Abstract
Female differentiation of Drosophila germ cells is induced by cell-nonautonomous signals generated in the gonadal soma that work with germ-cell-autonomous signals determined by germ-cell X chromosome dose. Generation of the nonautonomous feminizing signals was known to involve female-specific protein encoded by the master sex-determination gene Sex-lethal (Sxl) acting on its switch-gene target transformer (tra) to produce TraF protein. However, it was not known whether Sxl's action on tra alone would suffice to trigger a fully feminizing nonautonomous signal. We developed a constitutively feminizing tra transgene that allowed us to answer this question. In gynanders (XX//XO mosaics) feminized by this TraF transgene, functionally Sxl− haplo-X (chromosomally male) somatic cells collaborated successfully with diplo-X (chromosomally female) germ cells to make functional eggs. The fertility of such gynanders shows not only that TraF is sufficient to elicit a fully feminizing nonautonomous signal, but also that haplo-X somatic cells can execute all other somatic functions required for oogenesis, despite the fact that their genome is not expected to be dosage compensated for such diplo-X-specific functions. The unexpected observation that some TraF-feminized gynanders failed to lay their eggs showed there to be diplo-X cells outside the gonad for which TraF-feminized haplo-X cells cannot substitute.
THE defining distinction between females and males is the remarkably different investment that they make in their gametes, arguably the most sexually dimorphic animal cell type. For the model organism Drosophila melanogaster, much is known at the molecular level about how somatic cells choose between female and male alternative developmental pathways, but far less is known about how germ cells make the analogous choice to generate eggs vs. sperm. This asymmetry in understanding stems from the fact that so little of what has been learned about Drosophila somatic sex determination applies to the fly's germ cells. Moreover, the differences between somatic and germline sex determination make germ cells the less experimentally tractable system for sex-determination studies.
Unlike germ cells, most somatic cells autonomously choose their sex very early in development by transiently counting their X chromosomes to initiate a heritable developmental commitment to the appropriate sexual pathway (reviewed in Cline and Meyer 1996). Sex-lethal (Sxl) is the master switch gene that counts X chromosomes and maintains the resulting sexual commitment. The two X chromosomes in chromosomally female cells generate a level of X-chromosome signal element (XSE) gene products that triggers engagement of a positive feedback loop for Sxl pre-mRNA splicing, thereby locking Sxl into an actively feminizing expression mode that generates and is maintained by female-specific Sxl protein (SxlF). Because the level of XSE proteins generated by the single X chromosome in chromosomally male cells is not sufficient to trigger this autoregulatory circuit, Sxl remains in a passively masculinizing state by default, and no SxlF is produced. SxlF elicits female somatic sexual differentiation by directing the alternative splicing of transcripts from the switch gene transformer (tra) so that a female-specific mRNA encoding the actively feminizing protein TraF is produced. TraF then acts on multiple downstream targets to elicit their female-specific expression. Since male cells lack SxlF, all their tra transcripts are processed into mRNA encoding a nonfunctional protein, leaving the downstream regulatory targets of tra in their male expression mode by default. TraF can feminize only in the presence of its protein partner, Tra2, which is made in both sexes. Unlike TraF, SxlF also controls the vital process of X chromosome dosage compensation. Diplo-X individuals require SxlF to block the hyperactivation of dosage-compensated X-linked genes. That hyperactivation, which occurs only in the absence of SxlF, enables haplo-X individuals to match the level of X-linked gene products generated by diplo-X cells (reviewed in Meller 2000; Straub et al. 2005; Wilhelm and Smilbert 2005). Because Sxl controls dosage compensation but tra does not, somatic expression of Sxl in a sexual mode that is not matched to the number of X chromosomes is lethal, while such sexually inappropriate expression of tra is not.
Drosophila germ cells acquire their sexual identity in a remarkably different way (reviewed in Oliver 2002). Germ cells seem not to employ individual switch genes to coordinately control all aspects of their sexual differentiation, and there is no evidence that they can ever maintain their full sexual identity independently of the signals that specify it. Although Sxl is sex-specifically regulated in this cell type, and SxlF protein does have important female-specific germline functions, SxlF is not sufficient to impose a female fate on germ cells, nor is it required for germ-cell viability. Indeed, Sxl− XX germ cells proliferate wildly in a Sxl+ XX somatic environment, generating germline “tumors” composed of cells whose differentiation is blocked and whose sexual phenotype is ambiguous. None of the XSE genes that act upstream of Sxl to determine its expression state in the soma do so in germ cells, and none of the downstream targets of SxlF in the soma appear to be targets in germ cells.
One difference between somatic and germline sex determination that is particularly relevant to this study involves the cellular source of the sex-determination signals. For somatic cells, sex determination is generally a cell-autonomous process, with only minor aspects of sexual differentiation relying on cell-nonautonomous sex signals (for examples, see Fung and Gowen 1957; Lawrence and Johnston 1986), and those nonautonomous signals do not appear to influence Sxl. In contrast, cell-nonautonomous signals are critically important for directing the sexually appropriate differentiation of germ cells and clearly do influence Sxl expression in the process (Nöthiger et al. 1989; Steinmann-Zwicky et al. 1989; Steinmann-Zwicky 1994; Janzer and Steinmann-Zwicky 2001; Wawersik et al. 2005). Sexual signaling from the soma to the germ line is only one aspect of the extensive crosstalk that occurs between these two cell types in the gonad during gametogenesis (Gilboa and Lehmann 2004).
Given that Drosophila gametogenesis is a collaborative effort between two cell types whose sex-determination systems differ in such fundamental ways, it is perhaps not surprising that no mutant genotype has been found that induces a sexual transformation so complete that functional gametes of the opposite sex are produced. Instead, in nearly all situations where gonadal sexual identity has been perturbed by genetic manipulation, the aberrant phenotypes generated have been extremely variable and difficult to interpret (Nöthiger et al. 1989; Janzer and Steinmann-Zwicky 2001). Such ambiguities are one factor of many that have hindered the development of a clear understanding of germline sex determination.
Because we were able in this study to base conclusions on unambiguously wild-type gonadal phenotypes, we could obtain a clear and simplifying answer to a fundamental question regarding the genetic control of the nonautonomous feminizing signals to which diplo-X germ cells respond. That question is whether, in the absence of SxlF, TraF can induce somatic cells of the gonad to transmit a fully feminizing signal to their diplo-X germ-cell neighbors. In other words, could tra be the sole somatic target of Sxl in the control of gonadal sexual differentiation, just as it was thought to be in nongonadal cells? Or, instead, must there be additional targets of Sxl that contribute to the feminizing signal? More than a decade ago, Steinmann-Zwicky (1994) addressed this question, but without the TraF transgene needed to generate a definitive answer.
Steinmann-Zwicky (1994) transplanted diplo-X pole cells (germ-cell precursors) into preblastoderm haplo-X (male) host embryos that lacked germ cells of their own and that carried a hsp-traF transgene whose constitutive generation of TraF feminized their soma. Two chimeras that exhibited unambiguously female-like germ-cell development were recovered, but only one survived long enough to establish that it could make what appeared to be mature eggs. As expected, those eggs were not laid, since the transgene used to generate TraF could not rescue egg laying even for tra− females under the conditions required for the transplantation experiments (McKeown et al. 1988; Arthur et al. 1998). Because the eggs were not laid, however, the conclusion that they were fully female could not be based on evidence of their functionality, but instead was simply inferred from their superficial appearance.
Another limitation of the Steinmann-Zwicky (1994) transplantation experiment was the lack of any direct evidence for the key point that SxlF was absent from the feminized haplo-X soma of the egg-producing chimera. This point was simply assumed on the basis of the knowledge that haplo-X cells do not normally make SxlF, and perhaps as well on the expectation that such cells would suffer lethal dosage compensation upsets if they did make SxlF. We were uncomfortable with that assumption, since the egg-producing chimera represented a highly unnatural situation unlike any that had been examined for Sxl expression before. Moreover, the many known complexities of Sxl regulation include the fact that somatic expression of TraF can nonautonomously induce SxlF expression even in haplo-X germ cells that do not engage in oogenesis (Janzer and Steinmann-Zwicky 2001). Hence, we believed that an experimental test of this key point was needed to exclude the possibility that diplo-X germ cells induced by TraF to produce high levels of SxlF might in turn have been able to induce SxlF in their haplo-X somatic-cell neighbors with which they were exchanging developmental signals in the course of making eggs. A retrograde, indirect autoregulatory effect of this sort would not necessarily be lethal to haplo-X gonadal cells. Cell-lethal effects of Sxl misexpression have been documented only for the precursors of the adult integument, and even those cells can survive if they are not forced to compete locally with cells that are not misexpressing Sxl (Cline 1976, 1979), if their misexpression occurs for less than the full period from embryo to adult (Cline 1984), or if their level of misexpression is below that required to trigger full engagement of the Sxl-positive autoregulatory feedback loop (Cline 1984; Cline et al. 1999).
In addition to being relevant to the mechanism of germline sex determination, an answer to the question of whether eggs produced with the help of TraF-feminized XO cells are functional is relevant to the subject of X chromosome dosage compensation in the gonad and the importance of gene balance in oogenesis. Even if fully feminized, would a somatic cell carrying only a single copy of each X-linked gene be able to successfully execute all aspects of a complex developmental process that evolved only for cells with two copies of those same genes? Because dosage compensation is a process that evolved to enable cells with different doses of X-linked genes to carry out the same developmental processes, one would not expect it to accommodate such sex-specific processes as oogenesis and spermatogenesis (Schüpbach 1982). For this reason, eggs generated with the help of TraF-expressing haplo-X somatic cells might be unable to support normal development, even if all cells participating in oogenesis had been fully feminized. Moreover, such functional defects might not be obvious from egg morphology alone. Schüpbach et al. (1978) showed that eggs rendered nonfunctional by upsets in X chromosome gene balance (caused in that case by an extra X chromosome in female germ cells) nevertheless appear normal, even when laid. The fact that this potential dosage compensation complication did not interfere with our effort of determining the feminizing power of TraF in the gonad is both fortunate and significant.
Here we report the development of an improved constitutive TraF transgene that, unlike previous transgenes used in studies of germline sex determination, can rescue the fertility of otherwise tra− females under standard culture conditions. We use this new transgene to explore the limits of the tra gene's feminizing abilities, expecting feminized genetic mosaics with haplo-X somatic cells and diplo-X germ cells to be fertile if they make eggs that are fully female and if the anticipated lack of dosage compensation of oogenesis-specific somatic genes does not preclude normal oogenesis. We included a test of the critical assumption that chromosomally male somatic cells are not induced to express SxlF when engaged in oogenesis with SxlF-expressing diplo-X germ cells. The results that we obtained are definitive regarding the feminizing power of TraF within the gonad, and the ability of haplo-X somatic cells to engage in functions for which their genome is not expected to be dosage compensated. However, our expectation that all TraF-feminized mosaics would be able to lay any eggs that they made was not met. We suggest that the failure of so many of the feminized mosaics to lay their eggs reflects an unanticipated but interesting limitation either in the feminizing powers of TraF in some nongonadal cells required for egg laying or in the ability of fully feminized haplo-X cells to execute some diplo-X-specific cell functions required for egg laying.
MATERIALS AND METHODS
Drosophila culture and genetics:
Flies were raised at 25° in uncrowded conditions on a standard cornmeal, yeast, sucrose, and molasses medium. Information on markers and balancers can be found at http://flybase.bio.indiana.edu. The X-linked transgene P{Ub∷GFP w+mC}33C (Davis et al. 1995) was kindly provided by P. O'Farrell.
U2af-traF transgene construction:
P{U2af50∷traF w+mW.hs}, hereafter referred to as U2af-traF, was generated by replacing a NheI–EcoRI fragment from the U2af50 in vivo expression vector pdr141 (Rudner et al. 1998) with a NheI–EcoRI cDNA fragment containing the complete traF ORF (McKeown et al. 1988), subcloning into the transformation vector P[W8] at NotI sites, and transforming Drosophila using standard techniques. The transgene contains 4.5 kb of U2af50 upstream genomic sequence, followed by the 216-bp U2af50 5′-UTR with an improved Cavener translation initiation sequence. The ORF begins with the tripeptide MAS, followed by the full traF ORF, which terminates at the U2af50 stop codon. The 77-bp U2af50 3′-UTR and adjacent 1.5-kb downstream genomic sequence follows.
Microscopy and immunostaining:
For Figures 1 and 3, gonads were dissected and photographed live using a Zeiss Axioskop (Plan-Neofluar lenses, including a ×100/1.3 oil) with a Hamamatsu (Bridgewater, NJ) C4742 digital camera controlled by Metamorph v. 4.5 (Universal Imaging, West Chester, PA). For Figure 2, gynander ovaries were fixed and stained as previously described (Bopp et al. 1993). Primary antibodies used were polyclonal mouse anti-Sxl (Bernstein et al. 1995) at 1:2000 and polyclonal rabbit anti-GFP at 1:1000, while secondary antibodies were Alexa-488-conjugated goat anti-rabbit and Cy3-conjugated goat anti-mouse, both diluted 1:1000 (Molecular Probes, Eugene, OR). Nuclei were stained with DAPI in Vectashield (Vector Labs, Burlingame, CA). Gonads were imaged using a Leica SP2 AOBS confocal microscope (HCX PL APO ×63/1.40 lens). All images were processed in Adobe Photoshop 7.0 and Adobe Illustrator 10.0.
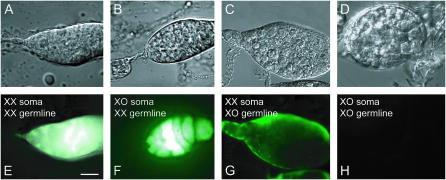
Figure 1.—
Four distinct ovariole genotypes from the gonads of XX//XO mosaics (gynanders) feminized by constitutive expression of TraF. All XX cells fluoresce green due to expression of a Ubi-GFP transgene on their maternal X chromosome, while all XO cells have lost this maternal X and are therefore nonfluorescent (from the cross in Table 2). All cells carry the feminizing U2af-traF transgene. (A–D) Nomarski images. (E–H) Corresponding GFP fluorescence images. (A and E) Germarium with XX soma and XX germ cells. All cells fluoresce and oogenesis is normal. (B and F) Germarium with nonfluorescent traF-feminized XO soma and fluorescent XX germ cells (longer exposure than E). The feminized XO cells support normal oogenesis for XX germ cells. (C and G) Abnormal germarium with fluorescent XX somatic cells and nonfluorescent tumorous XO germ cells. XO germ cells fail to develop normally even in a bona fide female somatic environment. (D and H) Abnormal tumorous cyst from an ovariole with feminized XO somatic cells and XO germ cells. No cells fluoresce. Consistent with G, XO germ cells fail to develop normally in a feminized somatic environment. Bar, 10 μm for A, B, E, and F and 20 μm for C, D, G, and H (anterior to the left).
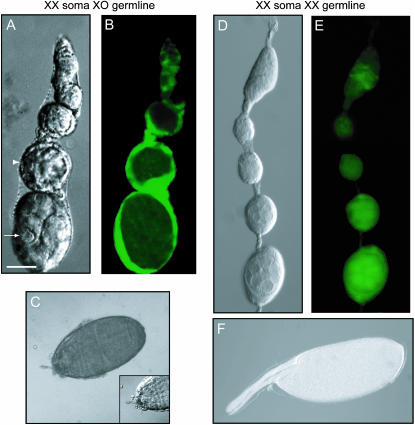
Figure 3.—
Only very rarely do germ cells marked as XO reach unambiguously female late stages of development when growing in a female somatic environment. Ovarioles and eggs are from U2af-traF-feminized gynanders from the cross described in the footnote to Table 2. (A, C, D, and F) Nomarski images. (B and E) GFP fluorescence images. (A–C) The one atypical ovariole of the only gynander ovary with apparently XO germ cells (nonfluorescent) and XX soma (fluorescent green) that showed unambiguously female differentiation. The white arrow indicates nurse-cell-like (female) differentiation, while the white arrowhead highlights a degenerating cyst. A nearly mature egg from this ovariole (C) had grossly abnormal dorsal appendages (inset) and was abnormally small. (D–F) A developmentally wild-type ovariole and egg from a nonmosaic XX gynander ovary for comparison. Bar, 25 μm for A, B, D, and E (anterior up) and 100 μm for C and F (anterior to left).
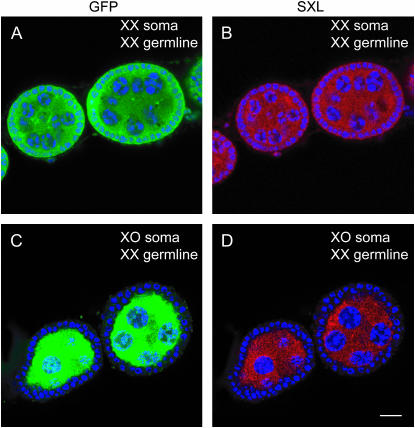
Figure 2.—
Sxl remains in its male expression state in TraF-feminized XO somatic cells that are engaged with XX germ cells in oogenesis. (A–D) Confocal micrographs of stages 4–6 oogenic cysts from the gonads of two different XX//XO mosaics (gynanders) feminized by U2af-traF (see Table 2). Green is anti-GFP staining that distinguishes XX cells from the nonstaining XO cells (A and C), red is anti-SxlF staining that indicates female Sxl expression (B and D), and blue is DAPI staining of DNA. (A and B) A control gynander with XX somatic and germ cells. All cells express SxlF proteins. (C and D) A gynander with TraF-feminized XO somatic cells engaged in oogenesis with XX germ cells. Note the absence of SxlF proteins in the XO somatic cells that surround germ cells producing SxlF. Bar, 20 μm.
RESULTS
A constitutively feminizing traF transgene that rescues tra− female fertility:
Studies of germline sex determination have been hampered by the lack of a constitutively expressing TraF transgene that would render otherwise tra− mutant females fertile. While previous TraF transgenes (McKeown et al. 1988; Finley et al. 1997; Waterbury et al. 2000) feminized XX tra− individuals in most respects, the rescued animals were invariably sterile when raised under standard culture conditions, at least in part because they failed to lay the eggs that they produced (see Table 1, cross D).
TABLE 1.
tra− females rescued by the U2af-traF transgene lay eggs
| For females that laid eggsa
|
|||||||
|---|---|---|---|---|---|---|---|
| Females characterized
|
Eggs laid per day
|
||||||
| Cross to generate femalesb | tra genotype | traF transgene | N | % of all females tested | Average ±SEM | Range of peak daily values | Adult progeny per egg for mated females (n eggs)b |
| A | −/− | U2af | 25 | 96 | 27 ± 5 | 2–91 | 0.82 (2470) |
| A | +/− | U2af | 23 | 100 | 59 ± 3 | 29–121 | 0.87 (6029) |
| B | −/− | U2af | 23 | 91 | 20 ± 4 | 1–84 | 0.73 (1388) |
| B | +/− | U2af | 14 | 100 | 82 ± 4 | 51–121 | 0.89 (4619) |
| C | −/− | U2af | 19 | 95 | 30 ± 5 | 5–90 | 0.91 (1895) |
| C | +/− | U2af | 14 | 100 | 59 ± 3 | 47–85 | 0.84 (3321) |
| D | −/− | hsp83 | 20 | 0 | 0 | — | — |
- A: w; P{U2AF∷traF w+mW.hs}2B/+; tra1/Df(3L)st-J7,tra−
 × ♂♂ w/YBs; Df(3L)st-J7,tra− Ki roe pp/TM3, Ser
× ♂♂ w/YBs; Df(3L)st-J7,tra− Ki roe pp/TM3, Ser
- B: w; P{U2AF∷traF w+mW.hs}2B/+; tra1/Df(3L)st-J7,tra−
 × ♂♂ w/YBs; tra1 e ca/TM6, Hu
× ♂♂ w/YBs; tra1 e ca/TM6, Hu
- C: w; P{U2AF∷traF w+mW.hs}2B/+; tra1/Df(3L)st-J7,tra−
 × ♂♂ w/YBs; trav2 kar ry red/TM6, Hu
× ♂♂ w/YBs; trav2 kar ry red/TM6, Hu
- D: w; P{hsp83-traF}5.4/ +; tra1 e ca/TM6, Hu
 × ♂♂ w/YBs; Df(3L)st-J7,tra− Ki roe pp/TM3, Ser.
× ♂♂ w/YBs; Df(3L)st-J7,tra− Ki roe pp/TM3, Ser.
Zero- to 1-day-old virgin females were mated individually to five w1118/Y males and transferred every day over a 5-day test period.
Individual females were deemed to have mated on the basis of their production of progeny, and data are for eggs laid only after the first egg collection that generated progeny.
We overcame this limitation by generating a transgene that expressed traF under the control of regulatory sequences belonging to the housekeeping gene U2af50. This new transgene rescued egg laying for tra− mutant females regardless of genetic background (Table 1, crosses A–C), and the rescued females were fertile. With this transgene, tra− alleles can now be maintained easily as homozygous mutant stocks, rather than as conventionally balanced lines, thereby minimizing the troublesome accumulation of spontaneous, closely linked recessive lethals.
Rescue was strong, but it was not complete for every animal. Overall, 94% of rescued tra− females laid at least one egg over the 5-day test period, while 100% of their tra+ control sisters met this criterion. There was considerable overlap between individual rescued females and control siblings with respect to peak daily egg output, but the egg output averaged over all females in a group was significantly lower (41% overall) for the rescued animals vs. controls in all three genetic backgrounds (P < 0.0001 by the Wilcoxon rank-sum test). On the basis of the production of progeny, we deduced that all 51 of the tra+ control females mated, while 3 of 63 feminized tra−experimentals that laid eggs (1, 10, and 144, respectively) may not have mated, since they produced no progeny. With respect to the proportion of eggs from mated females that developed into adults, for two of the three crosses in Table 1 (A and B), the experimentals were somewhat lower than the controls, but that difference does not seem to be functionally significant, since the opposite was true for cross C. For all three crosses, a large majority of the eggs from rescued tra− females developed into adults. Chromosomal males feminized by the U2af-traF transgene never made eggs (data not shown).
Chromosomally male somatic cells expressing TraF but not SxlF support unequivocally wild-type oogenesis:
We used a genetic approach to generate the haplo-X//diplo-X mosaic animals that would show whether transgene-derived TraF expression in somatic cells lacking SxlF is sufficient to support truly wild-type oogenesis with neighboring XX germ cells. We relied on the non-claret disjunctional mutant maternal effect of Df(3R)cand1 (cand1) to induce loss specifically of the maternally derived X chromosome in XX zygotes during the first mitotic division, thereby generating mosaics (gynanders) with huge, clonally related clusters of XX and XO cells (Sturtevant 1929; Lewis 1952; Yamamoto et al. 1989). XX cells were distinguished from XO cells by the presence (XPaternalXMaternal) or absence (XPaternalO) of the external marker alleles y+ and sn+ and the internal marker transgene Ubiquitin-GFP, carried on the maternal X chromosome. In scoring the somatic cells of the adult ovary, we considered only the genotype of the cells inside of the epithelial sheath that surrounds each ovariole from tip to base, and it is only to those cells that we refer when we mention “somatic cells of the gonad.” In this way we avoided complications from somatic cells in the ovary that are not in intimate contact with germ cells, are not relevant to germ-cell sex determination, and in some cases are much more difficult to score for X chromosome number. Heller and Steinmann-Zwicky (1998) showed that intimate contact between somatic and germ cells is required for the transmission of nonautonomous feminizing signals. Some cells in the adult ovary outside the region that we scored became associated with the ovary only during metamorphosis, having originated from primordia far from that for somatic cells inside the sheath. Such cells must not be relevant to germ-cell sex determination, since larval ovaries transplanted into male host larvae metamorphose into normal-appearing ovaries that generate fully developed eggs, even if they never connect to the host's genital disc derivatives (Fung and Gowen 1957).
Even if the minority genotype in a mosaic gonad arose from just a single founder cell at the blastoderm stage, that cell would have had ample opportunity to divide many times and produce an obvious clone. Consequently, the unfixed gonads of aged adults could be scored reliably for X chromosome number by standard fluorescence microscopy (Figure 1). The difference between germ cells and somatic cells with respect to morphology and location gave the gonads that were most critical to our analysis—those with all XX germ cells (light) and all XO somatic cells (dark)—a very distinctive appearance (Figure 1B).
Because the orientation of the plane dividing XX and XO halves of the embryo is nearly random among gynanders, tissue anlagen that are far apart on the blastoderm fate map are of different sexes more often than anlagen that are close (Garcia-Bellido and Merriam 1969; Hotta and Benzer 1972). Since all precursors of the somatic cells of the paired gonads arise from a single primordium that is relatively far from the single primordium for all germ cells, the sex of the somatic cells in gynander gonads is frequently opposite to that of the germ cells (Gehring et al. 1976). Of 168 gynanders carrying U2af-traF that we analyzed, the chromosomal sex of all somatic cells of both gonads was opposite to that of all germ cells in 36 cases. For addressing the question of TraF functionality, 14 of these 36 that had all XO somatic cells and all XX germ cells were useful, as well as 4 others whose somatic gonads were entirely XO but whose germline was mosaic.
For all 18 of the useful gynanders, constitutive expression of traF from the transgene was sufficient to induce the XO somatic cells to collaborate with XX germ cells and produce morphologically wild-type eggs. Moreover, in no case did we observe any XX germ cells that attempted to differentiate but failed to engage in morphologically normal oogenesis. Of these 18 feminized gynanders, 6 (33%) laid their eggs (Table 2, Somatic gonad, “both sides XO”), and, most significantly, all 6 were fertile. Over a 5-day test period, the 5 fertile gynanders with all XX germ cells had an average of 28 progeny (range 2–55), significantly fewer than the 60 average (range 3–146) for 41 fertile gynanders whose gonadal soma and germline were both entirely XX (P < 0.05 by the Wilcoxon rank-sum test). The lower relative fecundity of these 5 gynanders (47%) seems more likely to reflect a limitation of the U2af-traF transgene than an intrinsic limitation of feminized XO somatic cells, since it is so similar to the 41% figure mentioned earlier for tra−females rescued by the U2af-traF transgene compared to their tra+ sisters.
TABLE 2.
Correlations between the chromosomal sex of tissue landmarks and the ability of U2af-traF feminized gynanders to lay their eggs
| % of the specified class of egg-producing gynander (n = total in class) displaying the discordant egg-laying behavior indicateda
|
|||
|---|---|---|---|
| Structural landmark considered | A. One side XO, other side XX, laying eggs | B. Both sides XO, laying eggs | C. Both sides XX, not laying eggs |
| External | |||
| Eye | 80 (30) | 45 (40) | 5 (19) |
| Antenna | 62 (50) | 50 (44) | 13 (23) |
| Proboscis | 59 (54) | 52 (29) | 24 (34) |
| Thorax/wing | 62 (81) | 17 (6) | 39 (18) |
| Foreleg | 61 (72) | 50 (10) | 27 (26) |
| Midleg | 61 (82) | 43 (7) | 23 (22) |
| Hindleg | 60 (80) | 56 (9) | 19 (21) |
| Tergite 2 | 66 (50) | 56 (9) | 38 (52) |
| Tergite 3 | 64 (45) | 57 (7) | 40 (50) |
| Tergite 4 | 62 (45) | 67 (6) | 35 (54) |
| Tergite 5 | 59 (39) | 86 (7) | 38 (60) |
| Tergite 6 | 59 (29) | 67 (6) | 35 (69) |
| Tergite 7 | 56 (34) | 67 (6) | 35 (71) |
| Sternite 3 | 66 (62) | 33 (6) | 40 (48) |
| Sternite 4 | 66 (56) | 33 (6) | 39 (51) |
| Sternite 5 | 68 (60) | 0 (2) | 42 (52) |
| Sternite 6 | 68 (50) | 50 (2) | 41 (59) |
| Sternite 7 | 66 (41) | 0 (3) | 37 (73) |
| Genitalia | 79 (14) | 22 (9) | 37 (90) |
| Internal | |||
| Brain | 55 (22) | 53 (19) | 5 (22) |
| Thoracic ganglion | 63 (38) | 62 (21) | 8 (12) |
| Somatic gonad | 80 (5) | 33 (18) | 38 (71) |
Data for 117 U2af-traF feminized gynanders with eggs from the cross w P{Ub-GFP w+mC}33C; P{U2af50-traF w+mW.hs}2B/+; cand1  × ♂♂ y w sn/YBs. Gynanders were collected within 1 day of eclosion, individually mated to five Oregon-R wild-type males for 5 days, dissected, and examined for evidence of having mated (motile sperm in reproductive tract). Only gynanders that survived the 5-day egg-laying test were included in the analysis (∼15% did not). Not all gynanders were scored for brain and thoracic ganglion sex. Gynanders mosaic on either side for the particular landmark considered were not included.
× ♂♂ y w sn/YBs. Gynanders were collected within 1 day of eclosion, individually mated to five Oregon-R wild-type males for 5 days, dissected, and examined for evidence of having mated (motile sperm in reproductive tract). Only gynanders that survived the 5-day egg-laying test were included in the analysis (∼15% did not). Not all gynanders were scored for brain and thoracic ganglion sex. Gynanders mosaic on either side for the particular landmark considered were not included.
We immunostained mosaic gonads for SXL protein to establish the critical point that feminized XO somatic cells engaged in fully wild-type oogenesis with XX germ cells are not induced to express SXLF protein (compare Figure 2D with 2B). Hence, we can conclude that even in the absence of detectable SxlF protein, TraF protein in somatic cells of the gonad is sufficient to elicit all nonautonomous feminizing signals required by XX germ cells, as well as all other somatic-cell functions required for normal oogenesis.
Among the 168 feminized gynanders that survived the test for egg laying, only 2 were apparent exceptions to the rule that all germ cells marked as XX formed eggs but not tumorous egg chambers (Figure 1, A and B), while all germ cells marked as XO formed tumorous egg chambers but not eggs (Figure 1, C and D). The exceptions involved a few germ cells judged to be XO that nevertheless made eggs. They are described under Can haplo-X germ cells occasionally be induced to make eggs? The exceptions occurred in gynanders whose germline was entirely XO, and not in any of the 9 gynanders whose germlines were mosaic and who would therefore have provided an opportunity for germ cells of one chromosomal sex to influence the differentiation of germ cells of the opposite chromosomal sex, if such interactions could take place. The fact that such interactions did not occur is evidence that Drosophila germ cells do not receive sexual cues from each other, but instead interact with the gonadal soma as autonomous individual entities when determining their sex. The fact that all XX germ cells in these TraF-feminized gynanders exhibited normal development shows that there must not be any somatic cell type outside of the region of the ovary that we scored for X chromosome number that is required for female germ-cell differentiation but that is not fully feminized by TraF. If such a somatic cell type existed, all such cells would have had to have been XX in every gynander that had XX germ cells, a vanishingly unlikely possibility.
In four of the nine feminized gynanders with mosaic germlines, the germline in one ovary was entirely haplo-X while that in the other was entirely diplo-X—a “left-right” division of the germ cells within the mosaic animal. Among the other five, however, four had one ovary with a mosaic germline, and one was mosaic for the germline in both ovaries. On the basis of previously published work, we would not have expected to see haplo-X germ cells developing abnormally alongside normally developing diplo-X cells in the same adult ovary. Although Steinmann-Zwicky et al. (1989) had shown that haplo-X pole cells (germ-cell precursors) transplanted into female host embryos that had no germ cells of their own could contribute abnormally differentiating progeny to adult host ovaries, Schüpbach (1985) had shown earlier that no progeny of haplo-X germ cells could be found in adult host diplo-X ovaries if the female host embryos for haplo-X pole-cell donors had pole cells of their own. The apparent inability of transplanted haplo-X germ cells to compete with the host's own diplo-X germ cells in the 1985 study had to have been a consequence of the transplanted cells being sexually mismatched to their host's soma, since Marsh and Wieschaus (1978) had shown even earlier that if the diplo-X hosts had been masculinized by loss of tra, transplanted haplo-X pole cells competed effectively with the host's own pole cells and made (defective) sperm in the adult. In such a tra−diplo-X host, the host's own germ cells, rather than the donor haplo-X cells, were the sexually mismatched genotype.
Our observation that sexually mismatched haplo-X germ cells can compete for growth in a gonad with diplo-X germ cells that are not sexually mismatched cannot be a consequence of some inadequacy of the TraF transgene that reduces the magnitude of the sexual mismatch compared to that in the experiments cited, since, in three of the ovaries here that had mosaic germlines, all the somatic cells were diplo-X and must therefore have provided a fully feminizing gonadal environment. No study of gynander gonads either before (Gehring et al. 1976) or after (Szabad and Nothiger 1992) the classic pole-cell transplantation studies cited above presented data relevant to the question of whether sexually mismatched germ cells can compete for survival and growth with germ cells whose sex is matched to that of the soma.
Can haplo-X germ cells occasionally be induced to make eggs?
Among the 51 feminized U2af-traF gynanders whose gonads appeared to contain only XO germ cells, we found 2 individuals with female germ-cell differentiation in one ovariole of one of their two ovaries (Figure 3). Since wild-type ovaries each have 10–30 ovarioles (King 1970), these exceptions represented only a very small proportion of all XO cells, even in these 2 gynanders.
Both of the exceptional ovarioles contained multiple abnormal female cysts interspersed with degenerating cysts of ambiguous sexual phenotype (Figure 3, A and B; compare to Figure 3, D and E). Oogenic development as advanced as stage 14 (mature eggs) was apparent, but all eggs were unusually small and had abnormal dorsal appendages (compare Figure 3C with 3F). None were laid. The chromosomal sex of the somatic gonad did not seem to influence this phenomenon, since in one case the soma was entirely XX, while in the other entirely XO. Although these two exceptional ovarioles might seem to indicate that even haplo-X germ cells can be induced by TraF to engage in oogenesis, albeit at a very low frequency, we believe that the phenotype is more consistent with a rare, spontaneous change in germline stem-cell karyotype (see discussion).
A requirement for feminization by SxlF outside the gonad that TraF cannot satisfy:
The fertility of the 6 feminized gynanders that had entirely XO somatic gonads and XX germ cells answered the questions that we had raised regarding oogenesis, but the fact that the other 12 gynanders in this class were sterile was unexpected, since nearly all tra− nonmosaic females rescued by the same U2af-traF transgene had been fertile (Table 1). Sterility appeared to be caused by a defect in egg laying, rather than in egg function. Other categories of TraF-feminized egg-producing gynanders also failed to lay their eggs. Indeed, analysis of the entire set of 117 feminized gynanders that made eggs showed that there must be XX somatic cells outside the gonad that are required for egg laying for which XO cells feminized by TraF cannot substitute.
Of the 117 gynanders that made eggs, 49 were sterile. Only 5 of those 49 laid eggs, and their sterility was likely due to a mating or sperm-storage defect, since all 5 lacked sperm when dissected at the end of the 5-day fertility test. Of the 44 sterile gynanders that did not lay their eggs, 39% carried sperm at the end of the test and hence must have mated. Thus 38% (44/117) of all feminized gynanders that made eggs failed to lay them, compared with only 6% of tra− females rescued by U2af-traF. A simple explanation for this large difference is that there is a “tra-insufficient feminization” (TIF) pathway in which SxlF acts on a target other than tra to direct female-specific aspects of cell development or function required for egg laying in wild-type females. If these cells in the TraF-feminized gynanders are XO—and thus lacking SxlF—the gynanders cannot lay their eggs. We scrutinized the set of 117 egg-producing gynanders more carefully for clues as to where such a TIF pathway gene target for SxlF might be required.
Hotta and Benzer (1972) described how one could use gynanders to map a simple behavioral focus, i.e., determine the location on the blastoderm-stage fate map of the progenitors of the cells that require a particular gene's activity to produce animals with wild-type behavior. There will generally be at least two such foci as a consequence of the fly's bilateral symmetry. A bilateral behavioral focus is said to be domineering if the mutant phenotype (in this case, failure to lay) is observed even when the focus on only one side of the animal is mutant (in this case, XO). The focus is submissive if mutant behavior is observed only when the focus is mutant on both sides. The Hotta and Benzer (1972) approach is most useful and straightforward when the orientation of the plane dividing XX and XO cells is random among the set of gynanders considered. Unfortunately, in this case that orientation was strongly biased by the requirement that only gynanders making eggs be considered. Egg-producing gynanders had to be derived from embryos with at least some XX cells at their posterior pole—the germ-cell primordium. It is important to note, however, that the bias is only with respect to the distribution of XX tissue, not the actual amount of XX tissue (which is expected to be 50% in each gynander). Even with this biased set of gynanders, we hoped to be able to deduce at least whether the focus for egg laying was domineering or submissive and perhaps also to infer its general location by examining the correlations between egg-laying behavior and the sex of various specific regions of the egg-producing gynanders.
Table 2 lists the degree of discordance observed between the chromosomal sex of particular adult cuticular landmarks and the ability of that class of gynander to lay eggs. An XO genotype (TIF−) would be discordant with laying, and XX (TIF+) discordant with a failure to lay. The closer the primordium of a given landmark is to the behavioral focus being mapped, the less discordance one expects. Even for a simple behavioral focus, however, the lowest discordance that one observes may still be too high to be very informative if the analysis is limited to external adult cuticular landmarks, since all the primordia for such landmarks are relatively far from the primordia for elements of the adult central nervous system (Kankel and Hall 1976).
Egg-laying values in column A of Table 2 suggest that the TIF focus is unlikely to be domineering, since a majority of gynanders laid when the landmark on only one side of the animal was XO, regardless of where the landmark was. For locating a submissive focus, one considers gynanders in which the chromosomal sex of the landmark is the same on both sides. The closer the bilateral landmark is to the bilateral behavioral focus, the less frequent the discordant phenotype will be. Columns B and C of Table 2 show that, again, for every external landmark examined, a large fraction of gynanders displayed discordant behavior, suggesting that the focus is too far from any landmark to allow a conclusion to be drawn regarding the likely nature of the cells that might require TIF. Moreover, there was no obvious correlation between the discordance when both landmarks were XO and that when both were XX. In many cases, the usefulness of this comparison was limited by the low number of gynanders in the “both sides XO” class, but for some landmarks such as the eye, the large difference in discordance between these two classes cannot be dismissed as a statistical artifact. Such differences in discordance suggest that the behavioral focus is complex. The most that can be said from the data in columns B and C for external landmarks is that they favor a submissive model for the TIF behavioral focus, since for each landmark the average degree of discordance in the B and C columns combined was consistently lower than the discordance in column A.
Examination of the brains and thoracic ganglia of these gynanders supported the idea that the TIF focus is submissive and complex. In neither of these two neuronal regions is TIF required for egg laying: 53% of gynanders with an entirely XO brain laid eggs, as did 62% of gynanders with an entirely XO thoracic ganglion (bottom of Table 2, column B). On the other hand, gynanders with entirely XX brains and/or thoraxes (column C) only rarely failed to lay, as if either region might be nearly sufficient to provide TIF.
Although gynander analysis revealed relatively little affirmative about the TIF focus, it did show that the focus must be quite far from the primordium for the somatic gonad (Table 2, final row). The fact that these primordia are relatively far apart is what allowed the X chromosome loss method of generating mosaics to succeed for the purpose of determining whether TraF in the gonadal soma is sufficient to generate functional eggs. Ironically, if we had relied instead on the transplantation approach taken by Steinmann-Zwicky (1994) in her study of TraF, a “cleaner” approach in the sense that it generates animals with an entirely haplo-X soma and diplo-X germline, the animals would not have laid their eggs, even though they carried a TraF transgene capable of restoring fertility to tra− females.
DISCUSSION
The six fertile U2af-traF-feminized gynanders described here whose gonadal soma was entirely XO established an important, simplifying point regarding germline sex determination: TraF protein is sufficient to induce not only all the nonautonomous feminizing signals that XX germ cells need to make functional eggs, but also all the many other oogenesis-specific functions that the gonadal soma is called upon to supply when working with XX germ cells to make eggs. The conclusion that TraF can provide these functions without help from any other Sxl gene target rests not on assumptions, but rather on the direct experimental observation that no SxlF protein could be detected in the relevant TraF-feminized somatic cells engaged in oogenesis. Our demonstration that these female-specific functions seem to be carried out as effectively by feminized XO somatic cells as by XX somatic cells is surprising in light of the fact that one does not expect XO cells to have the benefit of X chromosome dosage compensation for genes specifically engaged in oogenesis.
While our study showed that TraF is sufficient for feminizing somatic cells in the gonad, ironically it also revealed that TraF may not be sufficient for feminizing some somatic cells elsewhere that are required for egg laying. It had been thought that all somatic sexual differentiation functions of Sxl outside the gonad were likely to be executed through tra. Now it seems that at least one such function required for egg laying might involve an unknown Sxl target in an unanticipated branch of the sex-determination gene regulatory hierarchy, although alternative possibilities are considered below.
The nature of sex-determination signals in the gonad:
Horabin et al. (1995) questioned the sufficiency of TraF for germ-cell feminization when they observed that loss of tra did not fully masculinize XX germ cells and that loss of tra2, a constitutively expressed gene whose protein product is a required partner for TraF, appeared to have a greater masculinizing effect than loss of tra. However, these workers did not account for the earlier data of Nöthiger et al. (1989) from which one could have argued the opposite: that loss of tra has a more masculinizing effect on the germline than loss of tra2. The apparent contradiction between these two studies stems from the fact that germ-cell development is abnormal and highly variable when the autonomous and nonautonomous germline sex signals are in conflict. What one concludes regarding germ-cell sexual identity in such developmentally confused situations depends on the particular molecular and/or morphological criteria for sexual identity that one employs and on the particular experimental conditions that one uses. Janzer and Steinmann-Zwicky (2001) showed that in such abnormal situations, one cannot even count on the germline sexual phenotype remaining constant as the mutant animals age. The variability is due not only to differences in genetic background, growth conditions, and age, but also to stochastic factors that can cause remarkable variability even between the two gonads within a single individual.
Such ambiguities are expected if, as it seems, there is no single master sex switch gene in germ cells that establishes and maintains all aspects of their sexual identity in a fashion analogous to how Sxl coordinates all aspects of sexual dimorphism in the soma. Moreover, if there is more than one regulatory target in germ cells for sex signals, what has been called, for convenience, “the autonomous sex signal,” may in fact be multiple, independent gene dose effects, some or perhaps all synergizing with the nonautonomous signal, but perhaps not controlling all aspects of germ-cell sexual dimorphism, even in the aggregate. Under these circumstances, one might well question the appropriateness of referring to the germ-cell autonomous X chromosome dose effect on sex-specific gene expression with the same “sex signal” terminology that one uses for the somatic X chromosome dose effect. However, it seems premature to dismiss the concept of germ-cell autonomous sex signals as completely as Waterbury et al. (2000) seemed to do in their article's bold title, and perhaps premature as well to dismiss, as these same authors did, the contribution of germline Sxl expression to germline sex determination without accounting for the fact that even though SxlF protein will not feminize a haplo-X germ cell in a male soma, it will feminize a diplo-X germ cell in that same masculine somatic environment (Steinmann-Zwicky et al. 1989).
An observation that we report here is directly relevant to the observation that seems most likely to have led Waterbury et al. (2000) to their extreme view of the gonadal soma as a sexual dictator, albeit one whose germ-cell subjects can seem remarkably unreceptive to dictation when their autonomous sex signal is in conflict. They reported that TraF could completely override a male germ-cell autonomous signal in 2–5% of XY animals feminized by their newly generated hsp83-traF transgene. Unfortunately, it was hard to know how much significance to attach to this striking observation, since it could not be repeated even by those reporting it.
Although we saw no such feminization of haplo-X germ cells among haplo-X animals carrying U2af-traF, we did observe eggs being made in 2 of 51 (4%) feminized gynanders that appeared to have only XO germ cells. As had been true for the feminized males in the Waterbury et al. (2000) study, the eggs made by these gynanders were grossly abnormal; however, our analysis of this feminized gonadal phenotype leads us to a very different interpretation of its origin than that by Waterbury et al. (2000). Although only a single ovariole in each of the two exceptional gynanders contained cysts that exhibited overtly female development, each of those ovarioles contained several such overtly female cysts. All other ovarioles in the 51 gynanders contained only tumorous cysts with no indication of female germ-cell differentiation. Since a mature adult female fruit fly has two ovaries, each with 10–30 ovarioles, and each of them with six to seven developing cysts (each cyst being the product of a single differentiating germ cell), the overall frequency of unambiguously female cysts among these gynanders was far lower than the 4% figure for egg-producing gynanders would suggest. The fact that such a low-probability event would occur as a cluster within a single ovariole is just what one would expect for a germ-cell clone that arose as a consequence of a rare mitotic error in an XO stem cell after the embryonic gonad had formed, an error that partially or fully diploidized the single X chromosome or reduced the cell's autosomal complement. The alternative explanation for such clustered feminization is that it arose as a rare epigenetic response of a germline stem cell to the nonautonomous feminizing signal induced by TraF. But that alternative explanation would seem to require the very thing that Waterbury et al. (2000) argued against: a master regulatory gene in the germline that responds to the nonautonomous signal to establish and then maintain female germline sexual identity in a heritable fashion.
The fact that the eggs made by these exceptional gynanders were grossly abnormal, while those made by bona fide XX germ cells in the same somatic environment were normal, does not preclude the possibility that the abnormal eggs were nevertheless derived from spontaneous XX or haploid clones. The developmentally normal eggs that the gynanders made were the product of germ cells that had been diplo-X throughout their development. The relatively small size of the presumptive clones that gave rise to GFP− abnormal eggs would argue that, in contrast, the cells that founded those clones were derived from progenitors that became XO after the first zygotic mitosis, losing their maternal GFP+ marker in the process, but then changed their karyotype again much later, after the gonad had formed and at a point in development where constraints on the reversibility of various relevant developmental processes might already have arisen. Until the frequency and predictability of these rare egg-producing events can be increased, it will be very difficult to obtain information on the germ-cell karyotype needed to determine their origin.
Sexually mismatched germ cells can compete, although perhaps rather poorly, with germ cells whose sex is matched to the gonadal soma:
On the basis of pole-cell (germ-cell precursor) transplantation experiments, Schüpbach (1985) concluded that haplo-X donor germ cells cannot survive in a female host when competing with the host's own diplo-X germ cells. Our experiments here with feminized gynanders show that they can. However, when our results are compared with those in Schüpbach's 1978 analysis of triplo-X//diplo-X mosaics, it is evident that the sexually mismatched germ cells in our experiment were at a significant competitive disadvantage relative to the host's sexually matched germ cells at some point in their development. Hence, we believe that the apparent discrepancy between our gynander results and those of the Schüpbach (1985) pole-cell transplantation study likely reflects a difference in the scope of the two experiments, with our set of gynanders providing a greater opportunity for rare events to be observed, rather than signaling a biological difference between sexually mixed germ-cell populations that are generated by preblastoderm X chromosome loss vs. those generated by pole-cell transplantation.
The triplo-X//diplo-X mosaics in Schüpbach's 1978 study were generated by early preblastoderm X chromosome loss, as were the feminized gynanders in this study. But in XXX//XX mosaics, all germ cells are female and all are in a female somatic environment. Moreover, the XXX germ cells were shown to grow nearly as well as their XX germ-cell neighbors. Among the XXX//XX mosaic females in the 1978 study, 22% (79/354) had mosaic germlines, a rate fourfold higher than the 5% (9/168) rate of germline mosaicism that we saw for feminized XX//XO mosaics. Not one of the 79 XXX//XX germline mosaics showed a strict left–right segregation of the two genotypes, while four of our nine (44%) XX//XO germline mosaics did. In the 1978 study, 75% (59/79) of the animals with mosaic germlines displayed germline mosaicism in both of their ovaries, while only 11% (1/9) did in this study. These three differences indicate that the haplo-X germ cells in our feminized gynanders were handicapped in their ability to compete with diplo-X cells in the same gonad, although not enough to preclude the possibility of at least some of them contributing progeny to the adult gonad.
The gonadal soma may be unusually tolerant of upsets in gene balance:
One does not expect the expression of X-linked Drosophila genes required for developmental programs such as oogenesis that are normally executed in only one sex to be dosage compensated (Schüpbach 1982). For that reason, the fertility of feminized gynanders with entirely XO somatic gonads is remarkable, and even more so considering the high rate at which they are able to make fully functional eggs. Although these gynanders produced only half as many eggs as their sister gynanders with XX somatic gonads, that difference seemed more likely to reflect a limitation in the U2af-traF transgene than a problem with dosage compensation. Either the assumption that most X-linked oogenesis-specific genes in the gonadal soma are not dosage compensated is invalid or, more likely, the ovary has regulatory mechanisms for accommodating the lack of dosage compensation, as appears to be true for yolk genes (Bownes et al. 1991).
A possible new branch in the somatic sex-determination pathway:
From the fact that 38% of TraF-feminized gynanders failed to lay their eggs, we infer that there are some XX somatic cells required for egg laying for which TraF-feminized XO cells cannot substitute. We can imagine three very different explanations for this limitation in the power of U2af-traF-feminized XO cells:
The cells in question are not fully feminized because a transformer-insufficient feminization (TIF) branch exists in the sex-determination pathway, downstream of Sxl and upstream of tra, in which SxlF is required to act on an unknown feminizing gene target needed for egg laying. TraF would have fully feminized all its targets in such XO cells, but because the cells have no SxlF, they would lack the TIF target function needed for egg laying.
The XO cells in question are fully feminized, but are not adequately dosage compensated for the female-specific functions that they are required to execute—a problem that did not arise in the gonad, where it was most expected.
The cells in question are incompletely feminized because, for some unknown reason, the U2af-traF transgene is less effective at generating TraF protein in haplo-X cells than in diplo-X cells, and that decreased effectiveness interferes only with this one small aspect of sexual dimorphism. It should be noted that because U2af50 itself is an X-linked gene, if the transgene has any of the sensitivity that the endogenous gene has to the dosage compensation system, one would expect it to be more highly expressed in haplo-X cells, not less.
TraF-feminized gynanders are not likely to be very useful for distinguishing among these alternatives, since one cannot generate large homogenous populations of the desired egg-laying-defective individuals. Fortunately, we have discovered an alternative to TraF-feminized gynanders for this purpose. Particular heteroallelic combinations of mutant Sxl alleles allow females to survive well, but as sterile phenotypic males, due to their failure to properly express tra. The U2af-traF transgene feminizes these individuals, allowing them to make eggs and mate; however, they remain sterile because they cannot lay their eggs, just like the sterile feminized gynanders described here. These TraF-feminized Sxl mutant females therefore should allow us to generate homogenous populations of egg-laying-defective females with which we can explore the possibility of an unanticipated TIF branch in the sex-determination pathway.
Acknowledgments
We are indebted to D. Rudner and M. Bell for generating U2af-traF, and we thank B. Meyer and current members of the Cline lab for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health grant GM23468 to T.W.C. and by a National Science Foundation predoctoral fellowship to D.S.E.
References
- Arthur, B. I., Jr., J. M. Jallon, B. Caflisch, Y. Choffat and R. Nothiger, 1998. Sexual behaviour in Drosophila is irreversibly programmed during a critical period. Curr. Biol. 8: 1187–1190. [DOI] [PubMed] [Google Scholar]
- Bernstein, M., R. A. Lersch, L. Subrahmanyan and T. W. Cline, 1995. Transposon insertions causing constitutive Sex-lethal activity in Drosophila melanogaster affect Sxl sex-specific transcript splicing. Genetics 139: 631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp, D., J. I. Horabin, R. A. Lersch, T. W. Cline and P. Schedl, 1993. Expression of the Sex-lethal gene is controlled at multiple levels during Drosophila oogenesis. Development 118: 797–812. [DOI] [PubMed] [Google Scholar]
- Bownes, M., K. Lineruth and D. Mauchline, 1991. Egg production and fertility in Drosophila depend upon the number of yolk-protein gene copies. Mol. Gen. Genet. 228: 324–327. [DOI] [PubMed] [Google Scholar]
- Cline, T. W., 1976. A sex-specific, temperature-sensitive maternal effect of the daughterless mutation of Drosophila melanogaster. Genetics 84: 723–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, T. W., 1979. A male-specific lethal mutation in Drosophila melanogaster that transforms sex. Dev. Biol. 72: 266–275. [DOI] [PubMed] [Google Scholar]
- Cline, T. W., 1984. Autoregulatory functioning of a Drosophila gene product that establishes and maintains the sexually determined state. Genetics 107: 231–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, T. W., and B. J. Meyer, 1996. Vive la différence: males vs females in flies vs worms. Annu. Rev. Genet. 30: 637–702. [DOI] [PubMed] [Google Scholar]
- Cline, T. W., D. Z. Rudner, D. A. Barbash, M. Bell and R. Vutien, 1999. Functioning of the Drosophila integral U1/U2 protein Snf independent of U1 and U2 small nuclear ribonucleoprotein particles is revealed by snf+ gene dose effects. Proc. Natl. Acad. Sci. USA 96: 14451–14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, I., C. H. Girdham and P. H. O'Farrell, 1995. A nuclear GFP that marks nuclei in living Drosophila embryos; maternal supply overcomes a delay in the appearance of zygotic fluorescence. Dev. Biol. 170: 726–729. [DOI] [PubMed] [Google Scholar]
- Finley, K. D., B. J. Taylor, M. Milstein and M. McKeown, 1997. dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 94: 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, S. T., and J. W. Gowen, 1957. Pigment-inducing potentialities of testes, ovaries and hermaphroditic (hr) gonads. J. Exp. Zool. 135: 5–18. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido, A., and J. R. Merriam, 1969. Cell lineage of the imaginal discs in Drosophila gynandromorphs. J. Exp. Zool. 170: 61–75. [DOI] [PubMed] [Google Scholar]
- Gehring, W. J., E. Wieschaus and M. Holliger, 1976. The use of “normal” and “transformed” gynandromorphs in mapping the primordial germ cells and the gonadal mesoderm in Drosophila. J. Embryol. Exp. Morphol. 35: 607–616. [PubMed] [Google Scholar]
- Gilboa, L., and R. Lehmann, 2004. How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development 131: 4895–4905. [DOI] [PubMed] [Google Scholar]
- Heller, A., and M. Steinmann-Zwicky, 1998. In Drosophila, female gonadal cells repress male-specific gene expression in XX germ cells. Mech. Dev. 73: 203–209. [DOI] [PubMed] [Google Scholar]
- Horabin, J. I., D. Bopp, J. Waterbury and P. Schedl, 1995. Selection and maintenance of sexual identity in the Drosophila germline. Genetics 141: 1521–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta, Y., and S. Benzer, 1972. Mapping of behaviour in Drosophila mosaics. Nature 240: 527–535. [DOI] [PubMed] [Google Scholar]
- Janzer, B., and M. Steinmann-Zwicky, 2001. Cell-autonomous and somatic signals control sex-specific gene expression in XY germ cells of Drosophila. Mech. Dev. 100: 3–13. [DOI] [PubMed] [Google Scholar]
- Kankel, D. R., and J. C. Hall, 1976. Fate mapping of nervous-system and other internal tissues in genetic mosaics of Drosophila melanogaster. Dev. Biol. 48: 1–24. [DOI] [PubMed] [Google Scholar]
- King, R. C., 1970. Ovarian Development in Drosophila melanogaster. Academic Press, New York.
- Lawrence, P. A., and P. Johnston, 1986. The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell 45: 505–513. [DOI] [PubMed] [Google Scholar]
- Lewis, E. B., and W. Gencarella, 1952. claret and non-disjunction in Drosophila melanogaster. Genetics 37: 600–601. [Google Scholar]
- Marsh, J. L., and E. Wieschaus, 1978. Is sex determination in germ line and soma controlled by separate genetic mechanisms? Nature 272: 249–251. [DOI] [PubMed] [Google Scholar]
- McKeown, M., J. M. Belote and R. T. Boggs, 1988. Ectopic expression of the female transformer gene product leads to female differentiation of chromosomally male Drosophila. Cell 53: 887–895. [DOI] [PubMed] [Google Scholar]
- Meller, V. H., 2000. Dosage compensation: making 1X equal 2X. Trends Cell Biol. 10: 54–59. [DOI] [PubMed] [Google Scholar]
- Nöthiger, R., M. Jonglez, M. Leuthold, P. M. Gerschwiler and T. Weber, 1989. Sex determination in the germline of Drosophila depends on genetic signals and inductive somatic factors. Development 107: 505–518. [DOI] [PubMed] [Google Scholar]
- Oliver, B., 2002. Genetic control of germline sexual dimorphism in Drosophila. Int. Rev. Cytol. 219: 1–60. [DOI] [PubMed] [Google Scholar]
- Rudner, D. Z., K. S. Breger and D. C. Rio, 1998. Molecular genetic analysis of the heterodimeric splicing factor U2AF: the RS domain on either the large or small Drosophila subunit is dispensable in vivo. Genes Dev. 12: 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach, T., 1982. Autosomal mutations that interfere with sex determination in somatic cells of Drosophila have no direct influence on the germ line. Dev. Biol. 89: 117–127. [DOI] [PubMed] [Google Scholar]
- Schüpbach, T., 1985. Normal female germ cell differentiation requires the female X chromosome to autosome ratio and expression of Sex-lethal in Drosophila melanogaster. Genetics 109: 529–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach, T., E. Wieschaus and R. Nöthiger, 1978. A study of the female germ line in mosaics of Drosophila. Rouxs Arch. Dev. Biol. 184: 41–56. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky, M., 1994. Sex determination of the Drosophila germ line: tra and dsx control somatic inductive signals. Development 120: 707–716. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky, M., H. Schmid and R. Nöthiger, 1989. Cell autonomous and inductive signals can determine the sex of the germline of Drosophila by regulating the gene Sxl. Cell 57: 157–166. [DOI] [PubMed] [Google Scholar]
- Straub, T., I. K. Dahlsveen and P. B. Becker, 2005. Dosage compensation in flies: mechanism, models, mystery. FEBS Lett. 579: 3258–3263. [DOI] [PubMed] [Google Scholar]
- Sturtevant, A. H., 1929. The claret mutant type of Drosophila simulans: a study of chromosome elimination and of cell-lineage. Z. Wiss. Zool. 135: 323–356. [Google Scholar]
- Szabad, J., and R. Nothiger, 1992. Gynandromorphs of Drosophila suggest one common primordium for the somatic cells of the female and male gonads in the region of abdominal segments 4 and 5. Development 115: 527–533. [DOI] [PubMed] [Google Scholar]
- Waterbury, J. A., J. I. Horabin, D. Bopp and P. Schedl, 2000. Sex determination in the Drosophila germline is dictated by the sexual identity of the surrounding soma. Genetics 155: 1741–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawersik, M., A. Milutinovich, A. L. Casper, E. Matunis, B. Williams et al., 2005. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 436: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm, J. E., and C. A. Smilbert, 2005. Mechanisms of translational regulation in Drosophila. Biol. Cell 97: 235–252. [DOI] [PubMed] [Google Scholar]
- Yamamoto, A. H., D. J. Komma, C. D. Shaffer, V. Pirrotta and S. A. Endow, 1989. The claret locus in Drosophila encodes products required for eye color and for meiotic chromosome segregation. EMBO J. 8: 3543–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]