Abstract
A specific host–pathogen interaction exists between Caenorhabditis elegans and the gram-positive bacterium Microbacterium nematophilum. This bacterium is able to colonize the rectum of susceptible worms and induces a defensive tail-swelling response in the host. Previous mutant screens have identified multiple loci that affect this interaction. Some of these loci correspond to known genes, but many bus genes [those with a bacterially unswollen (Bus) mutant phenotype] have yet to be cloned. We employed Mos1 transposon mutagenesis as a means of more rapidly cloning bus genes and identifying new mutants with altered pathogen response. This approach revealed new infection-related roles for two well-characterized and much-studied genes, egl-8 and tax-4. It also allowed the cloning of a known bus gene, bus-17, which encodes a predicted galactosyltransferase, and of a new bus gene, bus-19, which encodes a novel, albeit ancient, protein. The results illustrate advantages and disadvantages of Mos1 transposon mutagenesis in this system.
ALL animals and plants coexist with potentially lethal pathogens. Whether or not a pathogen infects a particular host, and whether an infection proves lethal, depends on the response of both the pathogen and host to these encounters. Most multicellular organisms rely on only two lines of defense against pathogens: a mechanical defense provided by the epidermis and an innate immune defense provided by germ-line-encoded pathogen recognition and antimicrobial factors and responses. Pathogen success relies on being able to exploit or evade these defenses. For example, carbohydrates on host surfaces are often targets for binding by pathogens (for examples see Johnson 1999). As the outer surfaces of nematodes such as Caenorhabditis elegans are composed mainly of sugar-modified proteins and lipids (for review see Blaxter and Bird 1997), it is not surprising to find that the surface of the worm is a site of adherence for pathogens (Jannson 1994; Mendoza de Gives et al. 1999; Hodgkin et al. 2000; Joshua et al. 2003; Hoflich et al. 2004).
Many studies have shown that C. elegans mounts conserved innate immune responses against invading pathogens. Specifically, evolutionarily distant organisms, such as plants, mammals, and nematodes, employ a p38 MAP kinase cascade or a TGFb pathway in defense against bacterial and fungal pathogens (for reviews see Millet and Ewbank 2004; Schulenburg et al. 2004; Gravato-Nobre and Hodgkin 2005; Sifri et al. 2005). Further, a nematode DAF-2 insulin-like receptor/IGF signaling pathway contributes to protection against pathogens (Garsin et al. 2003). This pathway is also implicated in environmental stress response pathways and longevity in C. elegans as well as other organisms (Kenyon et al. 1993; Tatar et al. 2003; Baba et al. 2005; Gami and Wolkow 2006). These studies have identified conserved host factors used against virulent pathogens with a generalized host range. However, host factors that have coevolved in response to pathogens with a narrow host range may have been missed.
When C. elegans is exposed to Microbacterium nematophilum it becomes sick and develops a swollen tail region, referred to as the deformed anal region (Dar) phenotype. Previous work has shown that this swelling response is mediated through the ERK/MAPK pathway (Nicholas and Hodgkin 2004). Worms with mutations in this pathway fail to swell after exposure to M. nematophilum; instead, these mutants experience severe constipation and greatly increased rate of larval arrest, adult sterility, or death. This implicates the ERK/MAPK pathway as a defensive signaling mechanism in the context of this infection.
Previous forward genetic screens have identified many loci that are involved in this host–pathogen interaction. From ethyl methanesulfonate (EMS) and mut-7 transposon screens, >20 loci were found to affect tail swelling in the presence of M. nematophilum, which is a conspicuous and easily scored phenotype (Gravato-Nobre et al. 2005). Some of these loci correspond to known genes, but most have not been previously defined and were termed bus for bacterially unswollen—that is, worms exposed to the pathogen fail to swell, either because infection fails or because the swelling response is defective. These screens have not reached saturation. Specifically, there are many mut-7-induced loci for which an EMS allele was not obtained, and loci still remain that have only one EMS allele. Further, the molecular identities of most of these bus loci remain unknown. Five of the 20 loci had been identified in unrelated mutant screens and only 3 of these previously defined genes, egl-5, sur-2, and srf-3, have been cloned. Among the 15 novel bus genes identified, only 6 to date have been cloned (M. Gravato-Nobre, D. O'Rourke, F. Partridge and J. Hodgkin, unpublished results).
Although many mutants were recovered after mut-7-induced Tc transposon mutagenesis, we found that these bus mutants were not readily identified through Tc1 insertion analysis using transposon insertion display (TID) (Wicks et al. 2000). Although Tc1 transposons provide a molecular tag for their site of insertion, the Tc1 tags are not unique enough for the rapid detection of the insertion site because the C. elegans genome contains many of these elements. Further, the endogenous transposons induced to hop in the mut-7 background include Tc3 and other Tc family members. Moreover, mutations induced by EMS or Tc family transposon insertions that result in abnormal but variable responses to the pathogen have been little characterized hitherto, owing to the greater difficulty of working with less penetrant phenotypes.
As a means of more rapidly cloning rectal infection response genes, we explored the use of the Mos1 transposon mutagenesis tool (Bessereau et al. 2001; Williams et al. 2005). Since Mos1 transposons are not endogenous elements in the C. elegans genome, the Mos1 sequence provides a unique molecular tag that can be used to identify the site of insertion and hence any gene that it may be disrupting. Here, we describe the results of Mos1 screens for mutants with altered response to M. nematophilum and discuss the distinctive properties of mutants in four different genes, which affect various steps in the process of infection and host reaction. We also discuss the effectiveness of Mos1 mutagenesis as a forward mutagenesis technique for C. elegans.
MATERIALS AND METHODS
Strains and general techniques:
C. elegans nematodes were cultured as published (Brenner 1974). The Bristol isolate N2 was used as the wild-type strain. Pathogen susceptibility assays to M. nematophilum were carried out on mixed bacterial lawns of Escherichia coli (OP50) with 10% M. nematophilum (MBL) plates (Gravato-Nobre et al. 2005). For analysis of the Dar/Bus phenotype single L4 worms were picked to MBL or E. coli plates (four plates each) and adult progeny were scored for tail swelling. Scores are given as percentages plus or minus standard deviation. Worms on MBL plates were grown at 25° except when working with mutant alleles that result in a temperature-sensitive dauer-constitutive phenotype such as alleles of tax-4, tax-2, and daf-11. The pathogen response for these mutants was assayed at 20°. The following mutations and strains were used or generated in this study [unless otherwise noted, strains were obtained from the Caenorhabditis Genetics Center (CGC) and are described in WormBase at http://www.wormbase.org/]:
LGI: tax-2(p691, p694, p671) (ky139) (provided by C. Bargmann).
LGII: tag-175(gk278) (generated by the International C. elegans Gene Knockout Consortium, http://www.celeganskoconsortium.omrf.org).
LGIII: tax-4(e2861) (this study), (ks11, ks28) (provided by I. Mori), daf-2(e1368, e1370), daf-16(m26, mgDf50).
LGIV: him-8(e1489).
LGV: egl-8(e2917) (this study), (n488), bus-19(e2912, e2964, e2965, e2966) (this study), unc-42(e270), him-5(e1490), daf-11(m47), dpy-11(e224).
LGX: bus-17(e2923) (this study), (e2800, e2695) (Gravato-Nobre et al. 2005), (br2) (provided by C. Darby), (cxTi9043) (provided by L. Segalat, NemaGENETAG Consortium).
Mapping of single-nucleotide polymorphisms (SNP) was carried out using the C. elegans Hawaiian isolate CB4856 (Wicks et al. 2001). For Mos1 mutagenesis we used the array-containing strains EG1470 oxEx229[Mos1;myo-2p∷GFP] and EG2474 oxEx166[hspp∷Mos1Transposase;unc-122p∷GFP;lin-15(+)] (Bessereau et al. 2001). him-8(e1489); oxIs12[unc-47p∷GFP;lin-15(+)] was used for outcrossing tax-4(e2861). him-8(e1489); bgIs312 [a transgene expressing green fluorescent protein (GFP) in the excretory cell, kindly provided by T. Bogaert] was used to outcross bus-19(e2912). The deletion in tag-175(gk278) was verified by PCR on pooled lysates of mutants using GGAATGGACAAGCAAGGAAA and GACAGTTGGGTCGCTGATTT, primers that flank the deletion, and TCGGTTAAGAGGCTTTGTTG, a primer that is predicted to lie in the deleted area. OP50-GFP was obtained from the CGC.
Mutagenesis:
Mos1 mutagenesis was performed as published (Williams et al. 2005), using the extrachromosomal arrays oxEx166, which carries the Mos1 transposase under the control of a heat-shock promoter, and oxEx229, which carries multiple copies of the substrate Mos1 transposon. To facilitate construction of hermaphrodites carrying both arrays, in most of the selections EG1470 and EG2474 hermaphrodites were crossed with him-8(e1489) to generate strains that continually segregated array-carrying males. For each mutagenesis round, fresh double-array-carrying animals were generated. him-8(e1489);oxEx166 males or him8(e1489);oxEx229 males were crossed to L4 hermaphrodites carrying the other array. Progeny containing both arrays, recognized by the concurrent expression of GFP in the pharynx (myo-2p∷GFP, contained in oxEx229) and GFP in the coelomocytes (unc-122p∷GFP, contained in oxEx166), were propagated for three to six generations before heat-shocking. Double-transgenic animals were heat-shocked for 1 hr at 33°, 1 hr at 20°, and 1 hr at 33° and then allowed to recover overnight at 15° or 20° before picking onto MBL plates. Eggs from P0's were collected 12–40 hr after heat shock. Five to 10 young adult F1's were moved to MBL plates. Bus worms were selected in the F2 generation. From each Bus F2 4 F3's were picked to a MBL plate and rescored for bacterial resistance. Worms that continually produced Bus mutants were kept for further analysis. Hop rates were determined as outlined in Williams et al. (2005). Worm lysates were made from single worms in 2.5 μl or five worms in 10 μl lysis buffer as published with the additional step of freezing on dry ice and/or at −70° for at least 20 min before incubation at 60° (Wicks et al. 2001).
Mutants that lost the extrachromosomal Mos1-bearing array were outcrossed at least once before assaying for the presence of Mos1 elements and trying to locate the site of insertion (unless noted). For those mutants that still contained a Mos1 element, we determined the insertion site through inverse PCR on worm lysates as published (Bessereau et al. 2001). To identify Mos1 insertion sites, inverse PCR (iPCR) was performed on e2861 worm lysates digested with HaeIII or Sau3A, e2912 worm lysates digested with HaeIII or MseI, and e2917 and e2923 worm lysates digested with HaeIII or AluI. iPCR products were gel purified and then TA cloned using a TOPO-TA cloning kit (Invitrogen, San Diego). Products were amplified directly from colonies that were positive for transformation using oJL115/oJL116 and sequenced with oJL115. Sequences were considered true insertion flanking sequence if they contained the 5′ end of the Mos1 element adjacent to the C. elegans genomic sequence starting at a TA dinucleotide. Primers used to amplify flanking regions of Mos1 insertions are as follows: egl-8 (B0348.4), TGCCGAAAGTTATCAAAAAGTTA and GCCAGATAACGCCTCAGAAG; tax-4 (ZC84.2), TCGGAATTGTCTGGAAAGAAC and CTCACTCGGGTAAGCTCTGC; bus-17 (ZK678.8), CAAAAAGATCACCGCCAAAA and CCCCATCTTTGGAGAGAACA; and bus-19 (T07F10.4), ATGCCCTCCAACTTATCCTCTTG and TGCTTAACTTCTGCACATAGCACC.
EMS mutagenesis:
To isolate more alleles of bus-19, N2 males were mutagenized with EMS as published (Brenner 1974). Mutagenized N2 males were mated to bgIs312; unc-47(e270) bus-19(e2912) hermaphrodites on MBL and allowed to mate overnight. Mated animals were then transferred once to a new MBL plate. In total, 6062 F1's were scored for the Bus phenotype. Non-Unc F2 Bus progeny were picked to new MBL plates and were scored for Bus non-Unc. Four F2's bred true for the nonUnc Bus phenotype and were subsequently tested for the presence of the parental Mos1 insertion, to preclude recovery of a recombinant carrying the original allele rather than a novel EMS mutation. One of these lines was a recombinant on the basis of the ability to amplify Mos1 and flanking sequence of the array.
SNP mapping:
We used SNPs to map the mutants to a linkage group (Wicks et al. 2001). CB4856 Hawaiian males were mated to mutant strains and F1's were singled to MBL plates. Bus F2's were used for snipSNP mapping alongside the original Bus homozygotes and CB4856 worms as controls. Primers and SNPs used for this linkage analysis are available on request.
Transgenic experiments:
Transformation through micro-injection was performed as published (Mello et al. 1991). For indicating successful transformation, we co-injected plasmids pRF4 rol-6(su1006) (Mello et al. 1991) or pPD97/98 unc-122p∷GFP (a coelomocyte marker, gift of P. Sengupta), at 30 ng/μl while pTG96 sur-5p∷GFP (Yochem et al. 1998) was injected at 40–50 ng/μl. Rescuing DNA and reporter constructs were injected at concentrations between 10 and 30 ng/μl. tax-4(e2861) mutants were rescued and analyzed using [tax-4∷GFP] and [odr-4p∷tax-4∷GFP] constructs (kindly provided by M. de Bono).
For bus-17 rescue experiments, cosmid ZK678 with unc-122p∷GFP was injected into e2923 and cxTi9043 worms. We also amplified a 10-kb product from genomic DNA that extended from the last intron of the adjacent 3′ wrt-4 ORF to the last intron of Y7A5A.1 residing 5′ of ZK678.8. This product was co-injected with unc-122p∷GFP into e2923 or cxTi9043 homozygotes. In all of these experiments, lines were established on E. coli before testing transformants on MBL.
For bus-19 rescue experiments, him-8(e1489);lon-3(e2175) lines were established with arrays containing cosmid T07F10, cosmid R90, cosmids T07F10 and R90, fosmid clone WRM0640cD03, a 7.3-kb fragment amplified from fosmid clone WRM0623dE01, or a 13-kb fragment amplified from fosmid clone WRM0623dE01 and co-injection markers sur-5p∷GFP or unc-122p∷GFP. Fosmid clones were provided by J. Perkins and D. Moerman. These lines were obtained either through mating with N2 lines containing the arrays or through direct injection of him-8(e1489);lon-3(e2175) animals. Lon males carrying each array were crossed to bus-19(e2912) or bus-19(e2964) homozygous animals and F1's were transferred to MBL plates. Bus worms and Dar worms were scored for the presence of the GFP-expressing array.
Molecular analysis of bus-17:
bus-17 was amplified from N2-derived cDNA, which was prepared using RETROscript (Ambion, Austin, TX) following the manufacturer's recommendations. Primers ATCGTCGTCAGGTACTGTGTG with CCCCATCTTTGGAGAGAACA or TGAGACGTCGGAAACATGAA were used to amplify a 600- and a 1200-bp product, respectively, from cDNA template.
Molecular analysis of bus-19:
T07F10.4a and T07F10.4b were amplified and sequenced from cDNA clones yk1387f02 and yk1345b03, kindly provided by Y. Kohara. Primers are available on request. To identify the mutations in bus-19 EMS alleles, all exons and introns of T07F10.4 were sequenced from PCR-amplified genomic DNA (primer sequences are available on request). These products were sequenced on an Applied Biosystems (Foster City, CA) 3730xl DNA analyzer using Applied Biosystems Big Dye terminators.
Fluorescent staining and gross cuticle analysis:
To visualize bacteria associated with infected worms, we incubated worms with the fluorescently labeled dye SYTO 13 (Invitrogen) as published. Bleach sensitivity assays and lectin staining were carried out as published. (Nicholas and Hodgkin 2004; Gravato-Nobre et al. 2005).
Behavioral assays:
The bacterial choice assay was performed as published except that standard NGM were seeded with 40 μl of E. coli and 40 μl of M. nematophilum (Bargmann et al. 1993; Pujol et al. 2001). Plates were incubated overnight at 37° and cooled to room temperature before adding worms that had been grown on E. coli. These assays were performed at room temperature. We assessed the Skiddy (Skd) phenotype of bus-19 alleles by placing 10 young adult animals, spaced apart from one another, at the edge of a bacterial lawn and letting them crawl into the lawn. As soon as a worm traversed the lawn it was removed from the plate and the tracks were qualitatively scored as wild type or Skd.
RESULTS
Isolation of Mos1 insertion mutants:
From a series of six small Mos1 mutagenesis experiments, >7600 F1 progeny from heat-shocked hermaphrodites carrying the two arrays that are jointly needed for Mos1 mutagenesis (Williams et al. 2005) were collected and placed onto MBL containing 90% E. coli and 10% M. nematophilum and their progeny were examined for Bus (nonswollen) mutants (Table 1). From among the candidates picked, four Mos1-positive mutants exhibited a completely or largely penetrant Bus phenotype, while a fifth stable bus mutant did not carry a Mos1 insertion. The bus mutation in this strain, e2913, was found to be an allele of a known gene, bus-2. Overall, the Mos1 insertion rate into the genome was low (Table 1). However, the screening for loss of swelling and, in some cases, enhanced growth on pathogenic bacterial lawns allowed Bus mutants to be easily identified, such that screening large numbers of genomes was readily feasible.
TABLE 1.
Results of individual Mos1 screens and hop frequencies
| Screen | Transposition frequency (%) | No. of F1's | Bus allele | Notes |
|---|---|---|---|---|
| 1 | ND | 1061 | e2861 | More than one Mos1 insertion. |
| 2 | 20/57 (35) | 930 | e2912 | More than one Mos1 insertion. |
| 3 | 1/14 (7) | 1977 | e2913a | No Mos1 insertion. |
| 4 | 33/73 (45) | 1810 | e2917 | Only one insertion. |
| 5 | 1/25 (4) | 973 | e2923 | Only one insertion. |
| 6 | 11/23 (48) | 869 | No Bus mutant found. | |
| Total | 7620 |
The transposition frequency was measured as the percentage of progeny from heat-shocked double-transgenic animals that carry at least one Mos1 insertion (Williams et al. 2005). To measure the transposition frequency, F1 animals were picked blind from the progeny of heat-shocked double-transgenic P0's. For each F1 that had lost the Mos1 substrate array 5–10 F2 animals were tested by PCR for the Mos1 element. For each F1 that still carried the array, non-array-carrying F2 progeny were identified and F3 animals were tested for Mos1 insertions.
e2913 did not complement bus-2. As we already had a number of alleles of bus-2, and this strain did not contain a Mos1 insertion, we did not keep this allele for further analysis.
To identify the insertion site in the Mos1-containing strains, iPCR was performed on lysates of the mutant worms. In each case, a Mos1 insertion was identified in the coding region of a unique ORF. Mos1 insertions were identified in exon 2 of egl-8 (B0348.4), exon 4 of tax-4 (ZC84.2), and exon 2 and exon 1 of two uncharacterized ORFs, ZK678.8 on LGX and T07F10.4 on LGV, respectively (Figures 1 and 2). The close proximity and similar phenotypes of e2923 mutants to bus-17 mutants isolated previously suggested that e2923 might be an allele of bus-17. e2923 does not complement bus-17(e2800), confirming this locus assignment. Two of the four mutants, tax-4 and bus-19, had more than one insertion in their genome; however, these insertions were not in coding regions and were verified as irrelevant after loss of the insertions through outcrossing and/or mapping the Bus phenotype (see below).
Figure 1.—
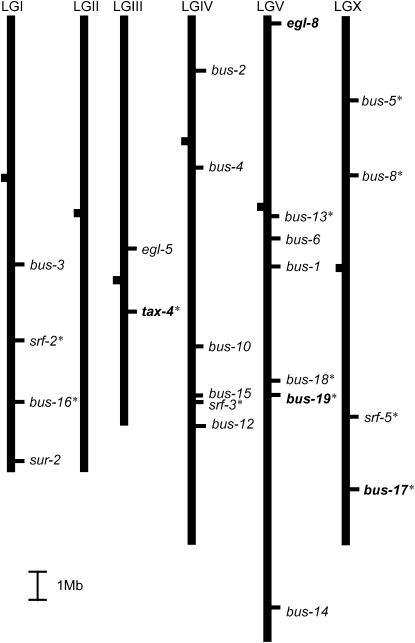
Genetic map of bus loci recovered from selections and screens for resistance to infection by M. nematophilum bacteria. The map has been scaled to the genome sequence. Alleles of genes shown in boldface type were identified in the present Mos1 mutagenesis selection. Alleles of other genes were identified in previous EMS and mut-7 screens, including bus-17 (Gravato-Nobre et al. 2005). bus-17 and bus-19 were cloned in this study. The asterisk indicates that fluorescently labeled lectins bind these mutants.
Figure 2.—
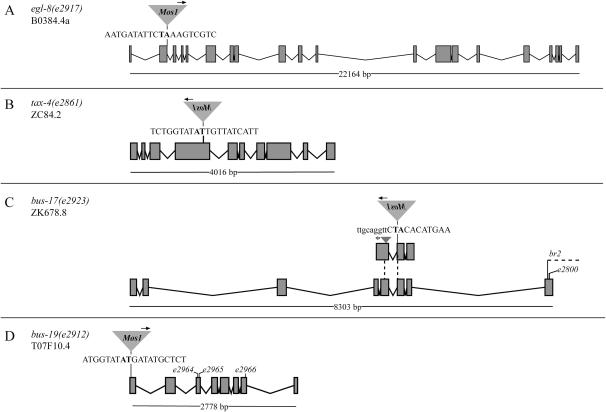
Insertion sites and polarities of Mos1 elements in bus mutants. Arrows above the Mos1 elements indicate the orientation of the Mos1 primer oJL115 used for sequencing iPCR products of mutant lysates. Uppercase letters signify exonic sequence. Gene models are taken or modified from WormBase Release WS154. The gene models are not drawn to scale with each other; the length of the ORF, however, is noted below each model. (A) egl-8(e2917) mutants have a Mos1 insertion in the second exon of B0384.4. This insertion would affect all three alternatively spliced transcripts, a, b, and c, of egl-8; only the B0384.4a transcript is modeled here. (B) tax-4(2861) mutants have a Mos1 insertion in the fourth exon of ZC84.2. (C) bus-17(e2923) mutants have an insertion in exon 2 of the WormBase (release WS154) ZK678.8 gene model (top gene model). This insertion site corresponds to exonic sequence of the sixth exon of a TWINSCAN prediction for the gene (bottom gene model). The arrowhead (asterisk) designates a Mos1 insertion in cxTi9043 mutants, which was generated by L. Segalat. The dotted lines indicate the positions of the Mos1 insertions in the corresponding TWINSCAN prediction. The br2 lesion is a 311-bp deletion that would affect the 3′ end of the bus-17 coding sequence, as denoted by the dotted line. The e2800 mutation is a missense mutation; see text and Figure 6 for more information. (D) bus-19(e2912) mutants have a Mos1 insertion in the first exon of T07F10.4. e2964, e2965, and e2966 are missense alleles of bus-19 that were isolated in this study.
Comparison of bacterial adherence of bus mutants:
Unlike several lethal pathogens of C. elegans, M. nematophilum does not colonize the intestine of the worm; instead, the vital dye SYTO 13 reveals M. nematophilum colonization in the rectum of infected worms (Gravato-Nobre et al. 2005) (Figure 3). This colonization correlates with tight adherence of the bacteria as shown by close examination of infected and extensively washed worms (Hodgkin et al. 2000). On the basis of the level of bacterial colonization relative to wild-type infected worms, the bus mutants isolated in previous screens were broadly classified into four groups (Gravato-Nobre et al. 2005). The mutants identified here also exhibit a range of rectal colonization by M. nematophilum (Figure 3). In particular, egl-8(e2917) mutants exhibit SYTO 13 bacterial staining in the distal portion of the rectum. This suggests that egl-8 is not required for the adherence of the pathogen and may therefore play a role in the postadherence swelling response. tax-4(e2861) mutants also exhibit bacterial staining; however, this staining is not consistent and is correlated with the presence of a Dar response. Although tax-4(e2861) mutants are sometimes Dar, many worms exhibit strongly attenuated swelling or are completely Bus with little or no rectal staining. No mutants reported in previous Bus screens share these exact characteristics; however, mutants that exhibit significant variability in Dar response have been little analyzed so far. The tax-4(e2861) mutation does not appear to affect the postadherence swelling response; instead, tax-4 wild-type activity appears to be variably required for the bacteria to efficiently establish a rectal infection.
Figure 3.—
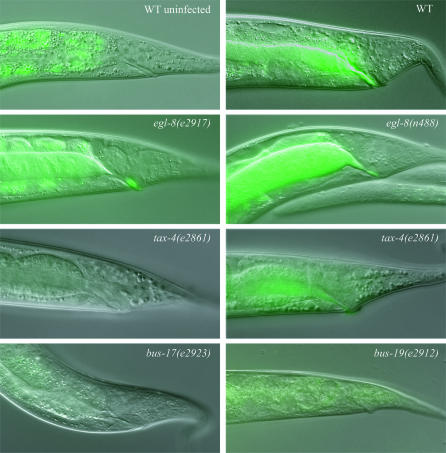
The swelling response to M. nematophilum is attenuated or absent in Bus mutants. M. nematophilum causes a deformed anal region (Dar) phenotype on infected WT worms. M. nematophilum adheres tightly to the rectal epithelium of WT worms and colonized the rectum as visualized by SYTO 13. egl-8(e2917), tax-4(e2861), bus-17(e2923), and bus-19(e2912) mutants do not swell in the presence of M. nematophilum and all mutants except egl-8 have a loss or attenuation of rectal colonization by the bacteria. Bacteria still colonize the rectum of egl-8 mutants e2917 and n488, the canonical null allele. tax-4 mutants exhibit variability in response to infection.
The other two mutants resembled the majority class of bus mutants isolated in previous screens, in that they do not exhibit any bacterial colonization and are 100% Bus. bus-17 alleles isolated in the previous screen fall into this category, as does e2923, the bus-17 allele isolated in this screen. Similarly, bus-19(e2912) mutants were invariably Bus and did not exhibit any rectal SYTO 13 bacterial staining. These observations suggest that bus-17 and bus-19 are required for bacteria to adhere to the rectal and postanal cuticle.
Cuticular defects of bus mutants:
The failure of bacteria to adhere to the rectal epithelium of bus-17(e2923) and bus-19(e2912) mutants may be due to alterations of the cuticle. A subset of the bus mutants previously identified did not show any rectal colonization of M. nematophilum and was demonstrated to have altered surface properties (Gravato-Nobre et al. 2005). Alterations in the surface properties of the worm can be observed by staining worms with labeled lectins such as Concanavalin A (ConA), wheat germ agglutinin (WGA), and soybean agglutinin (SBA) (Link et al. 1992). We examined bus-17(e2923) and bus-19(e2912) mutants for lectin binding and observed that these mutants exhibited strong lectin binding across the surface of the worm, in contrast to a lack of lectin binding by wild-type worms (Figure 4, A and C). The larvae of both bus-17 and bus-19 worms also exhibited significant levels of lectin binding, in some cases with severe disruptions in staining, signifying areas of the cuticle that had been physically damaged.
Figure 4.—
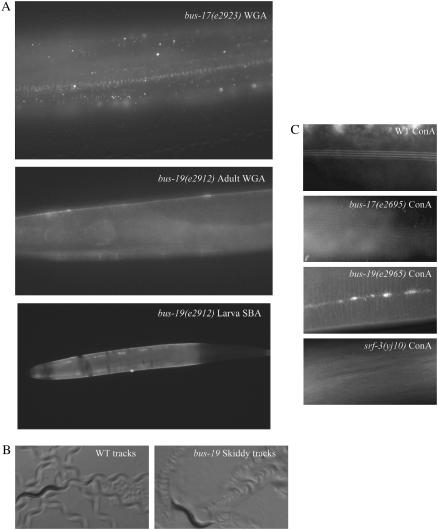
Skiddy (Skd) phenotype and cuticle defects of bus mutants. (A) Fluorescently labeled lectins bind to bus-17 and bus-19 mutant cuticles. Mutants were stained with WGA or SBA. Wild-type worms (not shown) do not exhibit cuticle lectin binding of WGA or SBA. (B) The Skd phenotype is characterized by a loss of traction on the agar. Wild-type worms propel themselves forward with little slippage, and each body bend leaves a characteristic wave pattern behind (left). bus and srf mutants are unable to propel themselves forward as easily as wild-type worms, which results in a high-frequency wave pattern in the food (right). (C) Alae, revealed by Concanavalin A (ConA) fluorescence, exhibit abnormalities in bus-19 mutants.
bus-17 and bus-19 exhibit a Skiddy (Skd) phenotype, an apparent loss of traction on the agar plate, which results in a characteristic high-frequency wave pattern in the bacterial lawn (Figure 4B). The Skd phenotype has been noted for many of the previously identified bus mutants including srf-3, bus-16, bus-17, and bus-18 (Gravato-Nobre et al. 2005). bus-19 mutants have more severe cuticle defects than bus-17 mutants. We observed that bus-19 mutants have disrupted alae, lateral ridges that run the length of the adult cuticle. ConA staining revealed the alae of wild-type worms as well as the bus mutants (this staining is most likely due to the trapping of the labeled lectin in the ridges). Although alae are distinct in wild type, bus-17, and srf-3, clearly demarcated lateral ridges in bus-19 mutants are not present. The fact that alae are present in srf-3 and bus-17 suggests that the Skd phenotype is not likely to be due to defects in these structures and that traction relies on other surface properties of the worm.
tax-4(e2861) mutants exhibited cuticle defects and lectin binding as well, but the lectin staining correlated with areas of the worm that had accumulated extra cuticle and may therefore be a result of trapped lectins rather than breaches in the cuticle (Figure 5). Further, the staining was localized to the anterior region of tax-4 mutants rather than along the whole body as observed for other bus mutants.
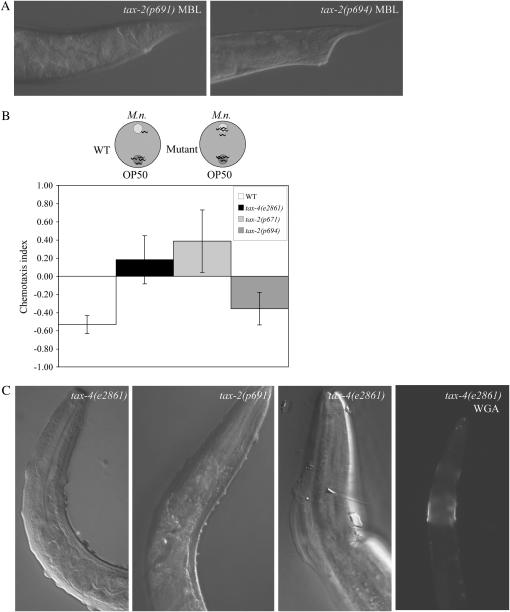
Figure 5.—
Behavioral and cuticle defects of tax-4 and tax-2 mutants. (A) Different tax-2 alleles exhibit varied responses to M. nematophilum. tax-2(p691) (left) is Bus like tax-4(e2861), whereas tax-2(p694) (right) is Dar. (B) TAX-4 is required for the avoidance behavior to M. nematophilum and for cuticle integrity. Chemotaxis index = (no. worms at M. nematophilum − no. worms at E. coli)/total no. worms. WT, N = 519, six trials; tax-4(e2861), N = 444, three trials; tax-2(p671), N = 154, two trials; tax-2(p694), N = 653, four trials). (C) tax-4 and tax-2 mutants exhibit cuticle defects when grown on E. coli, which include starvation-induced bumpy cuticles reminiscent of gherkins (left) and collars of extra cuticle, which can be highlighted by fluorescently labeled WGA lectin binding (right).
egl-8(e2917):
egl-8(e2917) mutants grow very slowly on MBL plates and are severely constipated (Con). On E. coli alone, these mutants exhibit visible egg-laying defective (Egl) and Con phenotypes. We identified only one Mos1 insertion in unoutcrossed mutants, which was an insertion in exon 2 of egl-8 (Figure 2); in further outcrosses the Bus phenotype cosegregated with the Mos1 insertion in egl-8. egl-8 encodes the β-subunit of phospholipaseC (PLCβ), which catalyzes the cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5 triphosphate (IP3) and diacyl glycerol (DAG), which are critical second messengers in cell signaling pathways (Lackner et al. 1999; Miller et al. 1999). EGL-8 is characteristic of the PLCβ family members in that it contains an N-terminal pleckstrin homology (PH) domain, an EF hand calcium-binding repeat region, X and Y catalytic domains, a C2 lipid-binding domain, and the Gqα-interacting G box (James and Downes 1997). The egl-8(e2917) insertion is predicted to disrupt the PH domain, which is required for binding to PIP2. This insertion would presumably affect all transcripts of egl-8 and is likely to be a null. We compared egl-8(e2917) mutants with worms homozygous for the egl-8(n488) null allele. egl-8(n488) mutants are also Bus, grow very slowly, and are severely Con on MBL. Further, egl-8(e2917) does not complement egl-8(n488) for the Bus phenotype. M. nematophilum bacteria adhere to distal rectal epithelium of both egl-8(e2917) and egl-8(n488) mutants but fail to elicit the swelling response (Figure 3). These mutants are severely Con so it is conceivable that the pressure inside the distended gut may force bacteria into the rectum and that bacteria leaked into the rectum may not be distinguishable from adherent bacteria by the SYTO 13 dye. However, this scenario is unlikely as egl-8 mutants fed E. coli OP50-GFP (E. coli expressing GFP), are also Con but do not exhibit patches of fluorescence in the rectum like that exhibited with SYTO 13-stained worms infected with M. nematophilum (data not shown). These observations imply that bacterial attachment factors are still present in these mutants and that the inability to swell in the presence of M. nematophilum is likely due to a defect in signaling the swelling response upon infection.
tax-4(e2861):
We identified three Mos1 insertions in a 2×-outcrossed version of e2861 Bus mutants. Two of these insertions were in intergenic regions on LGV, while a third insertion was in the fourth exon of ZC84.2 on LGIII, which encodes tax-4. We determined that the insertion of Mos1 in tax-4 was probably the relevant mutation by finding tight snpSNP linkage of e2861 to a Bristol polymorphism in F10E9, close to tax-4 on LGIII. As noted above, mutants homozygous for e2861 exhibit variability in their response to M. nematophilum. At 20°, a majority of the animals examined exhibited no or very slight swelling of their postanal region, but almost half (48 ± 8%, N = 175) of the population is Dar (distinctly swollen). Under the same 20° conditions, most N2 wild-type worms (81 ± 1%, N = 512) exhibited a Dar response. The strength of the tax-4(e2861) Dar response correlates with increased bacterial colonization, as shown by staining with SYTO 13 (Figure 3). We also looked at other tax-4 mutants and found that homozygous mutants of two other alleles of tax-4, ks11 and ks28, exhibited a variable Bus phenotype similar to e2861; for example, only 52 ± 10% (N = 311) of the tax-4(ks11) population was Dar when grown on MBL plates.
The TAX-4/TAX-2 cyclic nucleotide gated channel is required in a subset of neurons for full susceptibility to M. nematophilum:
tax-4 encodes the homolog of the cyclic nucleotide-gated (CNG) channel α-subunit (Komatsu et al. 1996). TAX-4 forms a channel with TAX-2, a homolog of the CNG channel β-subunit (Coburn and Bargmann 1996; Komatsu et al. 1999). TAX-4 and TAX-2 overlap in expression in 11 neurons and are responsible for modulating many behaviors of the worm, including chemosensation (to both soluble and volatile compounds), thermosensation, dauer formation, and social aggregation (Dusenbery et al. 1975; Hedgecock and Russell 1975; Bargmann and Horvitz 1991a,b; Bargmann et al. 1993; Komatsu et al. 1996; de Bono et al. 2002; Satterlee et al. 2004). Further, the TAX-4/TAX-2 channel acts downstream of the DAF-11 guanylate cyclase in many chemosensory behaviors and in dauer formation (Birnby et al. 2000). To test if the action of the channel is required for the swelling response, we exposed tax-2 and daf-11 mutants to the pathogen. Like tax-4(e2861) and tax-4(ks11) mutants, tax-2(p671), tax-2(p691), tax-2(ky139), and daf-11(m47) homozygotes are all variably Bus (Figure 5A). Surprisingly, tax-2(p694) worms were strongly Dar (100%, N = 124) whereas only 51 ± 10% (N = 131) of tax-2(p671) mutants were Dar. tax-2(p694) mutants carry a mutation that deletes the first exon of tax-2, resulting in altered expression of tax-2 and presumably a shorter TAX-2 product (Coburn and Bargmann 1996). This deletion in tax-2 has been demonstrated to affect TAX-2 function only in a subset of the neurons that require the TAX-4/TAX-2 channel (Coburn and Bargmann 1996). Specifically, the p694 deletion does not affect the expression or function of tax-2 in six amphid neurons, AWB, AWC, ASG, ASI, ASK, and ASJ. These neurons mediate chemosensation (AWB, AWC), taste (ASG, ASK), and dauer formation (ASI, ASJ). The fact that tax-2(p694) worms have a strong Dar response, whereas other tax-2 mutants are variably Bus, implicates one or more of these six amphid neurons in susceptibility to M. nematophilum.
TAX-4 mediates a choice between bacterial foods:
A possible explanation for the implication of tax-2 and tax-4 in the infection of worms by M. nematophilum is that these tax mutants are altered in their exposure to the pathogen, as a result of aberrant taxis behavior. C. elegans has been shown to be able to recognize and avoid other pathogens (Pujol et al. 2001). We therefore compared behavioral parameters between wild-type and mutant worms. Wild-type worms tend to avoid lawns contaminated with M. nematophilum. By contrast, tax-4(e2861) mutants can be found equally distributed inside and outside a mixed bacterial lawn. Mutants carrying a strong allele of tax-2, p671, displayed a comparable response to tax-4(e2861) (Table 2). When we challenged the worms with a direct choice between E. coli and M. nematophilum as a food source we found that wild-type worms exhibited a strong preference for E. coli. tax-4(e2861) mutants, however, exhibited no preference for one bacterium over the other (Figure 5B). tax-2(p694) mutants behaved like wild-type worms and exhibited a preference for E. coli. Comparable results were obtained in experiments using two Pseudomonas strains that are toxic to both wild-type and tax-4 mutant C. elegans, indicating that the defective avoidance behavior of tax-4 is general, rather than a result of its resistance to M. nematophilum (G. Preston and J. Hodgkin, unpublished results). As noted above, tax-2(p694) mutants have normal chemotaxis and taste functions as well as normal dauer formation regulation. These results indicate a paradoxical situation for tax-4 and tax-2 mutants: the strong tax mutants do not avoid the pathogenic food as much as wild-type worms do, yet they end up less sickened by it.
TABLE 2.
Fraction of animals in a population within a bacterial lawn
| Genotype | E. coli | M. nematophilum | Trials |
|---|---|---|---|
| WT | 0.71 (±0.09), N = 34 | 0.24 (±0.02), N = 89 | 2 |
| tax-4(e2861) | 0.72 (±0.08), N = 39 | 0.94 (±0.04), N = 52 | 3 |
| tax-2(p671) | 0.98 (±0.02), N = 38 | 0.94 (±0.06), N = 32 | 2 |
Two trials were performed for each bacterial lawn except in the case of tax-4(e2861), where three trials were done. SEM is indicated in parentheses; N, total numbers of worms tested over all trials.
TAX-4 and TAX-2 have been demonstrated to act downstream of DAF-11, a guanylate cyclase and group I dauer pathway gene (Thomas et al. 1993; Birnby et al. 2000). Like tax-4(e2861), daf-11(m47) mutants exhibit attenuated swelling in the presence of M. nematophilum. daf-11 mutants are abnormal in dauer formation and enter into dauer under nonpermissive conditions (Daf-c) at 25°, like most tax-4 and tax-2 mutants. In contrast, tax-4 and tax-2 mutants that do not have a Daf-c phenotype at 25° have a wild-type response to M. nematophilum, suggesting that defects in the entry or exit from the dauer state may be affecting the infection. However, activation or inhibition of the dauer program per se does not greatly alter the response of the worms to M. nematophilum. We assayed other dauer-regulation-defective mutants for the Dar response. Specifically, we tested both Daf-c mutants, daf-2(e1368) and daf-2(e1370), and dauer-formation-defective (Daf-d) mutants daf-16(m26) and daf-16(mgDf50). In addition to being Daf-c, daf-2(e1368) and daf-2(e1370) have recently been shown to exhibit increased resistance to killing by bacterial pathogens (Garsin et al. 2003). Specifically, daf-2(e1370) exhibited much stronger resistance than e1368 to killing by these pathogens, although e1368 still exhibited a marginal increase in resistance. e1368 is a class 1 allele of daf-2, which encompasses the Daf-c(ts), increased adult life span (Age), and increased thermotolerance (Itt) phenotypes. Class 2 alleles, such as e1370, exhibit all these phenotypes in addition to exhibiting gonad abnormalities, embryonic and L1 arrest, diminished adult body size, reduced pharyngeal pumping, and uncoordinated movement (Gems et al. 1998). The Daf-c and Age phenotypes of both class 1 and class 2 alleles can be suppressed by mutations in daf-16. daf-16 mutants are dauer defective and thus do not enter into or maintain the dauer state. Because dauer larvae do not become Dar in the presence of the pathogen, we assayed daf-2 mutants grown at 15° and shifted to 25° at L3, to avoid the temperature-sensitive Daf-c phenotype. We found that daf-2(e1368) mutants exhibited a normal Dar response to the pathogen after shifting to 25°. daf-2(e1370) mutants, on the other hand, exhibited a range of morphological phenotypes that prevent strong conclusions about the swelling response. In the case of daf-16, the inhibition of the dauer program does not alter the swelling response to the pathogen, as mutants homozygous for daf-16(m26) or daf-16(mgDf50) were 100% Dar as were wild-type worms at 25°.
tax-4 cuticle defects:
We observed that tax-4 and tax-2 mutants exhibit cuticle defects that have not been reported before. These defects include an accumulation of cuticle around the pharyngeal area of adult worms, which is present at a low level in the population (<10%) and can be observed under the dissecting scope or by staining with fluorescently labeled WGA (Figure 5C). This extra cuticle is suggestive of a molting defect. Worms that have the collars of extra cuticle cannot be tested for the swelling response to M. nematophilum since they are already adults when the collar is formed. However, the collars have been noted on Bus and Dar worms grown on infected plates at 15°. Unfortunately the swelling response of wild-type worms at 15° is variable and often attenuated, so we cannot conclude if the tax-4 mutants with molting defects are more or less susceptible to postanal swelling. In addition to these defects, tax mutant worms on starved plates exhibit an abnormal bumpy surface appearance, resembling a gherkin (Figure 5C), a phenotype that is not seen in starved wild-type worms. We interpret these observations to suggest that these mutants have subtle defects in their cuticles that impair initial bacterial adherence.
bus-17(e2923):
e2923 has a Mos1 insertion in ZK678.8 on the right arm of LGX (Figure 2). The location of the mutation responsible for the Bus phenotype was confirmed by snipSNP linkage to the Bristol polymorphism in F23D12 on LGXR. Another Mos1 insertion reported for ZK678.8, cxTi9043, was obtained from the Mos1 insertion consortium (Granger et al. 2004). This mutant is also Bus and did not complement e2923. Both mutants were rescued by the ZK678 cosmid and by a 10-kb fragment amplified from wild-type genomic DNA. The 10-kb rescuing fragment encompasses ZK678.8 and extends to the last introns of the adjacent 5′ and 3′ genes. Two EMS alleles of bus-17 (e2695 and e2800) were identified in a previous screen for bus mutants (Gravato-Nobre et al. 2005). Due to the close proximity of bus-17 to e2923 and to the similarities in phenotypes between the mutants, e.g., skiddiness and lectin binding, we performed a complementation test between these mutants. We found that e2923 does not complement bus-17(e2800). Two other alleles of bus-17 were identified in the lab of Creg Darby in a selection for mutants resistant to the accumulation of Yersinia sp. biofilm (C. Darby, personal communication). Yersinia pestis and Y. pseudotuberculosis form a biofilm on the head of C. elegans, covering the mouth of the worm and causing death by starvation (Darby et al. 2002). Two bah (biofilm absent from head) mutations, br2 and br11, failed to complement bus-17 for the Bus phenotype as well as for the Bah phenotype (C. Darby, personal communication).
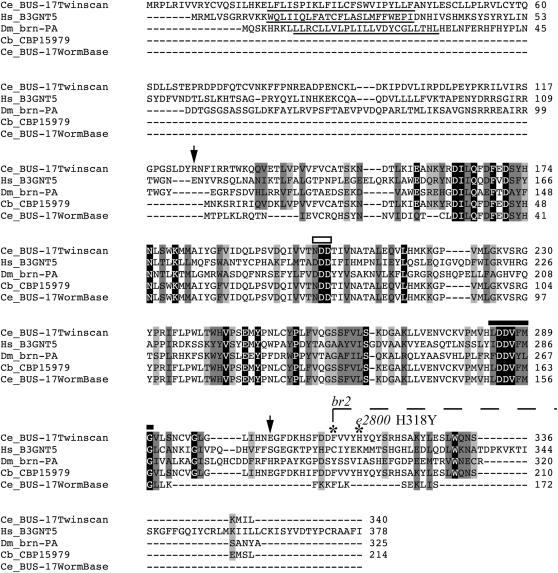
BUS-17 is predicted to be a member of the glycosyl transferase 31(GT31) family and is most closely related to the human β-1,3-N-acetylglucosaminyltransferase 5 (B3GNT5) (Figure 6). The predicted BUS-17 protein contains one conserved active site motif for this family but does not appear to have the DXD catalytic domain that is conserved in this family; instead an asparagine replaces the first aspartic acid, which is a mildly conserved substitution. The WormBase (release WS154) gene model of ZK678.8 predicted bus-17 to encode a noncanonical galactosyltransferase, which lacked the characteristic transmembrane domain. A nucleotide Blast search against the C. briggsae genome using sequences 5′ and 3′ of the C. elegans WS154 gene model revealed highly conserved sequences in both regions of CBG07766, the C. briggsae ortholog of ZK678.8. While the similar 5′ regions could be explained as conserved promoter elements, the similar 3′ region had been annotated as a coding sequence in the C. briggsae ortholog, suggesting probable omissions in the WS154 C. elegans gene model. Further, no mutations in the WS154 ZK678.8 sequence were identified in any of the non-Mos1 alleles of bus-17. Mutations in two of the alleles, e2800 and br2, were subsequently found in the conserved 3′ region, further supporting a larger gene model for ZK678.8. The obsolete gene model predicted by TWINSCAN, with additional 5′ and 3′ exons, appears to better annotate the gene (Korf et al. 2001). Finally, we verified that this gene model is correct by amplifying and sequencing bus-17 cDNA, which extended from within the first exon of the TWINSCAN prediction to the 3′-UTR.
Figure 6.—
ClustalW (v.1.83) alignment of BUS-17 with B3GALT family members. Light gray boxes signify semiconserved substitutions, dark gray boxes signify conserved substitutions, and black boxes signify identical residues. Species are as follows: Cb, Caenorhabditis briggsae; Hs, Homo sapiens; Dm, Drosophila melanogaster. Underlines denote TMHMM2 predicted TM domains for each protein. The boundaries of the BUS-17 pfam01762 galactosyltransferase domain are indicated by arrows. The DXD catalytic core motif (box) is characteristic for this family. BUS-17 and its C. briggsae ortholog have an N substitution of the first D. The N-terminal globular domain (thick line) is also conserved in family 31 galactosyltransferases. The br2 deletion lesion begins at amino acid 314 and would affect the remaining BUS-17 protein. The amino acid change caused by e2800 is noted. B3GNT5, β-1,3-N-acetylglucosaminyltransferase; Brn-PA, brainiac.
BLASTX of the TWINSCAN prediction reveals an intact galactosyltransferase, which includes an N-terminal transmembrane domain as well as an additional C-terminal sequence, which is altered in mutants that carry the bus-17(e2800) or bus-17(br2) mutations (Figures 2 and 6).
bus-19(e2912):
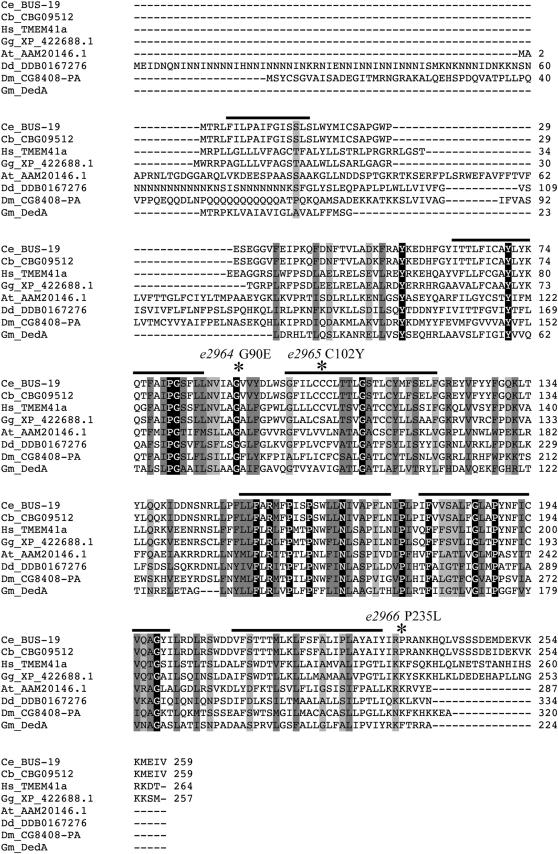
In addition to being Bus and Skd, bus-19(e2912) homozygous mutants are mild dumpy (Dpy), slow growing (Gro), hypersensitive to bleach, dauer defective (Daf-d), and segregate arrested larvae with rod-like lethality (<10%). Two Mos1 insertions were identified in an outcrossed bus-19(e2912) mutant, one in exon 1 of T07F10.4 on LGV and another in an intergenic region upstream of F54F12.2 on LGIII. In further outcrosses, the Bus phenotype was found to segregate only with the T07F10.4 insertion. We rescued bus-19 mutants with overlapping cosmids T07F10 and R90, but cosmid T07F10 alone did not rescue bus-19(e2912). Since T07F10.4 is at the end of the cosmid, it was possible that the end of T07F10.4 was deleted from the cosmid. The overlapping cosmid, R90, is likely to include most or all of the T07F10.4 open reading frames; however, it was not able to rescue the Bus phenotype of bus-19 mutants. Surprisingly, R90 did rescue the morphological Dpy and Skd phenotypes of bus-19(e2912). The two cosmids together fully rescued all phenotypes. This suggests that part of bus-19 is deleted from the T07F10 cosmid and that this missing region was provided by the R90 cosmid. Alternatively, bus-19(e2912) could have been another very close mutation, which is not tagged by the Mos1 transposon, but is responsible for the Bus phenotype. This possible scenario was supported by RNAi results in that knockdown of T07F10.4 in both N2 and rrf-3 mutant worms phenocopies the Dpy and Skd phenotypes of bus-19(e2912), but fails to phenocopy the Bus phenotype when the worms are exposed to M. nematophilum.
To clarify this situation, three more bus-19 alleles, e2964, e2965, and e2966, were recovered in a noncomplementation screen for the Bus phenotype. bus-19(e2964) is the most similar of the three new alleles to bus-19(e2912). bus-19(e2965) is variably Dpy but is a strong Bus mutant and is strong Skd. bus-19(e2966), however, is non-Dpy, non-Skd, and only weakly Bus. The Bus phenotype is stronger in a trans-heterozygote, genotype bus-19(e2912/e2966) although such heterozygotes still exhibit weak swelling. As with bus-19(e2912) mutants, all mutants were rescued by the simultaneous introduction of T07F10 and R90 cosmids; however, bus-19(e2966) mutants were rescued by the R90 cosmid alone. The three new alleles were sequenced and in all cases, changes were identified in the exonic sequence of T07F10.4 (Figure 7). Two alleles, bus-19(e2964) and bus-19(e2965), have missense mutations in exon 3 resulting in a G90E and a C102Y amino acid change, respectively. The G90E change in bus-19(e2964), the strongest mutation, affects a highly conserved residue. The bus-19(e2965) mutation affects a mildly conserved residue. The weak mutation, bus-19(e2966), has a missense mutation in exon 7, which causes a P235L amino acid change in a less conserved residue. These results indicate that bus-19 is indeed T07F10.4. The failure of RNAi experiments to phenocopy the Bus phenotype can be ascribed to the low efficiency of RNAi with respect to postembryonic phenotypes, which we have also observed with other bus genes. bus-19(e2964) was fully rescued with the fosmid clone WRM0640cD03. We achieved partial rescue of bus-19(e2964) with both a 7.3- and a 13-kb fragment amplified from fosmid clone WRM0623dE01, which spanned T07F10.4a and T07F10.4a and -b, respectively. This partial rescue corrected the Dpy and Skd phenotypes in both cases; however, the transgenic animals were still Bus on the pathogen. We confirmed the exon/intron boundaries of both T07F10.4a and T07F10.4b by sequencing two cDNA clones, yk1387f02 and yk1345b03, respectively, which were provided by Y. Kohara.
Figure 7.—
ClustalW (v.1.83) alignment of bus-19 and homologs. Light gray boxes signify semiconserved substitutions, dark gray boxes signify conserved substitutions, and black boxes signify identical residues. Species are as follows: Cb, Caenorhabditis briggsae; Hs, Homo sapiens; Gg, Gallus gallus; At, Arabidopsis thaliana; Dd, Dictyostelium discoideum; Dm, Drosophila melanogaster; Gm, Geobacter metallireducens. Bars above the sequence indicate transmembrane domains predicted for BUS-19 by TMHMM2. The amino acid changes caused by EMS in other bus-19 alleles are noted above the sequence.
bus-19 encodes a novel ancient protein:
BUS-19 belongs to the KOG3140/COG0398/DedA predicted membrane protein homology group, members of which exist in all taxa except Archaea and Viruses. No functional information is available for any homolog. The DedA domain occurs in many proteins concurrently with other well-characterized domains, such as a phospholipase D motif and a GlpE rhodanese sulfurtransferase domain. Although DedA-containing proteins are suspected to have transporter function, possibly involved in detoxification processes, the BUS-19/DedA group does not contain any characterized domain (Marchler-Bauer and Bryant 2004; Ledgham et al. 2005). ProtFun 2.2 (http://www.cbs.dtu.dk/services/ProtFun/) also predicts a transporter function for BUS-19. Other identifiable protein features of BUS-19 include six transmembrane domains (including a probable signal sequence) and a putative ER localization motif. Other members of KOG3140 include hypothetical proteins in human (Q96HV5), Drosophila (Q9VX39), Saccharomyces (P36164), and two C. elegans predicted proteins O62126 (tag-175; ORF D2013.10) and Q9XXB4 (ORF Y71A12C.2) (Figure 7). The C. elegans homologs of bus-19, tag-175, and Y71A12C.2 are located on LGII and LGI, respectively. The expression pattern and RNAi effects have been analyzed for tag-175; however, these analyses have not led to any further knowledge of its function (Henricson et al. 2004). We also examined tag-175(gk278), which is a putative null for this bus-19 homolog, and observed none of the multiple abnormal phenotypes of bus-19 mutants, including the Bus phenotype in the presence of M. nematophilum, suggesting that tag-175 is a less important member of this family.
DISCUSSION
Practicality of Mos1 mutagenesis:
Infection of C. elegans by the gram-positive bacterium M. nematophilum results in a defensive postanal swelling response through activation of an ERK/MAPK signaling pathway (Nicholas and Hodgkin 2004). Over 20 loci are required for infection and/or tail swelling, as revealed in previous EMS and mut-7 screens, which do not appear to have reached saturation (Gravato-Nobre et al. 2005). Three of these genes, egl-5, sur-2, and srf-3 were known from earlier unrelated studies, having been characterized for their roles in posterior cell fate, vulval development, and surface properties of the worm, respectively (Chisholm 1991; Link et al. 1992; Singh and Han 1995). To quickly identify more genes affecting the C. elegans/M. nematophilum interaction, we carried out screens using Mos1 mutagenesis. Mos1 insertions in four genes (egl-8, tax-4, bus-19, and bus-17) were found to result in a Bus (nonswollen tail) phenotype. Of the 19 bus loci identified in previous EMS and mut-7 mutagenesis screens, an allele of only one locus, bus-17, was identified here, which provides further indication that screens for Bus mutants are far from saturated.
Using Mos1 mutagenesis was therefore an attractive option, due to the large number of genes that can be mutated to resistance to M. nematophilum (Gravato-Nobre et al. 2005). At the time these screens were carried out, the rate of Mos1 mutagenesis was estimated to be significantly lower than that of EMS mutagenesis. Confirming this, we recovered Bus mutants at a much lower rate with Mos1 than with EMS. A clonal screen for EMS Bus mutants formed part of the previous study (Gravato-Nobre et al. 2005); this yielded six mutants among 639 F2 clones, or approximately one mutant for every 30 F1 animals. The overall efficiency of our Mos1 screens, yielding a total of four mutants for 7620 F1 animals, was ∼50-fold lower than this. This efficiency is comparable to that reported for a direct comparison of Mos1 mutagenesis with ENU mutagenesis to identify Osm mutants (Bessereau et al. 2001).
A previously published pilot experiment using Mos1 mutagenesis discusses the advantages of using Mos1 (Granger et al 2004). Although we experienced low efficiency and substantial variability in the rate of Mos1 transposition, these advantages still remain worthwhile. The variability may be due to a number of factors, such as the ancestry and the growth history of the double-array-carrying P0's, temperature fluctuations during the heat-shock procedure, or time of collection of animals after heat shock (Williams et al. 2005). However, since detection of the Bus phenotype is easy, and the number of target genes is quite high, the low efficiency of mutagenesis was offset by the ease and swiftness of identifying the molecular insertions, as demonstrated here by the case of bus-17. Further, two mutations, egl-8 and bus-19 severely impair the growth of the worm on the pathogen and thus would have been difficult to recover and map in previous screens, but were quickly cloned due to the presence of the Mos1 molecular tag.
Different stages of the host–pathogen interaction:
The genes identified in this and previous studies highlight the different defenses and vulnerabilities of C. elegans with respect to M. nematophilum. First, C. elegans is capable of recognizing and mounting a behavioral avoidance response to the presence of M. nematophilum in the environment. We show that this behavioral response involves the action of TAX-4 and TAX-2. C. elegans has been shown to mount a behavioral response to other pathogens, which includes learning to avoid them (Pujol et al. 2001; Zhang et al. 2005). Further, TAX-4 and TAX-2 are involved in the recognition of pathogenic Pseudomonas strains as well, suggesting that they play a general role in pathogen recognition and avoidance. tax-4 and tax-2 mutants are defective in many behaviors; in particular, chemosensory and gustatory behaviors are compromised (Dusenbery et al. 1975; Bargmann et al. 1993; Komatsu et al. 1996). However, this role of TAX-4 and TAX-2 in mediating avoidance to M. nematophilum does not explain the reduced infection and swelling of tax-4 and tax-2 mutants, because these tax mutants expose themselves to the contaminated food more than wild-type worms do and would therefore be expected to become sicker than wild-type worms, rather than the reverse. We observed other defects of tax-4 and tax-2 mutants, which suggest that these genes can affect the cuticle or surface composition of the worm, as discussed below.
Second, successful infection by M. nematophilum must involve elements of the rectal epithelial surface of C. elegans that can be recognized and adhered to by the pathogen. Colonization fails to occur in bus-17 and bus-19 mutants, suggesting that these surface factors are absent or masked in mutants of this type, perhaps as a result of alterations in cuticle formation. In support of this possibility, we observed cuticle defects in these mutants. The nematode cuticle is a multilayered structure composed of heavily cross-linked collagen and collagen-like molecules, overlaid by an electron-dense epicuticle and topped by a surface coat (glycocalyx) of carbohydrates. The carbohydrates of the surface coat probably provide major targets for pathogens. Cuticle and surface coat composition are molecularly distinct at each developmental stage of the worm (Cox et al. 1981). Further, changes in surface coat composition can be induced by environmental factors (Grenache et al. 1996).
bus-17 and bus-19 mutants exhibit cuticle defects similar to other bus mutants in the srf-3 mutant group (Gravato-Nobre et al. 2005). In particular, bus-17 and bus-19 mutants are Skd, do not allow bacterial adherence by M. nematophilum, and bind fluorescently labeled lectins, consistent with alterations in surface coat composition. srf-3 encodes a nucleotide sugar transporter, the activity of which is required in the synthesis of many glycoconjugates of the worm (Cipollo et al. 2004; Hoflich et al. 2004). In addition to preventing the adherence of M. nematophilum to the rectal epithelium of the worm, mutations in srf-3 also inhibit the accumulation of Yersinia biofilm (Darby et al. 2002; Tan and Darby 2004). Since M. nematophilum does not adhere to the head of the worm, it is unlikely that the same receptors are used by both pathogens. Most likely, the different receptors used by the pathogens are subject to the same glycosylation pathways. BUS-17 is predicted to encode a member of the GT31 family. There are 22 predicted C. elegans GT31 family members. Other members of this family include BRE-5 and BRE-2, which are required for the post-translational modification of the CRY5B receptor recognized by the Bacillus thuringiensis CRY5B toxin. Upon binding to this receptor, Bt toxin forms holes in the intestine of the worm, leading to death (Griffitts et al. 2001, 2003). Neither bre-2 nor bre-5 mutants appear to affect cuticular properties or susceptibility to M. nematophilum. Nevertheless, mutations in these two genes can affect many divergent processes, indicating functions beyond the modification of intestinal glycolipids (Katic et al. 2005).
Mutant defects are notably more severe in bus-19 than in bus-17. In addition to the strong lectin staining and Skd phenotypes, bus-19 mutants exhibit slow growth, dumpiness, and larval lethality and are Daf-d. Because of the pleiotropic nature of the defects of bus-19 mutants, it is likely that BUS-19 plays a more fundamental role in generating the cuticle of the worm and perhaps is involved in other processes. The biochemical functions of BUS-19 are entirely mysterious at present, despite the existence of related and equally mysterious proteins in many other organisms. It seems likely that these are integral membrane proteins, and the surface alterations we observe suggest that BUS-19 may be involved in trafficking or modification of extracellular molecules, but little more can be said. The C. elegans bus-19 mutant phenotypes represent the first reports of abnormalities associated with loss of a protein in this family in any organism.
tax-4 mutants also affect colonization by M. nematophilum, and the observations of cuticle abnormalities in both tax-2 and tax-4 mutants indicate that these genes influence the property of the nematode surface, but their Bus and cuticle phenotypes are variable, in contrast to the fully penetrant effects of strong mutations on bus-17 and bus-19. Moreover, the relevant action of these genes appears to be located in chemosensory neurons, which are unlikely to be directly involved in cuticle synthesis. We propose that TAX-4 and TAX-2 help to coordinate the secretion of components of the cuticle with the proper developmental age of the worm and that in tax-4 and tax-2 mutants, the impaired coordination results in abnormal cuticle that is less able to support infection. It is known that TAX-4 and TAX-2 play roles in determining entry and recovery from dauer formation, and changing to and from the dauer state requires alterations in metabolism as well as changes in the structure and composition of the cuticle and surface coat. tax-4 and tax-2 mutants exhibit visible and variable abnormalities in their cuticles, which may indicate more subtle defects that influence pathogen adherence. One aspect of the dauer decision that has not been fully explored is the signaling from neuronal to seam cells to coordinate the secretion of cuticle or coat components with the developmental stage of the worm. Changes in surface coat composition can be induced by environmental factors, in part through signaling by daf-c genes (Grenache et al. 1996). Mutations in daf-c genes, including daf-11, result in the expression of L1-specific antigens in older-stage animals. Further, it has been noted that surface antigen switching was reduced in tax-4(p678) mutants, suggesting a role for TAX-4 in mediating sensory input to cuticle output (Olsen et al. 2006).
We did not observe dauer-constitutive daf-2(e1368) mutants to have an altered response to M. nematophilum, in contrast to the variable Bus phenotype seen for Daf-c daf-11(m47) mutants, but the mutant cuticles may well be different in the two cases, such that daf-2(e1368) mutants remain fully susceptible to infection. Genes of the DAF-2 insulin/IGF group of the dauer pathway have also been demonstrated to influence the response to lethal pathogens, in part by affecting production of antimicrobial proteins. Specifically, mutations in daf-2 and age-1 result in enhanced resistance to killing by Pseudomonas aeruginosa, Enterococcus faecalis, and Staphylococcus aureus (Garsin et al. 2003). The DAF-2 insulin-like receptor regulates the expression of antimicrobial effectors, such as lys-7 and lys-8 (which encode lysozymes), and the saposin-like gene, spp-1, by derepressing the fork-head transcription factor DAF-16 (Murphy et al. 2003). In daf-2 mutants therefore, DAF-16 upregulates the transcription of a number of genes with proposed antimicrobial activities. We did not see any reduced infection or swelling of daf-2(e1368) mutants exposed to M. nematophilum at room temperature or at 25°. These results suggest that the induced upregulation of DAF-16 antimicrobials in daf-2 mutants is not protective against M. nematophilum. daf-16(m26) and daf-16(mgDf50) mutants survive well on the pathogen unlike hypersensitive sur-2 and other ERK/MAP kinase mutants, which suggests that DAF-16 does not play a major protective role against M. nematophilum infection. This may not be surprising since daf-16 mutants were not hypersensitive to killing by lethal pathogens in the study mentioned above (Garsin et al. 2003). Moreover, DAF-16 cannot be the only transcription factor contributing to the expression of antimicrobials such as lysozymes, because lys-7 mutants are severely affected by M. nematophilum, surviving exposure to the pathogen much less well than daf-16 mutants (O'Rourke et al. 2006).
Third, C. elegans induces a defensive response to the adherence of M. nematophilum to rectal epithelia and colonization of the rectum, which includes major swelling of rectal hypodermal cells. Previous work has demonstrated this swelling response to require the action of the members of the ERK/MAP kinase cascade. EGL-8 is most likely to function in this step of the pathogen response. Mutations in egl-8 result in little or no rectal swelling in infected worms despite bacterial colonization of the rectum. Although EGL-8 is expressed in most of the neurons of the worm, it is also expressed in the posterior intestine (Lackner et al. 1999; Miller et al. 1999). Recent studies have also indicated that EGL-8 functions in pharyngeal pumping, sperm transfer, and Ca2+ oscillation-associated posterior body contractions of the intestine, suggesting that it may function in nonneuronal tissues (Bastiani et al. 2003; Steger and Avery 2004; Espelt et al. 2005; Gower et al. 2005). Whether or not EGL-8 feeds into the ERK/MAP kinase cascade or acts in a parallel pathway remains to be determined. Moreover, egl-8 mutants are not as hypersensitive to M. nematophilum as are ERK/MAP kinase mutants, indicating that the kinase cascade probably contributes to defense in more ways than simply activating rectal swelling.
The analysis of these four mutants reveals the complexity of the interactions between host and pathogen and the multiple contributions of various genes both to immunity and to the normal development and behavior of the worm. If, as seems likely, there has been significant coevolution of C. elegans and M. nematophilum, such complexity is only to be expected.
Acknowledgments
We thank the Caenorhabditis Genetics Center for many strains, Ikue Mori for tax-4 mutants, Cori Bargmann for tax-2 mutants, Mario de Bono for the rescuing tax-4∷GFP and odr-4p∷tax-4∷GFP constructs, Yuji Kohara for cDNA clones, Don Moerman and Jaryn Perkins for fosmid clones, Gail Preston for Pseudomonas strains, members of the André Furger lab for help with the bus-17 molecular characterization, and anonymous reviewers for constructive criticisms. Jean-Louis Bessereau provided essential guidance with Mos1 mutagenesis, Creg Darby generously shared unpublished results on Yersinia, and Chris Ponting supplied useful thoughts on BUS-19. Members of the Hodgkin and Darby labs provided invaluable expertise and discussion. K.Y. was supported in part by a Ruth L. Kirschstein National Research Service Award from the National Institutes of Health. This work was supported by the Medical Research Council (United Kingdom).
References
- Baba, T., T. Shimizu, Y. Suzuki, M. Ogawara, K. Isono et al., 2005. Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice. J. Biol. Chem. 280: 16417–16426. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., and H. R. Horvitz, 1991. a Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., and H. R. Horvitz, 1991. b Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science 251: 1243–1246. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., E. Hartwieg and H. R. Horvitz, 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527. [DOI] [PubMed] [Google Scholar]
- Bastiani, C. A., S. Gharib, M. I. Simon and P. W. Sternberg, 2003. Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCbeta-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics 165: 1805–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessereau, J. L., A. Wright, D. C. Williams, K. Schuske, M. W. Davis et al., 2001. Mobilization of a Drosophila transposon in the Caenorhabditis elegans germ line. Nature 413: 70–74. [DOI] [PubMed] [Google Scholar]
- Birnby, D. A., E. M. Link, J. J. Vowels, H. Tian, P. L. Colacurcio et al., 2000. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics 155: 85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter, M. L., and D. Bird, 1997. Parasitic nematodes, pp. 851–878 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Plainview, NY. [PubMed]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm, A., 1991. Control of cell fate in the tail region of C. elegans by the gene egl-5. Development 111: 921–932. [DOI] [PubMed] [Google Scholar]
- Cipollo, J. F., A. M. Awad, C. E. Costello and C. B. Hirschberg, 2004. srf-3, a mutant of Caenorhabditis elegans, resistant to bacterial infection and to biofilm binding, is deficient in glycoconjugates. J. Biol. Chem. 279: 52893–52903. [DOI] [PubMed] [Google Scholar]
- Coburn, C. M., and C. I. Bargmann, 1996. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17: 695–706. [DOI] [PubMed] [Google Scholar]
- Cox, G. N., S. Staprans and R. S. Edgar, 1981. The cuticle of Caenorhabditis elegans. II. Stage-specific changes in ultrastructure and protein composition during postembryonic development. Dev. Biol. 86: 456–470. [DOI] [PubMed] [Google Scholar]
- Darby, C., J. W. Hsu, N. Ghori and S. Falkow, 2002. Caenorhabditis elegans: plague bacteria biofilm blocks food intake. Nature 417: 243–244. [DOI] [PubMed] [Google Scholar]
- de Bono, M., D. M. Tobin, M. W. Davis, L. Avery and C. I. Bargmann, 2002. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 419: 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenbery, D. B., R. E. Sheridan and R. L. Russell, 1975. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics 80: 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelt, M. V., A. Y. Estevez, X. Yin and K. Strange, 2005. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J. Gen. Physiol. 126: 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gami, M. S., and C. A. Wolkow, 2006. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell 5: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin, D. A., J. M. Villanueva, J. Begun, D. H. Kim, C. D. Sifri et al., 2003. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300: 1921. [DOI] [PubMed] [Google Scholar]
- Gems, D., A. J. Sutton, M. L. Sundermeyer, P. S. Albert, K. V. King et al., 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower, N. J., D. S. Walker and H. A. Baylis, 2005. Inositol 1,4,5-trisphosphate signaling regulates mating behavior in Caenorhabditis elegans males. Mol. Biol. Cell 16: 3978–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger, L., E. Martin and L. Segalat, 2004. Mos as a tool for genome-wide insertional mutagenesis in Caenorhabditis elegans: results of a pilot study. Nucleic Acids Res. 32: e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravato-Nobre, M. J., and J. Hodgkin, 2005. Caenorhabditis elegans as a model for innate immunity to pathogens. Cell Microbiol. 7: 741–751. [DOI] [PubMed] [Google Scholar]
- Gravato-Nobre, M. J., H. R. Nicholas, R. Nijland, D. O'Rourke, D. E. Whittington et al., 2005. Multiple genes affect sensitivity of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics 171: 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenache, D. G., I. Caldicott, P. S. Albert, D. L. Riddle and S. M. Politz, 1996. Environmental induction and genetic control of surface antigen switching in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93: 12388–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffitts, J. S., J. L. Whitacre, D. E. Stevens and R. V. Aroian, 2001. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science 293: 860–864. [DOI] [PubMed] [Google Scholar]
- Griffitts, J. S., D. L. Huffman, J. L. Whitacre, B. D. Barrows, L. D. Marroquin et al., 2003. Resistance to a bacterial toxin is mediated by removal of a conserved glycosylation pathway required for toxin-host interactions. J. Biol. Chem. 278: 45594–45602. [DOI] [PubMed] [Google Scholar]
- Hedgecock, E. M., and R. L. Russell, 1975. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 72: 4061–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricson, A., E. L. Sonnhammer, D. L. Baillie and A. V. Gomes, 2004. Functional characterization in Caenorhabditis elegans of transmembrane worm-human orthologs. BMC Genomics 5: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, J., P. E. Kuwabara and B. Corneliussen, 2000. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr. Biol. 10: 1615–1618. [DOI] [PubMed] [Google Scholar]
- Hoflich, J., P. Berninsone, C. Gobel, M. J. Gravato-Nobre, B. J. Libby et al., 2004. Loss of srf-3-encoded nucleotide sugar transporter activity in Caenorhabditis elegans alters surface antigenicity and prevents bacterial adherence. J. Biol. Chem. 279: 30440–30448. [DOI] [PubMed] [Google Scholar]
- James, S. R., and C. P. Downes, 1997. Structural and mechanistic features of phospholipases C: effectors of inositol phospholipid-mediated signal transduction. Cell Signal 9: 329–336. [DOI] [PubMed] [Google Scholar]
- Jannson, H. B., 1994. Adhesion of conidia of Drechmeria coniospora to Caenorhabditis elegans wild type and mutants. J. Nematol. 26: 430–435. [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. F., 1999. Synthesis of oligosaccharides by bacterial enzymes. Glycoconj. J. 16: 141–146. [DOI] [PubMed] [Google Scholar]
- Joshua, G. W., A. V. Karlyshev, M. P. Smith, K. E. Isherwood, R. W. Titball et al., 2003. A Caenorhabditis elegans model of Yersinia infection: biofilm formation on a biotic surface. Microbiology 149: 3221–3229. [DOI] [PubMed] [Google Scholar]
- Katic, I., L. G. Vallier and I. Greenwald, 2005. New positive regulators of lin-12 activity in Caenorhabditis elegans include the BRE-5/Brainiac glycosphingolipid biosynthesis enzyme. Genetics 171: 1605–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon, C., J. Chang, E. Gensch, A. Rudner and R. Tabtiang, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464. [DOI] [PubMed] [Google Scholar]
- Komatsu, H., I. Mori, J. S. Rhee, N. Akaike and Y. Ohshima, 1996. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17: 707–718. [DOI] [PubMed] [Google Scholar]
- Komatsu, H., Y. H. Jin, N. L'Etoile, I. Mori, C. I. Bargmann et al., 1999. Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res. 821: 160–168. [DOI] [PubMed] [Google Scholar]
- Korf, I., P. Flicek, D. Duan and M. R. Brent, 2001. Integrating genomic homology into gene structure prediction. Bioinformatics 17(Suppl. 1): S140–S148. [DOI] [PubMed] [Google Scholar]
- Lackner, M. R., S. J. Nurrish and J. M. Kaplan, 1999. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335–346. [DOI] [PubMed] [Google Scholar]
- Ledgham, F., B. Quest, T. Vallaeys, M. Mergeay and J. Coves, 2005. A probable link between the DedA protein and resistance to selenite. Res. Microbiol. 156: 367–374. [DOI] [PubMed] [Google Scholar]
- Link, C. D., M. A. Silverman, M. Breen, K. E. Watt and S. A. Dames, 1992. Characterization of Caenorhabditis elegans lectin-binding mutants. Genetics 131: 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer, A., and S. H. Bryant, 2004. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 32: W327–W331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza de Gives, P., K. G. Davies, M. Morgan and J. M. Behnke, 1999. Attachment tests of Pasteuria penetrans to the cuticle of plant and animal parasitic nematodes, free living nematodes and srf mutants of Caenorhabditis elegans. J. Helminthol. 73: 67–71. [DOI] [PubMed] [Google Scholar]
- Miller, K. G., M. D. Emerson and J. B. Rand, 1999. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron 24: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet, A. C., and J. J. Ewbank, 2004. Immunity in Caenorhabditis elegans. Curr. Opin. Immunol. 16: 4–9. [DOI] [PubMed] [Google Scholar]
- Murphy, C. T., S. A. McCarroll, C. I. Bargmann, A. Fraser, R. S. Kamath et al., 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–283. [DOI] [PubMed] [Google Scholar]
- Nicholas, H. R., and J. Hodgkin, 2004. The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr. Biol. 14: 1256–1261. [DOI] [PubMed] [Google Scholar]
- Olsen, D. P., D. Phu, L. J. Libby, J. A. Cormier, K. M. Montez et al., 2006. Chemosensory control of surface antigen switching in the nematode Caenorhabditis elegans. Genes Brain Behav. (in press). [DOI] [PubMed]
- O'Rourke, D., D. Baban, M. Demidova, R. Mott and J. Hodgkin, 2006. Genomic clusters, putative pathogen recognition molecules and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 16: 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol, N., E. M. Link, L. X. Liu, C. L. Kurz, G. Alloing et al., 2001. A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr. Biol. 11: 809–821. [DOI] [PubMed] [Google Scholar]
- Satterlee, J. S., W. S. Ryu and P. Sengupta, 2004. The CMK-1 CaMKI and the TAX-4 cyclic nucleotide-gated channel regulate thermosensory neuron gene expression and function in C. elegans. Curr. Biol. 14: 62–68. [DOI] [PubMed] [Google Scholar]
- Schulenburg, H., C. L. Kurz and J. J. Ewbank, 2004. Evolution of the innate immune system: the worm perspective. Immunol. Rev. 198: 36–58. [DOI] [PubMed] [Google Scholar]
- Sifri, C. D., J. Begun and F. M. Ausubel, 2005. The worm has turned—microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13: 119–127. [DOI] [PubMed] [Google Scholar]
- Singh, N., and M. Han, 1995. sur-2, a novel gene, functions late in the let-60 ras-mediated signaling pathway during Caenorhabditis elegans vulval induction. Genes Dev. 9: 2251–2265. [DOI] [PubMed] [Google Scholar]
- Steger, K. A., and L. Avery, 2004. The GAR-3 muscarinic receptor cooperates with calcium signals to regulate muscle contraction in the Caenorhabditis elegans pharynx. Genetics 167: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, L., and C. Darby, 2004. A movable surface: formation of Yersinia sp. biofilms on motile Caenorhabditis elegans. J. Bacteriol. 186: 5087–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar, M., A. Bartke and A. Antebi, 2003. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351. [DOI] [PubMed] [Google Scholar]
- Thomas, J. H., D. A. Birnby and J. J. Vowels, 1993. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics 134: 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks, S. R., C. J. de Vries, H. G. van Luenen and R. H. Plasterk, 2000. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev. Biol. 221: 295–307. [DOI] [PubMed] [Google Scholar]
- Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterston and R. H. Plasterk, 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164. [DOI] [PubMed] [Google Scholar]
- Williams, D. C., T. Boulin, A. F. Ruaud, E. M. Jorgensen and J. L. Bessereau, 2005. Characterization of Mos1-mediated mutagenesis in Caenorhabditis elegans: a method for the rapid identification of mutated genes. Genetics 169: 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem, J., T. Gu and M. Han, 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., H. Lu and C. I. Bargmann, 2005. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438: 179–184. [DOI] [PubMed] [Google Scholar]