Abstract
N-ethyl-N-nitrosourea (ENU)-induced mutagenesis provides a powerful approach for identifying genes involved in immune regulation and diseases. Here we describe a new mutant strain, HLB368, with hereditary leukopenia. At necropsy, the mutant mice had very small thymuses and spleens. All but the inguinal nodes were absent and there were no Peyer's patches. By flow cytometry, the ratios of T-cell subsets were normal, but B-cell development was blocked at the pre-pro-B-cell stage. The development of B1 and marginal zone B cells was relatively normal. The mutation was mapped to chromosome 3 between D3Mit221 and D3Mit224, a region that contains the Il7 gene. cDNA and genomic DNA sequences of Il7 revealed a T-to-C missense transition resulting in a change of Leu to Pro within the leader peptide that would be predicted to inhibit secretion. In keeping with this concept, we found that in vitro treatment of B-cell progenitors from mutant mice with IL-7 induced them to differentiate into pre-BII cells. Phenotypic comparisons of HLB368 with genetically targeted Il7 null mice showed many similarities along with a few differences, indicating that this ENU-induced mutant carries a novel allele. This new strain thus provides a new model for studying the functions of IL-7 on a pure C57BL/6 background.
THE cytokine interleukin seven (IL-7) was discovered in 1988 by Namen et al. (1988a) as a growth factor for B-cell progenitors. Extensive studies have found a central role for IL-7 in development of both B and T lymphocytes. IL-7 is a member of the type I cytokine family and binds to the IL-7 receptor (IL-7R), which is expressed only at certain stages of lymphoid cell development. The IL-7R is composed of two elements, the common γ-chain, γc, encoded by Il2rg, and the IL-7Rα chain, encoded by Il7r. The γc chain is a shared component of the receptors for IL-2, IL-4, IL-9, IL-15, and IL-21. The IL-7Rα chain is also a component of the receptor for thymic stromal lymphopoietin (TSLP). The paired components of the IL-7R pair transmit signals regulating the proliferation of early lymphocytes in the bone marrow (BM) and thymus and homeostasis of peripheral T cells (Fry and Mackall 2005). IL-7 is produced primarily by nonhematopoietic cells including stromal cells in the BM and thymus and epithelial cells in the skin and intestine (Namen et al. 1988b; Sakata et al. 1990; Heufler et al. 1993; Watanabe et al. 1995).
The requirement for IL-7 in development of lymphocytes has been demonstrated in experiments using blocking antibodies and with genetically engineered mice with a null allele of Il7. Neutralization of IL-7 or blocking of the IL-7R in vivo results in rapid depletion of BM B-lineage cells and thymic T cells (Grabstein et al. 1993; Sudo et al. 1993). In addition, IL-7- or IL-7R-deficient mice exhibit severe lymphopenia of both B and T lineages (Peschon et al. 1994; von Freeden-Jeffry et al. 1995; Rich 1997). The identification of IL-7Rα chain mutations in patients with T−B+NK+ severe combined immunodeficiency disease showed that IL-7 is also essential for human T-cell development, but not for B-cell differentiation (Puel et al. 1998). However, because one of the functions of IL-7 is to induce clonal expansion, it is highly possible that IL-7 signaling may contribute to the diversity of the human B-cell repertoire (Monroe and Allman 2004).
N-ethyl-N-nitrosourea (ENU)-induced mutagenesis has provided a powerful approach for identifying genes involved in normal biological processes and diseases. ENU introduces single-point mutations at a rate ∼100-fold higher than the rate of spontaneous mutations (Beier 2000; Coghill et al. 2002). Mice with phenotypically discernible point mutations in single genes are being generated in large numbers through several ongoing ENU mutagenesis screens, including one at the Jackson Laboratory funded by the National Heart, Lung and Blood Institute as part of the Mouse Heart, Lung, Blood and Sleep Disorders Center. Progeny mice are tested for a large number of physiologic parameters, including peripheral white blood cell (WBC) counts. A phenotypic deviant with a decreased WBC count was bred and the trait was found to be heritable. The characteristics of the mutant mice and genetic analysis identify the mutant as occurring in the Il7 gene. The availability of this mutation on a pure C57BL/6 background provides a new opportunity for studying the roles played by IL-7 in the development and function of the immune system and in various disease conditions.
MATERIALS AND METHODS
Animals:
C57BL/6J (B6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). A mutant B6 mouse, HLB368, was generated as part of an ENU project in the Mouse Heart, Lung, Blood and Sleep Disorders Center at the Jackson Laboratory. A detailed description of the project can be found at http://pga.jax.org/about/index.html. HLB368 mice exhibited heritable recessive leukopenia (http://pga.jax.org/reports/43691.html). Except as indicated in supplemental data (at http://www.genetics.org/supplemental/), 5-month-old mice were used for fluorescence-activated cell sorting (FACS) analysis.
Flow cytometric analyses:
Single-cell suspensions were prepared from bone marrow, spleen, thymus, lymph node (LN), and peritoneum washouts as described previously (Wang et al. 2001). Cells were stained with FITC-, phycoerythrin-, allophycocyanin-, or biotin-conjugated antibodies specific for mouse B220, CD3, CD4, CD5, CD8a, CD11b, CD11c, CD19, CD21, CD23, CD24 (HSA), CD25, CD40, CD43, CD44, CD69, CD80, CD86, MHC class II, immunoglobulin (IgM), Gr-1, and NK1.1 (BD-Biosciences, San Diego). Biotinylated antibodies were revealed with streptavidin-conjugated Percp (BD-Biosciences). Cells were analyzed using a FACSCalibur (Becton Dickinson, Mountain View, CA). Data were analyzed by WinMDI software (Scripps Institute, La Jolla, CA).
Mutation mapping:
Genetic mapping was performed using an F2 intercross with C3H/HeJ mice and the mutation was mapped to an interval of 14 cM on chromosome 3 bounded by D3Mit221 and D3Mit224 (http://pga.jax.org/reports/43691.html).
Identification of the Il7 mutation:
Total RNA was extracted from spleens of B6 and HLB368 mice using a RNeasy mini kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. A DNA digestion step was included before elution of RNA. cDNA was synthesized with SuperScript III reverse transcriptase and oligo(dT) primers (Invitrogen Life Technologies, Carlsbad, CA). PCR primers for cloning were Il7 sense, 5′-AGTTATGGCAAAGCCAGAGCG-3′, and Il7 antisense, 5′-ATTTGAGCAGGAGGCATCCAG-3′. Genomic DNA was prepared from the tails of B6 and HLB368 mice. PCR primers for cloning were Il7 sense, 5′-CCTCCCCATCGGATACCTC-3′; Il7 antisense, 5′-TGCCTCCTTTCTTGTCAGC-3′. Amplicons of both cDNA and genomic DNA were cloned into pGEM-T Easy vector (Promega, Madison, WI) and sequenced by the Research Technology Branch, National Institute of Allergy and Infectious Diseases/National Institutes of Health (Hamilton, MT).
Bone marrow culture:
Red blood cells in bone marrow cells of B6 and HLB368 mice were lysed with ACK lysing buffer (Cambrex Bio Science, Walkersville, MD). Cells were cultured at 1 × 106/ml in Iscove's modified Dulbecco's media medium supplemented with 10% fetal bovine serum and 10 ng/ml of IL-7 (R & D Systems, Minneapolis) for 6 days. The cells were then harvested, stained with antibodies recognizing IgM, B220, and CD25, and analyzed by FACS.
RESULTS
Identification of mutant HLB368 and genetic mapping studies:
WBC counts performed as part of the screening of third-generation mutagenized mice identified a phenotypic deviant with a significantly decreased WBC count on nonfasted whole-blood analysis (WBC was 1.5 × 103 cells/μl). The heritability of the phenotype was confirmed and mapped to an interval of 14 cM on chromosome 3 between D3Mit221 and D3Mit224 (http://pga.jax.org/reports/43691.html).
Characteristics of lymphoid organs are abnormal in mutant mice:
At necropsy, HLB368 mice were seen to have significantly smaller thymuses and spleens. While inguinal LN were detectable at markedly reduced size, all other peripheral LN (popliteal, cervical, axillary), mesenteric nodes, and Peyer's patches were not present. Histological analysis of spleens from mutant mice revealed grossly normal structures including the well-organized white pulp and follicles. The marginal zone of the follicle was well demarcated by the marginal sinus (not shown). The thymuses of mutant mice had normal medullary and cortical zones (data not shown).
ENU mutant mice exhibit reduced T-cell numbers but normal T subsets in thymus and spleen:
The cellularity of thymuses and spleens of HLB368 mice was reduced to 10 and 40% of normal mice, respectively (Figure 1A). In the thymus, FACS analysis indicated that the percentages of both CD4 and CD8 lineage cells were normal. However, the ratios of CD4 to CD8 cells of mutant mice were slightly but consistently increased compared with those of normal mice (Figure 1B). In the spleen, the ratios of CD4 to CD8 cells were normal even though the absolute number of T cells was reduced by nearly 80% (Figure 1C). These data suggested that the mutation primarily affected T-cell proliferation and expansion rather than differentiation.
Figure 1.—
Analysis of cellularity and T-cell phenotype in lymphoid organs. (A) Total cell numbers of thymus and spleen from mice aged 2–3 months. The data are means ±SD of four mice from each group. (B) Thymocytes were stained with antibodies against CD4 and CD8 and analyzed by FACS. The numbers are percentages of cells falling in each quadrant. The data are representative of six mice analyzed. (C) The absolute numbers of lymphocyte subsets in the spleen of B6 and HLB368 mice. The data are means ±SD of four mice from each group.
Abnormal B-cell development in ENU mutant mice:
In the BM, the cellularity was comparable between normal and mutant mice (data not shown). However, the numbers of IgM+ and IgM− B cells were reduced to 10 and 39% of normal, respectively (Figure 2). Among the few IgM− B cells of mutants, there were no HSA+ and BP-1+ B cells, indicating that the B-cell development was blocked at the transition from Hardy fraction (Fr.) A (pre-pro-B) to Fr. B/C cells (early/late pro-B) (Figure 2). The lack of typical CD25+ and CD43− Fr. D (pre-BII) cells (Figure 2) was consistent with a block before the pre-BII cell stage.
Figure 2.—
FACS analysis of BM B cells. BM cells from B6 and HLB368 mice were stained with the indicated antibodies to identify Hardy Fr. A to Fr. F subsets. The numbers are percentages of cells falling in each gate. The data are representative of four mice analyzed.
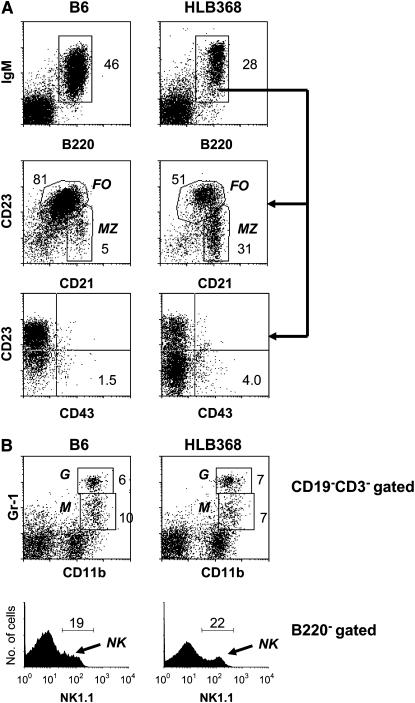
In the spleens of mutant mice, the absolute number of B220+IgM+ B cells was reduced by 70% (Figure 1C). Among the B cells, the number of follicular B2 cells (CD23+CD21low) was reduced by 87% whereas the number of marginal zone (MZ) B cells (CD23−CD21high) was slightly increased (Figure 3A and Figure 1C). This result was consistent with the view that impaired early B-cell development is associated with a disproportionate filling of the MZ B-cell compartment (Martin and Kearney 2002). Interestingly, the proportions of follicular (FO), MZ cells, and B1 cells (CD43+, CD23−, CD5+, and CD11b+) changed progressively with age (supplemental Figure 1S at http://www.genetics.org/supplemental/). The spleens of 18-month-old mutant mice had essentially no FO or MZ B cells while the frequency of B1-like cells (CD43+, CD23−, CD5±, and CD11b±) increased dramatically. This may be due to the self-replenishing nature of B1 cells and the diminished production of BM immature B cells required to maintain the MZ and FO B-cell pools in aged mice.
Figure 3.—
FACS analysis of spleen cells. Spleen cells from B6 and HLB368 mice were stained with the indicated antibodies and analyzed by FACS. (A) IgM, CD23, CD21, and CD43 expression on gated B cells. The numbers are percentages of cells falling in each gate. FO, follicular; MZ, marginal zone. (B) Staining patterns of granulocytes, macrophages, and natural killer (NK) cells. The numbers are percentages of cells falling in each gate. The data are representative of four mice analyzed. G, granulocytes; M, macrophages.
In the peritoneal cavity, the numbers of B1 cells, including both the CD5+ B1a and the CD5−CD11b+ B1b subsets, were relatively normal whereas those of CD23+ B2 cells were decreased in mutant mice (Figure 4, A and B). The reduction of B2 cells in the peritoneum was in keeping with impaired follicular B2 cell development in the spleen (Figure 3). Together, these analyses indicated that the mutation had a profound impact on B-cell development.
Figure 4.—
FACS analysis of B-cell development in the peritoneum and spleen. (A) Peritoneal cells from B6 and HLB368 mice were stained with antibodies against CD19, IgM, CD23, CD5, and CD11b. The numbers are percentages of cells falling in each gate. Each subset is indicated. The data are representative of five mice analyzed. (B) Absolute numbers of peritoneal B-cell subsets. Data are means ±SD of three mice aged 2–3 months. (C) Splenic cells were stained and analyzed by FACS to reveal activation marker expression. The cells are gated on B220+IgM+ B cells. Data are representative of three mice from each group.
Splenic B cells of ENU mutant mice exhibit an activated phenotype:
To determine whether B-cell lymphopenia is associated with peripheral B-cell activation, as previously reported (Kitamura et al. 1992; Agenes and Freitas 1999), splenic B cells were stained with antibodies recognizing several activation markers and analyzed by FACS. As shown in Figure 4C, splenic B cells of mutant mice expressed higher levels of CD40, CD80, CD86, and CD69 than those from normal mice. The expression levels of MHC class II were comparable between mutant and normal B cells (data not shown). These data suggested that the majority of splenic B cells of mutant mice were activated, similar to that seen in other mice with B-cell lymphopenia (Kitamura et al. 1992; Agenes and Freitas 1999; Carvalho et al. 2001).
Normal myeloid cell development in ENU mutant mice:
FACS analysis of myeloid cells in the spleen indicated that the numbers of Gr1hiCD11bhi granulocytes, Gr1intCD11bhi macrophages, and NK1.1+CD3-CD11c− NK cells were comparable between HLB368 and B6 mice (Figure 3B). These data indicated that the mutation had no effect on the development of myeloid lineage cells.
Identification of a point mutation in the Il7 gene:
The HLB368 mutation was mapped to chromosome 3 between 2 and 18 cM. An analysis of genes lying within this interval suggested Il7 as a candidate, as mice with knockouts of this gene (von Freeden-Jeffry et al. 1995; Rich 1997) exhibited phenotypes similar to HLB368 (Table 1). Sequencing of Il7 genomic and cDNA from the mutant mice revealed a T-to-C exchange resulting in a substitution of Leu to Pro at amino acid 15 of the leader signal peptide (Figure 5). This mutation could be predicted to impair IL-7 translocation through the endomembrane system leading to failed secretion.
TABLE 1.
Phenotypes of HLB368 and IL-7 knockout mice
| Strains | HLB368 | IL-7−/− (von Freeden-Jeffery et al. 1995) | IL-7−/− (Rich 1997) |
|---|---|---|---|
| Genomic clone | NA | 129/SV | 129/Sv |
| ES cells | NA | 129P2/OlaHsd | 129S6/SvEvTac |
| Strain | B6 | B6;129 | 129; NIH Black Swiss |
| Neo cassette | NA | + | + |
| Thymus and spleen | Reduced in size and weight | Reduced in size and weight | Reduced in size and weight |
| LN | Inguinal nodes only | One | NR |
| WBC | Decreased | Decreased | NR |
| Cellularity | |||
| BM | Normal | Normal | Decreased by 40% |
| Spleen | Reduced by 60% | Reduced by 85% | Reduced by 70% |
| Thymus | Reduced by 90% | Reduced by 95% | Reduced by 95% |
| B-cell development | |||
| Early | Blocked at Fr. A to B/C transition | Blocked at Fr. C to D or Fr. A to B/C transition | NR |
| Late | Decreased FO subset; B1a and MZ B cells are normal; splenic B cells are CD43− but express activation markers. | Decreased FO subset; B1a and MZ B cells are normal; splenic B cells express CD43 and activation markers. | NR |
| T-cell development | Decreased in numbers; the percentages of CD4 and CD8 cells are normal. | Decreased in numbers; the percentages of CD4 and CD8 cells are normal. | Decreased in numbers; the percentages of CD4 and CD8 cells are normal. |
| Reference | This study | von Freeden-Jeffry et al. (1995); Carvalho et al. (2001) | Rich (1997) |
NA, not applicable; NR, not reported.
Figure 5.—
DNA sequence of Il7. The highlight indicates a T-to-C transition in the Il7 gene. This single-base-pair substitution caused a change in amino acid 15 of the leader peptide sequence, which would affect IL-7 translocation in the endomembrane system, leading to failed secretion.
Exogenous IL-7 rescued pre-B-cell development in vitro:
Pro-B-cell survival and differentiation in vitro requires IL-7 (reviewed in Milne and Paige 2006). To determine whether B-cell progenitors from mutant mice can survive and differentiate in the presence of exogenous IL-7, we cultured BM cells from HLB368 and B6 mice with high concentrations of IL-7 for 6 days. FACS analysis indicated that BM cells from mutant mice gave rise to CD25+ pre-BII cells, which were not seen in freshly isolated BM cells (Figure 6 and Figure 2). These data suggested that exogenous IL-7 promoted the development of earlier B-lineage cells of mutant mice. This was in agreement with previous studies in which injection of IL-7 into IL-7−/− mice induced B-cell differentiation (Wei et al. 2000).
Figure 6.—
Enforced differentiation of mutant BM B-cell progenitors by exogenous IL-7 in vitro. BM cells from B6 and HLB368 mice were cultured in the presence of 10 ng/ml of IL-7 for 6 days. Cells were stained with antibodies to IgM, CD25, and B220 and analyzed by FACS. The numbers are percentages of cells falling in each gate. The data represent one of two independent experiments.
DISCUSSION
In this report, we describe a new mutant strain, HLB368, recovered from an ENU-induced mutagenesis screen of B6 mice. We found that HLB368 mice exhibited heritable leukopenia due to a T-to-C missense transition in the Il7 gene that resulted in a Leu-to-Pro change at amino acid 15 of the leader peptide. This could cause the IL-7 protein to be retained intracellularly.
Despite many similarities between HLB368 and IL-7 null mice (Table 1), such as severe lymphopenia, impaired pro-B-cell development in the BM, and normal development of MZ and B1 cells in the periphery, there were inconsistencies between the two strains. First, HLB368 mice have inguinal LN, albeit at a reduced size, whereas IL-7−/− mice do not (von Freeden-Jeffry et al. 1995). Second, the stage at which B-cell differentiation was blocked in HLB368 mice was shown to be at the transition from Fr. A to B/C. Previous studies of a single strain of Il7 knockout mice came to the same conclusion (Carvalho et al. 2001) or suggested that the arrest occurred at a later stage, either between Fr. B/C and D or between Fr. C and C′ (von Freeden-Jeffry et al. 1995; Wei et al. 2000). Third, when analyzed at a relatively young age, only ∼4% of splenic B cells of HLB368 mice expressed CD43, whereas up to 20% of splenic B cells in IL-7−/− mice expressed high levels of CD43, a phenotype similar to that of B1 cells (Carvalho et al. 2001). The reasons for these discrepancies are unknown. This could be due to differences in genetic background, methods of analysis, and/or the effect of gene-targeting events. For example, engineered strains harbor a linked neomycin resistance gene (Neor) used for selection of engineered embryonic stem (ES) cells. Insertion of the Neor gene into different sites within the immunoglobulin heavy chain locus has been found to result in profound effects on expression of genes located as far as 100 kb upstream of the insert (Cogne et al. 1994; Seidl et al. 1999; Manis et al. 2003). Similar effects of the Neor gene were also found in other targeted deletion models (Fiering et al. 1995; Nagy 2000). However, the single base mutation found in HLB368 would not be expected to have such effects. Thus, the HLB368 strain has clear advantages over engineered Il7 null strains for cleanly determining the functions of IL-7.
Even though the splenic B cells of HLB368 mice were CD43−, they expressed high levels of activation markers. Splenic T cells of HLB368 mice exhibited upregulation of activation markers (data not shown). A similar activation phenotype for B and T cells was also found in IL-7−/− mice (Carvalho et al. 2001). The mechanisms responsible for lymphocyte activation in IL-7-deficient mice are currently unknown, but the enriched MZ B cells in HLB368 mice may account, at least partially, for the activation phenotype of splenic B cells. MZ B cells are a specialized population located outside the follicles of the white pulp. Their origins are not well defined. Antigens are likely a main driving force for MZ B-cell development (Wen et al. 2005). Unlike resting follicular B2 cells, MZ B cells are relatively large in size and have many similarities with B1 cells (Martin and Kearney 2002). Their activation state and unique location at the blood–lymphoid interface render them able to quickly react to blood-borne pathogens (Martin et al. 2001). This study and others (Carvalho et al. 2001) support the conclusion that the development of MZ B cells is unlikely to be IL-7 dependent.
B1 cells are another specialized subset of B cells that are predominantly localized in the pleural and peritoneal cavities of mice. Unlike follicular B2 cells, which are generated from the BM throughout adult life, B1 cells are generated during fetal and neonatal development and are maintained throughout life by self-renewal (Hardy 2006). The relatively normal numbers of B1 cells, including both B1a and B1b subsets in HLB368 mice, strongly indicate that the development of B1 cells is IL-7 independent. A similar conclusion was reached from studies of IL-7−/− mice (Carvalho et al. 2001). These results differ from those obtained with studies of IL-7Rα knockout mice, which showed that the numbers of B1 cells were reduced (Erlandsson et al. 2004; Hesslein et al. 2006). Because IL-7Rα is also a component of the receptor for TSLP, a cytokine contributing to B-cell development early in life (Vosshenrich et al. 2003), deficiency of IL-7Rα would be expected to abrogate the function of both IL-7 and TSLP and to induce more severe defects in fetal B-cell development than those observed in IL-7-deficient mice.
The point mutation affecting the leader peptide of IL-7 may have abrogated secretion of the cytokine by blocking its release from the endoplasmic reticulum. By intracellular fluorescence staining and ELISA analysis using cultured BM stromal cells or freshly isolated BM cells, we failed to detect an increase of intracellular IL-7 in HLB368 stromal cells compared with normal controls (data not shown). This raises the possibility that the mutant peptide may interfere with translation of IL-7 possibly through a feedback inhibition mechanism. Further studies will be necessary to determine if this is the case or if the defect lies simply in a failure to secrete the protein. Nonetheless, the similarities between the phenotypes of HLB368 mutant mice and mice with a null mutation of IL7 indicate that the block to IL-7 expression in the mutants is complete.
In summary, the phenotype of HLB368 mice is consistent with that of mice with a genetically engineered null mutation of Il7. Genetic analyses identified a point mutation affecting the sequences of the leader peptide of the Il7 gene, resulting in a seemingly complete block in IL-7 secretion. Thus, this new mutant on a pure C57BL/6 background should provide a valuable model for understanding the biology and functions of IL-7.
Acknowledgments
We thank Karen L. Svenson at the Jackson Laboratory for providing us with the HLB368 mice and for a critical reading of the article. This work was supported by a National Heart, Lung and Blood Institute Programs for Genomic Application grant (HL66611) (to the Jackson Laboratory) and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Genotyping to identify the genomic location of HLB368 was performed by the Fine Mapping Laboratory of Jackson Laboratory Scientific Services. The authors have no conflicts of interest.
References
- Agenes, F., and A. A. Freitas, 1999. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J. Exp. Med. 189: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier, D. R., 2000. Sequence-based analysis of mutagenized mice. Mamm. Genome 11: 594–597. [DOI] [PubMed] [Google Scholar]
- Carvalho, T. L., T. Mota-Santos, A. Cumano, J. Demengeot and P. Vieira, 2001. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J. Exp. Med. 194: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill, E. L., A. Hugill, N. Parkinson, C. Davison, P. Glenister et al., 2002. A gene-driven approach to the identification of ENU mutants in the mouse. Nat. Genet. 30: 255–256. [DOI] [PubMed] [Google Scholar]
- Cogne, M., R. Lansford, A. Bottaro, J. Zhang, J. Gorman et al., 1994. A class switch control region at the 3′ end of the immunoglobulin heavy chain locus. Cell 77: 737–747. [DOI] [PubMed] [Google Scholar]
- Erlandsson, L., S. Licence, F. Gaspal, S. Bell, P. Lane et al., 2004. Impaired B-1 and B-2 B cell development and atypical splenic B cell structures in IL-7 receptor-deficient mice. Eur. J. Immunol. 34: 3595–3603. [DOI] [PubMed] [Google Scholar]
- Fiering, S., E. Epner, K. Robinson, Y. Zhuang, A. Telling et al., 1995. Targeted deletion of 5′HS2 of the murine beta-globin LCR reveals that it is not essential for proper regulation of the beta-globin locus. Genes Dev. 9: 2203–2213. [DOI] [PubMed] [Google Scholar]
- Fry, T. J., and C. L. Mackall, 2005. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 174: 6571–6576. [DOI] [PubMed] [Google Scholar]
- Grabstein, K. H., T. J. Waldschmidt, F. D. Finkelman, B. W. Hess, A. R. Alpert et al., 1993. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J. Exp. Med. 178: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, R. R., 2006. B-1 B cell development. J. Immunol. 177: 2749–2754. [DOI] [PubMed] [Google Scholar]
- Hesslein, D. G., S. Y. Yang and D. G. Schatz, 2006. Origins of peripheral B cells in IL-7 receptor-deficient mice. Mol. Immunol. 43: 326–334. [DOI] [PubMed] [Google Scholar]
- Heufler, C., G. Topar, A. Grasseger, U. Stanzl, F. Koch et al., 1993. Interleukin 7 is produced by murine and human keratinocytes. J. Exp. Med. 178: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura, D., A. Kudo, S. Schaal, W. Muller, F. Melchers et al., 1992. A critical role of lambda 5 protein in B cell development. Cell 69: 823–831. [DOI] [PubMed] [Google Scholar]
- Manis, J. P., J. S. Michaelson, B. K. Birshtein and F. W. Alt, 2003. Elucidation of a downstream boundary of the 3′ IgH regulatory region. Mol. Immunol. 39: 753–760. [DOI] [PubMed] [Google Scholar]
- Martin, F., and J. F. Kearney, 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2: 323–335. [DOI] [PubMed] [Google Scholar]
- Martin, F., A. M. Oliver and J. F. Kearney, 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14: 617–629. [DOI] [PubMed] [Google Scholar]
- Milne, C. D., and C. J. Paige, 2006. IL-7: a key regulator of B lymphopoiesis. Semin. Immunol. 18: 20–30. [DOI] [PubMed] [Google Scholar]
- Monroe, J. G., and D. Allman, 2004. Keeping track of pro-B cells: a new model for the effects of IL-7 during B cell development. Eur. J. Immunol. 34: 2642–2646. [DOI] [PubMed] [Google Scholar]
- Nagy, A., 2000. Cre recombinase: the universal reagent for genome tailoring. Genesis 26: 99–109. [PubMed] [Google Scholar]
- Namen, A. E., S. Lupton, K. Hjerrild, J. Wignall, D. Y. Mochizuki et al., 1988. a Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature 333: 571–573. [DOI] [PubMed] [Google Scholar]
- Namen, A. E., A. E. Schmierer, C. J. March, R. W. Overell, L. S. Park et al., 1988. b B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J. Exp. Med. 167: 988–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon, J. J., P. J. Morrissey, K. H. Grabstein, F. J. Ramsdell, E. Maraskovsky et al., 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180: 1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel, A., S. F. Ziegler, R. H. Buckley and W. J. Leonard, 1998. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat. Genet. 20: 394–397. [DOI] [PubMed] [Google Scholar]
- Rich, B. E., 1997. Autocrine expression of interleukin-7 rescues lymphoid expansion in interleukin-7-deficient mice. Immunology 92: 374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata, T., S. Iwagami, Y. Tsuruta, H. Teraoka, Y. Tatsumi et al., 1990. Constitutive expression of interleukin-7 mRNA and production of IL-7 by a cloned murine thymic stromal cell line. J. Leukoc. Biol. 48: 205–212. [DOI] [PubMed] [Google Scholar]
- Seidl, K. J., J. P. Manis, A. Bottaro, J. Zhang, L. Davidson et al., 1999. Position-dependent inhibition of class-switch recombination by PGK-neor cassettes inserted into the immunoglobulin heavy chain constant region locus. Proc. Natl. Acad. Sci. USA 96: 3000–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo, T., S. Nishikawa, N. Ohno, N. Akiyama, M. Tamakoshi et al., 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA 90: 9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Freeden-Jeffry, U., P. Vieira, L. A. Lucian, T. McNeil, S. E. Burdach et al., 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181: 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich, C. A., A. Cumano, W. Muller, J. P. Di Santo and P. Vieira, 2003. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat. Immunol. 4: 773–779. [DOI] [PubMed] [Google Scholar]
- Wang, H., J. Ye, L. W. Arnold, S. K. McCray and S. H. Clarke, 2001. A VH12 transgenic mouse exhibits defects in pre-B cell development and is unable to make IgM+ B cells. J. Immunol. 167: 1254–1262. [DOI] [PubMed] [Google Scholar]
- Watanabe, M., Y. Ueno, T. Yajima, Y. Iwao, M. Tsuchiya et al., 1995. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Invest. 95: 2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, C., R. Zeff and I. Goldschneider, 2000. Murine pro-B cells require IL-7 and its receptor complex to up-regulate IL-7R alpha, terminal deoxynucleotidyltransferase, and c mu expression. J. Immunol. 164: 1961–1970. [DOI] [PubMed] [Google Scholar]
- Wen, L., J. Brill-Dashoff, S. A. Shinton, M. Asano, R. R. Hardy et al., 2005. Evidence of marginal-zone B cell-positive selection in spleen. Immunity 23: 297–308. [DOI] [PubMed] [Google Scholar]