kstE17A.1 (I. K. Hariharan, unpublished results); PTENdj189 (Gao et al. 2000); TSC2192 (Ito and Rubin 1999); TSC129 (Gao and Pan 2001); scrib2, lgl4w3 (Bilder and Perrimon 2000); lgl4 (Gateff and Schneiderman 1974); lgdd7 (Buratovich and Bryant 1995); gug35 (Erkner et al. 2002); matsroo, matse235 (Lai et al. 2005); and dad271-68 (Tsuneizumi et al. 1997). The eyFLP transgene has been described (Newsome et al. 2000). DsmurfKG07014 is a P-element insertion also known as P{SUPor-P}lackKG07014 that interrupts the second exon of the Dsmurf transcript (FlyBase). For heat-shock-induced clones, we used a flp122 insertion on the X chromosome. Larvae were subjected to a 60-min heat pulse at 37°, 24–72 hr after egg deposition. Dissection was performed 72 hr after clone induction unless otherwise stated. To assess potential effects of heat shock on cell competition, larvae of genotypes ey-FLP/M(1)Bld; P[RpL36+ w+] arm-lacZ FRT40/FRT40 or +/M(1)Bld; P[RpL36+ w+] arm-lacZ FRT40/FRT40; Dpp-GAL4, UAS-FLP/+ were subjected to a 1-hr heat pulse 72 hr before dissection and fixation, as would typically be done in experiments where clones are induced by heat shock.
Mutagenesis and screening:
RpL36 transgene insertions were recombined with FRT sites for the appropriate arm and used to rescue the M/+ phenotype of Df(1)R194, a deletion of the M(1)Bld and dredd genes. In combination with the Ey-FLP system to generate mosaic eyes (Newsome et al. 2000), clones of cells lacking RpL36 transgenes were effectively M/+ if heterozygous for Df(1)R194 (Figure 1).
Figure 1.—
A screen for survival of M/+ cells in a wild-type background. (A) Crossing scheme for screening chromosome arm 2L for mutations; analogous schemes were used to screen the other autosome arms. Males with isogenized FRT40 chromosomes were mutagenized with EMS. The mutagenized chromosomes of interest are depicted in red. Mutagenized males were mated to females carrying both a deficiency that deletes the M(1)Bld gene encoding RpL36 and an FRT40 chromosome bearing a genomic DNA rescue construct (P[RpL36+ w+]). F1 females of the appropriate genotype were screened for surviving white clones. (B) A mosaic eye from the genotype yweyf/+; FRT40/P[RpL36+ w+] FRT40. The adult eye is divided into two genotypes, distinguishable by the presence (P[RpL36+ w+] FRT40/P[RpL36+ w+] FRT40) or absence (FRT40/FRT40) of red pigment. (C) Cell competition eliminated the white M/+ eye cells of the genotype yweyf/Df(1)R194; FRT40/FRT40 from this yweyf/Df(1)R194; FRT40/P[RpL36+ w+] FRT40 fly. (D) A mosaic eye imaginal disc from the genotype yweyf/Df(1)R194; P[RpL36+ w+] FRT40/P[RpL36+ w+] arm-lacZ FRT40, labeled for β-galactosidase expression. The disc contains P[RpL36+ w+]FRT40 homozygous clones that lack β-galactosidase and P[RpL36+ w+] arm-lacZ FRT40 homozygous clones that express it. All cells have the same RpL36 gene dose, so no cell competition occurs. (E) Cell competition eliminated most P[RpL36+ w+] arm-lacZ FRT40 homozygous cells from this yweyf/Df(1)R194; FRT40/P[RpL36+ w+] arm-lacZ FRT40 eye disc, because they lack the P[RpL36+ w+] transgene and are therefore M/+. Some small M/+ clones persist in the posterior part of the eye field, but M/+ cells were almost entirely eliminated from the more anterior parts of the disc. (Anterior is to the left, posterior to the right.) (F) In an adult eye of genotype yweyf/Df(1)R194 ; FRT80/P[RpL36+ w+] FRT80, white cells of the FRT80/FRT80 genotype are eliminated by cell competition (the paler cells visible are the unrecombined heterozygous genotype).(G) In an adult eye of genotype yweyf/Df(1)R194; FRT80 M(3R)/P[RpL36+ w+] FRT80, an unidentified M mutation on the mutagenized chromosome arm 3R renders all cells M/+, irrespective of their dosage of M(1)Bld, so that there is no competition between the 3L recombinant genotypes.
Male flies were fed 25 mm EMS in sucrose solution as described (Newsome et al. 2000) and subsequent crosses were performed as described in Figure 1A. All crosses were performed at 25° on standard medium. Because the F1 flies screened were females, meiotic recombination could occur, and multiple F2 males were bred and the F3 generation rescreened to establish each mutant stock because it proved even more difficult to recover strains through the F2 M/+ females. The numbers of F1 females screened were the following: 2L, 4535; 2R, 4362; 3L, 2728, 3R, 4853.
To test whether the mutations recovered were dominant or recessive, we asked whether heterozygosity for the newly induced mutation would protect M/+ clones induced by recombination on a different chromosome. None did, indicating that they are recessive mutations that protect cells from competition cell autonomously. None of the six mutations had any dominant effect on the time of development from egg laying to eclosion, as would be expected from a second hit to the ribosomal synthesis machinery.
Immunohistochemistry:
Imaginal discs were dissected on 0.1 m sodium phosphate (pH 7.2) and fixed in paraformaldehyde–lysine–periodate (Tomlinson and Ready 1987) for 45 min at 4°. Antibodies were diluted and washes performed in 0.1 m sodium phosphate, 0.3% sodium deoxycholate, and 0.3% Triton X-100. Antibodies used were CM1 (Yu et al. 2002), rabbit anti-Salm (Kuhnlein et al. 1994), rat anti-Brk (Campbell and Tomlinson 1999), and mAb40-1a (Developmental Studies Hybridoma Bank). Apoptotic cells were visualized using CM1, a polyclonal antiserum raised against activated caspase 3 (Yu et al. 2002). DRAQ5 (Alexis) was used at 5 μm to stain DNA. Samples were mounted in 75% glycerol/2% n-propyl gallate and imaged using a Bio-Rad (Hercules, CA) Radiance 2000 confocal microscope. Subsequent image analysis was performed using ImageJ (National Institutes of Health) and Photoshop (Adobe Systems).
RESULTS
A screen for autosomal mutations that inhibit cell competition:
To identify mutations that prevent Minute heterozygous cells (M/+) from being eliminated by cell competition, we engineered a system in which clones of cells homozygous for newly induced mutations and heterozygous for a deletion of the RpL36 gene were generated in a background wild type for RpL36 (Figure 1A). Genomic RpL36+ transgene insertions were obtained, recombined with FRT sites for the appropriate arm, and used to rescue the M/+ phenotype of Df(1)R194, a deletion of the M(1)Bld and dredd genes (loss of dredd does not affect cell competition; W. Li, unpublished results). In combination with the Ey-FLP system of generating mosaic eyes (Newsome et al. 2000), the eyes of F1 female flies contained two genotypes: pigmented cells in which haplo-insufficiency for RpL36 is rescued by P[RpL36+ w+] and unpigmented Df(1)R194/+ cells (Figure 1). As unpigmented Df(1)R194/+ cells were eliminated by cell competition, they were not seen in adult eyes (Figure 1, B and C). In eye imaginal discs, Df(1)R194/+ clones were small and fragmented (Figure 1, D and E). The M/+ clones survived longer in the posterior of the eye, which is the first to become postmitotic as the wave of eye differentiation begins, but were rare in the anterior of the eye, which proliferates for longer (Figure 1, D and E).
After mutagenesis, 16,478 F1 females were screened for surviving white eye tissue, resulting in the recovery of six recessive, cell-autonomous mutations in five complementation groups that rescued M/+ eye clones (Figure 2). In principle, dominant mutations might also have been recovered. Since starvation rescues M/+ cells (Simpson 1979), if there were haplo-insufficient genes that regulated processes such as growth rate, feeding behavior, or digestive function, we might have recovered their dominant mutations. No dominant mutations other than Minutes were recovered, however. Dominant rescue by unlinked Minute mutations was expected because differences in RpL36 gene dose are masked if all cells lack a copy of another Minute gene (Figure 1, F and G) (Schultz 1929; Simpson and Morata 1981). Such Minute mutations were recognized in the F1 by their bristle phenotype and discarded.
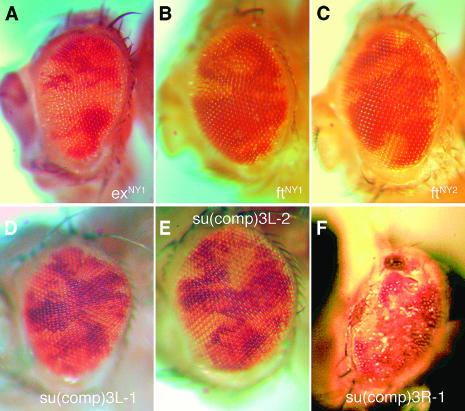
Figure 2.—
Mutations recovered in a screen for rescue of cell competition. (A–F) These heads contain white eye tissue composed of cells that are RpL36/+ rescued by homozygosity for the following mutations: (A) exNY1, (B) ftNY1, (C) ftNY2, (D) su(comp)3R-1, (E) su(comp)3L-2, and (F) su(comp)3L-1. In the case of su(comp)3R-1 (D), the mutant cells fail to differentiate as eye tissue, resulting in a rough, scarred appearance.
We anticipated that mutations that rescue M/+ cells from competition during growth but subsequently cause defects in differentiation or survival might be recovered through effects on eye morphology . Unlike other mosaic screens (Xu and Rubin 1993; Newsome et al. 2000), ours recovered only nine mutations with defective eye morphology, because in most cases the mutant tissue would be eliminated by cell competition before adult morphology could be affected. We found that none of the nine mutations prevented the elimination by competition of M/+ cells in imaginal discs, however (data not shown). For example, an allele of Star was recovered, but M/+ S/S cells were eliminated as efficiently as M/+ cells during larval development (not shown). A rough-eye phenotype must be due to the very few surviving S/S M/+ cells. S mutations have strong, nonautonomous effects through failure of cells to secrete the signaling molecule Spitz (Freeman 2002; Klambt 2002).
An account of the recessive mutations that were isolated follows. Although the screen has not saturated for such mutations, analysis of these initial mutations led to the testing and identification of other similarly acting genes.
expanded mutations protect cells from competition:
Mutation 2L-19 mapped to the left of al and failed to complement the deficiency Df(2)al. This deficiency includes the gene expanded (ex), and 2L-19 failed to complement the mutations ex1 and exe1. The null allele exe1 was also able to rescue M/+ cells from competition in our assay (data not shown). We therefore conclude that 2L-19 is a new ex allele and have named it exNY1. In heteroallelic combinations, exNY1 behaves similarly to a deficiency and is slightly stronger phenotypically than exe1. The ex gene encodes a FERM-domain protein belonging to the Band 4.1 superfamily (Boedigheimer et al. 1993).
ex, M/+ mutant cells are able to survive and differentiate as eye tissue, contributing to ∼50% of the adult eye (Figure 2A). ex has been previously described as a tumor suppressor (Boedigheimer and Laughon 1993; Boedigheimer et al. 1997). When clones of exNY1 were induced in the absence of M mutations, they occupied most of the eye and caused overgrowth similar to the null allele exe1 (Blaumueller and Mlodzik 2000).
If cell competition was the result of differential abilities of cells to compete for extracellular Dpp, leading to cell death (Moreno et al. 2002), then either ex could affect the relative ability of cells to compete for Dpp or ex could be required to induce apoptosis. To test whether ex mutations increased Dpp capture, we used an antibody against Spalt major (Salm), a protein that is expressed in response to high levels of Dpp signaling in the wing primordium (de Celis et al. 1996). Salm levels were compared in clones of exNY1 mutant cells and adjacent ex/+ tissue (no cells are M/+ in this experiment). Salm protein levels were higher in exNY1 clones (Figure 3A). Interestingly, Salm levels were reduced in the wild-type (+/+) twin spots, indicating that the effect of ex on Dpp signaling was dose sensitive.
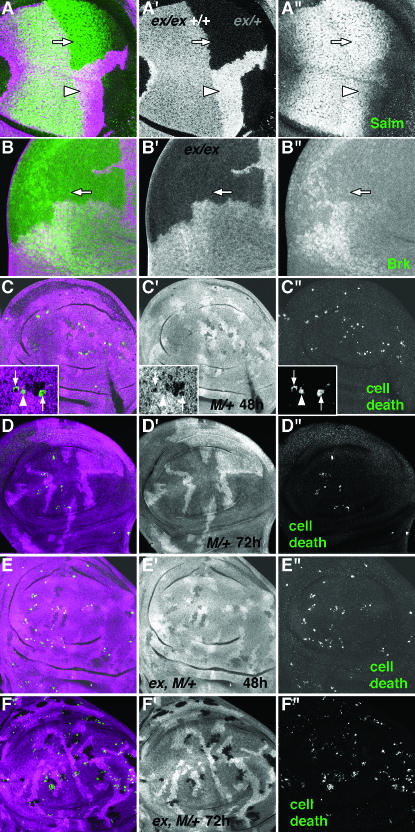
Figure 3.—
Dpp signaling and apoptosis of M/+ cells in expanded mutants. (A and B) Wing discs containing exNY1 mutant clones induced in the genotype hsflp/+; arm-lacZ FRT40/exNY1 FRT40. Clones that lack β-galactosidase are highlighted by magenta. (A) Salm levels (green) are increased in exNY1 tissue (arrow). This effect is most notable in the posterior part of the Salm expression domain, which corresponds to the region between the presumptive L4 and L5 veins (de Celis and Barrio 2000). This is the region of the wing that is most strongly affected by viable mutations in ex (Boedigheimer and Laughon 1993). In addition, Salm levels are decreased in +/+ tissue (highest levels of β-galactosidase signal are indicated by the arrowhead), compared to heterozygous exNY1/+ tissue. (B) Brk expression (green) is decreased in exNY1 mutant clones (arrow). (C and D) Wing discs containing M/+ clones fixed 48 hr (C) or 72 hr (D) after clone induction (genotype hsflp/M(1)Bld; P[RpL36+ w+] armlacz FRT40/FRT40). M/+ cells lack β-galactosidase (a single representative confocal section is shown in magenta). Apoptotic cells within the disc epithelium are projected in green. There are often more apoptotic cells beneath the disc epithelium, presumably having been expelled. These have not been illustrated or quantified here, as their origin cannot be precisely determined. (C) The majority (≥60%) of CM1-positive cells that could be scored were M/+. Inset shows an enlargement of a single confocal through a typical M/+ clone, so that the genotype of dying cells can be determined more precisely. Two of the three apoptotic cells clearly lack β-galactosidase and so are M/+ (arrows in inset). Both are single cells, surrounded by β-galactosidase-positive neighbors. The third dying cell (arrowhead in inset) may be β-galactosidase positive, although it is difficult to rule out the presence of a M/+ cell. (D) M/+ clones are almost entirely eliminated 72 hr after induction of clones. A few dying cells are left. (E and F) ex, M/+ clones fixed 48 hr (E) or 72 hr (F) after clone induction (genotype hsflp/M(1)Bld; P[RpL36+ w+] armlacz FRT40/exNY1 FRT40). ex, M/+ cells lack β-galactosidase (magenta). Apoptotic cells are labeled in green. (E) The number of apoptotic ex, M/+ cells is similar to the control (compare with C). The ratio of homozygous ex, apoptotic M/+ cells to clone size after 48 hr was compared to that for the M/+ control and was not significantly different. (F) ex, M/+ clones still survive 72 hr after clone induction (contrast with D). The high level of apoptosis indicates that loss of ex accelerates the growth of M/+ cells without protecting them from apoptosis.
Another target of Dpp signaling is brk. Brk transcription is repressed by Dpp signaling (Campbell and Tomlinson 1999; Jazwinska et al. 1999; Minami et al. 1999; Muller et al. 2003; Martin et al. 2004). Brk protein levels were reduced in exNY1 mutant clones, consistent with increased Dpp-signaling activity (Figure 3B).
Increased Dpp signaling could be a result of either an increased quantity of available Dpp or an increased sensitivity of the cells to Dpp. The quantity of available Dpp could be increased if ex mutant cells secreted more Dpp than wild-type cells, or removed less Dpp from the extracellular space. In either case, one would expect wild-type cells neighboring ex mutant cells to be exposed to increased Dpp levels. Another possibility is that ex mutant cells are able to sequester Dpp more effectively than wild-type cells. In this case, the wild-type cells immediately adjacent to ex cells would receive less Dpp than cells farther away from the mutant clone. In contrast to these predictions, changes in Salm and Brk appeared cell autonomous (Figure 3, A and B). This indicated that ex mutant cells had increased sensitivity to Dpp but did not change its extracellular distribution. These results are consistent with the hypothesis that ex mutations protect M/+ cells from competition by increasing the sensitivity of cells to Dpp, so that Dpp signaling in ex, M/+ cells is restored to a level comparable with adjacent wild-type cells.
Elevated Dpp signaling might prevent apoptosis of cells undergoing competition (Moreno and Basler 2004). We compared levels of apoptosis in M/+ and ex, M/+ clones in third instar wing imaginal discs to determine whether ex, M/+ cells survive better, because unlike eye imaginal discs, most of the wing imaginal disc is still growing in third instar eye discs. Forty-eight hours after inducing wing clones, M/+ clones were small and contained dying cells with activated caspases (Figure 3C). At least 60% of the dying cells were M/+ (Figure 3C). This was an underestimate, as it was more difficult to see the absence of the β-galactosidase label in a single M/+ cell than its presence in a single +/+ cell, and it is most often single M/+ cells that are in greatest contact with wild-type cells that die (W. Li, unpublished data). By 72 hr after clone induction, M/+ cells had been almost entirely eliminated from the wing disc (Figure 3D). Mosaic discs containing ex, M/+ clones contained similar numbers of apoptotic cells to control discs containing M/+ clones (Figure 3E); the apoptotic cells were predominantly the ex M/+ genotype and adjacent to the clone boundary (Figure 3E). ex, M/+ clones continued to grow 72 hr after clone induction (Figure 3F). Thus loss of ex did not prevent apoptosis caused by cell competition, but did rescue growth.
fat mutations protect cells from competition:
Mutations 2L-13 and 2L-42 were lethal and failed to complement one another. 2L-13 was mapped near the fat gene (ft) by mitotic recombination and failed to complement the lethal alleles ft8 and ftGrv. Furthermore, ftGrv was able to rescue M/+ cells in our assay (data not shown). 2L-13 and 2L-42 were renamed ftNY1 and ftNY2, respectively. The ft gene encodes a large, cadherin-like protein (Mahoney et al. 1991).
M/+, ft/ft mutant cells were able to survive and differentiate as eye tissue, contributing to ∼50% of the adult eye (Figure 2B). ft has been previously described as a tumor suppressor, and our new alleles caused overgrowth of mutant tissue in the absence of M mutations similar to the strong loss-of-function allele ftGrv (Bryant et al. 1988). From the degree of overgrowth of mutant clones, we suggest that ftNY1 is a stronger allele than ftGrv and that ftNY2 is a weaker one (data not shown).
As was also true for ex, Salm expression was increased in ftNY1 clones compared to adjacent heterozygous tissue and reduced in the twin spots where cells had two wild-type copies of the ft gene, consistent with dose-dependent effects on Dpp signaling (Figure 4A). Like M/+ clones, ftNY1, M/+ clones contained dying cells 48 hr after clone induction (Figure 4, B and C). Seventy-two hours after clone induction, ftNY1, M/+ clones had been eliminated from wing imaginal discs (Figure 4D). Because ft mutations did not prevent loss of M/+ clones in the wing imaginal disc, we wondered how they did so in eyes (Figure 2B). Both exNY1, M/+ and ftNY1, M/+ clones survived at more anterior locations in the eye disc than did M/+ clones and grew larger before being “frozen” by the arrest in proliferation that accompanies the morphogenetic furrow. However, ftNY1, M/+ clones did not grow as large as did exNY1, M/+ clones did (Figure 4, E–G). If the posterior eye, which differentiated first, were subject to less competition, then the most anterior position of clone survival in the eye would provide an estimate of the degree of competition. Our data were consistent with the notion that ft rescues M/+ cells to a lesser extent than ex does, so that ftNY1, M/+ cells survive in parts of the eye that are less sensitive to competition than the wing; our data were also consistent with previous conclusions that competition in developing wings is more severe than elsewhere in the fly (Morata and Ripoll 1975).
Figure 4.—
Dpp signaling and apoptosis of M/+ cells in fat mutants. (A) ftNY1 mutant clones induced in the genotype hsflp/+; arm-lacZ FRT40/ ftNY1 FRT40. Mutant clones are marked by the absence of β-galactosidase (magenta). Salm levels (green) are increased in ftNY1 clones (arrow). In addition, Salm levels are decreased in +/+ tissue (highest levels of β-galactosidase signal are indicated by an arrowhead).(B) M/+ clones fixed 48 hr after induction (genotype hsflp/M(1)Bld; P[RpL36+ w+] arm-lacZ FRT40/FRT40). M/+ cells that lack β-galactosidase immunofluorescence are in magenta. Only small patches of M/+ cells remain (smaller than their associated twin spots) and apoptotic cells can be seen within these clones (green). (C) ftNY1, M/+ mutant clones fixed 48 hr after induction (genotype hsflp/M(1)Bld; P[RpL36+ w+] arm-lacZ FRT40/ftNY1 FRT40). Clones are smaller than their twin spots and contain dying cells (green). The ratio of homozygous ft, apoptotic M/+ cells to clone size after 48 hr was compared to that for the M/+ control and was not significantly different. (D) ft, M/+ mutant clones fixed 72 hr after clone induction. Few ftNY1, M/+ cells remain.(E–G) Eye imaginal discs were labeled for galactosidase 72 hr after clone induction. In E–G, anterior is to the left and the arrowhead marks the position of the morphogenetic furrow. (E) M/+ clones are almost entirely eliminated; some small clones persist at the posterior edge of the disc. Reciprocal +/+ clones (arrow) show that recombination occurred and M/+ clones must have been eliminated anteriorly to the morphogenetic furrow. (F) exNY1, M/+ clones grow much larger than control clones and are able to survive in much more anterior positions in the eye disc. (G) ftNY1, M/+ clones grow larger and persist more anteriorly than control clones (compare with E).
su(comp)3R-1 is a novel tumor suppressor:
One of the recovered mutations produced rough eyes and tissue that did not differentiate correctly, so that eye color could not be assessed (Figure 2F). su(comp)3R-1 was mapped by recombination with a rucuca chromosome to the interval between sr and e and fails to survive in trans to Df(3R)H-B79, suggesting that su(comp)3R-1 corresponds to an essential gene in the region 92B3–92F13.
M/+, su(comp)3R-1 clones survived eye development (Figure 2F). su(comp)3R-1 clones that were not hampered by the M/+ genotype grew even more to exhibit dramatic overgrowth both in eye tissue and in the wing (Figure 5, A and B). In the wing imaginal disc, the hyperplastic tissue formed convoluted folds (Figure 5B).
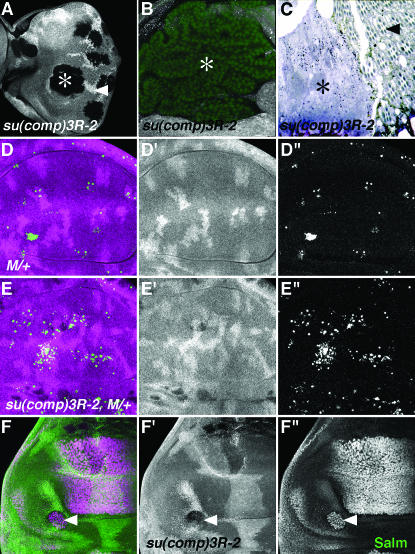
Figure 5.—
Dpp signaling, apoptosis of M/+ cells, and differentiation in su(comp)3R-1 mutants. (A) su(comp)3R-1 clones in eye imaginal discs 72 hr after clone induction (gentoype hsflp/M(1)Bld; FRT82/FRT82 P[RpL36+ w+] arm-lacZ). Clones (asterisk) are larger than twin spots (arrowhead).(B) su(comp)3R-1 mutant clone (asterisk) in the wing; mutant tissue folds to accommodate the dramatic overgrowth. Nuclei of all cells are labeled in green with DRAQ-5. (C) In a section through the adult eye containing su(comp)3R-1 mutant (asterisk) and wild-type (arrowhead) tissue, the mutant tissue is amorphous and lacks the regular array of rhabdomeres. (D) Control M/+ clones in wing discs labeled 48 hr after clone induction (gentoype hsflp/M(1)Bld; FRT82/FRT82 P[RpL36+ w+] arm-lacZ). M/+ cells lack β-galactosidase (shown in magenta), but few are seen. Apoptotic cells are in green. (E) su(comp)3R-1, M/+ clones in wing discs labeled 48 hr after clone induction (genotype hsflp/M(1)Bld; FRT82 3R-12/FRT82 P[RpL36+ w+] arm-lacZ). 3R-12, M/+ clones are present, despite abundant cell death. (F) Salm expression (green and F′′) is upregulated in su(comp)3R-1 clones (arrowhead) (genotype hsflp/+; FRT82 su(comp)3R-1 /FRT82 arm-lacZ).
su(comp)3R-1 mutant cells gave rise to amorphous tissue in the adult eye, without the photoreceptor rhabdomeres characteristic of eye tissue (Figure 5C). su(comp)3R-1 clones caused tumor-like outgrowths in the adult wing and other body parts, and eye differentiation was affected at the imaginal disc stage (data not shown). Although these phenotypes were reminiscent of those caused by mutations in neoplastic tumor suppressors such as scribble (Brumby and Richardson 2003), labeling with an antibody against Fat, which recognizes the apical junctions of cells, revealed that unlike scrib, the su(comp)3R-1 mutant cells retained apical–basal polarity.
In the wing imaginal disc, su(comp)3R-1, M/+ clones survived 72 hr after clone induction, when M/+ clones had almost disappeared (Figure 5, D and E). The su(comp)3R-1, M/+ clones contained many dying cells, but this was difficult to compare quantitatively with those in M/+ clones, because the latter have largely disappeared by this stage and because su(comp)3R-1 mutant clones tended to bulge in the epithelium so that it was difficult to assess their size accurately (Figure 5, D and E). Spalt levels were dramatically upregulated in su(comp)3R-1 wing clones; large clones that originate in salm-expressing territory autonomously express Salm throughout the overgrown tissue, even where the clone extends beyond the normal Salm domain (Figure 5F).
Novel mutations on 3L that permit survival of M/+ cells:
Two mutations recovered on 3L were homozygous viable and morphologically normal so that neither complementation testing nor deficiency mapping was possible. su(comp)3L-1 was mapped to the interval between the SNPs 3L105 and 3L120 (Berger et al. 2001), corresponding to the cytogenetic location 69C2-70D1. su(comp)3L-2 was not mapped, and we cannot say whether these two mutations are in different genes; the phenotypes, however, are similar so the two mutations are described together.
Whereas rescue of M/+ cells was dramatic (Figure 2, D and E), clones of su(comp)3L-1 or su(comp)3L-2 cells generated in wild-type backgrounds did not outgrow their twin spots (Figure 6, A–C). This differentiated su(comp)3L-1 and su(comp)3L-2 from the hyperplastic ex, ft, and su(comp)3R-1 mutations. Another difference was that Salm levels were not increased in su(comp)3L-1 or su(comp)3L-2 mutant clones (Figure 6, D and E).
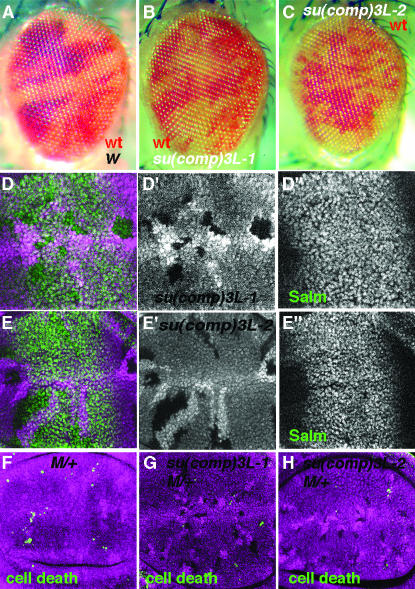
Figure 6.—
Dpp signaling and apoptosis of M/+ cells in su(comp)3L-1 and su(comp)3L-2 mutants. (A) The eye is divided roughly equally into red and white tissue in a control (genotype y w Ey-FLP; FRT80/w+ FRT80). (B and C) Proportions of mutant (white) and control (red) tissue are also similar when clones are homozygous for su(comp)3L-1 (B) or su(comp)3L-2(C). (D) Salm levels in the wing disc (D″ and green in D) are not affected in su(comp)3L-1 clones. (E) Salm levels in the wing disc (D″ and green in D) are are not affected in su(comp)3L-2 clones. (F) M/+ clones (those lacking the magenta labeling for β-galactosidase) 48 hr after induction (genotype hsflp/M(1)Bld; P[RpL36+ w+] arm-lacZ FRT80/FRT80). Most have been lost; only a few apoptotic corpses remain (green). (G) M/+, su(comp)3L-1 clones persist with little apoptosis. (H) M/+, su(comp)3L-1 clones persist with little apoptosis.
We reasoned that su(comp)3L-1 and su(comp)3L-2 might rescue M/+ cells from competition through inhibition of apoptosis. Consistent with this hypothesis, cell death was much reduced in su(comp)3L-1, M/+ or su(comp)3L-2, M/+ clones compared to M/+ clones (Figure 6, F–H). This suggested that the mutations acted on M/+ cell survival downstream of, or in parallel to, any role of Dpp signaling. Neither su(comp)3L-1 nor su(comp)3L-2 affected the developmentally regulated burst of apoptosis that accompanies eye morphogenesis in the pupa (data not shown). Thus the mutations do not affect all apoptotic processes and might be specific for cell competition.
Dpp signaling and cell competition:
Most mutations that rescued cell competition were either tumor suppressors that elevated Dpp signaling, as measured by Salm or Brk protein levels, or mutations that affected neither Dpp outputs nor growth. This suggested that Dpp signaling and/or hyperplastic growth might rescue cell competition and also that other pathways could be involved.
If elevated Dpp signaling were sufficient to rescue M/+ cells from competition, we would predict that mutations in negative regulators of the Dpp signaling would rescue M/+ eye cells. Dsmurf encodes a ubiquitin ligase that negatively regulates Dpp signaling (Podos et al. 2001). M/+, Dsmurf cells survived in the adult eye (Figure 7, A and B). When Ey-FLP was used to generate DsmurfKG07014 mutant clones in a wild-type background, the DsmurfKG07014 mutant cells outgrew their wild-type counterparts and contributed to >90% of the adult eye (Figure 7C). In clones homozygous for the DsmurfKG07014 allele, the extent of Salm expression was expanded in the wing disc, confirming an increased response of cells to moderate levels of Dpp (Figure 7D). Apoptosis was not suppressed in DsmurfKG07014, M/+ clones in the wing, although such clones persisted after control M/+ clones had been eliminated (Figure 7, E–H). These findings showed that Dsmurf was a negative regulator of Dpp signaling in imaginal discs, as well as in embryogenesis, and negatively regulated growth of both wild-type and M/+ cells although, unlike brk (Moreno et al. 2002), it does not appear to affect competitive apoptosis. The Dsmurf mutation may affect Dpp-signaling levels less than brk does, or absolute levels of Dpp signaling may not be what is relevant for survival.
Figure 7.—
Dpp signaling and apoptosis of M/+ cells in Dsmurf mutants. (A) No white M/+ cells remain in the y w Ey-FLP/Df(1)R194; FRT42/FRT42 P[RpL36+ w+] P[armLacZ w+] eye. (B) DsmurfKG07014, M/+ cells do survive in the adult eye (genotype y w Ey-FLP/Df(1)R194; FRT42 DsmurfKG07014/FRT42 P[RpL36+ w+]). Because the DsmurfKG07014 mutation is caused by a w+ P-element insertion, DsmurfKG07014 homozygous cells are the more pigmented genotype (e.g., arrowhead).(C) Pigmented DsmurfKG07014 homozygous clones (e.g., arrowhead) predominate in a mosaic with wild-type cells (genotype y w Ey-FLP/+; FRT42 DsmurfKG07014/FRT42).(D) Salm protein levels (green) are upregulated in DsmurfKG07014 homozygous cells (those lacking β-galactosidase are in magenta). Both subtle increases in expression level and lateral expansion of the expression domain occur (e.g., arrows). DsmurfKG07014 hyperplasia is less certain in the wing than in the eye, perhaps reflecting differing growth effects of Dpp signaling in distinct regions of the wing disc (Rogulja and Irvine 2005). (E and F) M/+ clones are small and apoptotic after 48 hr (E) and mostly eliminated after 72 hr (F) (genotype hsflp/M(1)Bld; FRT42 P[RpL36+ w+] arm-lacZ /FRT42). (G and H). M/+, DsmurfKG07014 clones are small and apoptotic 48 hr after induction (G), but many survive 72 hr after induction (H) (genotype hsflp/M(1)Bld; FRT42 P[RpL36+ w+] arm-lacZ /FRT42 DsmurfKG07014). (I) exNY1 Mad10, M/+ clones can survive in the adult eye (e.g., arrows) (genotype y w Ey-FLP/Df(1)R194; P[RpL36+ w+] P[armLacZ w+] FRT40/exNY1 Mad10 FRT40). Some clones also have differentiation defects. Control M/+ clones are shown in Figure 1C.
By contrast to Dsmurf, we found that a mutation in the inhibitory Smad Daughters against Dpp (Dad) (Tsuneizumi et al. 1997) did not protect M/+ cells (data not shown). Dad271-68, M/+ cells were effectively competed in the eye. Dad271-68 clones, however, themselves grow poorly and could not be recovered in the adult eye or in the wing imaginal disc (data not shown).
In addition to examining whether Dpp signaling could rescue M/+ clones, we also tested whether Dpp signaling was necessary. If the mutations such as ex and ft that rescued M/+ cells did so through elevated Dpp signaling, then the Dpp signal transduction pathway would be required in these cells. A null allele of the Mad gene was used to test this for ex. M/+, ex Mad clones were found in adult eyes, although they were smaller than M/+, ex clones (Figures 2A and 7I). This showed that ex mutants could rescue M/+ clones independently of Dpp signaling, although Dpp signaling did contribute to their size.
Jun N-terminal kinase pathway and cell competition:
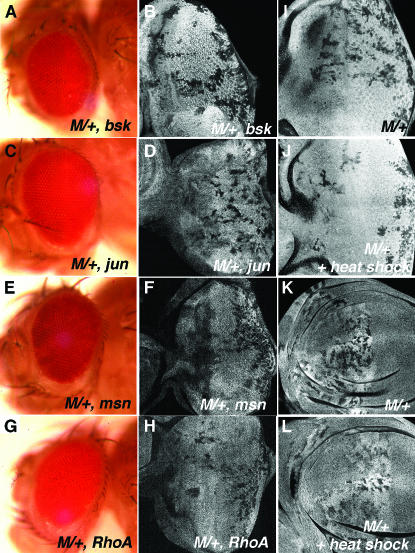
It has been proposed that JNK-mediated apoptosis is essential for cell competition (Moreno et al. 2002). Our screen did not isolate known JNK pathway mutations. We tested alleles of misshapen (msn102) (a Ste20 kinase required for JNK activation), basket (bsk2 and bsk170B) (the Drosophila JNK), RhoABH, and jun2 (Weston and Davis 2002), but none rescued M/+ cells. We did not detect surviving M/+ cells in the adult eye, nor any increase in the proportion of M/+ cells in the larval eye disc (Figure 8, A–H).
Figure 8.—
The JNK pathway and heat shock (A–H). M/+ clones also homozygous for JNK components were induced using eyFLP. None of the JNK mutations protected M/+ clones in adults (A: bsk2; C: jun2; E: msn102; and G: RhoABH; compare the negative control in Figure 1C and the positive controls in Figure 2). None rescued in eye discs either (B: bsk2; D: jun2; F: msn102; and H: RhoABH; compare the negative control in Figure 1E and the positive controls in Figure 4, F and G). None of the mutations affected clonal growth adversely by themselves (data not shown). (I–L) M/+ clones were induced continuously in eyes by eyFLP (I and J) or in anterior wings by dppGal4>UAS:FLP (K and L). M/+ clones were eliminated more efficiently in tissues that had been heat-shocked 72 hr earlier (J and L) than in those that had not (I and K).
Heat shock and cell competition:
Because the JNK pathway can be activated by heat shock (Gibson and Perrimon 2005), it was possible that inducing mosaicism with eyFlp rather than hsFLP diminished the importance of JNK activity for cell competition. Conversely, heat shock might enhance competition among clones. Consistent with this notion, heat shock enhanced loss of M/+ cell clones induced by eyFlp-mediated recombination (Figure 8, I and J). Similarly, although M/+ cell clones induced during wing development by Dpp>Flp-mediated recombination were competed, their loss was accelerated by heat shock (Figure 8, K and L; see materials and methods for details).
Role of hyperplastic tumor suppressors in cell competition:
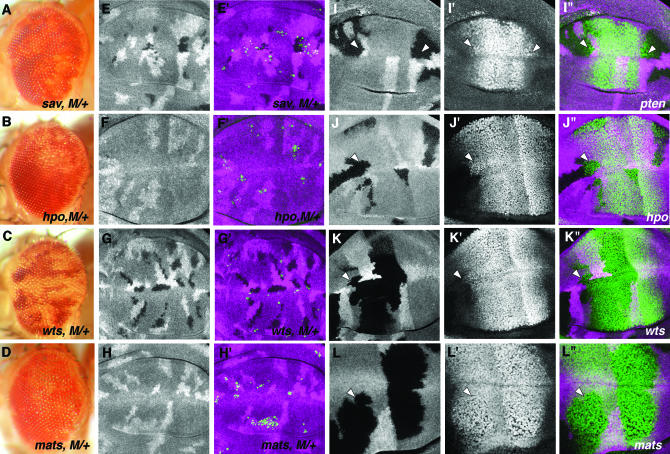
Several of the mutations that we recovered in the screen inactivate tumor-suppressor genes. We wondered whether all mutations that induce overgrowth might be able to prevent the loss of M/+ cells through cell competition. The genes dacapo (dap), ft, ex, argos (aos), gap1, TSC1, TSC2, PTEN, warts (wts), salvador (sav), hippo (hpo), archipelago (ago), and karst (kst) were identified in a mosaic screen for mutations that cause overgrowth of mutant tissue (de Nooij et al. 2000; Moberg et al. 2001; Tapon et al. 2002; Harvey et al. 2003; I. K. Hariharan, personal communication). We tested mutations in these genes to see whether they were able to rescue M/+ eye cells from competition, whether they affected Dpp signaling as assayed by Salm expression, and whether they affected the apoptosis of M/+ cells in the wing disc (results summarized in Table 1). The mutations dap4, aosΔ7, gap1E21F.2, kstE17A.1, PTENdj189, ago3, TSC2192, and TSC129 were unable to rescue M/+ eye cells, but mutations in salvador (sav1, sav2, sav3), hippo (hpoMGH4), and warts (wtsMGH1) did (Figure 9, A–C). sav, hpo, and wts function in a common pathway that regulates growth and apoptosis (Harvey et al. 2003; Pantalacci et al. 2003; Udan et al. 2003; Wu et al. 2003). A fourth gene that functions in the same pathway, mats, was recently described (Lai et al. 2005). We found that the matsroo and matse235 mutations also protected M/+ cells from competition (Figure 9D).
TABLE 1.
Mutations and their effects on cell competition and Dpp signaling
| Mutation | M/+ cells survive in adult eye | Prevent apoptosis of M/+ cells in wing | Increase Salm levels wing | Hyperplastic |
|---|---|---|---|---|
| ex | + | − | + | + |
| ft | + | − | + | + |
| sav | + | (+) | − | + |
| hpo | + | − | + | + |
| wts | + | (+) | + | + |
| mats | + | − | + | + |
| su(comp)3R-1 | + | − | + | + |
| su(com)3L-1 | + | + | − | − |
| su(com)3L-2 | + | + | − | − |
| aos | − | − | − | + |
| Kst | − | − | − | + |
| Gap1 | − | − | − | + |
| PTEN | − | − | + | + |
| dap | − | − | − | + |
| ago | − | − | − | + |
| TSC1 | − | − | − | + |
| TSC2 | − | − | − | + |
| Dad | − | ND | ND | − |
| Dsmurf | + | − | + | + |
Mutations in the following genes were also tested and found not to rescue M/+ cells in the adult eye: msn, jun, RhoA, bsk, lgl, lgd, scrib, ds, fz, gug, fj.
Figure 9.—
A subset of hyperplastic tumor suppressors prevent cell competition or affect Dpp signaling. (A–D) Mutations in the Warts pathway allow survival of M/+ cells. The following mutations allowed white, M/+ clones to survive in the adult eye: (A) sav3, (B) hpoMGH4, (C) wtsMGH1, and (D) matsroo. (E–H) M/+ clones also homozygous for sav3 (E), hpoMGH4 (F), wtsMGH1 (G), and matsroo (H) were fixed 48 hr after clone induction. Apoptosis (green) is reduced by sav and wts compared to controls, but not by hpo or mats. See Figure 5 for FRT82 control for wts, mats, and sav. The ratio of homozygous mutant, apoptotic cells to clone size was compared, except that too few matsroo, M//+ cells or hpoMGH4, M/+ cells were found for quantification. (I–L) Salm protein levels are upregulated in mutant cells: PTENdj189 (I), hpoMGH4 (J), wtsMGH1 (K), and matsroo(L). Note either an increase in the intensity of labeling relative to the adjacent tissue or the lateral expansion of label within the clone (arrowheads). Clones of mutant cells are marked by the absence of the β-galactosidase label (magenta, I′′–L′′). Salm protein is labeled green in merges (I′′–L′′). Homozygous mats cells have notably larger nuclei (L′), but this cannot account for the intensity of Salm labeling, which is increased in single confocal sections.
We additionally tested mutations in the neoplastic tumor suppressors lethal giant discs (lgdd7), scribble (scrib2), and lethal giant larvae (lgl4, lgl4w3) and found that they could not rescue M/+ cells (data not shown).
In most cases, there was a correlation between the mutations that elevated Dpp signaling and those that interfered with cell competition. Salm protein levels were increased in clones of cells mutant for hpoMGH4, matsroo, wtsMGH1, and PTENdj189 (Figure 9, I–L) but not for sav3, dap, aos, gap1, TSC1, TSC2, ago, or kst (data not shown). Thus sav3 was the only mutation that rescued competition without elevating Salm, and PTENdj189 was the only mutation elevating Salm but not rescuing competition (Table 1).
We tested whether sav, hpo, wts, or mts mutations rescued M/+ clones by preventing apoptosis of M/+ cells (Figure 9, E–H). Although none did so completely, some had quantitative effects. Although hpoMGH1, M/+ cells and matsroo, M/+ cells were mostly eliminated from the wing imaginal disc by 48 hr after clone induction, and entirely lost after 72 hr, by contrast, sav3, M/+ cells and wtsMGH1 M/+ cells remained 72 hr after clone induction and showed significantly reduced numbers of dying cells compared to M/+ clones (Figure 9, E–H). It is difficult to draw very strong quantitative conclusions from these data: differences in sizes, shapes, and persistence over time of mutant clones make comparison to precisely matched M/+ control clones difficult because of the problems in genotyping all apoptotic cells as described earlier for M/+ clones and because clones mutant for Warts pathway genes themselves induce cell death in surrounding cells (see below). Nevertheless, it seems likely that mutations in the Warts pathway affect competitive cell death without preventing it completely.
These studies showed that a subset of tumor-suppressor mutations rescued M/+ clones from cell competition. This subset includes all known members of the Warts pathway. While our study was underway, ex and mer were found to be upstream members of this same pathway (Hamaratoglu et al. 2006). We were not able to examine the effect of mer on cell competition because it is X linked.
Planar polarity and cell competition:
In addition to its role in growth suppression, ft is also required for the establishment of correct planar polarity, i.e., the orientation of epithelial cells within the plane of the epithelium (Saburi and McNeill 2005). Loss of ex function leads to polarity defects in the developing eye, characterized by random orientation of the R3/R4 photoreceptors and leading to inversions of chirality and misrotation of ommatidia. Therefore ex has also been described as a planar polarity gene (Blaumueller and Mlodzik 2000). It has been suggested that planar cell polarity might be related to the sensing of Dpp gradients (Rogulja and Irvine 2005).
To determine whether cell competition depends on a pathway common to planar polarity, we tested mutations in each of these planar polarity genes: dachsous (dsUA071), frizzled (fzR52), four-jointed (fjd1), and atrophin (gug35) (Yang et al. 2002; Fanto et al. 2003; Cho and Irvine 2004; Simon 2004). None of the mutations was able to rescue M/+ cells (data not shown). Thus the planar polarity signaling pathway that is downstream of ft and ex is not required for competition of M/+ cells.
Warts pathway mutations render cells supercompetitors:
Because not all tumor suppressors rescued M/+ clones, we wondered what distinguished the subset that did so. Because it is already known that only certain mechanisms of growth difference induce cell competition (de la Cova et al. 2004), we wondered whether Warts pathway mutations were another example of mutations that caused cell competition directly, contributing to the overgrowth that such mutants exhibit in the presence of +/+ cells. Cell death was examined in wing discs containing various mutant clones to test whether wild-type cells nearby were killed. Dying cells were seen adjacent to homozygous ex, ft, sav, hpo, or wts clones, although not as many as in M/+ clones adjacent to wild type (Figure 10). The number of dying cells was higher when eyFlp was used, resulting in eye discs mostly comprising mutant cells (Figure 10, E and F). In addition, cell death also occurred in wild-type cells within the recombinant twin-spot clone adjacent to ex/+ cells, indicating that a different ex gene dose is sufficient to initiate cell competition (Figure 10A). Cell death was not altered from normal levels when clones of cells homozygous for control FRT chromosomes were induced, whether by heat-inducible or eyFlp methods (data not shown). Thus, the tumor-suppressor mutations that rescued M/+ cell competition were themselves supercompetitor mutations.
Figure 10.—
Warts pathway mutants compete with wild type. All panels show cell death (green) in discs containing clones of homozygous mutant cells lacking β-galactosidase (magenta). Some other cells die next to clones for each of the Warts pathway mutants (arrowheads). In addition, for ex, some wild-type cells (+/+, twice the β-galactosidase level) die next to heterozygous ex/+ cells (example in A).
DISCUSSION
We isolated mutations that protect clones of RpL36/+ cells from cell competition during Drosophila eye development. Except for mutations of other Minute loci, the mutations acted recessively (Table 1). We recovered mutations at three tumor-suppressor loci, each of which elevated Dpp signaling, and two of which also affect planar cell polarity. Another pair of mutations completely prevented competitive cell death, but did not affect Dpp signaling or cause hyperplastic growth. We did not recover mutations in known negative regulators of Dpp signaling or in the JNK pathway. Because they could have been missed by chance, these candidates were tested directly, along with known tumor-suppressor and planar polarity genes, to evaluate the role of the Dpp, JNK, planar polarity, and tumor-suppressor pathways in cell competition. Our results identify new components of competitive cell death and implicate the Warts pathway in cell competition.
Dpp signaling:
Most tumor-suppressor mutations that rescue M/+ cells also elevated Dpp signaling to some degree, and mutating Dsmurf to elevate Dpp signaling rescued M/+ cells, consistent with the existing notion that cells compete for Dpp to survive (Moreno et al. 2002; Moreno and Basler 2004). However, M/+ clone survival was rescued despite continuing cell death, although it is difficult to be certain that rates of cell death were not reduced. Since Dpp signaling also enhances growth (Rogulja and Irvine 2005), an alternative basis for rescue may be that cell death is insufficient to eliminate M/+ clones that are growing faster due to elevated Dpp signaling. However, ex mutations protected M/+ cells during eye development even in the absence of the Mad gene, providing a demonstration that M/+ clones can be rescued independently of the Dpp pathway (Figure 7I).
Evaluating the contribution of Dpp is further complicated by recent findings that Dpp affects growth through multiple mechanisms. Even within the wing imaginal disc, Dpp signaling autonomously promotes growth proximally and suppresses growth distally, while discontinuities in Dpp-signaling levels promote growth nonautonomously (Rogulja and Irvine 2005). Assessing the contribution of Dpp through loss-of-function experiments in the wing is made difficult by the role of vestigial, a gene required for proliferation and survival of cells in the wing pouch, and specifically expressed there as a target of Dpp signaling (Cohen 1996; Kim et al. 1996, 1997; Martin-Castellanos and Edgar 2002). In the wing, cells unable to receive Dpp die secondarily to mechanical exclusion of these cells from the wing imaginal disc epithelium (Gibson and Perrimon 2005; Shen and Dahmann 2005). Exclusion may depend on vg, which has been shown to regulate the cell adhesion properties of wing cells (Liu et al. 2000). Thus the wing may differ from the eye and other tissues where the vg gene is neither expressed nor required and where cells unable to receive Dpp are not automatically excluded (Fu and Baker 2003). These complications make deeper understanding of the relationship of Dpp signaling to cell survival and growth during cell competition challenging.
The overall conclusion from our genetic screen is that the broad correlation between mutations that rescue M/+ clones and elevated Dpp signaling is generally consistent with the notion that competition between M/+ and wild-type cells affects Dpp signaling, but that other mechanisms and signaling pathways may also be involved (see below).
JNK signaling:
The failure of several mutations in the JNK pathway to rescue M/+ clones was surprising in view of the rescue of M/+ clones by mutation of the JunKK hep (Moreno et al. 2002). It has also been reported that the JNK pathway is not essential for competition among cells expressing different Myc levels (de la Cova et al. 2004). One possible technical issue concerns the genotypes used to generate M/+ clones experimentally, each of which also affects other genes. Although the principle of using translocation loss to uncover an unlinked Minute genotype (Moreno et al. 2002) is the same as our use of a transgene, heterozygosity for Df(2R)M60E renders some 20–30 genes haploid, whereas heterozygosity for Df(1)R194 removed one copy of the RpL36 and dredd genes only. We do not think dredd plays a significant role in cell competition (W. Li, unpublished results), but have not studied genes deleted by Df(2R)M60E. Perdurance is a further concern. If clones of homozygous mutant cells lose msn, bsk, RhoA, or jun function less rapidly than hep function, then the importance of the former genes may be underestimated by mosaic analysis.
Other data raise the possibility that JNK may contribute to cell competition indirectly. Heat shock potentiates JNK activity and apoptosis in cells with impaired Dpp signaling (Gibson and Perrimon 2005). We found that heat shock also enhanced the elimination of M/+ clones in eye and wing discs (Figure 8). It is possible that, when M/+ and wild-type cells compete, genotypes and conditions that alter the threshold of cell death modify the rate at which cell competition occurs. Since our screen made use of an eye-specific recombinase rather than a heat-shock-induced recombinase, no contribution of heat-shock-induced JNK activity would be expected.
Evidence for a novel death pathway:
Instead of known autosomal components of the Dpp and JNK pathways, our screen identified two novel mutations that prevented competitive cell death. It is not yet possible to determine whether the su(comp)3L-1 and su(comp)3L-2 mutations are allelic, as the effect on cell competition is scored only in homozygous clones and as both mutations lack any other obvious phenotype. Neither detectably affected Dpp signaling. These results suggest the existence of a distinct mechanism that activates apoptosis in cells undergoing competition. The mechanism may be relatively specific for this purpose, since these mutations were viable and did not affect apoptosis that occurs during normal eye development. Consistent with this hypothesis, we find that competitive apoptosis occurs independently of several genes with functions in other forms of apoptosis and in part depends on specific interactions with neighboring cells (W. Li and N. E. Baker, unpublished results). Further work will be required to determine whether su(comp)3L-1 and su(comp)3L-2 affect components of a novel death pathway.
Warts pathway:
Many of the recessive mutations that permitted M/+ cells to survive and to contribute to adult tissues behaved as “tumor suppressors” in the absence of cell competition (Table 1). That is, homozygous cells showed hyperplastic growth compared to the twin-spot controls when no M mutation was present. All known members of the Warts pathway rescued M/+ cells. It is possible that the ft and su(comp)3R-1 mutations might be unrecognized members of the Warts pathway, as it has been suggested that this pathway might be regulated by a receptor acting upstream of ex (Hamaratoglu et al. 2006). More recent studies confirm the notion that fat regulates the Warts pathway (Cho et al. 2006; Silva et al. 2006; D. M. Tyler and N. E. Baker, unpublished results).
The simplest explanation for the recovery of tumor-suppressor mutations that rescue M/+ clones is that growth of the competed cells was promoted to such an extent that apoptosis was insufficient to remove them. In this view, tumor-suppressor mutations would affect growth independently of cell competition. This model predicts that all tumor-suppressor mutations should rescue M/+ clones, depending on their quantitative effects on growth, and does not predict that such mutations will necessarily affect Dpp signaling or competitive cell death.
Our results contrasted with this simple model. Not all tumor-suppressor mutations rescued cell competition (Table 1). Those that did so generally elevated Dpp signaling, and some reduced apoptosis but did not eliminate it. In addition to rescuing M/+ cells from competition, Warts pathway mutations converted wild-type cells into supercompetitors. This is significant, because juxtaposition of cells with different growth rates does not always result in the elimination of the slower cells by competition (de la Cova et al. 2004). This indicates that the Warts pathway is one of a few growth pathways able to induce cell competition by itself and raises the possibility that rescue of M/+ clones is related to this.
One feature common to most Warts pathway mutants is enhanced Dpp signaling. However, ex mutations protected M/+ clones even in the absence of the Mad gene. The Warts pathway may activate growth and survival not only through Dpp signaling, but also through multiple signaling pathways, perhaps through a general effect on endocytosis (Maitra et al. 2006; D. M. Tyler and N. E. Baker, unpublished results; B. Pellock and I. K. Hariharan, unpublished results). In addition, it is possible that Warts pathway mutations that promote survival do so independently of any effect on Dpp signaling, because many upregulate DIAP1 transcription (Harvey et al. 2003; Pantalacci et al. 2003; Udan et al. 2003; Wu et al. 2003; Huang et al. 2005).
The finding that Warts pathway mutations are supercompetitors indicates that changes in Warts pathway activity itself result in competition. Our results raise the possibility that activity of the Warts pathway might be altered at boundaries between M/+ and wild-type cells, contributing either to death and elimination of M/+ cells or to compensating proliferation by wild-type cells that replace them. One challenge to this model is that Warts pathway mutants did not completely suppress M/+ cell death. It remains possible that the mutations used did not remove all Warts pathway activity or that Warts pathway activity is not the only factor promoting M/+ cell death. Alternatively, Warts pathway mutations might identify a parallel mechanism of competition, independent of that induced at M/+ clone boundaries. In this case, Warts pathway mutants rescue M/+ clones because adjacent wild-type and M/+ cells compete with one another through competing mechanisms, resulting in a stalemate.
Mammalian homologs of Warts family members are implicated in cancers (St. John et al. 1999; Tapon et al. 2002; McClatchey and Giovanni 2005; Takahashi et al. 2005). This provides further evidence that cell competition may contribute to the development and progression of cancer, in addition to the ability of the proto-oncogene Myc to induce cell competition (de la Cova et al. 2004; Moreno and Basler 2004). Because most cancers have a genetic basis (Hanahan and Weinberg 2000), the affected individuals are genetic mosaics, so that competition between tumor and wild-type cells for survival and growth could occur at tumor boundaries. The effects might include the progressive elimination of normal cells by cancerous ones, similar to the supercompetitive effect of Warts mutant cells in Drosophila, or the protection of tumor cells from competition with normal cells, similar to the protection of M/+ cells in Drosophila.
Acknowledgments
We thank I. Hariharan, K. Irvine, L. Johnston, and H. McNeill for discussions and comments on an earlier version of the manuscript. We also thank J. Axelrod, I. Hariharan, T. Klein, Z. Lai, H. McNeill, M. Mlodzik, D. J. Pan, N. Perrimon, J. Triesman, T. Xie, and the Bloomington Drosophila Stock Center for Drosophila strains; G. Campbell and B. Mollereau for antisera used in this study; and R. Rodriguez for RpL36 DNA. We thank W. Fu, A. Koyama-Koganeya, and S.-Y. Yu for assistance. This work was supported by the National Institutes of Health (GM61230). B.P. was supported by the Jack A. Davis, M.D., postdoctoral fellowship from the American Cancer Society (PF-02-234-01) and by the Massachusetts Biomedical Research Council Tosteson postdoctoral fellowship. N.E.B. is a Scholar of the Irma T. Hirschl Trust for Biomedical Research. Some data in this article are from a thesis to be submitted in partial fulfillment of the requirement for the degree of Doctor of Philosophy in the Graduate Division of Biomedical Sciences, Albert Einstein College of Medicine, Yeshiva University.
References
- Adler, P. N., J. Charlton, K. H. Jones and J. Liu, 1994. The cold-sensitive period for frizzled in the development of wing hair polarity ends prior to the start of hair morphogenesis. Mech. Dev. 46: 101–107. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., J. Charlton and J. Liu, 1998. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development 125: 959–968. [DOI] [PubMed] [Google Scholar]
- Berger, J., T. Suzuki, K. A. Senti, J. Stubbs, G. Schaffner et al., 2001. Genetic mapping with SNP markers in Drosophila. Nat. Genet. 29: 475–481. [DOI] [PubMed] [Google Scholar]
- Bilder, D., and N. Perrimon, 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680. [DOI] [PubMed] [Google Scholar]
- Blaumueller, C. M., and M. Mlodzik, 2000. The Drosophila tumor suppressor expanded regulates growth, apoptosis, and patterning during development. Mech. Dev. 92: 251–262. [DOI] [PubMed] [Google Scholar]
- Boedigheimer, M., and A. Laughon, 1993. Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development 118: 1291–1301. [DOI] [PubMed] [Google Scholar]
- Boedigheimer, M., P. Bryant and A. Laughon, 1993. Expanded, a negative regulator of cell proliferation in Drosophila, shows homology to the NF2 tumor suppressor. Mech. Dev. 44: 83–84. [DOI] [PubMed] [Google Scholar]
- Boedigheimer, M. J., K. P. Nguyen and P. J. Bryant, 1997. Expanded functions in the apical cell domain to regulate the growth rate of imaginal discs. Dev. Genet. 20: 103–110. [DOI] [PubMed] [Google Scholar]
- Brumby, A. M., and H. E. Richardson, 2003. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22: 5769–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, P. J., B. Huettner, L. I. Held, Jr., J. Ryerse and J. Szidonya, 1988. Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev. Biol. 129: 541–554. [DOI] [PubMed] [Google Scholar]
- Buratovich, M. A., and P. J. Bryant, 1995. Duplication of l(2)gd imaginal discs in Drosophila is mediated by ectopic expression of wg and dpp. Dev. Biol. 168: 452–463. [DOI] [PubMed] [Google Scholar]
- Campbell, G., and A. Tomlinson, 1999. Transducing the Dpp morphogen gradient in the wing of Drosophila: regulation of Dpp targets by brinker. Cell 96: 553–562. [DOI] [PubMed] [Google Scholar]
- Chen, P., W. Nordstrom, B. Gish and J. M. Abrams, 1996. grim, a novel cell death gene in Drosophila. Genes Dev. 10: 1773–1782. [DOI] [PubMed] [Google Scholar]
- Cho, E., and K. D. Irvine, 2004. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development 131: 4489–4500. [DOI] [PubMed] [Google Scholar]
- Cho, E., Y. Feng, C. Rauskolb, S. Maitra, R. G. Fehon et al., 2006. Delineation of a Fat tumor suppressor pathway. Nature Genetics 38: 1142–1150. [DOI] [PubMed] [Google Scholar]
- Cohen, S. M., 1996. Controlling growth of the wing: vestigial integrates signals from the compartment boundaries. BioEssays 18: 855–858. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., and R. Barrio, 2000. Function of the spalt/spalt-related gene complex in positioning the veins in the Drosophila wing. Mech. Dev. 91: 31–41. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., R. Barrio and F. C. Kafatos, 1996. A gene complex acting downstream of dpp in Drosophila wing morphogenesis. Nature 381: 421–424. [DOI] [PubMed] [Google Scholar]
- de la Cova, C., M. Abril, P. Bellosta, P. Gallant and L. A. Johnston, 2004. Drosophila myc regulates organ size by inducing cell competition. Cell 117: 107–116. [DOI] [PubMed] [Google Scholar]
- de Nooij, J. C., K. H. Graber and I. K. Hariharan, 2000. Expression of the cyclin-dependent kinase inhibitor Dacapo is regulated by cyclin E. Mech. Dev. 97: 73–83. [DOI] [PubMed] [Google Scholar]
- Erkner, A., A. Roure, B. Charroux, M. Delaage, N. Holway et al., 2002. Grunge, related to human Atrophin-like proteins, has multiple functions in Drosophila development. Development 129: 1119–1129. [DOI] [PubMed] [Google Scholar]
- Fanto, M., L. Clayton, J. Meredith, K. Hardiman, B. Charroux et al., 2003. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development 130: 763–774. [DOI] [PubMed] [Google Scholar]
- Freeman, M., 2002. A fly's eye view of EGF receptor signalling. EMBO J. 21: 6635–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, M., C. Klambt, C. S. Goodman and G. M. Rubin, 1992. The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye. Cell 69: 963–975. [DOI] [PubMed] [Google Scholar]
- Fu, W., and N. E. Baker, 2003. Deciphering synergistic and redundant roles of Hedgehog, Decapentaplegic and Delta that drive the wave of differentiation in Drosophila eye development. Development 130: 5229–5239. [DOI] [PubMed] [Google Scholar]
- Gao, X., and D. Pan, 2001. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 15: 1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X., T. P. Neufeld and D. Pan, 2000. Drosophila PTEN regulates cell growth and proliferation through PI3k-dependent and -independent pathways. Dev. Biol. 221: 404–418. [DOI] [PubMed] [Google Scholar]
- Gateff, E., and H. A. Schneiderman, 1974. Developmental capacities of benign and malignant neoplasms of Drosophila. Rouxs Arch. Dev. Biol. 176: 23–26. [DOI] [PubMed] [Google Scholar]
- Gibson, M. C., and N. Perrimon, 2005. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science 307: 1785–1789. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu, F., M. Willecke, M. Kango-Singh, R. Nolo, E. Hyun et al., 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 8: 27–36. [DOI] [PubMed] [Google Scholar]
- Hanahan, D., and R. Weinberg, 2000. The hallmarks of cancer. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]
- Harvey, K. F., C. M. Pfleger and I. K. Hariharan, 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467. [DOI] [PubMed] [Google Scholar]
- Huang, J., S. Wu, J. Barrera, K. Matthews and D. Pan, 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434. [DOI] [PubMed] [Google Scholar]
- Ito, N., and G. M. Rubin, 1999. gias, a Drosophila homolog of tuberous sclerosis gene product 2 regulates the cell cycle. Cell 96: 529–553. [DOI] [PubMed] [Google Scholar]
- Jazwinska, A., N. Kirov, E. Wieschaus, S. Roth and C. Rushlow, 1999. The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell 96: 563–573. [DOI] [PubMed] [Google Scholar]
- Kim, J., A. Sebring, J. J. Esch, M. E. Kraus, K. Vorwerk et al., 1996. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382: 133–138. [DOI] [PubMed] [Google Scholar]
- Kim, J., J. Magee and S. B. Carroll, 1997. Intercompartmental signaling and the regulation of vestigial expression at the dorsoventral boundary of the developing Drosophila wing. Cold Spring Harbor Symp. Quant. Biol. 62: 283–291. [PubMed] [Google Scholar]
- Klambt, C., 2002. EGF receptor signalling: roles of star and rhomboid revealed. Curr. Biol. 12: R21–R23. [DOI] [PubMed] [Google Scholar]
- Kuhnlein, R. P., G. Frommer, M. Friedrich, M. Gonzalez-Gaitan, A. Weber et al., 1994. spalt encodes an evolutionarily conserved zinc finger protein of novel structure which provides homeotic gene function in the head and tail region of the Drosophila embryo. EMBO J. 13: 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, Z. C., X. Wei, T. Shimizu, E. Ramos, M. Rohrbaugh et al., 2005. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120: 675–685. [DOI] [PubMed] [Google Scholar]
- Lambertsson, A., 1998. The minute genes in Drosophila and their molecular functions. Adv. Genet. 38: 69–134. [DOI] [PubMed] [Google Scholar]
- Lane, M. E., K. Sauer, K. Wallace, Y. N. Jan, C. F. Lehner et al., 1996. Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell 87: 1225–1235. [DOI] [PubMed] [Google Scholar]
- Lawrence, P. A., 1982. Cell lineage of the thoracic muscles of Drosophila. Cell 29: 493–503. [DOI] [PubMed] [Google Scholar]
- Lindsley, D., and G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, New York.
- Liu, X., M. Grammont and K. D. Irvine, 2000. Roles for scalloped and vestigial in regulating cell affinity and interactions between the wing blade and the wing hinge. Dev. Biol. 228: 287–303. [DOI] [PubMed] [Google Scholar]
- Mahoney, P. A., U. Weber, P. Onofrechuk, H. Biessmann, P. J. Bryant et al., 1991. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell 67: 853–868. [DOI] [PubMed] [Google Scholar]
- Maitra, S., R. Kulikauskas, H. Gavilan and R. Fehon, 2006. The tumor suppressors Merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 16: 702–709. [DOI] [PubMed] [Google Scholar]
- Martin, F. A., A. Perez-Garijo, E. Moreno and G. Morata, 2004. The brinker gradient controls wing growth in Drosophila. Development 131: 4921–4930. [DOI] [PubMed] [Google Scholar]
- Martin-Castellanos, C., and B. A. Edgar, 2002. A characterization of the effects of Dpp signaling on cell growth and proliferation in the Drosophila wing. Development 129: 1003–1013. [DOI] [PubMed] [Google Scholar]
- McClatchey, A. I., and M. Giovanni, 2005. Membrane organization and tumorigenesis: the NF2 tumor suppressor. Genes Dev. 19: 2265–2277. [DOI] [PubMed] [Google Scholar]
- Minami, M., N. Kinoshita, Y. Kamoshida, H. Tanimoto and T. Tabata, 1999. brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature 398: 242–246. [DOI] [PubMed] [Google Scholar]
- Moberg, K. H., D. W. Bell, D. C. Wahrer, D. A. Haber and I. K. Hariharan, 2001. Archipelago regulates cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 413: 311–316. [DOI] [PubMed] [Google Scholar]
- Morata, G., and P. Ripoll, 1975. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 42: 211–221. [DOI] [PubMed] [Google Scholar]
- Moreno, E., and K. Basler, 2004. dMyc transforms cells into super-competitors. Cell 117: 117–129. [DOI] [PubMed] [Google Scholar]
- Moreno, E., K. Basler and G. Morata, 2002. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416: 755–759. [DOI] [PubMed] [Google Scholar]
- Muller, B., B. Hartmann, G. Pyrowolakis, M. Affolter and K. Basler, 2003. Conversion of an extracellular Dpp/BMP morphogen gradient into an inverse transcriptional gradient. Cell 113: 221–233. [DOI] [PubMed] [Google Scholar]
- Newsome, T. P., B. Asling and B. J. Dickson, 2000. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127: 851–860. [DOI] [PubMed] [Google Scholar]
- Oertel, M., A. Menthena and D. A. Shafritz, 2006. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology 130: 507–520. [DOI] [PubMed] [Google Scholar]
- Pantalacci, S., N. Tapon and P. Leopold, 2003. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5: 921–927. [DOI] [PubMed] [Google Scholar]
- Podos, S. D., K. K. Hanson, Y. C. Wang and E. L. Ferguson, 2001. The DSmurf ubiquitin-protein ligase restricts BMP signaling spatially and temporally during Drosophila embryogenesis. Dev. Cell 1: 567–578. [DOI] [PubMed] [Google Scholar]
- Rogulja, D., and K. D. Irvine, 2005. Regulation of cell proliferation by a morphogen gradient. Cell 123: 449–461. [DOI] [PubMed] [Google Scholar]
- Saburi, S., and H. McNeill, 2005. Organising cells into tissues: new roles for cell adhesion molecules in planar cell polarity. Curr. Opin. Cell Biol. 17: 482–488. [DOI] [PubMed] [Google Scholar]
- Schultz, J., 1929. The Minute reaction in the development of Drosophila melanogaster. Genetics 14: 366–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J., and C. Dahmann, 2005. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science 307: 1789–1790. [DOI] [PubMed] [Google Scholar]
- Silva, E., Y. Tsatskis, L. Gardano, N. Tapon and H. McNeill, 2006. The tumor-suppressor gene fat controls tissue growth upstream of Expanded in the Hippo signalling pathway. Curr. Biol. 16: 2081–2089. [DOI] [PubMed] [Google Scholar]
- Simon, M. A., 2004. Planar cell polarity in the Drosophila eye is directed by graded four-jointed and Dachsous expression. Development 131: 6175–6184. [DOI] [PubMed] [Google Scholar]
- Simpson, P., 1979. Parameters of cell competition in the compartments of the wing disc of Drosophila. Dev. Biol. 69: 182–193. [DOI] [PubMed] [Google Scholar]
- Simpson, P., and G. Morata, 1981. Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Dev. Biol. 85: 299–308. [DOI] [PubMed] [Google Scholar]
- Sluss, H. K., Z. Han, T. Barrett, R. J. Davis and Y. T. Ip, 1996. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 10: 2745–2758. [DOI] [PubMed] [Google Scholar]
- St. John, M. A., W. Tao, X. Fei, R. Fukumoto, M. L. Carcanglu et al., 1999. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumors and pituitary dysfunction. Nat. Genet. 21: 182–186. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y., Y. Miyoshi, C. Takahata, N. Irahara, T. Taguchi et al., 2005. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin. Cancer Res. 11: 1380–1385. [DOI] [PubMed] [Google Scholar]
- Tapon, N., K. Harvey, D. Bell, D. Wahrer, T. Schiripo et al., 2002. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110: 467. [DOI] [PubMed] [Google Scholar]
- Tearle, R., and C. Nusslein-Volhard, 1987. Tubingen mutants and stock list. Dros. Inf. Serv. 66: 209–269. [Google Scholar]
- Tomlinson, A., and D. F. Ready, 1987. Neuronal differentiation in the Drosophila ommatidium. Dev. Biol. 120: 336–376. [DOI] [PubMed] [Google Scholar]
- Treisman, J. E., N. Ito and G. M. Rubin, 1997. misshapen encodes a protein kinase involved in cell shape control in Drosophila. Gene 186: 119–129. [DOI] [PubMed] [Google Scholar]
- Tsuneizumi, K., T. Nakayama, Y. Kamoshida, T. B. Kornberg, J. L. Christian et al., 1997. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389: 627–631. [DOI] [PubMed] [Google Scholar]
- Udan, R. S., M. Kango-Singh, R. Nolo, C. Tao and G. Halder, 2003. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5: 914–920. [DOI] [PubMed] [Google Scholar]
- Weston, C. R., and R. J. Davis, 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12: 14–21. [DOI] [PubMed] [Google Scholar]
- Wu, S., J. Huang, J. Dong and D. Pan, 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456. [DOI] [PubMed] [Google Scholar]
- Xu, T., and G. M. Rubin, 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237. [DOI] [PubMed] [Google Scholar]
- Yang, C. H., J. D. Axelrod and M. A. Simon, 2002. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108: 675–688. [DOI] [PubMed] [Google Scholar]
- Yu, S. Y., S. J. Yoo, L. Yang, C. Zapata, A. Srinivasan et al., 2002. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development 129: 3269–3278. [DOI] [PubMed] [Google Scholar]