Abstract
The receptor tyrosine kinase ErbB2 plays a crucial role in tumorigenesis. We showed previously that the molecular chaperone Hsp90 protects ErbB2 from proteasome-mediated degradation by binding to a short loop structure in the N-lobe of the kinase domain. Here we show that loss of Hsp90 binding correlates with enhanced ErbB2 kinase activity and its transactivating potential, concomitant with constitutively increased phosphorylation of Tyr877, located in the activation loop of the kinase domain. We show further that Tyr877 phosphorylation is mediated by Src and that it is necessary for the enhanced kinase activity of ErbB2. Finally, computer modeling of the kinase domain suggests a phosphorylation-dependent reorientation of the activation loop, denoting the importance of Tyr877 phosphorylation for ErbB2 activity. These findings suggest that Hsp90 binding to ErbB2 participates in regulation of kinase activity as well as kinase stability.
The erbB2 gene encodes a 185-kDa glycoprotein, which is a member of the ErbB family of receptor tyrosine kinases that also includes the epidermal growth factor receptor (EGFR/ErbB1), ErbB3, and ErbB4. They are transmembrane proteins, comprising an N-terminal extracellular domain that is involved in ligand binding, a single transmembrane domain, and an intracellular domain that includes a tyrosine kinase domain and a C-terminal tail containing multiple phosphorylation sites. Crystal structures show that the extracellular domains of ErbB1, ErbB3, and ErbB4 adopt a tethered conformation, with the dimerization loop buried by intramolecular interactions (5, 6, 20). Ligand binding induces conformational change of the extracellular domain, exposing the dimerization loop and resulting in receptor dimerization. Homodimerization and heterodimerization activate intrinsic receptor kinase activity, leading to autophosphorylation of tyrosine residues in the C-terminal tail. Unlike the other family members, no soluble ligand has been found for ErbB2. On the other hand, the ErbB2 extracellular domain adopts a conformation with the dimerization loop exposed in the absence of ligand binding, raising the question of how ErbB2 dimerization and activation are regulated.
One way to regulate receptor tyrosine kinase activity is via phosphorylation of its activation loop (A-loop). Phosphorylation stabilizes the A-loop in an open and extended conformation that is permissive for substrate binding (15). This phosphorylation is often mediated by intermolecular action between the protomers of a receptor dimer, but sometimes it is carried out by Src kinase, as shown for focal adhesion kinase and ZAP-70 kinase (8, 30). Src-dependent phosphorylation of ErbB1 on Tyr845 in the A-loop has been observed (34). Surprisingly, however, Tyr845 phosphorylation seems not to affect ErbB1 kinase activity, as ErbB1 carrying a Y845F mutation is fully active (34). Phosphorylation, in tumor cells, on the analogous site in ErbB2, Tyr877, was reported recently (12, 27), but the functional implication is not clear. In contrast to ErbB1, data reported for the oncogenic rat protein ErbB2/neu, which carries an activating point mutation in the transmembrane domain, suggest that A-loop phosphorylation is important for intrinsic kinase activity, as mutation of the analogous Tyr882 to phenylalanine compromised kinase activity (39).
The molecular chaperone Hsp90 is a key mediator of ErbB2 stability (10). Pharmacologic disruption of Hsp90 association results in ErbB2 polyubiquitination that is detectable within 15 min (23). Recently, Hsp90 was proposed also to modulate ErbB2 dimerization and kinase activity (11). These investigators observed enhanced ErbB2 phosphorylation 1 min after treating cells with the Hsp90 inhibitor geldanamycin. However, because Hsp90 inhibition results in rapid ErbB2 degradation and may also contribute additional confounding effects by simultaneously affecting multiple Hsp90 clients, it is difficult to use this approach to understand the specific role of Hsp90 in regulating ErbB2 activity. We and others have shown that Hsp90, together with its kinase-specific cochaperone p50cdc37, binds to the ErbB2 kinase domain, depending on the hyrdropathic properties of the loop (M5 loop) between the αC helix and the β4 strand in the N-lobe of the kinase domain (11, 38). Disruption of Hsp90 binding by mutation of the M5 loop (ErbB2-5M) neither destabilizes ErbB2 nor alters the predicted three-dimensional structure of its kinase domain (38). ErbB2-5M thus provides an opportunity to further investigate Hsp90 regulatory effects on ErbB2. In this study, concomitant with loss of Hsp90 binding, we observed an augmentation of ErbB2 kinase activity mediated by Src family kinases.
MATERIALS AND METHODS
Chemicals and antibodies.
Geldanamycin was from Kosan Biosciences (Hayward, CA). Src inhibitor PP1 was from Biomol (Plymouth Meeting, PA). Epidermal growth factor (EGF) and heregulin-β1 (HRG) were from Lab Vision (Fremont, CA). Rabbit antibodies for ErbB2 and ErbB3, goat antibody for p50cdc37, and biotinylated monoclonal antibody for phosphorylated tyrosine (Y99) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for tubulin, Src (Ab-1), and ErbB2 (Ab-2 and Ab-5) were from Calbiochem (La Jolla, CA). Fyn and Yes monoclonal antibodies were from BD Bioscience (San Jose, CA). Hsp90 rat monoclonal antibody (SPA-835) was from Stressgen Biotechnologies (Victoria, British Columbia, Canada). Rabbit antibody specific for phosphorylated Tyr877 of ErbB2 was from Cell Signaling (Beverly, MA), and monoclonal antibody for phosphorylated Tyr1248 was from Lab Vision.
Plasmid constructs.
pcDNA3-ErbB2, pcDNA3-ErbB2-5M, pcDNA3-ErbB2-K753A, and chimeric ErbB1/2 were previously described (37, 38). ErbB3-expressing vector pEV7-HER3 was a kind gift from Ke Zhang at Amgen, Inc. (40). Plasmids expressing wild-type and dominant-negative Src were from Upstate Biotechnology (Lake Placid, NY). Y877F and Y877D point mutations of ErbB2 were made by using the QuickChange XL kit (Stratagene, La Jolla, CA).
Cell culture and transient transfection.
Mouse SFY, SFY-Src, HEK-293, NIH 3T3, and COS7 cells were purchased from American Type Culture Collection (Manassas, VA) and cultured as directed by the provider. Transient transfection of COS7 and HEK-293 cells was performed by using FuGene 6 (Roche Diagnostics, Indianapolis, IN), following the manufacturer's instructions. Transfected cells were continuously cultured in the same medium for 24 to 48 h before lysis. Transfection of SFY and SFY-Src cells was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
Colony-forming assay.
NIH 3T3 cells were transfected with wild-type ErbB2, ErbB2-5M, or the pcDNA3 vector using FuGene 6. Stably transfected cells were selected in medium containing 800 μg/ml G418. A total of 1.0 × 104 cells were plated in triplicate in 6 ml of 0.35% agarose (SeaPlaque; Cambrex, Rockland, ME) in complete growth medium overlaid on a 0.7% agarose base, also in complete growth medium. Three weeks after incubation, colonies were visualized after staining with 1.0 mg/ml p-iodonitrotetrazolium violet overnight and photographed by bright-field microscopy at a magnification of ×200.
RNA interference.
Small interfering RNAs (siRNAs) for Src, Fyn, and Yes (siRNA SMARTpool) were from Upstate, and control irrelevant RNA was from QIAGEN (Valencia, CA). HEK-293 cells at 40 to 50% confluence were transfected, using Lipofectamine 2000, at a final concentration of 60 nM siRNA. Seven hours after addition of siRNA, cells were transfected again with pcDNA3-ErbB2-5M, using FuGene as the transfection reagent. Three days after transfection, cells were lysed with modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 7.5, 1% NP40, 0.5% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 2 mM sodium orthovanadate, 2 mM NaF) for the examination of ErbB2 phosphorylation.
Immunoprecipitation and Western blotting.
ErbB2 was immunoprecipitated as described previously (37); ErbB3 was precipitated in the same way, except that the lysis buffer was modified RIPA buffer. Western blotting was performed as described previously (10).
In vitro kinase assay.
COS7 cells transiently expressing wild-type ErbB2 protein were lysed with RIPA buffer containing Complete protease inhibitors (Roche Diagnostics), and ErbB2 protein was immunoprecipitated. The pellet was washed twice with RIPA buffer and twice with kinase buffer (25 mM Tris, pH 7.5, 0.1 mM sodium orthovanadate, 20 mM MgCl2, 5 mM MnCl2) and then resuspended in kinase buffer supplemented with 2 mM diothiothreitol and 10 mM ATP. The mixture was aliquoted, added with or without purified Src protein (9 U/reaction; Upstate), PP1 (final concentration at 4 μM), and CI-1033 (final concentration at 0.2 μM). The reaction was carried out at 30°C for 30 min, with intermittent mixing, and stopped with the addition of sodium dodecyl sulfate sample buffer and heating at 100°C for 5 min. ErbB2 phosphorylation was detected by Western blotting.
Structure modeling.
The conformation of ErbB2 with phosphorylated Tyr877 was modeled based on the crystal structure of ErbB1 (PDB code 1m14) using the NEST program (http://cmm.cit.nih.gov/∼xiang) (35, 36). The conformation of the activation loop for ErbB2 with unphosphorylated Tyr877 was modeled with the loop prediction program LOOPY (36). Specifically, LOOPY starts with 10,000 randomly generated initial conformations, plus a number of conformations from corresponding segments of other kinases, including the phosphorylated conformation of ERBB2. All of the candidate conformations were subjected to energy minimization, and the top 3,000 candidates with the lowest energy were retained. These candidates were “fused” with each other: i.e., two loops (root-mean-square distance larger than 4 Å) exchange conformation with each other from the middle. The screening process was repeated with the newly generated and parent conformations. The conformation of the lowest energy was chosen after 5 rounds of loop fusion. The predicted conformation has a root-mean-square distance of 3.5 Å to the corresponding conformation of inactivated CDK6 (see Fig. 5f).
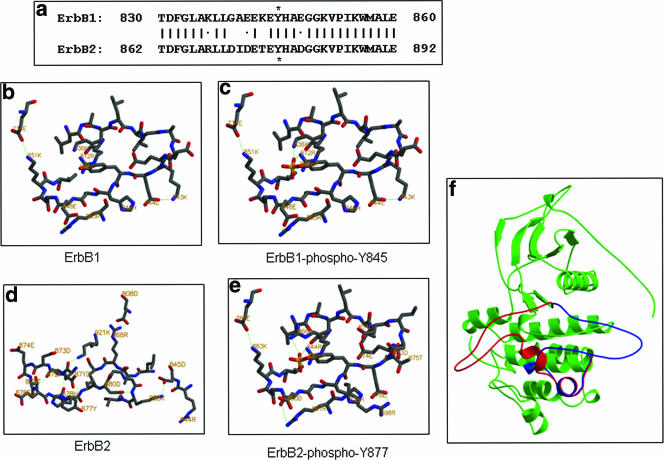
FIG. 5.
The A-loop of the ErbB2 kinase domain adopts a different conformation from that of ErbB1. (a) Sequence alignment of the A-loops of ErbB1 and ErbB2. Identical residues are indicated by a vertical bar, and similarity is indicated by a dot. Asterisks indicate the phosphorylated tyrosine residues. Panels b, c, d, and e are conformations of the A-loops of ErbB1 and ErbB2 in the unphosphorylated and phosphorylated states, respectively. Green dotted lines indicate salt bridges or hydrogen bonds. (f) Superimposition of the modeled structures of the ErbB2 kinase domain with the A-loop in the phosphorylated or unphosphorylated states. Red denotes the unphosphorylated A-loop, and blue denotes the phosphorylated A-loop.
RESULTS
ErbB2-5M is constitutively hyperphosphorylated and is a potent transactivator of ErbB3.
ErbB2-5M (whose M5 loop is mutated to ablate Hsp90 binding, see Fig. 1a) was examined for its ability to transactivate ErbB3, whose kinase domain is naturally inactive. HRG treatment of COS7 cells expressing ErbB3 alone resulted in moderate ErbB3 phosphorylation, likely mediated by endogenously expressed ErbB2 (Fig. 1b, lanes 1 and 2). Coexpression of wild-type ErbB2 substantially increased phosphorylation of ErbB3 by HRG (Fig. 1b, compare lanes 2 and 4). ErbB2-5M promoted efficient phosphorylation of ErbB3 in the presence of HRG (Fig. 1b, compare lanes 2 and 6 in the “p-Tyr” panel). In the absence of HRG, ErbB2-5M promoted phosphorylation of ErbB3 more efficiently than did wild-type ErbB2 (Fig. 1b, compare lanes 3 and 5 in the “p-Tyr” panel and the amount of ErbB2 expressed in the lower panel). These data indicate that loss of Hsp90 association does not compromise the ability of ErbB2-5M to serve as a dimerization partner for ErbB3; loss of chaperone binding actually promotes ErbB2-mediated transactivation.
FIG. 1.
Mutations in the M5 loop that dissociate Hsp90 promote ErbB2 hyperphosphorylation. (a) Mutations in the M5 loop disrupt ErbB2 association with Hsp90 complex. Wild-type (wt) and M5 mutant ErbB2 proteins transiently expressed in COS7 cells were immunoprecipitated (IP) with mouse antibodies. The association of Hsp90 and p50cdc37 was detected by Western blotting with rat and goat antibodies, respectively. ErbB2 protein was detected with rabbit antibody. (b) ErbB2-5M has higher activity in transactivating ErbB3. COS7 cells were transfected with ErbB3 plasmid together with either wild-type or mutant ErbB2 or empty vector pcDNA3, switched to serum-free medium 24 h after transfection, and continued in culture overnight. Half of the cells were treated with 1 nM HRG for 15 min at 37°C. ErbB3 proteins were precipitated with mouse anti-ErbB3 antibodies, and phosphorylation was detected with biotinylated mouse antiphosphotyrosine antibody and horseradish peroxidase-conjugated streptavidin. ErbB3 protein levels were detected with rabbit antibody. ErbB2 protein levels in total cell lysates were detected with mouse antibody. (c) ErbB2-5M is hyperphosphorylated. The phosphorylation of wild-type ErbB2 and ErbB2-5M in total cell lysates of transiently transfected COS7 cells was detected by Western blotting with specific antibodies. ErbB2 protein levels were confirmed by Western blotting. (d) Phase-contrast images of wild-type ErbB2- and ErbB2-5M-expressing NIH 3T3 cells demonstrate anchorage-independent growth. Stably transfected NIH 3T3 cells were plated in soft agar-containing six-well plates and incubated for 3 weeks. Acini were stained with p-iodonitrotetrazolium violet and photographed by bright-field microscopy. Note the larger size of ErbB2-5M acini and the absence of peripheral projections in the smaller ErbB2 acini.
To confirm that ErbB2-5M has a higher intrinsic kinase activity than wild-type ErbB2, we examined the autophosphorylation level on Tyr1248 by using a site-specific antibody. Tyr1248 phosphorylation was substantially higher in ErbB2-5M as compared to wild-type ErbB2 (Fig. 1c, middle panel), indicating that ErbB2-5M indeed has a higher intrinsic kinase activity.
Tyr882 phosphorylation of the A-loop of rat ErbB2/neu is important for its intrinsic kinase activity (39). We determined whether the analogous Tyr877 is phosphorylated to a higher level in ErbB2-5M than in the wild-type kinase, and we found that it was dramatically increased (Fig. 1c, bottom panel). Taken together, these data suggest that dissociation of Hsp90 from ErbB2 correlates with increased kinase activity, concomitant with enhanced phosphorylation of the A-loop.
We examined the in vivo consequence of ErbB2-5M's apparently increased activity by monitoring anchorage-independent growth in soft agar of 3T3 cells stably transfected with either wild-type ErbB2 or ErbB2-5M (Fig. 1d). After 3 weeks of growth, acini were stained and photographed. Representative images reveal that ErbB2-5M-expressing acini grow to a larger size and display more peripheral projections than do acini formed from wild-type ErbB2-expressing cells. The larger, more irregular acini formed by ErbB2-5M-expressing cells suggest a more aggressive phenotype.
Src kinase mediates ErbB2 Tyr877 phosphorylation and more readily associates with ErbB2-5M than with wild-type ErbB2.
Phosphorylation of Tyr845, the analogous site of ErbB1 to Tyr877, is mediated by Src (34). To investigate whether Src also phosphorylates Tyr877 in ErbB2, we first tested whether the Src inhibitor PP1 would block constitutive phosphorylation of this site in ErbB2-5M. We found that PP1 treatment dramatically decreases Tyr877 phosphorylation (Fig. 2a). Similarly, dominant-negative Src markedly reduced Tyr877 phosphorylation when coexpressed with ErbB2-5M (Fig. 2b). In addition, coexpression of wild-type Src further elevated the already high level of phosphorylation on this site (Fig. 2b). While moderately enhancing Tyr877 phosphorylation in ErbB2-5M, wild-type Src has a much greater effect on wild-type ErbB2 (Fig. 2c, lanes 2 and 3). It also substantially increased Tyr877 phosphorylation of kinase activity-deficient ErbB2-K753A (Fig. 2c, lanes 4 and 5), eliminating the possibility that Tyr877 is an autophosphorylation site. Together, these data suggest that Src promotes phosphorylation of ErbB2 on Tyr877, directly or indirectly.
FIG. 2.
Src kinase mediates hyperphosphorylation of Tyr877 in ErbB2-5M. (a) Src kinase-specific inhibitor PP1 inhibits ErbB2 Tyr877 phosphorylation and reduces Tyr1248 phosphorylation. COS7 cells expressing ErbB2-5M were treated with 10 μM PP1 for the indicated times or with the same amount of vehicle (dimethyl sulfoxide) for 1 h. ErbB2 protein and phosphorylation were determined as described in Fig. 1c. (b) Dominant-negative (DN) Src inhibits ErbB2-5M phosphorylation on Tyr877. COS7 cells transiently expressing ErbB2-5M together with dominant-negative or wild-type (WT) Src were lysed with modified RIPA buffer. Total cell lysates were used to detect ErbB2 protein and Tyr877 phosphorylation levels by Western blotting. The expression level in cell lysate of DN and wild-type Src protein is shown. The ratio of intensities of the bands corresponding to phospho-Y877 and total ErbB2, as determined by densitometry, are displayed below the blots. (c) Wild-type Src increases ErbB2 phosphorylation on Tyr877. COS7 cells were transfected with wild-type or kinase-deficient ErbB2, together with wild-type Src or empty vector (v). ErbB2 protein and phosphorylation were determined as described in the legend to Fig. 1c. (d) Src phosphorylates ErbB2 on Tyr877 in an in vitro assay. Wild-type ErbB2 protein expressed in COS7 cells was immunoprecipitated (IP) from total cell lysate, aliquoted into four tubes, and mixed with purified Src proteins as indicated. The in vitro kinase assay was carried out at 30°C for 30 min in the presence or absence of Src inhibitor PP1 and ErbB inhibitor CI-1033. Samples were examined by Western blotting. Duplicate blots were used to detect ErbB2 phosphorylation on Tyr877 and the protein levels of ErbB2 and Src. (e) Src associates to a higher level with ErbB2-5M than with wild-type ErbB2. COS7 cells were transfected with wild-type Src together with wild-type ErbB2 or ErbB2-5M. Src was immunoprecipitated from total cell lysate, and coprecipitation of ErbB2 was examined by Western blotting. Expression levels of Src and ErbB2 were also examined by Western blotting.
To determine whether Src can directly phosphorylate Tyr877, we performed an in vitro kinase assay, adding purified, activated Src protein to wild-type ErbB2 that was immunoprecipitated from transiently transfected COS7 cells. In the presence of exogenous Src protein, a significant increase in ErbB2 Tyr877 phosphorylation was seen (Fig. 2d, compare lanes 1 and 2), and this increase was largely abolished by PP1 (Fig. 2d, lane 3), but not by the ErbB inhibitor CI-1033 (Fig. 2d, lane 4). Although it is formally possible that Src activation of an unidentified coprecipitating kinase (which then phosphorylated ErbB2 Tyr877) was responsible for this observation, these findings more likely represent direct phosphorylation of Tyr877 by Src.
Our model proposes that loss of Hsp90 binding favors Src association with ErbB2. To test this hypothesis, we cotransfected Src with either wild-type ErbB2 or ErbB2-5M and assessed Src association with ErbB2 by coprecipitation (Fig. 2e). The data clearly show more ErbB2-5M coprecipitating with an equivalent amount of Src, compared to wild-type ErbB2.
The Src family members Fyn and Yes are involved in Tyr877 phosphorylation.
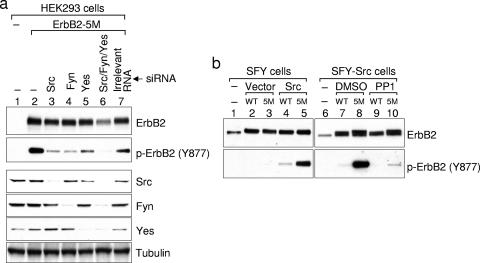
There are 10 members of the Src family. While many of them are expressed primarily in hematopoietic cells, three of them, Src, Fyn, and Yes, are expressed in cells that may also express ErbB2 (33). To investigate their involvement in phosphorylating ErbB2 on Tyr877, we examined ErbB2 phosphorylation in HEK293 cells with these three Src kinases knocked down by siRNA (Fig. 3a, lanes 3, 4, and 5). As expected, knockdown of Src markedly reduced Tyr877 phosphorylation (Fig. 3a, compare lanes 2 and 3). Noticeably, individual knockdowns of Fyn or Yes also reduced Tyr877 phosphorylation (Fig. 3a, lanes 4 and 5). Consistent with a contribution from all three Src kinases, knockdown of all three together reduced Tyr877 phosphorylation to an even lower level than did individual knockdowns (Fig. 3a, lane 6).
FIG. 3.
All three ubiquitously expressed Src kinases are involved in ErbB2 phosphorylation on Tyr877. (a) Reducing the expression of Src kinases by siRNA decreases ErbB2 Tyr877 phosphorylation. HEK-293 cells in six-well plates were transfected with siRNAs specific for Src, Fyn, or Yes using Lipofectamine 2000, followed by the transfection of ErbB2-5M plasmid with FuGene. On the third day after transfection, cells were lysed with modified RIPA buffer and total cell lysates were subjected to Western blotting analysis. Individual blots were probed for phosphorylated Tyr877 and for ErbB2, Src, Fyn, and Yes expression. A Western blot for tubulin in the cell lysates is included as a loading control. (b) ErbB2 phosphorylation on Tyr877 is diminished in SFY cells and is rescued by the expression of Src. SFY cells were transfected with wild-type ErbB2 or ErbB2-5M, together with wild-type Src or empty vector. Two days after transfection, cells were lysed and subjected to Western blotting analysis. For SFY-Src cells, single transfection of wild-type ErbB2 or ErbB2-5M was performed. One hour before lysis, cells were treated with 10 μM PP1 or the same amount of vehicle (dimethyl sulfoxide [DMSO]). ErbB2 protein and phosphorylation were determined as described in the legend to Fig. 1c.
The hypothesis that Src kinases phosphorylate ErbB2 on Tyr877 was further supported by data from SFY cells, which are deficient for Src, Fyn, and Yes. In contrast to results obtained with COS7 cells, Tyr877 in ErbB2-5M was not hyperphosphorylated in SFY cells (Fig. 3b, lane 3). However, transient expression of wild-type Src restored Tyr877 phosphorylation (Fig. 3b, lane 5). Importantly, Tyr877 was phosphorylated more highly in ErbB2-5M than in wild-type ErbB2 (Fig. 3b, lanes 4 and 5). Similar results were seen in SFY cells stably expressing wild-type Src (Fig. 3b, lanes 7 and 8), and in these cells, Tyr877 phosphorylation was markedly inhibited by PP1 (Fig. 3b, lanes 9 and 10).
Tyr877 Phosphorylation increases ErbB2 intrinsic kinase activity.
To test whether Tyr877 phosphorylation modulates ErbB2 intrinsic kinase activity, we examined ErbB2-5M autophosphorylation in the presence or absence of PP1. We found that, concomitant with decreased Tyr877 phosphorylation caused by PP1, autophosphorylation on Tyr1248 also decreased. Noticeably, the decrease in Tyr1248 phosphorylation lagged behind the loss of Tyr877 phosphorylation (Fig. 2a), suggesting that the former may occur as a result of the latter.
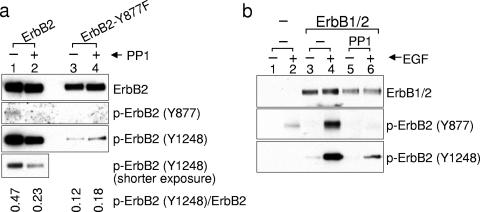
Kinase activity of wild-type ErbB2 is also inhibited by PP1 (Fig. 4a, bottom panel). If Tyr877 phosphorylation is important for ErbB2 kinase activity, its mutation to phenylalanine should decrease autophosphorylation of Tyr1248. Indeed, we found Tyr1248 phosphorylation of ErbB2-Y877F to be significantly lower than that of wild-type ErbB2 (Fig. 4a, lanes 1 and 3). Furthermore, if Tyr877 is phosphorylated by Src kinases, residual activity of the Y877F mutant should not be sensitive to PP1. The observed lack of effect of PP1 on Tyr1248 phosphorylation of ErbB2-Y877F confirms this prediction (Fig. 4a, lanes 3 and 4). Together, these results support the notion that Src-mediated Tyr877 phosphorylation augments ErbB2 kinase activity.
FIG. 4.
Tyr877 phosphorylation increases ErbB2 intrinsic kinase activity. (a) Y877F mutation compromises ErbB2 kinase activity and confers resistance to inhibition by Src antagonist PP1. COS7 cells expressing wild-type or Y877F mutant ErbB2 were treated with or without 10 μM PP1 for 2 h. ErbB2 protein and phosphorylation in total cell lysate were examined by Western blotting with specific antibodies. The ratio of p-ErbB2 (Y1248) to total ErbB2 in the presence and absence of PP1 is shown below the blots. (b) Src inhibitor PP1 decreases ligand-induced ErbB2 autophosphorylation on Tyr1248. COS7 cells were transfected with the plasmid expressing chimeric ErbB1/2. Eight hours after transfection, cells were switched to serum-free medium and continued in culture overnight. After pretreatment with 10 μM PP1 or the same amount of dimethyl sulfoxide for 1 h, cells were stimulated with 100 ng/ml EGF for 5 min. Protein and phosphorylation levels were determined as described in the legend to Fig. 1c.
The role of Src-mediated Tyr877 phosphorylation in upregulating ErbB2 kinase activity is further supported by the inhibitory effects of PP1 on EGF-stimulated activation of an ErbB1/ErbB2 chimeric protein. For these experiments, we used the EGF-responsive chimeric molecule ErbB1/2, which contains the extracellular and transmembrane domains of ErbB1 and the intracellular domain of ErbB2 (37). In the absence of PP1, EGF induced dramatic phosphorylation of Tyr1248 and Tyr877 (Fig. 4b, lanes 3 and 4). However, PP1 markedly suppressed EGF-dependent Tyr1248 phosphorylation and almost completely inhibited Tyr877 phosphorylation (Fig. 4b, lanes 5 and 6). These data further support a model in which Src-mediated Tyr877 phosphorylation serves to amplify the intrinsic kinase activity of ErbB2.
Tyr877 phosphorylation may induce a conformational change in the ErbB2 A-loop.
Comparing our data to previously published studies on ErbB1, it is apparent that ErbB1 and ErbB2 kinase activities are differentially sensitive to A-loop phosphorylation. To understand this difference, we used computational methods to examine the kinase domains of ErbB1 and ErbB2. The unphosphorylated A-loop of ErbB1 is reported to adopt an extended configuration, which is similar to that of the phosphorylated and activated insulin receptor (14). Energetic analysis suggested that this configuration is mainly stabilized by three salt bridges (Lys851/Glu734, Glu848/Arg865, and Lys843/Glu844; Fig. 5a) and by favorable interactions of the A-loop with the solvent. Phosphorylation of Tyr845 establishes additional interactions, with the phosphoryl group making salt bridges with Arg812 and Lys836 (Fig. 5b), further stabilizing the active configuration but without introducing conformational changes.
In contrast, it is energetically unfavorable for the unphosphorylated A-loop of ErbB2 to adopt the same configuration, because the different amino acid sequence inflicts charge-charge repulsion and desolvation penalties (Fig. 5a). The repulsion stems from the close proximity of Asp871 to Asp873 and Glu874 (OD2 atom of Asp871 is 3.7 Å to OD1 of Asp873 and 4.0 Å to OE1 of Glu874). The desolvation penalty is due to decreased solvent access of multiple charged residues (e.g., Asp873, Glu874, Glu876, Asp880, and Lys883 each enjoy a smaller surface area than their corresponding residues in ErbB1) and increased solvent exposure of the hydrophobic Ile872. Computational modeling suggests that the unphosphorylated A-loop of ErbB2 would be more energetically stable, adopting a strand-like conformation that swings to the opposite direction with respect to its location in ErbB1 (Fig. 5f, depicted in red). The strand-like conformation would be stabilized by hydrogen bonds within the A-loop, and the position of the loop would be upheld by two salt bridges and one hydrogen bond between the A-loop and residues from other parts of ErbB2: i.e., Arg868/Asp808, Lys883/Asp845, and Lys921/Asp871 (Fig. 5d). In this conformation, the aforementioned repulsion coming from Asp871 disappears because of increased distance from both Asp873 and Glu874. Further, the buried Arg844 and partially buried Glu874 and Glu876 residues are exposed to solvent, increasing favorable solvent interactions. Additionally, the otherwise exposed Ile872 moves to the core of the protein, contributing to stabilization while decreasing unfavorable solvent interactions.
Phosphorylation of Tyr877 is expected to significantly alter the interaction of residues in ErbB2's A-loop. The phosphoryl group establishes interactions with charged residues, especially with buried Arg844 and Arg868 (Fig. 5e). These interactions prompt the A-loop to undergo a dramatic conformational change, allowing it to attain an extended and activated configuration similar to that of ErbB1 (Fig. 5f, depicted in blue).
DISCUSSION
The stability of ErbB2 is critically dependent on Hsp90, and this dependence is solely determined by the kinase domain of the ErbB2 protomer. We recently identified the M5 loop between the αC helix and the β4 strand of the kinase domain N-lobe as a key mediator of this interaction. In contrast to pharmacologic approaches, mutation of the M5 loop to disrupt Hsp90 binding does not promote ErbB2 destabilization. Using this mutant ErbB2 (ErbB2-5M), we demonstrate that loss of Hsp90 binding correlates with enhanced ErbB2 activity that is mediated by Src kinases.
It is noteworthy that, once mature, only ErbB2 among the four-member ErbB family associates with Hsp90. This association may have arisen as an important mechanism for modulating ErbB2 function due to its unique structure. Unlike other members in the family, ErbB2's extracellular domain spontaneously adopts a configuration ready to dimerize in the absence of ligand binding, thus necessitating additional regulatory mechanisms. Hsp90 binding may have evolved to function as a negative modulator of ErbB2, apart from its role in stabilizing the protein. Our results are in agreement with and further extend the proposal put forth by Citri et al., who observed that for wild-type ErbB2 the immediate, albeit transient, consequence of geldanamycin-induced Hsp90 dissociation is receptor activation (11). Indeed, a similar inhibitory role has been observed for non-receptor serine-threonine kinases (13), raising the possibility that this may be a common function of the Hsp90 complex.
Multiple lines of evidence suggest that Src plays a role in ErbB2 activation. First, both ErbB2 and Src are located at the plasma membrane and are present in the same signaling particle (17). Second, ErbB2 and Src exist as a heterocomplex in tumor cells (3, 25, 26). Third, Src mediates ErbB2 activation induced by G protein-coupled receptors (7, 29). Our data suggest the underlying molecular mechanism by which Src activates the ErbB2 receptor: i.e., by phosphorylating Tyr877 in the A-loop. This phosphorylation is predicted to facilitate a conformational change, switching the A-loop into the active conformation. It is noticeable that Src-mediated ErbB2 activation is analogous to that of many other kinases, including Src itself, where autophosphorylation of Tyr416 leads to kinase activation (28). Another example is the cytosolic kinase ZAP-70, which is phosphorylated on Tyr493 in the A-loop by Lck, a Src kinase expressed exclusively in lymphocytes (8). Tyr877 in ErbB2 was originally thought to be an autophosphorylation site, yet supporting experimental evidence is lacking. Our data indicate that Tyr877, like the analogous Tyr845 in ErbB1, is instead phosphorylated by Src kinases. These data suggest Src to be a viable molecular target in tumors expressing elevated ErbB2.
We observed a dramatic decrease in the kinase activity of both wild-type ErbB2 and ErbB2-5M when Tyr877 was replaced with nonphosphorylatable phenylalanine. This is in contrast to ErbB1, where Y845F mutation exerts no effect on kinase activity (34). The lack of impact of this mutation on ErbB1 can be explained by the unique conformation of its A-loop, which adopts an extended, activated configuration in the absence of Tyr845 phosphorylation (31). However, computational studies suggest that the unphosphorylated ErbB2 A-loop adopts a different configuration from that of ErbB1. When Tyr877 is unphosphorylated, the A-loop is predicted to flip away from the ATP-binding cleft, taking a position similar to that of inactive Cdk6 (16), incapable of aligning ATP and substrate. Upon Tyr877 phosphorylation, the phosphoryl group establishes strong salt bridges with two arginine residues, which we suggest induces a conformational change in the A-loop, enabling it to attain the active conformation. Consistent with our observations, which were made with the human ErbB2 protein, Src dependence has also been observed for the constitutively active rat ErbB2/neu protein (39). Importantly, the amino acid sequence of the A-loop of rat ErbB2/neu is identical to that of human ErbB2, suggesting it may also undergo a conformational change when the corresponding Tyr882 is phosphorylated. Further, since Src is capable of activating ErbB2 but not ErbB1, and since ErbB2 is the preferred ErbB dimerization partner, the frequently observed Src dependence of ErbB1 activation by lateral signaling pathways (22) is likely a consequence of Src-mediated ErbB2 activation and not a direct effect of Src on ErbB1.
How might Hsp90 binding oppose Src-mediated ErbB2 activation? One possibility is that the Hsp90 complex physically prevents Src association with ErbB2. A key presumption of this hypothesis, formation of a preferably phosphorylation-independent Src-ErbB2 complex, is supported by a recent report (18). Although Hsp90 and Src do not bind to the same region of ErbB2's kinase domain, it remains possible that the Hsp90 complex may sterically interfere with Src association.
Hsp90 might also indirectly affect Src association with ErbB2 by inhibiting the phosphorylation of putative Src docking sites in ErbB2's C-terminal tail, achieved by preventing ErbB2 dimerization. Two lines of evidence support a role for the Hsp90 complex in regulating ErbB2 dimerization. First, the ErbB2 kinase domain is directly involved in receptor dimerization, as demonstrated by both experimental and computer modeling studies (9, 24). Second, the M5 loop is located in the interface of the ErbB2 kinase domain dimer, according to a recent model built on a nearly full-length ErbB2 molecule using information obtained from X-ray and nuclear-magnetic resonance data (2). A similar orientation was also proposed for an ErbB1 dimer (1, 19). Conceivably, binding of the Hsp90 complex at the M5 loop would prevent direct contact between the kinase domain of one ErbB2 protomer and that of another or the protomer of another ErbB family member. Thus, dislodging Hsp90 would release this impediment to dimerization of ErbB2's kinase domain. However, this model suffers from the requirement that the process of kinase domain dimerization must itself alter the conformation of the unphosphorylated A-loop. Further, several groups have reported that Src fails to bind to the C-terminal tail of autophosphorylated ErbB2 (18, 32).
These concerns would be satisfied were Src to create its own docking site by phosphorylating specific tyrosine residues in ErbB2's C-terminal tail, thus positioning itself to phosphorylate Tyr877. A similar scenario has been proposed for Src interaction with ErbB1 (4), which is supported by the finding that a putative Src binding site in the ErbB1 C-terminal tail is a substrate for Src phosphorylation and not for autophosphorylation (21, 32). Indeed, one study demonstrated that Src bound to ErbB1 and ErbB2 only if they were previously phosphorylated by Src, but not if they were autophosphorylated (32). Release of Hsp90 from the kinase domain may induce a conformational change in the C-terminal tail, exposing an Src phosphorylation/binding site.
Analysis of the functional regulation of client proteins by Hsp90 has often been complicated by the intrinsic instability of these proteins once Hsp90 is dissociated by pharmacologic means. To minimize the effects of protein loss, some investigators have tried to expose cells to Hsp90 inhibitors for very short times. We solved this problem instead by exploiting mutants that no longer rely on Hsp90 for protein stability. This approach may be applicable to the study of other Hsp90 client proteins.
Footnotes
Published ahead of print on 9 October 2006.
REFERENCES
- 1.Aifa, S., N. Miled, F. Frikha, M. R. Aniba, S. P. Svensson, and A. Rebai. 27. December 2006. Electrostatic interactions of peptides flanking the tyrosine kinase domain in the epidermal growth factor receptor provides a model for intracellular dimerization and autophosphorylation. Proteins. 62:1036-1043. [DOI] [PubMed]
- 2.Bagossi, P., G. Horvath, G. Vereb, J. Szollosi, and J. Tozser. 2005. Molecular modeling of nearly full-length ErbB2 receptor. Biophys. J. 88:1354-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belsches-Jablonski, A. P., J. S. Biscardi, D. R. Peavy, D. A. Tice, D. A. Romney, and S. J. Parsons. 2001. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene 20:1465-1475. [DOI] [PubMed] [Google Scholar]
- 4.Biscardi, J. S., M. C. Maa, D. A. Tice, M. E. Cox, T. H. Leu, and S. J. Parsons. 1999. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 274:8335-8343. [DOI] [PubMed] [Google Scholar]
- 5.Bouyain, S., P. A. Longo, S. Li, K. M. Ferguson, and D. J. Leahy. 2005. The extracellular region of ErbB4 adopts a tethered conformation in the absence of ligand. Proc. Natl. Acad. Sci. USA 102:15024-15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess, A. W., H. S. Cho, C. Eigenbrot, K. M. Ferguson, T. P. Garrett, D. J. Leahy, M. A. Lemmon, M. X. Sliwkowski, C. W. Ward, and S. Yokoyama. 2003. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell 12:541-552. [DOI] [PubMed] [Google Scholar]
- 7.Cabioglu, N., J. Summy, C. Miller, N. U. Parikh, A. A. Sahin, S. Tuzlali, K. Pumiglia, G. E. Gallick, and J. E. Price. 2005. CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activation. Cancer Res. 65:6493-6497. [DOI] [PubMed] [Google Scholar]
- 8.Chan, A. C., M. Dalton, R. Johnson, G. H. Kong, T. Wang, R. Thoma, and T. Kurosaki. 1995. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 14:2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chantry, A. 1995. The kinase domain and membrane localization determine intracellular interactions between epidermal growth factor receptors. J. Biol. Chem. 270:3068-3073. [PubMed] [Google Scholar]
- 10.Chavany, C., E. Mimnaugh, P. Miller, R. Bitton, P. Nguyen, J. Trepel, L. Whitesell, R. Schnur, J. Moyer, and L. Neckers. 1996. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J. Biol. Chem. 271:4974-4977. [DOI] [PubMed] [Google Scholar]
- 11.Citri, A., J. Gan, Y. Mosesson, G. Vereb, J. Szollosi, and Y. Yarden. 2004. Hsp90 restrains ErbB-2/HER2 signalling by limiting heterodimer formation. EMBO Rep. 5:1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diermeier, S., G. Horvath, R. Knuechel-Clarke, F. Hofstaedter, J. Szollosi, and G. Brockhoff. 2005. Epidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activation. Exp. Cell Res. 304:604-619. [DOI] [PubMed] [Google Scholar]
- 13.Donze, O., T. Abbas-Terki, and D. Picard. 2001. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 20:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard, S. R. 1997. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 16:5572-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huse, M., and J. Kuriyan. 2002. The conformational plasticity of protein kinases. Cell 109:275-282. [DOI] [PubMed] [Google Scholar]
- 16.Jeffrey, P. D., L. Tong, and N. P. Pavletich. 2000. Structural basis of inhibition of CDK-cyclin complexes by INK4 inhibitors. Genes Dev. 14:3115-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juang, S. H., M. E. Carvajal, M. Whitney, Y. Liu, and C. A. Carraway. 1996. Tyrosine phosphorylation at the membrane-microfilament interface: a p185neu-associated signal transduction particle containing Src, Abl and phosphorylated p58, a membrane- and microfilament-associated retroviral gag-like protein. Oncogene 12:1033-1042. [PubMed] [Google Scholar]
- 18.Kim, H., R. Chan, D. L. Dankort, D. Zuo, M. Najoukas, M. Park, and W. J. Muller. 2005. The c-Src tyrosine kinase associates with the catalytic domain of ErbB-2: implications for ErbB-2 mediated signaling and transformation. Oncogene 24:7599-7607. [DOI] [PubMed] [Google Scholar]
- 19.Landau, M., S. J. Fleishman, and N. Ben-Tal. 2004. A putative mechanism for downregulation of the catalytic activity of the EGF receptor via direct contact between its kinase and C-terminal domains. Structure 12:2265-2275. [DOI] [PubMed] [Google Scholar]
- 20.Leahy, D. J. 2004. Structure and function of the epidermal growth factor (EGF/ErbB) family of receptors. Adv. Protein Chem. 68:1-27. [DOI] [PubMed] [Google Scholar]
- 21.Lombardo, C. R., T. G. Consler, and D. B. Kassel. 1995. In vitro phosphorylation of the epidermal growth factor receptor autophosphorylation domain by c-src: identification of phosphorylation sites and c-src SH2 domain binding sites. Biochemistry 34:16456-16466. [DOI] [PubMed] [Google Scholar]
- 22.Luttrell, L. M., B. E. Hawes, T. van Biesen, D. K. Luttrell, T. J. Lansing, and R. J. Lefkowitz. 1996. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 271:19443-19450. [DOI] [PubMed] [Google Scholar]
- 23.Mimnaugh, E. G., C. Chavany, and L. Neckers. 1996. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J. Biol. Chem. 271:22796-22801. [DOI] [PubMed] [Google Scholar]
- 24.Murali, R., P. J. Brennan, T. Kieber-Emmons, and M. I. Greene. 1996. Structural analysis of p185c-neu and epidermal growth factor receptor tyrosine kinases: oligomerization of kinase domains. Proc. Natl. Acad. Sci. USA 93:6252-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthuswamy, S. K., and W. J. Muller. 1995. Activation of Src family kinases in Neu-induced mammary tumors correlates with their association with distinct sets of tyrosine phosphorylated proteins in vivo. Oncogene 11:1801-1810. [PubMed] [Google Scholar]
- 26.Muthuswamy, S. K., and W. J. Muller. 1995. Direct and specific interaction of c-Src with Neu is involved in signaling by the epidermal growth factor receptor. Oncogene 11:271-279. [PubMed] [Google Scholar]
- 27.Pero, S. C., G. S. Shukla, A. L. Armstrong, D. Peterson, S. P. Fuller, K. Godin, S. L. Kingsley-Richards, D. L. Weaver, J. Bond, and D. N. Krag. 2004. Identification of a small peptide that inhibits the phosphorylation of ErbB2 and proliferation of ErbB2 overexpressing breast cancer cells. Int. J. Cancer 111:951-960. [DOI] [PubMed] [Google Scholar]
- 28.Roskoski, R., Jr. 2005. Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 331:1-14. [DOI] [PubMed] [Google Scholar]
- 29.Sabri, A., J. Guo, H. Elouardighi, A. L. Darrow, P. Andrade-Gordon, and S. F. Steinberg. 2003. Mechanisms of protease-activated receptor-4 actions in cardiomyocytes. Role of Src tyrosine kinase. J. Biol. Chem. 278:11714-11720. [DOI] [PubMed] [Google Scholar]
- 30.Schlaepfer, D. D., and S. K. Mitra. 2004. Multiple connections link FAK to cell motility and invasion. Curr. Opin. Genet. Dev. 14:92-101. [DOI] [PubMed] [Google Scholar]
- 31.Stamos, J., M. X. Sliwkowski, and C. Eigenbrot. 2002. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 277:46265-46272. [DOI] [PubMed] [Google Scholar]
- 32.Stover, D. R., M. Becker, J. Liebetanz, and N. B. Lydon. 1995. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J. Biol. Chem. 270:15591-15597. [DOI] [PubMed] [Google Scholar]
- 33.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 34.Tice, D. A., J. S. Biscardi, A. L. Nickles, and S. J. Parsons. 1999. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA 96:1415-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang, Z., and B. Honig. 2001. Extending the accuracy limits of prediction for side-chain conformations. J. Mol. Biol. 311:421-430. [DOI] [PubMed] [Google Scholar]
- 36.Xiang, Z., C. S. Soto, and B. Honig. 2002. Evaluating conformational free energies: the colony energy and its application to the problem of loop prediction. Proc. Natl. Acad. Sci. USA 99:7432-7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu, W., E. Mimnaugh, M. F. Rosser, C. Nicchitta, M. Marcu, Y. Yarden, and L. Neckers. 2001. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem. 276:3702-3708. [DOI] [PubMed] [Google Scholar]
- 38.Xu, W., X. Yuan, Z. Xiang, E. Mimnaugh, M. Marcu, and L. Neckers. 2005. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat. Struct. Mol. Biol. 12:120-126. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, H. T., D. M. O'Rourke, H. Zhao, R. Murali, Y. Mikami, J. G. Davis, M. I. Greene, and X. Qian. 1998. Absence of autophosphorylation site Y882 in the p185neu oncogene product correlates with a reduction of transforming potential. Oncogene 16:2835-2842. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, K., J. Sun, N. Liu, D. Wen, D. Chang, A. Thomason, and S. K. Yoshinaga. 1996. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J. Biol. Chem. 271:3884-3890. [PubMed] [Google Scholar]