Abstract
The CBP/p300 family of proteins comprises related acetyltransferases that coactivate signal-responsive transcription. Recent evidence suggests that p300/CBP may also interact directly with complexes that mediate different aspects of DNA metabolism such as replication and repair. In this report, we show that loss of dCBP in Drosophila cells and eye discs results in a defect in the cell cycle arrest induced by stalled DNA replication. We show that dCBP and the checkpoint kinase Mei-41 can be found together in a complex and, furthermore, that dCBP has a genetic interaction with mei-41 in the response to stalled DNA replication. These observations suggest a broader role for the p300/CBP acetyltransferases in the modulation of chromatin structure and function during DNA metabolic events as well as for transcription.
The protein acetyltransferases p300 and CBP are transcriptional coactivators for signal transduction cascades that regulate cell cycle progression, cellular growth, differentiation, and apoptosis. Studies in mice indicate that both p300 and CBP are required for normal development in a gene dose-dependent manner (37, 60), indicating that p300 and CBP have at least some overlapping functions. In addition to forming a physical link between activated transcription factors and the basal transcriptional machinery, p300/CBP can enhance signal-responsive transcription by acetylating chromatin-associated proteins and consequently modifying chromatin structure and function (16, 26).
In addition to transcriptional activity, recent evidence suggests that p300/CBP may also interact directly with complexes that mediate chromatin metabolism. For example, p300 has been shown to bind PCNA, associate with newly synthesized DNA, and stimulate DNA synthesis in vitro (30). In addition, acetylation of Fen1, the endonuclease important for the removal of RNA primers during Okazaki fragment maturation, by p300/CBP inhibits its DNA binding and nuclease activity (31). p300 also binds and acetylates DNA polymerase β, which is involved in base excision repair (29). p300/CBP is also in a complex with and acetylates thymine DNA glycosylase, the enzyme that recognizes and repairs mispaired thymine and uracil groups (57). Further evidence that p300 plays a role during the response to DNA damage comes from the observation that acetylation of the RecQ helicase WRN by p300 facilitates the translocation of WRN protein from the nucleolus to nucleoplasmic foci (9). The WRN protein is critical for the resolution and restart of stalled DNA replication forks (47). Taken together, these observations suggest that p300/CBP plays an important role during DNA synthesis following DNA damage or stalled replication.
Chromosome replication is accomplished by initiating DNA replication forks at many origins along each chromosome. The cell cycle ensures that the new DNA strands are replicated only once per cell cycle by strictly regulating the temporal and spatial firing of these origins (36). The cell cycle checkpoints continuously monitor the genome to prevent the irreversible event of mitosis until DNA synthesis has been completed or until DNA aberrations have been resolved (28). In eukaryotic cells, the DNA replication checkpoint, which is thought to ensure that mitosis does not occur until the completion of S phase, is dependent upon protein kinases that are related to the ATR/ATM family. ATR is a mammalian gene with homology to the gene mutated in the human genetic disease ataxia telangiectasia (ATM) (17, 35). ATR and ATM share significant functional and sequence homology with Schizosaccharomyces pombe rad3 (6), Saccharomyces cerevisiae ESR1/MEC1 (50), and the Drosophila mei-41 (27) and telomere fusion (tefu) (45, 48) genes. Here we show that loss of dCBP in Drosophila cells results in a defect in the DNA replication checkpoint.
MATERIALS AND METHODS
Plasmids.
The dCBP transgene is the 10.6-kb V5 epitope-tagged dCBP cDNA (41) fused to 5.2 kb of genomic DNA that is 5′ to the putative dCBP start site and cloned into the Yellow Carnegie 4 vector (provided by Pam Geyer). The pUAST-dCBP small interfering RNA (siRNA) vector was generated by inserting intron 7 of dCBP between inverted segments of dCBP coding sequence. The dCBP PCR fragment from bp 231 to 1139 was tagged with EcoRI and NotI, the dCBP PCR fragment corresponding to the seventh dCBP intron was tagged with NotI and XhoI, and the inverse 231- to 1131-bp dCBP PCR fragment was tagged with XhoI and XbaI. These fragments were cloned sequentially into the pUAST transformation vector (10). The pPacB mei-41V5 expression construct was generated by cloning two mei-41 PCR fragments from cDNA into pPacB (Invitrogen). The mei-41 PCR fragment from bp 1 to 4415 was generated using the oligonucleotides 5′ mei-41 NotI-AACAGCGGCCGCATGTCGACACAACGGAAGGAT and 3′ mei-41(4415)-TTCCTTAATGGCTCCAAGCTCCTG, which uses the single XhoI site found in mei-41. It was cloned into a pPacB vector that was cut with NotI and XhoI. The second mei-41 PCR fragment was generated with the oligonucleotides 5′ mei-41(4319)-TTCATGGCAGTTCTGCAAGCCAAC and 3′ mei-41(7752) SacII-AACACCGCGGAAGAAACGCTCCCCAGCCAAT. This fragment was then cloned into the pPacB mei-41(1-4415) vector that was cut with XhoI and SacII.
Cell culture.
The Drosophila Kc cells were grown at 25°C in 1× Schneider's Drosophila medium (GIBCO) and supplemented with 5% fetal bovine serum (FBS) and 1% penicillin and streptomycin. Kc cell transfections were performed using a calcium phosphate transfection kit from Invitrogen according to the manufacturer's protocol.
Fly strains and culture.
The UAS-dCBP-siRNA and P{yt7.7 dCBPt + 16}transgenic lines were generated in y* w* flies using standard techniques and a helper pπΔ2-3 vector (49, 56). Two UAS-dCBP-siRNA constructs, hp12 and hp12.3, were generated and used in these studies. Two lines carrying the P{yt7.7 dCBPt + 16} transgene were also generated, and both complement the dCBP deletion mutations that have been characterized previously (1). For ease of crosses, the third chromosome insertion, Tr31, was used in these studies. The dCBP3 allele is the nej3 allele previously described by us (1) and is used for clarity. The UAS-dCBPV5 and UAS-dCBPm5V5 flies have the wild-type and mutant dCBP genes tagged with the V5 epitope (Invitrogen) and driven by the gal4 enhancer (41). The hp12.3 UAS-dCBPm5V5 chromosome was generated using standard recombination techniques. The dCBPm5V5 (dCBP F2161A) mutant cannot bind acetyl-coenzyme A and has no acetyltransferase activity (41). The mei-41RT allele, the P{w[+mC] = GAL4-Hsp 70.PB}89-2-1 (referred to as hsgal4) chromosome, FM7c, P{w+mC = GAL4-Kr.C}DC1, P{w+mC = UAS-GFP.S65T}DC5,sn+ (referred to as FM7G), CyO, P{w+mC = GAL4-Kr.C}DC3, P{w+mC = UAS-GFP.S65T}DC7 (referred to as CyOG), and all other balancer chromosomes were obtained from the Bloomington Stock Center and are described in Flybase (23) or Lindsley and Zimm (39). Flies were cultured on standard Drosophila cornmeal-yeast source medium in 8-oz plastic bottles or 28- by 95-mm plastic shell vials. All crosses were reared at 25°C.
Determination of S phase checkpoint and G2 timing.
y* w*; hp12.3; hsgal4 flies were mated to y* w*; hp12.3, y* w*; hp12.3; UAS-dCBPV5 and hp12.3UAS-dCBPm5V5 flies and the y* w*; hp12.3; hsgal4/+, y* w*; hp12.3; hsgal4/UAS-dCBPV5 and hp12.3UAS-dCBPm5V5/+; hsgal4/+ progeny were reared at 25°C until the end of the second instar/beginning of the third instar, when they were heat shocked at 37°C for 1 h. The larvae were then reared at 29°C for 16 to 20 h when wandering third-instar larvae were collected. y* w* flies were mated to hsgal4 flies and the hsgal4/+ and hp12.3 larvae were treated in the same fashion and used as controls. Non-heat shock controls were also shifted to 29°C until the wandering third instar stage. At this stage of development, even larvae staged at hatching can differ in age by as much as 6 h. Thus, we enriched for a similar age by dissecting discs from larvae that had very little or no secretions in the salivary gland and/or had not everted their anterior spiracles. Discs were dissected into 1× Schneider's medium (control) or 1× Schneider's medium that was made 0.05 M for hydroxyurea (HU). The discs were incubated on a nutator at room temperature for 2.5 h, when they were fixed and stained with a 1:1,000 dilution of the antibody directed against the mitosis marker, phosphorylated histone H3 (Upstate Biotechnology), as described previously (49). The stained discs were incubated in a glycerol series, mounted in 70% glycerol, and visualized with Nomarsky optics. Serial optical sections for each disc were collected and merged in Photoshop (Adobe) so that all of the mitotic cells were visible in one image. The age enrichment resulted in over 80% of the non-HU-treated discs' having a detectible first mitotic wave. We counted the total number of mitotic cells in at least five of the discs that were at the same point in development with regard to the morphogenic furrow. The HU-treated discs had no discernible mitotic wave. Thus, we counted the mitotic cells in at least five of the discs. Each experiment was performed at least three times.
Discs from the same hsgal4 larval populations were also dissected into 1× Schneider's medium supplemented with 75 μg/ml bromodeoxyuridine (BrdU) to detect DNA synthesis and incubated for 1, 2, 3.5, 4.5, and 6 h. They were then fixed, treated with 2 N HCl for 30 min, and neutralized with three washes of phosphate-buffered saline (PBS)-0.3% Triton X-100 (PBT). The discs were incubated overnight at 4°C with antibodies against BrdU (BD Biosciences) at a dilution of 1:2.5 and with the anti-phosphorylated histone H3 antibody at a dilution of 1:1,000. The antibodies were detected with anti-mouse fluorescein-conjugated (Vector) and anti-rabbit Alexa Fluor 594-conjugated (Molecular Probes) secondary antibodies used at a 1:200 dilution. The stained discs were washed and mounted in SlowFade (Molecular Probes) and visualized with a confocal laser scanning microscope (Bio-Rad 1024ES laser and Nikon Eclipse TE300 microscope). To assess the incorporation of BrdU in the presence of HU, the heat shocked hp12.3; hsgal4/+ and hsgal4/+ control discs were treated with or without 0.05 M HU for 2.5 h as above and then supplemented with 75 μg/ml BrdU for 30 min, after which they were processed as above. Almost all of the non-HU-treated discs in these experiments had the same pattern of BrdU incorporation, showing that the discs were assayed at about the same time in development. To determine whether BrdU-labeled DNA could escape the HU-induced replication checkpoint, the heat-shocked hp12.3; hsgal4/+ and hsgal4/+ control discs were treated with 75 μg/ml BrdU for 30 to 45 min, after which half of the control and experimental discs were supplemented with 0.05 M HU for 4.5 to 5 h. The discs were then processed as above.
dCBP3/FM7G females were mated to mei-41RT males. The female third instar larvae were sorted on the basis of the green fluorescence marker (mei41RT/FM7G control and dCBP3/mei41RT). The discs were dissected from the two groups and stained with the anti-phosphorylated H3 antibody as described above. The mei-41RT controls were discs from green and nongreen female third instar larvae from a mei-41RT/FM7G stock. For the rescue experiments, dCBP3; Tr31/TM6b, Tb males were mated to mei41RT/FM7G females. The discs from female larvae that were green or nongreen or tubby or nontubby were scored for mitotic cells as described above.
Kc cells were grown to a density of 1 × 106 cells/ml and then treated with water (control) or double-stranded RNA (dsRNA) homologous to dCBP or mei-41 following the protocol described by Clemens et al. (19). At 24, 48, and 72 h posttreatment, the cells were incubated in 20 mM HU for 12 and 24 h. Each siRNA treatment time point was performed in triplicate. The control cells were treated with HU at the 48- or 72-h time point. The cells were washed with 1× PBS, fixed in 4% paraformaldehyde-PBS for 20 min, washed two times in PBS, and air dried. At the end of the last HU time point, the cells were blocked for 1 h in 10% horse serum in 1× PBT and stained with a 1:100 dilution of anti-phosphorylated histone H3 antibody overnight at 4°C. After washes in PBT, the cells were treated with a 1:100 dilution of an anti-rabbit fluorescein-conjugated secondary antibody (Vector) for 1 h at room temperature. The cells were washed, mounted in SlowFade (Molecular Probes; 0.05 μg/ml propidium iodide), and visualized by fluorescence microscopy. At least five randomly chosen fields for each slide were photographed for quantification. Each dCBP and mei-41 siRNA experiment was performed at least three times.
DNA synthesis was assessed by BrdU incorporation. Control and dCBP dsRNA-treated Kc cells were generated in triplicate as above, but at each time point they were incubated with 10 μM BrdU for 30 min. The cells were fixed, dried, and denatured in 0.07 N NaOH for 2 min. They were neutralized in 1× PBS, pH 8.5, washed, and stained as described using an anti-BrdU antibody (BD Biosciences) at a dilution of 1:2.5. The anti-mouse fluorescein-conjugated secondary antibody (Vector) was used at a dilution of 1:200. The cells were washed, mounted in SlowFade (Molecular Probes), and visualized by fluorescence microscopy. At least 5 randomly chosen fields for each slide were photographed for quantification.
Statistical analysis.
In all of the experiments, statistical differences among the means were determined by standard two-tailed Student's t test analysis.
Generation of siRNAs and treatment of cells.
The dsRNAs were generated as described by Clemens et al. (19). The dCBP dsRNAs were made to the 5′ and 3′ untranslated regions of the dCBP cDNA. The 5′ dCBP sequences were amplified using two 5′ primers (forward primer dCBP 5, 5′-GAA TTA ATA CGA CTC ACT ATA GGG AGA ACC AAA CAA ACA ACC TGT GCA ACA-3′; reverse primer dCBP 261, 5′-GAA TTA ATA CGA CTC ACT ATA GGG AGA GTT TGA TTC TTT CGA TTC GGA AAA AAA CAG-3′) tagged with the T7 polymerase binding site. The 3′ dCBP sequences were amplified using two 3′ primers (forward primer dCBP 10145, 5′-GAA TTA ATA CGA CTC ACT ATA GGG AGA TAC AAT GGT TGG TAG GCG ATG TGG CTA GAG-3′; reverse primer dCBP 10591, 5′-GAA TTA ATA CGA CTC ACT ATA GGG AGA TAT ATC TGC TTA GTG GAA ACG-3′) tagged with the T7 polymerase binding site. The mei-41 dsRNAs were made to the mei-41 coding sequence. The T7 polymerase binding site tagged the forward mei-41 primer, mei-41 5′ 586 (5′-GAA TTA ATA CGA CTC ACT ATA GGG AGA TTC ATC AGG CTC TGC TAA TCC-3′) and reverse primer, mei-41 3′ 1148 (5′-GAA TTA ATA CGA CTC ACT ATA GGG AGA CAA GAT TTC AAC GAG ACT TTG-3′). The Kc cells were treated as previously described (19).
Immunoprecipitations, Western analysis, and RT-PCR.
Kc cells were grown to a density of 1 × 107 cells/ml, and immunoprecipitations of dCBP were performed as described (4). The dCBP antibody (3) was used at a 1:10 dilution. For Western analysis of cell proteins, 1 × 105 cells were homogenized in 2× loading buffer, and the proteins were separated by 3 to 8% gradient or 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred to Immobilon-P membrane (Millipore) using a wet transfer apparatus. dCBP was detected with the anti-dCBP antibody used at a 1:1,000 dilution, and Mei-41 was detected with an anti-Mei-41 antibody (provided by T. Su, University of Colorado, Boulder, CO) at a 1:1,000 dilution. The filters were then reacted with horseradish peroxidase-conjugated secondary antibodies to chicken or mouse (Promega) according to the protocol provided with the SuperSignal kit (Pierce). To increase detection of Mei-41 in the dCBP immunoprecipitations, the filters were also incubated with IRDye 700DX-conjugated anti-rabbit secondary antibodies and visualized with the LI-COR Biosciences Odyssey imaging system. For Western analysis of larval tissues, the brains and discs from 10 third instar larvae were dissected into Drosophila Ringer's solution and homogenized in 2× loading buffer, and the proteins were separated by a 3 to 8% gradient or 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred and processed as described for the Kc cells. For the reverse transcription-PCR (RT-PCR), RNAs were isolated from 5 × 106 Kc cells using Tri Reagent (Molecular Research Center, Inc.) according to the manufacturer's protocol. We used 2 μg of the RNAs to make cDNAs, and the PCRs were performed on equal amounts of the cDNAs using the following primers: mei-41 (forward, 5′-CTA ATC CTC AAG GAG TGC GAC-3′; reverse, 5′-TCC AAG TGT ATG TGT CTT CGG-3′), grp (forward, 5′-AAC AGG TAC CAA CTT GTT GAC AGT GTA GCT G-3′; reverse, 5′-AAC AGC GGC CGC GTT CCT CCT TCA TTC ATT GAA-3′), and actin (forward, 5′-CAA GAA TCA GGA TTA CAA CTG CAG-3′; reverse, 5′-GGC TGG TGA ATG TTG AAT GCC AGT-3′). These primers are homologous to sequences in the coding region of each of the genes. The concentration of cDNAs used was determined by a dilution gradient. We used the concentration in which we could detect loss of mei-41 RNA in the mei-41 dsRNA-treated cells and the loss of grp from cells treated with dsRNA directed against grp.
RESULTS
dCBP is required for the DNA replication checkpoint in developing eye discs and cell culture.
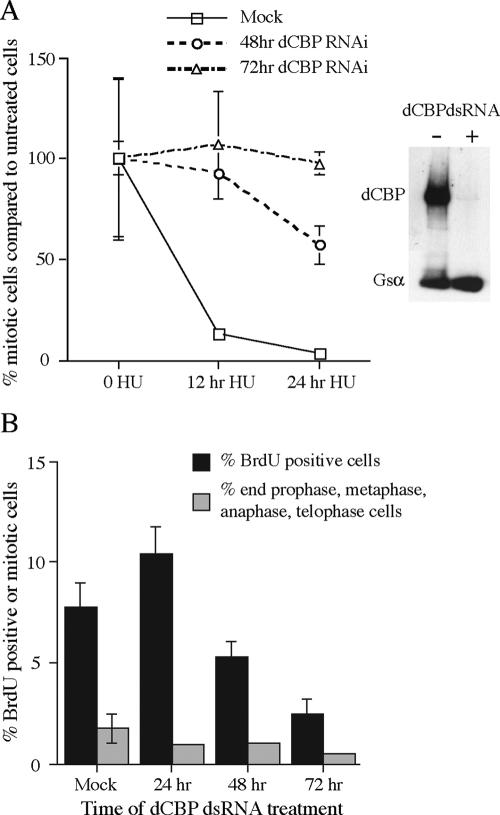
Drosophila Kc cells treated with dsRNA directed against dCBP lose dCBP protein (see Fig. 4A) and begin to die after 48 h of treatment. Karyotypic analysis of the dCBP dsRNA-treated cells indicates that at least 50% of the metaphase chromosomes have defects in condensation and ultimately shred (Fig. 1B and C). To determine whether loss of dCBP function could cause the same defect in flies, we analyzed the metaphase chromosomes from the brains of third instar larvae that express dCBP siRNAs. The two insertion chromosomes, hp12 and hp12.3, carry a dCBP dsRNA construct that is driven by the Gal4 UAS enhancer. Flies homozygous for the stronger of these two insertions, hp12.3, and heterozygous for a gal4 gene under the control of the heat shock promoter (hsgal4) were grown at 25°C until the end of the second instar/beginning of the third instar and incubated at 37°C for 1 h. Control hsgal4/+ and hp12.3 larvae were treated in the same fashion. The larvae were allowed to develop at 29°C for 16 to 24 h when the wandering third instar larvae could be collected and staged. The non-heat shock controls were shifted to 29°C at the same time as the experimental flies. The brains were dissected, and metaphase chromosomes were visualized as described in Materials and Methods. As shown in Fig. 1, the heat shock regimen and expression of gal4 do not affect normal metaphase chromosome morphology (Fig. 1D to F). However, approximately 50% of the metaphase chromosomes from brains expressing dCBP siRNAs fail to condense properly and appear to shred (Fig. 1G to J). The expression of the dCBP siRNAs severely reduces the levels of dCBP while neither the heat shock nor the expression of gal4 affects dCBP levels (Fig. 1K). Because this type of chromosomal defect can result from a defect in the DNA replication checkpoint, we determined whether a loss of dCBP function results in a deficiency in the cell cycle delay that can be induced by stalled DNA replication. For this analysis we expressed dCBP dsRNAs in developing eye discs and determined whether the disc cells could arrest the cell cycle in response to HU, which results in stalled replication. The hp12.3; hsgal4/+, control hsgal4/+, and hp12.3 larvae were treated as described above, and the eye discs were dissected as described in Materials and Methods. The discs were incubated for 2.5 h in the presence or absence of HU and assayed for mitotic cells using an antibody against the mitosis-specific phosphorylation of histone H3 (serine 10). Only eye discs that were wild type in size were assessed for mitotic cells. The number of mitotic cells observed in the dCBP mutant discs was reduced only two- to threefold by HU while the number of mitotic cells observed in the discs from control larvae was reduced between 10- and 16-fold by HU treatment (Fig. 2A and B). Non-heat-shocked control discs all retained a robust checkpoint response to HU treatment (Fig. 2B). Thus, dCBP function is required for the cell cycle arrest induced by HU in vivo. An additional 10 discs were assayed by Western blotting for expression of dCBP (Fig. 2B). The same analysis for the hp12 insertion chromosome resulted in a similar though less robust HU response (data not shown).
FIG. 4.
Loss of dCBP function causes a defect in the Kc cell replication checkpoint. (A) Kc cells treated with water (mock) or dCBP dsRNAs for 48 and 72 h were also treated with HU for 12 or 24 h before staining with the mitosis marker anti-phospho-H3 antibody. The mitotic index is expressed as a percentage of mitotic cells observed in the absence of HU treatment. Western analysis of mock-treated Kc cells or Kc cells treated with dCBP dsRNAs for 48 h shows that dCBP is depleted in the dsRNA-treated cells. Gsα was used as an internal control for gel loading and protein transfer. (B) Cells that are depleted of dCBP continue to incorporate BrdU, at least for 72 h. In addition, the frequency of mitotic cells (metaphase, anaphase, and telophase) was determined following phospho-H3 and propidium iodide staining.
FIG. 1.
Loss of dCBP function causes defects in chromosomal condensation and integrity. Chromosome spreads of Kc cells treated with water (A) or dsRNAs for dCBP (B and C). The chromosomes were visualized by staining with propidium iodide. Control chromosomes are evenly and tightly condensed while the chromosomes from the dCBP dsRNA-treated cells are only partially condensed (B) or shredded (C). Mitotic chromosomes from heat-shocked hsgal4/+ (D to F) and hp12.3; hsgal4/+ (G to J) third instar larvae are shown. The heat-shocked hsgal4/+ larval brain cells have normally condensed mitotic chromosomes (D to F), demonstrating that the heat shock treatment does not affect the morphology of the chromosomes. The heat-shocked hp12.3 larval brains are identical to the heat-shocked hsgal4/+ (not shown). The hp12.3; hsgal4/+ larval brain cells contained chromosomes that failed to condense properly and appeared shredded (G to J). (K) A Western analysis of the gal4/+ and hp12.3; hsgal4/+ larval brains. dCBP is significantly reduced in the heat-shocked hp12.3; hsgal4/+ brains. Heat shock does not affect the expression of dCBP in the hsgal4/+ controls. Gsα is an internal loading and transfer control.
FIG. 2.
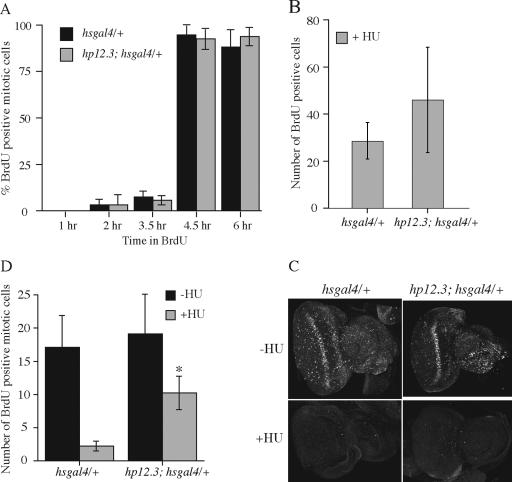
Loss of dCBP function in mitotically active eye discs results in loss of the replication checkpoint. (A) hp12.3; hsgal4/+ and hsgal4/+ second and third instar larvae were left at 25°C as a control group or subjected to heat shock to induce dCBP dsRNAs. The hp12.3/hp12.3 UAS-dCBPm5V5; hsgal4/+ and hp12.3; hsgal4/UAS-dCBPV5 larvae were subjected to the same heat shock protocol. The control and experimental discs were separated into two groups, and one set was treated with HU. Mitotic cells were detected with the phospho-H3 antibody. Discs from the heat-shocked larvae are shown. The exogenously expressed wild-type and mutant dCBPs are tagged with the V5 epitope and their expression is robust. (B) At least 5 discs were assessed for each group, and the number of mitotic cells was quantified. The discs from hsgal4/+ and hp12.3 larvae responded identically, and the results for the hsgal4/+ discs are shown here. Student's t tests determined the differences among the means. An asterisk indicates a significant difference (P < 0.001). Western analysis of the discs shows that the dCBP dsRNAs greatly reduced the level of dCBP expression. Fasciclin is an internal control for protein levels and transfer. Coexpression of a wild-type dCBP gene (V5 in panel B) can titrate the dCBP siRNAs and ameliorate the replication checkpoint defect in discs expressing dCBP siRNAs. However, coexpression of dCBP siRNAs and a mutant of dCBP that has no acetyltransferase activity (m5V5 in panel B) does not rescue the defective HU response. Thus, the replication checkpoint defect generated by the dCBP siRNAs is due specifically to loss of dCBP function. Expression of the wild-type and mutant dCBP constructs alone does not affect the HU-mediated cell cycle arrest.
Specificity of the dCBP siRNAs was confirmed by demonstrating that the defective replication checkpoint caused by the dCBP siRNAs could be ameliorated by coexpressing a wild-type dCBP gene. In these assays, hp12.3; UAS-dCBPV5 flies were mated to the hp12.3; hsgal4 flies and the hp12.3; UAS-dCBPV5/hsgal4 larvae were heat shocked, and the eye discs assayed as described. The hsgal4/+ and hp12.3; hsgal4/+ flies were used as controls. The rescued discs have wild-type levels of mitosis, and, as shown in Fig. 2A and B, HU treatment reduces the number of their mitotic cells to the levels seen in the wild-type control discs. In addition, the coexpression of a mutant dCBP that has no detectable acetyltransferase activity (UAS-dCBPm5V5) with the dCBP siRNAs does not rescue the defective HU response (Fig. 2A and B). In these experiments, hp12.3 UAS-dCBPm5V5 flies were mated to the hp12.3; hsgal4 flies, the hp12.3/hp12.3 UAS-dCBPm5V5; hsgal4/+ larvae were heat shocked, and the discs were assayed for mitosis as described. Overexpression of the wild-type and mutant dCBP genes alone did not affect the HU-mediated cell cycle arrest (Fig. 2B). Both the UAS-dCBPm5V5 and UAS-dCBPV5 chromosomes express full-length dCBP that is able to bind known acetylation targets, and they are expressed to the same extent (41). Thus, the failure of the UAS-dCBPm5V5 insertion to rescue the loss of dCBP mediated by the dCBP siRNAs is due specifically to its loss of acetyltransferase activity.
One interpretation of the relative nonresponsiveness of dCBP-deficient cells to HU is that loss of dCBP affects G2 timing. To determine the length of G2 in control and dCBP loss-of-function eye disc cells, we determined the minimum time required for DNA synthesized with BrdU to enter mitosis. We incubated eye discs from the same populations that we used to assess the HU-mediated checkpoint response, with BrdU for 1, 2, 3.5, 4.5, and 6 h and fixed and processed them for both BrdU incorporation and mitosis (Fig. 3A). At 1 h, the cells from both the control and dCBP siRNA-treated discs that are in mitosis had not incorporated BrdU. At 2 and 3.5 h, only a very few (on average, 2 to 6%) of the mitotic cells had incorporated BrdU. By 4.5 h almost all of the mitotic cells were BrdU positive in both groups. Thus, at a level of dCBP expression where the replication checkpoint is perturbed, G2 is unaffected.
FIG. 3.
Loss of dCBP function does not affect G2 timing, and HU suppresses BrdU incorporation. (A) Discs from the heat-shocked hp12.3; hsgal4/+ and hsgal4/+ larvae were incubated in BrdU for 1, 2, 3.5, 4.5, and 6 h and were then assayed for mitotic cells that had incorporated the BrdU. There is no detectable, significant difference in the lengths of G2 seen in the two populations. (B) Quantification of the HU-treated discs pooled from three experiments. A Student's t test determined that there are no differences between the means of the control and experimental discs. (C) Discs from the heat-shocked hp12.3; hsgal4/+ and hsgal4/+ larvae were treated with HU for 2.5 h and then treated with BrdU for 30 min. HU treatment suppresses BrdU incorporation in control and dCBP siRNA-expressing cells. (D) Discs from the heat-shocked hp12.3; hsgal4/+ and hsgal4/+ larvae were incubated in BrdU for 30 min, and then half of the control and experimental discs were treated with HU for 4.5 to 5 h. Significantly more mitotic cells are BrdU positive in the HU-treated hp12.3; hsgal4/+ discs than in the HU-treated hsgal4/+ discs. The data represent three pooled experiments. A Student's t test shows that the mean between the two populations is significantly different. The asterisk indicates a significant difference (P < 0.01).
While many groups have shown that HU induces the replication checkpoint in mammalian and insect cell lines, we determined whether HU could affect the incorporation of BrdU in discs. Thus, we treated the heat-shocked hsgal4/+ and hp12.3; hsgal4/+ discs with HU for 2.5 h as described above or left them untreated. We then added BrdU to the discs for 30 min, after which they were fixed and assayed for BrdU incorporation. As shown in Fig. 3B and C, BrdU incorporation in the control and dCBP siRNA-expressing disc cells is greatly reduced. Although there are slightly more BrdU-positive cells in the dCBP siRNA-expressing discs, this is not significantly different from the control discs. Thus, as with other cell types, HU inhibits replication in Drosophila disc cells and induces a replication checkpoint. Furthermore, the reduction in dCBP function that allows some escape from the S phase checkpoint does not affect the ability of HU to suppress replication. We confirmed this result by following BrdU-labeled DNA into mitosis in the presence and absence of HU. We treated the heat-shocked hsgal4/+ and hp12.3;hsgal4/+ discs with BrdU for 30 min and then treated half of the control and experimental discs with HU for 4.5 to 5.0 h, a little more than the length of G2. As shown in Fig. 3D, significantly more mitotic cells are BrdU positive in the dCBP siRNA-expressing discs than in control disks. Thus, these experiments also demonstrate that in these studies, changes in mitotic cell counts reflect a change in the replication checkpoint.
We also determined whether loss of dCBP function could affect the HU response in Drosophila Kc cell culture. We exposed dCBP-dsRNA-treated Kc cells to HU and, in this case, assayed the frequency of mitotic cells at 12 and 24 h of treatment. In mock-treated Kc cells, the cell cycle arrest induced by HU occurs at both 12 and 24 h of treatment. In contrast, cells treated with dsRNA for dCBP no longer respond to HU and continue to enter mitosis (Fig. 4A). Figure 4A shows Western blot analysis of dCBP expression in Kc cells treated for 48 h of dCBP siRNA. Figure 4B shows that the dCBP-depleted cells continue to cycle as seen by their continuing to incorporate BrdU and maintain detectable, though lower, levels of mitosis.
To determine whether the defect in the HU response of dCBP-depleted cells was similar to depletion of the Drosophila ATR homologue, Mei-41, we analyzed discs from the mei-41RT mutant larvae and demonstrated that mei-41 is required for the cell cycle arrest induced by HU in the eye discs (Fig. 5A and B). This is consistent with previous findings, demonstrating that mei-41 is required for the replication checkpoint in Drosophila embryos (25, 51, 52).
FIG. 5.
Loss of Mei-41 function in mitotically active eye discs and Kc cells results in loss of the replication checkpoint. (A and B) Discs from mei-41RT heterozygotes or homozygotes were treated with HU, and the mitotic cells of the eye discs were quantitated. (C) Kc cells treated with water (mock) or dsRNAs directed against mei-41 for 48 and 72 h were also treated with HU for 12 or 24 h before staining with the mitosis marker anti-phospho-H3 antibody. The mitotic index is expressed as a percentage of mitotic cells observed in the absence of HU treatment. RT-PCR analysis of the Kc cells treated with water or mei-41 dsRNAs after 48 h shows that mei-41 is not expressed in these cells. Actin was used as internal control for the integrity of the cDNAs. (D) Kc cells that are depleted of mei-41 continue to incorporate BrdU, at least for 72 h. In addition, the frequency of mitotic cells (metaphase, anaphase, and telophase) was determined following phospho-H3 and propidium iodide staining.
We also treated Kc cells with dsRNA directed against the coding region of the mei-41 gene and determined the HU response. Figure 5C and D show that mei-41 is also required for the cell cycle arrest induced by HU in Kc cells. Figure 5C also shows RT-PCR analysis of Kc cells treated with dsRNA against mei-41 and demonstrates that mei-41 mRNA is no longer detectable. As with Kc cells treated with dsRNA directed against dCBP, cells treated with dsRNAs against mei-41 continue in the cell cycle, as assayed by BrdU incorporation and phospho-H3 staining, for at least 72 h (Fig. 5D).
Because dCBP is a coactivator of transcription, it is possible that the replication checkpoint genes require dCBP for their expression. In this case, loss of the replication checkpoint in dCBP-loss-of-function cells would reflect a loss of checkpoint gene transcription. RT-PCR analysis of Kc cells treated with dCBP dsRNAs demonstrated that the steady-state levels of the transcripts of two genes involved in the replication checkpoint, grapes (grp), a Chk1 homologue (24), and mei-41 are unaffected by loss of dCBP function (Fig. 6), indicating that loss of the checkpoint is not due to loss of grp or mei-41 transcription. We would have detected any loss in transcript levels in these experiments because we used the same concentrations of starting RNAs, and a concentration gradient of the cDNA product was used in the PCRs. This approach assured us that we were in the linear range and could detect loss. This approach allowed us to detect loss of mei-41 transcripts in the cells treated with dsRNAs directed against mei-41 (Fig. 5C).
FIG. 6.

Loss of dCBP function does not affect the steady-state levels of mei-41 or grp. Kc cells treated with dCBP dsRNAs deplete the cells of dCBP protein (A). Gsα is used as an internal loading and protein transfer control. The dsRNAs are directed against the 5′ and 3′ untranslated sequences of dCBP. RT-PCR analysis indicates that loss of dCBP function does not affect the steady-state expression of mei-41, grp, or actin mRNA (B). The cDNA dilutions are given at the bottom.
The replication checkpoint is affected by the relative doses of dCBP and Mei-41.
dCBP is involved in a number of different signaling pathways that affect development. Thus, while a reduction of dCBP does not affect the transcription of mei-41 or grp, it may affect the transcription of other genes that have a secondary effect on the replication checkpoint. In order to assess the role of dCBP that is specific to the Mei-41-mediated replication checkpoint pathway, we determined whether changes in the relative dosages of dCBP and mei-41 could affect HU-induced cell cycle arrest. When the dosages of two genes acting in the same complex or pathway are lowered, the function of the complex/pathway can be reduced, and this often leads to a phenotype. In contrast, altering the levels of two genes in parallel or secondary pathways would not be particularly destabilizing and would not be expected to give a phenotype. Therefore, we crossed mei-41RT males with dCBP3/FM7G females and determined whether the replication checkpoint was compromised in mei-41RT/dCBP3 transheterozygotes. As seen in Fig. 7A, the number of mitotic cells is slightly but significantly higher in the HU-treated discs from mei-41RT/dCBP3 transheterozygotes compared to the mei-41RT/FM7G controls. Furthermore, the expression of a dCBP transgene, Tr31, can suppress this effect, demonstrating that the dosage effect is due to dCBP levels and not secondary, genetic effects of the dCBP3 chromosome (Fig. 7B).
FIG. 7.
The HU-dependent cell cycle delay is sensitive to changes in the relative doses of dCBP and mei-41. (A) Eye discs heterozygous for mei-41RT are sensitive to HU treatment and arrest in S phase, while dCBP3/mei-41RT transheterozygotes have a significant increase in mitotic cells following HU treatment. Student's t tests determined the differences among the means. An asterisk indicates a significant difference (P < 0.01). Two different experiments from two separate matings gave the same qualitative result. (B) A duplication for dCBP (Tr31) restores the replication checkpoint in the dCBP3/mei-41RT transheterozygotes to wild-type levels. These are pooled results from three experiments (P < 0.001). (C) The genetic interactions suggest that dCBP and Mei-41 might interact directly. Coimmunoprecipitations show that dCBP and Mei-41 can be found together in complexes in Kc cells. The top panel is a Western analysis of mock-treated Kc cells that have been left untreated or treated with HU, Kc cells treated with siRNAs against dCBP, and Kc cells treated with siRNAs against mei-41. Gsα was used as an internal control for loading and protein transfer. The samples are 1/10 input of the extracts used for the immunoprecipitations. Results of dCBP immunoprecipitations (IP) from the extracts used in Western blotting are shown in the bottom panel. Although at low levels, Mei-41 is detected in the dCBP immunocomplexes from the control Kc cells but not in the dCBP immunoprecipitates from cells depleted of either dCBP or Mei-41. The 250-kDa marker (lane 1) cross-reacts with the fluorescent-labeled secondary antibodies used in the analysis of the immunoprecipitations.
In addition to the genetic interaction, we determined that dCBP and Mei-41 could be found together in a complex. Figure 7C shows that dCBP complexes immunoprecipitated from Kc cells with antibodies against dCBP contain endogenous Mei-41. It is interesting that Mei-41 is detected in the dCBP immune coprecipitates isolated from cells in the presence and absence of HU.
DISCUSSION
The regulation of chromatin structure and function is essential for proper gene transcription, DNA replication, recombination, and repair. Much of this regulation is effected through the posttranslational acetylation of histones and other chromatin-associated proteins. Mutations in the p300/CBP family of acetyltransferases cause lethal developmental defects in mouse, humans, and Drosophila (26), demonstrating that this type of regulation is highly conserved. How acetylation of chromatin proteins modulates chromosome structure and function to affect these cellular functions is still poorly understood.
dCBP is part of the Mei-41 replication checkpoint pathway.
Most of the accumulated data imply that p300/CBP affects the cell cycle indirectly through its ability to stimulate the transcription of genes encoding proteins required for replication and other cell cycle regulatory processes (26). However, recent reports provide evidence that p300/CBP can interact directly with and acetylate DNA metabolic enzymes (29, 30, 57). In this report we describe evidence suggesting that dCBP may also mediate cell cycle arrest in response to stalled DNA replication through interactions with the Drosophila ATR homologue, Mei-41.
In Drosophila, Mei-41, mediates both the replication and DNA repair checkpoints during embryogenesis and in proliferating imaginal disc cells (11, 25, 27, 34, 38, 51). The mei-41 pathway is also required to increase the interphase of the final 12 to 14 syncytial mitoses of the early blastoderm, which is important for establishing zygotic transcription and initiating embryonic cellularization (27, 51, 52). Interestingly, both the mei-41 and dCBP mutant discs are defective for the replication checkpoint, suggesting that they may be part of the same pathway. However, the reduced response to HU seen in the dCBP loss-of-function cells might be due to a G2 timing defect rather than a replication checkpoint defect. This is a very reasonable explanation since p300/CBP interacts with E2F1, a transcription factor that regulates entry into S (58, 59). In Drosophila, dE2F regulates the initiation of both S and M by affecting the expression of Cyclin E and string, the fly cdc25 homologue (44). We do not rule out the possibility that dCBP can affect G2 timing, possibly through its interaction with dE2F. However, our assays show that the dCBP siRNAs reduce dCBP to levels that do affect the replication checkpoint but do not affect G2 timing. The length of G2, measured by following BrdU-labeled DNA into mitosis, in the cells of the eye disc is similar to the G2 determined for mammalian cultured cells and the G2 previously measured in fly wing discs (44).
A reduction in dCBP function allows a significant number of disc cells to escape the replication checkpoint; significantly more BrdU-labeled cells enter mitosis in the HU-treated dCBP loss-of-function discs than in control discs. However, a reduction in dCBP function does not affect the ability of HU to retard replication. In both control and dCBP siRNA-expressing discs, HU inhibits the incorporation of BrdU. Although the data show a slight increase in BrdU-positive cells in the dCBP siRNA-expressing cells, it is not statistically significant.
While HU can reduce the number of mitotic eye disc cells over 10-fold in 2.5 h, the same reduction in Kc cell mitosis requires at least 12 h of HU treatment. This is consistent with previous data showing that HU-mediated cell cycle arrest requires a 15-h treatment in Drosophila S2 cells (20). We cannot rule out the possibility that this longer HU treatment is initiating a DNA repair checkpoint, which is known to require the Mei-41 pathway (34). In this case, our Kc cell data would suggest that dCBP is also a part of the DNA repair checkpoint as well. It may be that in Drosophila, stalled replication and DNA damage initiate the same checkpoint pathway.
The reduction of dCBP via the expression of dCBP siRNAs affects all dCBP functions. Thus, the defect in the replication checkpoint of dCBP siRNA-expressing cells could be a secondary effect of defects in other developmental processes. We can rule out the pleiotropic effects caused by a loss of dCBP function by assessing the mei-41RT/dCBP3 transheterozygotes for changes in the replication checkpoint. The mei-41RT/dCBP3 transheterozygotes show a small but significant defect in the HU response, suggesting that the two proteins interact specifically in the same pathway. However, this result does not address whether the interaction is direct. It could be that a reduction of dCBP suppresses the transcription of replication checkpoint genes and thus affects the mei-41 pathway indirectly. We have not assayed all the genes known to regulate the DNA damage checkpoint, and that may also affect a checkpoint in response to stalled replication; so we cannot absolutely rule out this possibility. Had we done so and found no changes, we still could not rule out the possibility that a reduction in dCBP activity might suppress the expression of unknown genes that are part of the Mei-41 pathway. However, our observations argue against it. First, expression of two of the replication checkpoint genes, mei-41 and grp, is unaffected in the dCBP-depleted Kc cells. Recently, we have also found that the expression of the ATRIP homologue mus304 (12) as well as the cdc25 homologue string (stg) (21) is unaffected by a reduction in dCBP (K. Jones, unpublished observation).
Second, coimmunoprecipitation analysis demonstrates that dCBP is detectable in a complex with Mei-41. We detected the dCBP-Mei-41 interaction in the both the absence and presence of HU, which suggests that their activities are stimulated by a checkpoint signal. However, because the immunoprecipitations are not quantitative, we cannot rule out the possibility that there is quantitative change in the dCBP-Mei-41 interaction upon induction of the checkpoint. Whether the dCBP-Mei-41 interaction affects replication or the monitoring of normal forks is not known. Although CBP/p300 is known to bind PCNA and may mediate the acetylation that regulates PCNA binding to DNA (30, 42), suggesting a role in mammalian replication, we detected no changes in BrdU incorporation or mitotic index in dCBP-depleted disc cells. This result suggests that the dCBP function is not a necessary component of the replication machinery or replication fork monitoring in Drosophila. However, it may be that the activated checkpoint is more sensitive to a reduction in the dosage of dCBP than fork monitoring or replication. In any event, these data suggest a more direct role for dCBP in mediating the replication checkpoint through interactions with Mei-41. It will be very interesting to determine whether dCBP is also required for the Mei-41 regulation of the midblastula transition as well.
The Drosophila tefu gene encodes the fly ATM homologue (45, 48). Mutations in tefu cause not only telomere fusions in dividing cells but also a defect in the checkpoint induced by low levels of ionizing irradiation (7, 45, 48, 53, 55). In contrast, the checkpoint induced by both high and low levels of ionizing irradiation requires mei-41 (12, 27). Recent data suggest that the tefu and mei-41 pathways act uniquely and in parallel to protect telomere integrity (8, 46). Whether the HU-induced checkpoint requires tefu and whether the tefu-mediated telomere protection requires dCBP function await further studies.
To confirm that the HU-mediated checkpoint defect seen in the dCBP siRNA-expressing cells was specific to loss of dCBP function, we rescued the defect by overexpressing a wild-type dCBP gene. Furthermore, we showed that overexpression of a dCBP acetyltransferase mutant could not rescue the checkpoint defect. We showed previously (41) that these exogenous genes express to similar levels and encode full-length proteins that bind known target proteins. Thus, this result shows that the dCBP acetyltransferase activity is required for the checkpoint. Initial in vitro acetyltransferase assays suggest that Mei-41 is not a dCBP target (K. Jones, unpublished observation). However, two well-conserved ATR/ATM phosphorylation sites in dCBP suggest that Mei-41 may regulate dCBP activity. In this model, Mei-41 activation may cause the phosphorylation of dCBP. This phosphorylation event may activate (or inactivate) dCBP activity. The targets of dCBP acetyltransferase may be histones and/or other members of the checkpoint pathway, and their acetylation status may determine whether the replication checkpoint is established or maintained. This may be since CBP/p300 is known to acetylate and possibly activate p53 in response to stalled replication (32, 40, 43), to regulate WRN localization to the extranucleolar DNA in response to DNA damage (9), and to acetylate Fen1 and other members of the base excision repair pathway to regulate its activity (5, 29).
CBP affects genomic stability.
In our earlier studies, we found it difficult to generate somatic clones mutant for dCBP in adult fly tissues (1). This result suggested that, in addition to mediating developmental programs of gene transcription, dCBP activity might be required for cell viability. The results described here indicate that dCBP depletion in Kc cells and third instar larval brains results in a defect in chromosome condensation and chromosome shredding during mitosis and, ultimately, cell death. Consistent with these observations, loss of the replication checkpoint in flies, by mutation in grp, causes defects in chromosome condensation and a metaphase delay by reducing the time between the initiation of condensation and metaphase (61). Therefore, loss of dCBP may cause a defect similar to that observed in the grp mutants. However, further studies are needed to determine if these two phenotypes are mechanistically similar or distinct. It may be that dCBP indirectly affects chromosome integrity by regulating the expression of other genes needed to maintain chromosome structure. The dCBP checkpoint activity may or may not be involved in this process.
The disruption of the DNA replication checkpoint by depletion of ATR in human cells results in chromosome fragmentation during mitosis (15) and in mice leads to chromosome fragmentation and caspase-dependent apoptosis that then leads to early embryonic lethality (13). However, unlike the Mei-41 pathway in flies, the mammalian ATR pathway does not appear to be the only component of the replication checkpoint. ATR-depleted, as well as ATR- and ATM-depleted, murine embryonic fibroblasts exhibit an intact HU-dependent cell cycle arrest (14). Furthermore, this ATR/ATM-independent HU-induced cell cycle arrest is defective in some human tumor cell lines (22). One interpretation of these results is that higher organisms have evolved separate and redundant checkpoint pathways. For example, in Drosophila, p53 is required only for apoptosis and has no detectable checkpoint function (11, 54) while in mammals, p53 is involved in both apoptosis and checkpoint functions. Mutations in mei-41 affect the regulation of DNA repair and the DNA repair and replication checkpoint pathways (25, 51, 52), while mammalian cells depleted of ATR have an intact HU-mediated checkpoint (14). In fact, perturbations in DNA metabolism at any time during the cell cycle may stimulate the same checkpoint pathway in flies, while that is not the case in mammalian cells.
Studies from yeast also indicate that acetyltransferases are critical components of pathways that maintain chromatin structure. For example, genetic studies of the Gcn5-containing N-acetyltransferases (GNATs) and MYST (named for yeast and human members MOZ, YBF2, SAS2, and Tip60) acetyltransferases demonstrate that histone tails are differentially acetylated to bring about activation of transcription, DNA repair, and cell proliferation. Yeast mutant for both the GNAT Gcn5 and the MYST acetyltransferase Sas3 have a global reduction in histone H3 acetylation, are nonviable, and are blocked at the G2/M transition (33). In addition, mutation in the MYST acetyltransferase Esa1 results in a global reduction of histone H4 acetylation, cell lethality, and a defect in the Rad9 DNA repair checkpoint (2, 18). Similarly, we previously showed that mutations in fly dCBP cause a global reduction in the acetylation of histone H4 (41). Taken together, these results support a model in which acetyltransferases in both yeast and higher eukaryotes modulate chromatin structure in response to signal transduction pathways that regulate transcription, DNA repair, recombination, and replication. The fact that loss of p300/CBP function results in checkpoint defects and decondensed and shredded chromosomes suggests that these acetyltransferases are critically important for the maintenance of genome stability.
Acknowledgments
We are very grateful to A. Snyder (MMI Department, Oregon Health and Sciences University, and the Oregon Hearing Research Center) for assistance with the confocal analysis. We are also indebted to Mathew Thayer for help with karyotyping and discussions throughout the development of this study.
This work was supported by NIH grant GM61837 (S.M.S.).
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Akimaru, H., Y. Chen, P. Dai, D. X. Hou, M. Nonaka, S. Smolik, S. Armstrong, R. Goodman, and S. Ishii. 1997. Drosophila CBP is a co-activator of cubitus interruptus in hedgehog signalling. Nature 386:735-738. [DOI] [PubMed] [Google Scholar]
- 2.Allard, S., R. T. Utley, J. Savard, A. Clarke, P. Grant, C. J. Brandl, L. Pillus, J. L. Workman, and J. Cote. 1999. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 18:5108-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bantignies, F., R. H. Goodman, and S. M. Smolik. 2000. Functional interaction between the coactivator Drosophila CREB-binding protein and ASH1, a member of the trithorax group of chromatin modifiers. Mol. Cell. Biol. 20:9317-9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bantignies, F., R. H. Goodman, and S. M. Smolik. 2002. The interaction between the coactivator dCBP and Modulo, a chromatin-associated factor, affects segmentation and melanotic tumor formation in Drosophila. Proc. Natl. Acad. Sci. USA 99:2895-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartek, J., C. Lukas, and J. Lukas. 2004. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell. Biol. 5:792-804. [DOI] [PubMed] [Google Scholar]
- 6.Bentley, N. J., D. A. Holtzman, G. Flaggs, K. S. Keegan, A. DeMaggio, J. C. Ford, M. Hoekstra, and A. M. Carr. 1996. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 15:6641-6651. [PMC free article] [PubMed] [Google Scholar]
- 7.Bi, X., M. Gong, D. Srikanta, and Y. S. Rong. 2005. Drosophila ATM and Mre11 are essential for the G2/M checkpoint induced by low-dose irradiation. Genetics 171:845-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi, X., D. Srikanta, L. Fanti, S. Pimpinelli, R. Badugu, R. Kellum, and Y. S. Rong. 2005. Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance. Proc. Natl. Acad. Sci. USA 102:15167-15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blander, G., N. Zalle, Y. Daniely, J. Taplick, M. D. Gray, and M. Oren. 2002. DNA damage-induced translocation of the Werner helicase is regulated by acetylation. J. Biol. Chem. 277:50934-50940. [DOI] [PubMed] [Google Scholar]
- 10.Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky, M. H., W. Nordstrom, G. Tsang, E. Kwan, G. M. Rubin, and J. M. Abrams. 2000. Drosophila p53 binds a damage response element at the reaper locus. Cell 101:103-113. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky, M. H., J. J. Sekelsky, G. Tsang, R. S. Hawley, and G. M. Rubin. 2000. mus304 encodes a novel DNA damage checkpoint protein required during Drosophila development. Genes Dev. 14:666-678. [PMC free article] [PubMed] [Google Scholar]
- 13.Brown, E. J., and D. Baltimore. 2000. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14:397-402. [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, E. J., and D. Baltimore. 2003. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17:615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casper, A. M., P. Nghiem, M. F. Arlt, and T. W. Glover. 2002. ATR regulates fragile site stability. Cell 111:779-789. [DOI] [PubMed] [Google Scholar]
- 16.Chan, H. M., and N. B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363-2373. [DOI] [PubMed] [Google Scholar]
- 17.Cimprich, K. A., T. B. Shin, C. T. Keith, and S. L. Schreiber. 1996. cDNA cloning and gene mapping of a candidate human cell cycle checkpoint protein. Proc. Natl. Acad. Sci. USA 93:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke, A. S., J. E. Lowell, S. J. Jacobson, and L. Pillus. 1999. Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol. Cell. Biol. 19:2515-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 97:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vries, H. I., L. Uyetake, W. Lemstra, J. F. Brunsting, T. T. Su, H. H. Kampinga, and O. C. Sibon. 2005. Grp/DChk1 is required for G2-M checkpoint activation in Drosophila S2 cells, whereas Dmnk/DChk2 is dispensable. J. Cell Sci. 118:1833-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar, B. A., and P. H. O'Farrell. 1989. Genetic control of cell division patterns in the Drosophila embryo. Cell 57:177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florensa, R., O. Bachs, and N. Agell. 2003. ATM/ATR-independent inhibition of cyclin B accumulation in response to hydroxyurea in nontransformed cell lines is altered in tumour cell lines. Oncogene 22:8283-8292. [DOI] [PubMed] [Google Scholar]
- 23.FlyBase. 1999. The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 27:85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogarty, P., S. D. Campbell, R. Abu-Shumays, B. S. Phalle, K. R. Yu, G. L. Uy, M. L. Goldberg, and W. Sullivan. 1997. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 7:418-426. [DOI] [PubMed] [Google Scholar]
- 25.Garner, M., S. van Kreeveld, and T. T. Su. 2001. mei-41 and bub1 block mitosis at two distinct steps in response to incomplete DNA replication in Drosophila embryos. Curr. Biol. 11:1595-1599. [DOI] [PubMed] [Google Scholar]
- 26.Goodman, R. H., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14:1553-1577. [PubMed] [Google Scholar]
- 27.Hari, K. L., A. Santerre, J. J. Sekelsky, K. S. McKim, J. B. Boyd, and R. S. Hawley. 1995. The mei-41 gene of D. melanogaster is a structural and functional homolog of the human ataxia telangiectasia gene. Cell 82:815-821. [DOI] [PubMed] [Google Scholar]
- 28.Hartwell, L. H., and T. A. Weinert. 1989. Checkpoints: controls that ensure the order of cell cycle events. Science 246:629-634. [DOI] [PubMed] [Google Scholar]
- 29.Hasan, S., N. El-Andaloussi, U. Hardeland, P. O. Hassa, C. Burki, R. Imhof, P. Schar, and M. O. Hottiger. 2002. Acetylation regulates the DNA end-trimming activity of DNA polymerase beta. Mol. Cell 10:1213-1222. [DOI] [PubMed] [Google Scholar]
- 30.Hasan, S., P. O. Hassa, R. Imhof, and M. O. Hottiger. 2001. Transcription coactivator p300 binds PCNA and may have a role in DNA repair synthesis. Nature 410:387-391. [DOI] [PubMed] [Google Scholar]
- 31.Hasan, S., M. Stucki, P. O. Hassa, R. Imhof, P. Gehrig, P. Hunziker, U. Hubscher, and M. O. Hottiger. 2001. Regulation of human flap endonuclease-1 activity by acetylation through the transcriptional coactivator p300. Mol. Cell 7:1221-1231. [DOI] [PubMed] [Google Scholar]
- 32.Ho, C. C., W. Y. Siu, A. Lau, W. M. Chan, T. Arooz, and R. Y. Poon. 2006. Stalled replication induces p53 accumulation through distinct mechanisms from DNA damage checkpoint pathways. Cancer Res. 66:2233-2241. [DOI] [PubMed] [Google Scholar]
- 33.Howe, L., D. Auston, P. Grant, S. John, R. G. Cook, J. L. Workman, and L. Pillus. 2001. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 15:3144-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaklevic, B. R., and T. T. Su. 2004. Relative contribution of DNA repair, cell cycle checkpoints, and cell death to survival after DNA damage in Drosophila larvae. Curr. Biol. 14:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keegan, K. S., D. A. Holtzman, A. W. Plug, E. R. Christenson, E. E. Brainerd, G. Flaggs, N. J. Bentley, E. M. Taylor, M. S. Meyn, S. B. Moss, A. M. Carr, T. Ashley, and M. F. Hoekstra. 1996. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev. 10:2423-2437. [DOI] [PubMed] [Google Scholar]
- 36.Kelly, T. J., and G. W. Brown. 2000. Regulation of chromosome replication. Annu. Rev. Biochem. 69:829-880. [DOI] [PubMed] [Google Scholar]
- 37.Kung, A. L., V. I. Rebel, R. T. Bronson, L. E. Ch'ng, C. A. Sieff, D. M. Livingston, and T. P. Yao. 2000. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 14:272-277. [PMC free article] [PubMed] [Google Scholar]
- 38.Laurencon, A., A. Purdy, J. Sekelsky, R. S. Hawley, and T. T. Su. 2003. Phenotypic analysis of separation-of-function alleles of MEI-41, Drosophila ATM/ATR. Genetics 164:589-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsley, D. L., and G. G. Zimm. 1992. The genome of Drosophila melanogaster. Academic Press, San Diego, CA.
- 40.Livengood, J. A., K. E. Scoggin, K. Van Orden, S. J. McBryant, R. S. Edayathumangalam, P. J. Laybourn, and J. K. Nyborg. 2002. p53 Transcriptional activity is mediated through the SRC1-interacting domain of CBP/p300. J. Biol. Chem. 277:9054-9061. [DOI] [PubMed] [Google Scholar]
- 41.Ludlam, W. H., M. H. Taylor, K. G. Tanner, J. M. Denu, R. H. Goodman, and S. M. Smolik. 2002. The acetyltransferase activity of CBP is required for wingless activation and H4 acetylation in Drosophila melanogaster. Mol. Cell. Biol. 22:3832-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naryzhny, S. N., and H. Lee. 2004. The post-translational modifications of proliferating cell nuclear antigen: acetylation, not phosphorylation, plays an important role in the regulation of its function. J. Biol. Chem. 279:20194-20199. [DOI] [PubMed] [Google Scholar]
- 43.Nayak, B. K., and G. M. Das. 2002. Stabilization of p53 and transactivation of its target genes in response to replication blockade. Oncogene 21:7226-7229. [DOI] [PubMed] [Google Scholar]
- 44.Neufeld, T. P., A. F. de la Cruz, L. A. Johnston, and B. A. Edgar. 1998. Coordination of growth and cell division in the Drosophila wing. Cell 93:1183-1193. [DOI] [PubMed] [Google Scholar]
- 45.Oikemus, S. R., N. McGinnis, J. Queiroz-Machado, H. Tukachinsky, S. Takada, C. E. Sunkel, and M. H. Brodsky. 2004. Drosophila atm/telomere fusion is required for telomeric localization of HP1 and telomere position effect. Genes Dev. 18:1850-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oikemus, S. R., J. Queiroz-Machado, K. Lai, N. McGinnis, C. Sunkel, and M. H. Brodsky. 2006. Epigenetic telomere protection by Drosophila DNA damage response pathways. PLOS Genet. 2:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pichierri, P., and A. Franchitto. 2004. Werner syndrome protein, the MRE11 complex and ATR: menage-a-trois in guarding genome stability during DNA replication? Bioessays 26:306-313. [DOI] [PubMed] [Google Scholar]
- 48.Queiroz-Machado, J., J. Perdigao, P. Simoes-Carvalho, S. Herrmann, and C. E. Sunkel. 2001. tef: a mutation that causes telomere fusion and severe genome rearrangements in Drosophila melanogaster. Chromosoma 110:10-23. [DOI] [PubMed] [Google Scholar]
- 49.Rose, R. E., N. M. Gallaher, D. J. Andrew, R. H. Goodman, and S. M. Smolik. 1997. The CRE binding protein dCREB-A is required for Drosophila embryonic development. Genetics 146:595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez, Y., B. A. Desany, W. J. Jones, Q. Liu, B. Wang, and S. J. Elledge. 1996. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 271:357-360. [DOI] [PubMed] [Google Scholar]
- 51.Sibon, O. C., A. Laurencon, R. Hawley, and W. E. Theurkauf. 1999. The Drosophila ATM homologue Mei-41 has an essential checkpoint function at the midblastula transition. Curr. Biol. 9:302-312. [DOI] [PubMed] [Google Scholar]
- 52.Sibon, O. C., V. A. Stevenson, and W. E. Theurkauf. 1997. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature 388:93-97. [DOI] [PubMed] [Google Scholar]
- 53.Silva, E., S. Tiong, M. Pedersen, E. Homola, A. Royou, B. Fasulo, G. Siriaco, and S. D. Campbell. 2004. ATM is required for telomere maintenance and chromosome stability during Drosophila development. Curr. Biol. 14:1341-1347. [DOI] [PubMed] [Google Scholar]
- 54.Sogame, N., M. Kim, and J. M. Abrams. 2003. Drosophila p53 preserves genomic stability by regulating cell death. Proc. Natl. Acad. Sci. USA 100:4696-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song, Y. H., G. Mirey, M. Betson, D. A. Haber, and J. Settleman. 2004. The Drosophila ATM ortholog, dATM, mediates the response to ionizing radiation and to spontaneous DNA damage during development. Curr. Biol. 14:1354-1359. [DOI] [PubMed] [Google Scholar]
- 56.Spradling, A. 1986. P element-mediated trasfromation, p. 175-198. In D. B. Roberts (ed.), Drosophila: a practical approach. IRL Press, New York, NY.
- 57.Tini, M., A. Benecke, S. J. Um, J. Torchia, R. M. Evans, and P. Chambon. 2002. Association of CBP/p300 acetylase and thymine DNA glycosylase links DNA repair and transcription. Mol. Cell 9:265-277. [DOI] [PubMed] [Google Scholar]
- 58.Trouche, D., A. Cook, and T. Kouzarides. 1996. The CBP co-activator stimulates E2F1/DP1 activity. Nucleic Acids Res. 24:4139-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trouche, D., and T. Kouzarides. 1996. E2F1 and E1A(12S) have a homologous activation domain regulated by RB and CBP. Proc. Natl. Acad. Sci. USA 93:1439-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao, T. P., S. P. Oh, M. Fuchs, N. D. Zhou, L. E. Ch'ng, D. Newsome, R. T. Bronson, E. Li, D. M. Livingston, and R. Eckner. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361-372. [DOI] [PubMed] [Google Scholar]
- 61.Yu, K. R., R. B. Saint, and W. Sullivan. 2000. The Grapes checkpoint coordinates nuclear envelope breakdown and chromosome condensation. Nat. Cell Biol. 2:609-615. [DOI] [PubMed] [Google Scholar]