Abstract
Eukaryotes produce a number of noncoding transcripts from intergenic regions. In Saccharomyces cerevisiae, such cryptic unstable transcripts (CUTs) are thought to be degraded in the nucleus by a process involving polyadenylation and 3′-to-5′ degradation by the nuclear exosome. In this work, we examine the degradation pathway of the RNA SRG1, which is produced from an intergenic region and contributes to the regulation of the SER3 gene by promoter occlusion during SRG1 transcription. Although there is some effect on SRG1 transcript levels when the nuclear exosome is compromised, the bulk of the SRG1 RNA is degraded in the cytoplasm by decapping and 5′-to-3′ exonucleolytic digestion. Examination of other CUTs suggests that individual CUTs can be degraded by a variety of different mechanisms, including nuclear decay, cytoplasmic decapping and 5′-to-3′ decay, and nonsense-mediated decay. Moreover, some CUTs appear to be associated with polyribosomes. These results indicate that some CUTs can be exported from the nucleus and enter translation before being degraded, identifying a potential mechanism for the evolution of new protein-encoding genes.
Historically, RNAs transcribed by RNA polymerase II (RNA Pol II) in eukaryotic cells included functional transcripts such as the mRNAs, snRNAs, and snoRNAs. However, it is now clear that RNA Pol II also produces a number of promiscuous or “dark matter” transcripts: intergenic and noncoding RNAs, pseudogenes, antisense transcripts, or transcripts resulting from adventitious initiation sites (16). In the yeast Saccharomyces cerevisiae, one class of such promiscuous transcripts are capped, intergenic transcripts referred to as cryptic unstable transcripts (CUTs), which are thought to be noncoding and extremely unstable (8, 28). Some of these intergenic transcripts may be functional. For example, the yeast noncoding transcript SRG1 is involved in the transcriptional control of its downstream neighbor SER3 by promoter occlusion during SRG1 transcription (1, 20, 21). An emerging model is that yeast CUTs, including the SRG1 RNA, are recognized and degraded in the nucleus in a process that involves polyadenylation of the RNA by the poly(A) polymerase Trf4p, followed by degradation by the nuclear exosome (8, 24, 28). However, the possible role of cytoplasmic RNA degradation mechanisms in the decay of these RNAs has not been explored.
To determine if cytoplasmic RNA decay pathways might also function in the degradation of the CUTs and SRG1, we first focused on examining the mechanisms by which the SRG1 RNA is degraded. We found that the SRG1 locus produces two RNAs with different 3′ ends (referred to as SRG1-s and SRG1-l), which are both primarily degraded in the cytoplasm by decapping and 5′-to-3′ decay in a process independent of nonsense-mediated decay (NMD). In addition, a SRG1-SER3 read-through transcript (referred to as SRG1-rt) is also degraded by cytoplasmic decapping and 5′-to-3′ decay, although this RNA is targeted for decapping by NMD. Furthermore, closer investigation of other CUTs shows that a number of them may be degraded by both cytoplasmic and nuclear pathways, and some can enter translation. This supports the idea that different intergenic RNA Pol II transcripts may be processed and degraded by a variety of mechanisms. In addition, these results identify a possible mechanism for the evolution of new protein-encoding genes.
MATERIALS AND METHODS
Yeast strains and culture.
Yeast strains are listed in Table 1. Strains were constructed using standard methods. Euroscarf strains (27) can be obtained from Open Biosystems; the strain BY4741 was used as the wild type (WT). Yeast cells were routinely grown in yeast extract-peptone medium or selective minimal medium supplemented with 2% dextrose.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference and/or source |
|---|---|---|
| yRP 582 | matarpb1-1 ura3 leu2 | 9 |
| yRP 926 | matα ura3 xrn1Δ::LEU2 rpb1-1 leu2 | 22 |
| yRP 1200 | matahis4-539 leu2-3 leu-112 trp1-Δ1 ura3-52 dcp1Δ::URA3 | 2 |
| yRP 1358 | matahis4 leu2 lys2 trp1 ura3 dcp2Δ::TRP1 | 10 |
| yRP 1805 | matα ura4 dcp2Δ::TRP1 rpb1-1 trp1 lys2 ura3 leu2 | 22 |
| yRP 2156 | matα xrn1Δ::LEU2 rrp6Δ::URA3 leu2 ura3 rpb1-1 | This study |
| yRP 2157 | matarpb1-1 ura3 leu2 trf4Δ::kanMX4 | This study |
| yRP 2158 | mataura3 leu2 rpb1-1 rrp6Δ::URA3 | This study |
| Y0 1777 | matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rrp6Δ::kanMX4 | 27/Euroscarf |
| Y0 3858 | matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 dhh1Δ::kanMX4 | 27/Euroscarf |
| Y0 4540 | matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 xrn1Δ::kanMX4 | 27/Euroscarf |
| Y0 5307 | matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ski2Δ::kanMX4 | 27/Euroscarf |
| Y0 6265 | matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 trf4Δ::kanMX4 | 27/Euroscarf |
| Y0 6214 | matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 upf1Δ::kanMX4 | 27/Euroscarf |
| BY4741 | matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | 27/Euroscarf |
RNA analysis.
Isolation of total cellular RNA was done as previously described (5). Transcriptional shutoffs and half-life analyses were done essentially as described previously (9), except that the carbon source (dextrose) was not changed. For agarose Northern blots, 50 μg of RNA was run on 1.5% agarose-formaldehyde gels and blotted onto a Nytran membrane. Blots were probed using either random-primed, [α-32P]dATP-labeled probes or 5′-end [γ-32P]ATP- labeled oligonucleotide probes and scanned using a PhosphorImager (Typhoon 9410; Amersham Biosciences). RNase H reactions were performed basically as described previously (9), using 40 μg RNA and 600 ng oligo(dT) per reaction (for Fig. 1B) or 300 μg RNA and 7 μg oligo(dT) per reaction (for Fig. 2C). Products were resolved on 6% polyacrylamide gels, blotted onto a Nytran membrane, and probed with a [γ-32P]ATP-labeled SRG1 5′-end-specific oligonucleotide probe (5′-GGTTCTTCCTTTATATACATAAC-3′; oRP 1304) (Fig. 1) or a random-primed SRG1 probe spanning −470 to −125 (relative to SER3 start; using primers oRP 1308 and oRP 1309) (Fig. 2). CUT probes were PCR amplified using the primers NEL025c (F, 5′-CGTACAGTGCAGGTTTTGAC-3′; R, 5′-GCTCGAGACATTTTGGGTAGA-3′) (oRP 1310 and oRP 1311) and NMR026w (F, 5′-CAATTTTATCAAGACCGCAC-3′; R, 5′-TAAACGTTAGCGTGTTCTTC-3′) (oRP 1312 and oRP 1313) and random-prime labeled. A 5′-end, γ-32P-labeled 7s oligonucleotide probe (oRP 100) was used for normalization.
FIG. 1.
Three forms of SRG1 RNA. A. Agarose Northern blot showing that three transcripts are produced from the SRG1 locus. B. RNase H-directed cleavage of SRG1 using oligo(dT). The probe used and the expected sizes of cleavage products following treatment with oligo(dT) are shown in panel C. L, SRG1-l; S, SRG1-s. Positions of the size markers (M) are indicated in nucleotides. This experiment was repeated three times; a representative blot is shown. C. Schematic of SRG1 transcripts, their approximate sizes, and their 3′ ends (black octagons) relative to SER3. The SER3 promoter is represented by the gray arrow. The schematic is not to scale.
FIG. 2.
Accumulation of SRG1 transcripts in mutant strains. A. Accumulation of SRG1 RNAs in various mutant strains at steady state. The short (S), long (L), and read-through (RT) forms of SRG1 are indicated. 7s is used as a loading control. At least three independent samples per mutant strain were examined; a representative blot is shown. B. Accumulation of SRG1-l and SRG1-s RNAs in various mutant strains. Bars represent the averages of at least three independent experiments, normalized to the 7s loading control. C. RNase H-directed cleavage with (+) or without (−) oligo(dT) of SRG1 RNA in indicated strains. 7s is used as a loading control. Positions of the size markers (M) are indicated in nucleotides. “S” denotes SRG1-s following cleavage; “C” indicates a constant band which does not change in size. This experiment was repeated three times; a representative blot is shown. D. Accumulation of SRG1-rt RNA in various mutant strains. Bars represent the averages of at least three independent experiments, normalized to the 7s loading control.
Polysome analysis.
Cell lysis and polysome analysis were done essentially as described previously (4), with some modifications. Briefly, yeast were grown in 250 ml of rich medium (yeast extract-peptone with 2% dextrose) at 30°C to an optical density at 600 nm of 0.3 to 0.5, collected, and grown a further 30 min in the presence or absence of 1 M KCl. Cells were collected by centrifugation at 4,000 rpm for 2 min at 4°C, washed in 1 ml of lysis buffer (20 mM Tris, pH 8, 140 mM KCl, 5 mM MgCl2, 0.5 mM dithiothreitol, 1 mg/ml heparin), and frozen. For cell lysis, the pellet was resuspended in 400 μl of lysis buffer. A 200-μl volume of glass beads was added, and the reaction mixture was vortexed at full speed for 2 min followed by incubation on ice, repeated a total of three times. The lysates were clarified by centrifugation for 2 min at 4000 rpm at 4°C. Approximately 20 A260 units was loaded on a 15 to 50% sucrose gradient containing lysis buffer. The samples were sedimented in a Beckman SW41 rotor at 4°C for 2.5 h at 39,000 rpm and collected while the A260 value was monitored using a continuous flow cell UV detector. Fraction collection and RNA extraction were performed as described previously (4); RNA was analyzed as described above.
Analysis of previously published microarray experiments.
Experimental data from previous studies using Affymetrix Yeast S98 arrays (Affymetrix, Santa Clara, CA) were downloaded from http://jacobsonlab.umassmed.edu/publications/2003-Feng/supplementarydata/(NMD(26)-Raw Data-9335.txt) (11) and http://www.ncbi.nih.gov/geo, accession number GSE2579 (28). All probes annotated as “nonannotated serial analysis of gene expression (SAGE) open reading frames (ORFs)” were considered; we used the absent/present spot calling from the primary data set to include only those probes that detected an RNA. Analysis was performed using GeneSpring (Agilent Technologies, Palo Alto, CA) and Microsoft Excel. For the cytoplasmic decay mutant arrays (11), a t test comparison of mutant and wild-type readings for a given target was performed, followed by filtering on confidence for increases in level using the Benjamini and Hochberg false discovery rate adjustment, with a P value cutoff of 0.01. Targets with significant changes in the xrn1Δ or dcp1Δ strain or changes in all three NMD mutant strains (upf1Δ, upf2Δ, and upf3Δ strains) compared to wild type were selected. The nuclear arrays were not performed enough times to allow for this type of analysis. Instead, for each target, each rrp6Δ reading was compared to the mean of the two wild-type values, and the average of the two readings was used to give an rrp6Δ/WT comparison. Targets with an rrp6Δ/WT ratio of at least 3 were selected. Next, SAGE ORF coordinates were acquired from Affymetrix and used to annotate the yeast genome viewer gBrowse (http://db.yeastgenome.org/cgi-bin/gbrowse/yeast/). Each SAGE ORF was checked on gBrowse; those overlapping annotated genomic elements were not considered CUTs and not included in the analysis.
RESULTS
The SRG1 locus produces three classes of transcripts.
As a first step in our analyses, we examined the transcripts produced by the SRG1 locus. These experiments were done in the presence of high levels of serine in the medium, which increases the transcription of SRG1 (20, 21; also data not shown) but does not alter its decay rate (data not shown). Examination of the SRG1 transcripts on an agarose Northern blot reveals that three RNAs are produced from the SRG1 locus (Fig. 1A). As previously described (20), the longest transcript derived from the SRG1 locus is a ∼1,900-bp read-through transcript that continues to the 3′ end of the SER3 gene (referred to as SRG1-rt).
The SRG1 locus also produces a smaller transcript (20), which we were able to resolve into two transcripts on an agarose Northern blot (Fig. 1A). An RNase H-directed cleavage using an SRG1-specific oligonucleotide and SRG1 3′-end probe showed that these transcripts have different 3′ ends (data not shown). To map the positions of the 3′ ends, treatment with oligo(dT) and RNase H was used to remove any poly(A) tail and the RNAs were analyzed using a polyacrylamide Northern blot assay (Fig. 1B). This revealed that the smaller band, referred to herein as SRG1-s, corresponds to a 3′ end approximately 400 nucleotides downstream of the initiation site (Fig. 1B, “S”). Further, since this smaller SRG1 RNA becomes approximately 60 nucleotides smaller following RNase H treatment with oligo(dT), it is apparently polyadenylated (Fig. 1B). The larger SRG1 band on the agarose Northern blot corresponds to a set of closely spaced 3′ ends (referred to collectively as SRG1-l) located approximately 480 to 520 nucleotides from the initiation site (Fig. 1B, “L”) which also appear to be polyadenylated (Fig. 1B). Our finding that the SRG1 RNAs can be polyadenylated agrees with previous work that showed enrichment of SRG1 in a poly(A)+ RNA fraction (20), although a second group did not observe a strong enrichment of SRG1 following oligo(dT) selection of poly(A)+ RNA (8). Therefore, to further confirm that SRG1 RNAs are polyadenylated, we performed 3′ rapid amplification of cDNA ends and were successful in amplifying both SRG1-s and SRG1-l, suggesting that at least some fraction of SRG1 is indeed polyadenylated (data not shown). In conclusion, the SRG1-s and SRG1-l RNAs are a mixture of polyadenylated molecules with different 3′ ends, all of which map within the SER3 promoter or coding region (Fig. 1C).
SRG1-s and SRG1-l accumulate in mutant strains defective for cytoplasmic decapping or 5′-to-3′ exonuclease activity.
To determine how the SRG1 transcripts are degraded, we first examined the steady-state accumulation of the SRG1-s and SRG1-l RNAs in strains with defects in various nuclear and cytoplasmic RNA decay mechanisms. We saw at least a fivefold accumulation of both the SRG1-s and SRG1-l transcripts in an xrn1Δ strain, which lacks the major cytoplasmic 5′-to-3′ exonuclease, as well as three- to fivefold accumulation in the dcp1Δ and dcp2Δ decapping mutant strains (Fig. 2A and B). These observations suggest that the SRG1-s and SRG1-l RNAs can be exported and degraded in the cytoplasm by decapping and 5′-to-3′ degradation. Consistent with this possibility, both SRG1-s and SRG1-l also accumulated in a dhh1Δ strain, which is defective in mRNA decapping (Fig. 2A and B) (7). SRG1-s and SRG1-l are unlikely to be degraded by the cytoplasmic exosome, as they do not accumulate in the ski2Δ, ski4-1, and ski7Δ exosome mutant strains (Fig. 2A and B; also data not shown) (2, 25).
Although the SRG1-s and SRG1-l RNAs lack a long ORF, they do contain very small potential reading frames of 9 to 22 codons, which suggested that the SRG1 RNAs could be targets of the NMD pathway (reviewed in reference 3). However, the levels of SRG1-s and SRG1-l are similar in upf1Δ, upf2Δ, and upf3Δ strains compared to wild type (Fig. 2A and B and data not shown). Since Upf1p, Upf2p, and Upf3p are required for NMD, this suggests that these short SRG1 RNAs are not targets of NMD. SRG1-s and SRG1-l also do not accumulate in the pop2Δ, ccr4Δ, pan2Δ, and ccr4Δ pan2Δ deadenylation mutant strains (data not shown), suggesting that the decay of SRG1-s and SRG1-l RNAs is independent of deadenylation. Taken together, the above observations argue that both the SRG1-s and SRG1-l RNAs can be exported to the cytoplasm and degraded by deadenylation-independent decapping, followed by 5′-to-3′ exonuclease digestion by Xrn1p.
To examine whether SRG1 transcripts could also be degraded in the nucleus, we examined the accumulation of the SRG1-s and SRG1-l transcripts in a strain defective for Trf4p, a nuclear poly(A) polymerase that can adenylate substrates and target them for degradation by the nuclear exosome, or Rrp6p, a 3′-to-5′ exonuclease associated with the nuclear exosome (Fig. 2A and B) (14, 17, 18, 24, 28). The SRG1-s RNA did not significantly accumulate in the rrp6Δ or trf4Δ strain (Fig. 2A and B). In contrast, the SRG1-l RNAs accumulated approximately fourfold over wild-type levels in both the rrp6Δ and trf4Δ strains (Fig. 2A and B) (8). Moreover, a comparison of the sizes of the SRG1-l transcripts with and without removal of their poly(A) tails by oligo(dT) treatment indicated that the transcripts were hyperadenylated in the rrp6Δ strain but not in the trf4Δ strain (Fig. 2C). These observations are similar to what has been seen for CUTs that are thought to degrade in the nucleus (8, 24, 28), which raises the possibility either that the SRG1-l RNAs are also degraded in the nucleus or that Trf4p or Rrp6p can specifically limit SRG1-l levels by an alternative mechanism (see Discussion).
SRG1 decays more slowly in cytoplasmic decay mutant strains.
The observations that SRG1-s and/or SRG1-l levels increased in strains defective in either nuclear or cytoplasmic RNA decay mechanisms raise three possibilities, which can be distinguished by directly measuring the decay rates of the SRG1 RNAs in various mutant strains. First, SRG1-s and/or SRG1-l could be primarily degraded in the cytoplasm and accumulate in rrp6Δ and trf4Δ mutant strains for indirect reasons. In this case, SRG1-s and SRG1-l RNAs should decay more slowly in dcp2Δ or xrn1Δ mutant strains but not in rrp6Δ and trf4Δ strains. Alternatively, SRG1-s and/or SRG1-l RNAs could be degraded in the nucleus and Xrn1p and Dcp2p could indirectly affect their production. In this case, the SRG1-s and/or SRG1-l RNAs should decay more slowly in rrp6Δ and trf4Δ strains but not in the xrn1Δ and dcp2Δ strains. Finally, some of the SRG1-s and/or SRG1-l RNAs could be degraded in each compartment, which would predict that they would decay more slowly in xrn1Δ, dcp2Δ, rrp6Δ, and trf4Δ mutant strains. To distinguish between these possibilities, we utilized a temperature-sensitive allele of RNA Pol II (rpb1-1) to measure the decay rates of SRG1-s and SRG1-l in the various mutant strains (Fig. 3).
FIG. 3.
Decay of SRG1 transcripts in mutant strains. A to E. Representative Northern blots showing SRG1 RNA levels in indicated strains following transcriptional shutoff. 7s is used as a loading control. Half-lives indicated are averages calculated from at least three experiments. The half-lives of SRG1-l and SRG1-s (relative to one another) did not significantly differ in any mutant strain tested. F. SRG1-l and SRG1-s decay curves, semilog plot. Curves are calculated from normalized values of at least three experiments per strain. Error bars represent 1 standard deviation from the mean.
Measurement of SRG1-s and SRG1-l decay rates yielded three important observations. First, the decay rates of both SRG1-s and SRG1-l transcripts were similar and quite high (half-life of approximately 2 min) in the rpb1-1 strain, which is wild type for RNA decay pathways (Fig. 3A). Second, in an xrn1Δ rpb1-1 or dcp2Δ rpb1-1 strain, the half-life of both SRG1-s and SRG1-l increased to slightly more than 5 min (Fig. 3B and C). This is consistent with the accumulation data and suggests that at least some fraction of both SRG1-s and SRG1-l is degraded in the cytoplasm through the predominant yeast degradation pathway of decapping followed by 5′-to-3′ digestion by the nuclease Xrn1p.
A third, and surprising, observation from measuring decay rates was that both the SRG1-s and SRG1-l RNAs decayed more rapidly in the rrp6Δ rpb1-1 strain than in the wild-type strain, with a reproducibly shorter half-life of ∼1 min, a statistically significant difference (P = 0.008 by two-tailed t test) (Fig. 3D). The half-life of both SRG1-s and SRG1-l in a trf4Δ rpb1-1 mutant strain was intermediate between those in the wild-type and rrp6Δ rpb1-1 strains (∼1.6 min) (Fig. 3E). The increased decay rates of the SRG1-s and SRG1-l RNAs in the rrp6Δ strain are inconsistent with a model in which these RNAs are primarily substrates for nuclear decay. Based on this fact and the decreased decay rates observed in the xrn1Δ rpb1-1 and dcp2Δ rpb1-1 strains, we conclude that the SRG1-s and SRG1-l RNAs are primarily degraded in the cytoplasm by decapping and 5′-to-3′ digestion. However, Trf4p and Rrp6p clearly can affect SRG1 RNA accumulation, either by extremely rapid nuclear decay of a subpopulation of these RNAs or by modulation of their transcription (see Discussion).
The SRG1-rt RNA is a substrate for NMD.
To determine how the SRG1-rt transcript is degraded, we also examined its steady-state accumulation in strains defective in various RNA decay mechanisms within both the nucleus and the cytoplasm. The SRG1-rt transcript accumulated in dcp1Δ, dcp2Δ, xrn1Δ, upf1Δ, upf2Δ, and upf3Δ strains (Fig. 2A and D and data not shown). Moreover, the half-life of the SRG1-rt RNA increased in the dcp2Δ rpb1-1 and xrn1Δ rpb1-1 strains, from 2.5 min in the wild type to 13.9 and 25 min, respectively (Fig. 3). These results indicate that the read-through RNA can be exported to the cytoplasm, enter translation, and be degraded by NMD. This is consistent with earlier results showing that bicistronic transcripts can be substrates for NMD in yeast (11).
We also observed a higher level of the SRG1-rt RNA in both trf4Δ and rrp6Δ strains (Fig. 2A and D). Similarly to the SRG1-l and SRG1-s transcripts, however, the decay rate of SRG1-rt is slightly higher in these strains than in the wild type, with a half-life of 1.8 min in the trf4Δ rpb1-1 strain and 1.2 min in the rrp6Δ rpb1-1 strain (Fig. 3). The trend of higher decay rates in these strains, together with the observations suggesting that the SRG1-rt RNA can undergo NMD in the cytoplasm, suggests that the level or processing of the read-through transcript is limited by Rrp6p and Trf4p in a manner yet to be determined (see Discussion).
CUTs are degraded by a variety of pathways.
The observation that SRG1 transcripts can be degraded in the cytoplasm raises the possibility that other intergenic transcripts are also exported from the nucleus and degraded in the cytoplasm. Previous results concluding that CUTs are primarily degraded in the nucleus were largely based on observations that their steady-state expression levels were increased in an rrp6Δ or trf4Δ strain compared to wild type (8, 28). Indeed, only in the case of the CUT NEL025c has the loss of Rrp6p been shown to affect the decay rate of the intergenic transcript (28). The possibility that CUTs other than SRG1 may be degraded in the cytoplasm has not been investigated. Given this, we examined how various CUTs are affected by defects in nuclear or cytoplasmic decay mechanisms.
To obtain a general overview of CUT degradation, we utilized Affymetrix microarray data both from the earlier CUT study (28) and from a genome-wide yeast study of xrn1Δ, dcp1Δ, and NMD (upf1Δ, upf2Δ, and upf3Δ) mutant strains (11). We began with 14 “canonical CUTs,” which accumulated in an rrp6Δ strain and thus were used as canonical examples in early work (28). Interestingly, three of these CUTs—NOR077w, NGR104c, and NHL005c—also accumulated significantly in an xrn1Δ or dcp1Δ strain (t test with P value cutoff of 0.01; see Materials and Methods), similarly to SRG1 RNAs; two of those three also accumulated in all three NMD mutant strains. These data preliminarily suggested not only that CUTs decay in the nucleus but also that some may be exported to the cytoplasm, enter translation, and be degraded by decapping followed by 5′-to-3′ exonucleolytic digestion.
To more broadly examine how intergenic transcripts might be degraded, we expanded our analysis to include additional RNAs. All of the 14 CUTs investigated above are characterized by Affymetrix array documentation as “nonannotated SAGE ORFs,” meaning that the sequence was found during a genome-wide serial analysis of a gene expression study (26) but was not associated with a known ORF. Thus, the 1,004 such “SAGE probes” on the array, provided that they were still unannotated, were potentially capable of detecting a pool of “SAGE targets,” or candidate CUTs. We eliminated 141 (14%) of the SAGE probes from our analysis because they now recognize annotated genomic features (tRNAs, Ty elements, sn/snoRNAs, or newly recognized ORFs).
To examine whether SAGE targets or candidate CUTs might be decayed within the cytoplasm, we selected SAGE targets whose levels changed significantly (t test with P value cutoff of 0.01; see Materials and Methods) in the xrn1Δ or dcp1Δ strain or in all three NMD mutant strains (upf1Δ, upf2Δ, and upf3Δ strains). As the rrp6Δ arrays were not replicated enough times for this type of analysis, to find candidate CUTs that might be decayed in the nucleus, we selected SAGE targets whose rrp6Δ/WT signal ratio was at least 3 (see Materials and Methods). In all, these selections identified 207 SAGE targets or candidate CUTs that appeared to accumulate in at least one of the decay mutants.
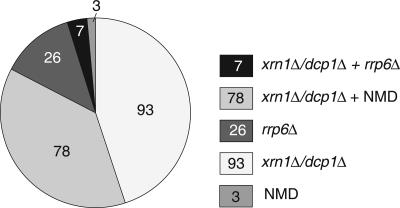
The microarray data indicate that only 13% (26/207) of the CUTs investigated accumulate solely in the rrp6Δ strain, suggesting that they are affected predominantly by nuclear processes (Fig. 4). In contrast, 83% of the CUTs accumulated in mutants with defects in cytoplasmic mRNA degradation. Specifically, 45% of the CUTs (93/207) increased only in the dcp1Δ or xrn1Δ strain, and 38% (78/207) of the CUTs increased in either the dcp1Δ or xrn1Δ strain as well as in all three NMD mutant strains, suggesting that this latter class of RNAs is exported and degraded by NMD. An additional three CUTs increased only in the NMD mutant strains. Note that because NMD requires translation, it implies that these classes of RNAs may be able to enter translation, despite their lack of an annotated ORF. The remaining 3% (7/207) of CUTs accumulated in both the rrp6Δ strain and one or more cytoplasmic decay mutant strains, suggesting that they may undergo decay in both compartments. Therefore, analysis of these microarray data strongly suggests that intergenic transcripts may be substrates for a variety of decay pathways.
FIG. 4.
Microarray predictions of CUT accumulation. The pie chart shows results for 207 CUTs that showed increased accumulation compared to wild type in the indicated decay mutant strains or strain combinations by microarray analysis.
To determine the reliability of the microarray data, we chose 14 CUTs predicted to accumulate in either the xrn1Δ and/or rrp6Δ strain and examined their levels in xrn1Δ, rrp6Δ, and wild-type strains by Northern blot analysis. The results, summarized in Table 2, revealed several interesting observations. First, the majority of the CUTs were undetectable in the wild-type strain, which prevents us from defining a baseline expression level for these CUTs. Second, only two out of nine transcripts predicted to accumulate in an xrn1Δ strain were detectable by Northern blot analysis in an xrn1Δ strain (NJL004c and NIR005c). However, both did indeed accumulate to higher levels in the xrn1Δ strain than in the wild-type strain (Table 2 and data not shown). This demonstrates that some CUTs do accumulate in an xrn1Δ mutant strain and are therefore likely to undergo cytoplasmic decay. It should be noted that because we were unable to detect the other seven transcripts by Northern blotting in either the wild-type or the xrn1Δ strain, it is possible that these RNAs do increase in level in the xrn1Δ strain but remain at very low levels.
TABLE 2.
Array and Northern blot analyses of CUTs
| CUT | Value for strain type and analysis
|
|||||
|---|---|---|---|---|---|---|
| WT
|
rrp6Δ
|
xrn1Δ
|
||||
| Arraya | Northern blotb | Arraya | Northern blotb | Arraya | Northern blotb | |
| NBL001c | 1 | ND | 6.7 | ++ | 0.68 | ND |
| NLR021w | 1 | ND | 5.1 | ++ | NA | ND |
| NKL034c | 1 | ND | 1.7 | ++ | 14.1 | ND |
| NJL004c | 1 | ND | 1.8 | + | 8.3 | + |
| NOR077w | 1 | ND | 2.2 | ND | 3.9 | ND |
| NMR061c | 1 | ND | 5.6 | ++ | 2.7 | ND |
| NMR026w | 1 | + | 4.1 | + | 1.6 | +++ |
| NMR020w | 1 | ND | 0.79 | ND | 8.4 | ND |
| NIR005c | 1 | + | 0.62 | ++ | 3.2 | ++ |
| NER004c | 1 | ND | 1.2 | +++ | 12.0 | ND |
| NML002w | 1 | ND | 1.3 | + | 2.9 | ND |
| NKL014w | 1 | ND | 0.72 | +++ | NA | ND |
| NLR029w | 1 | ND | 1.0 | + | 3.8 | ND |
| NEL025c | 1 | + | 25.4 | +++ | NA | + |
Microarray data are represented as accumulation (fold) compared to that of wild type. NA, data not available.
ND, not detected. +, weak signal; ++, good signal; +++, strong signal.
In contrast, we observed that four of the five CUTs predicted by microarrays to accumulate in an rrp6Δ strain did so (Table 2). In addition, several of the CUTs (e.g., NIR005c, NML002w, NKL014w, NLR029w, and NKL034c) were detectable by Northern blot analysis at higher levels in the rrp6Δ strain than in the wild type (Table 2 and data not shown), although on the microarrays they showed either modest or no accumulation in an rrp6Δ strain. These observations verify the idea that a significant number of CUTs increase in levels in an rrp6Δ mutant strain and further suggest that microarray analysis may underestimate the number of transcripts affected by Rrp6p.
Decay rate measurements suggest that CUTs are not always highly unstable.
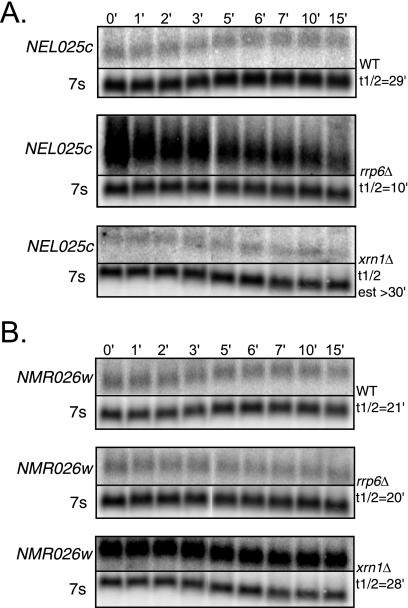
The results of the above analyses strongly suggested that CUTs are degraded by a variety of pathways. However, microarrays measure only the steady-state level of target RNAs, leaving open the possibility that indirect effects may affect CUT accumulation. Therefore, we again used the rpb1-1 mutant and Northern blot analysis to directly measure the decay rate of two CUTs predicted by array analysis to accumulate in the xrn1Δ and/or the rrp6Δ mutant strain (Fig. 5). The first, NEL025c, accumulated ∼25-fold in an rrp6Δ strain by microarray measurement (Table 2) (28). We found that NEL025c, while expressed at fairly low levels in the rpb1-1 (wild-type) and xrn1Δ rpb1-1 strains (Fig. 5A), was surprisingly stable, with a half-life of ∼29 min in the wild-type strain (Fig. 5A). In the rrp6Δ rpb1-1 strain, NEL025c accumulated to higher levels than in the wild type, yet its half-life was shorter than in the wild type (∼10 min). Therefore, our results are consistent with the previously reported accumulation of NEL025c in an rrp6Δ strain (28), but we do not observe the reported stabilization of NEL025c in the rrp6Δ strain. While the basis for this discrepancy is unclear, it could be due to the use of quantitative reverse transcription-PCR to detect the RNA in the earlier study (28), which measures the levels of a specific piece of the RNA, versus our use of Northern blot assays, which measure the levels of the full-length transcript.
FIG. 5.
Accumulation and decay of CUTs in the rrp6Δ or xrn1Δ mutant strain. A. Representative Northern blots showing NEL025c RNA levels in rpb1-1 (wild-type), rrp6Δ rpb1-1, and xrn1Δ rpb1-1 strains following transcriptional shutoff. 7s is used as a loading control. Decay in xrn1Δ rpb1-1 was not determined precisely, due to a low signal-to-noise ratio. B. The experiment was done as described for panel A but with a probe for NMR026w. Equal exposures of blot sets are shown to facilitate comparison of relative accumulation. Three independent samples from each mutant strain were analyzed; representative examples are shown.
The second CUT candidate that we examined, NMR026w, was predicted by array to accumulate significantly in a dcp1Δ strain and to accumulate nearly twofold in an xrn1Δ strain and at least fourfold in the rrp6Δ strain (Table 2). Northern blot analysis indicates that this CUT also appears to be fairly stable, as its half-life in the rpb1-1 (wild-type) strain was ∼21 min (Fig. 5B). It accumulates in the xrn1Δ rpb1-1 strain. However, while the transcript may increase in size slightly in the rrp6Δ rpb1-1 strain, it does not accumulate, contradicting the array data (Fig. 5B; Table 2). The half-life of NMR026w does not change significantly in the rrp6Δ rpb1-1 strain compared to wild type (∼20 min) but does increase slightly in the xrn1Δ rpb1-1 strain (∼28 min), consistent with this RNA being degraded primarily in the cytoplasm.
Some CUTs can enter translation.
The demonstration that SRG1 RNAs and other CUTs can be degraded in the cytoplasm suggested that these RNAs might be able to enter translation. To test this possibility, we used polysome gradients to determine if these RNAs could be associated with polyribosomes. While both SRG1 and NEL025c were observed only in the RNP portion of the gradient (Fig. 6A and data not shown), NMR026w was observed in polyribosome fractions (Fig. 6A). Moreover, because the NMR026w RNA shifts to the RNP fraction when polysomes are disrupted due to osmotic stress (27) (Fig. 6B; fractions 1 to 5 contain 28% of the NMR026w transcript in A but 51% in B), the presence of this RNA in the polyribosome fractions is due to its association with ribosomes.
FIG. 6.
Polysome analysis of the CUT NMR026w. A. Polysome profile of wild-type cells, with Northern blot of RNA isolated from indicated fractions, probed for NMR026w and SRG1. Twenty-eight percent of NMR026w transcript is present in fractions 1 to 5. B. Polysome profile of wild-type cells treated with 1 M KCl for 30 min to disrupt polysome formation, with Northern blot of RNA isolated from indicated fractions, probed for NMR026w. Fifty-one percent of NMR026w is present in fractions 1 to 5.
NMR026w contains three overlapping potential small reading frames of 21, 85, and 88 codons. However, these reading frames and their start codons are poorly conserved among closely related yeast species. This lack of conservation argues that the NMR026w RNA is not an uncharacterized yeast ORF. Instead, NMR026w represents a class of CUTs that are able to both exit the nucleus and enter translation. This suggests that fortuitous transcription of CUTs might be a mechanism for the evolution of new open reading frames (see Discussion).
DISCUSSION
Intergenic transcripts can be degraded in the cytoplasm.
In this work, we provide several lines of evidence that some intergenic transcripts in yeast are degraded in the cytoplasm by decapping and 5′-to-3′ exonucleolytic digestion. This was first revealed by our analysis of the SRG1 locus, which produces intergenic transcripts that overlap the SER3 promoter and can control SER3 transcription (Fig. 1C). We observed that both the SRG1-s and SRG1-l transcripts accumulated and/or showed higher decay rates in dcp2Δ, dhh1Δ, and xrn1Δ strains (Fig. 2 and 3). This suggests that the SRG1-s and SRG1-l RNAs can be exported from the nucleus and degraded by cytoplasmic decapping and 5′-to-3′ decay. In addition, Northern blot analysis demonstrated that the CUT NMR026w showed an increased steady-state level in an xrn1Δ strain (Fig. 5B), as well as a slightly lower decay rate (Fig. 5B). Finally, examination of available microarray data identified additional intergenic RNAs that accumulate in an xrn1Δ strain (Table 2; Fig. 4). Taken together, these results demonstrate that some intergenic transcripts are exported and degraded in the cytoplasm.
Intergenic transcripts can be affected by nuclear RNA turnover pathways.
In addition to being degraded in the cytoplasm, intergenic transcripts can also be affected by nuclear RNA turnover mechanisms. This has been suggested by several groups reporting the accumulation of intergenic RNAs in rrp6Δ and/or trf4Δ strains (8, 24, 28). Similarly, we also observed that numerous intergenic RNAs accumulated in rrp6Δ strains as assessed either by examination of preexisting microarray data sets or by direct Northern blot analysis (Table 2; Fig. 4 and 5). Moreover, the SRG1-l RNAs accumulated in both rrp6Δ and trf4Δ strains (Fig. 2), which indicates that despite the cytoplasmic decay of the SRG1-l RNAs, the level of these RNAs is somehow limited by Rrp6p and Trf4p. Interestingly, the level of the SRG1-rt transcript is also limited by Rrp6p and Trf4p, despite this transcript being a potential substrate for NMD (Fig. 2).
The effects of Rrp6p on intergenic transcripts.
An unresolved issue is how Rrp6p and Trf4p limit the production of the SRG1-l and SRG1-rt RNAs and potentially of other intergenic transcripts. The simplest model, based on recent findings and discussions in the literature, is that Trf4p adenylates these RNAs in the nucleus and targets them for Rrp6p-dependent 3′-to-5′ degradation by the nuclear exosome (8, 18, 24, 28). However, this model is not supported by our observation that the decay rates of the SRG1-l and SRG1-rt transcripts actually increase in the rrp6Δ strain (Fig. 3), a finding that suggests that Rrp6p is not rapidly degrading these RNAs but may instead be limiting their exposure to other degradative enzymes. Consistent with this latter possibility, we also observed that the decay rate of the CUT NEL025c increased to some extent in an rrp6Δ strain (Fig. 5A). Moreover, it has been previously observed that the loss of Rrp6p increases the decay rate of pre-mRNAs that accumulate as lariats and yet are degraded in the cytoplasm (13). These observations indicate that this perplexing contradiction, wherein RNAs accumulate yet have increased decay rates in an rrp6Δ strain, is not unique to SRG1 transcripts.
Two general types of alternative model might explain how loss of Rrp6p and Trf4p could increase SRG1-l and SRG1-rt levels and yet also increase their observed decay rate. In one model, the SRG1-l RNA is recognized as aberrant and retained at the site of transcription in an Rrp6p-dependent manner. This model is based on previous results showing that aberrant transcripts can be retained at the locus in a manner dependent on the nuclear exosome (12, 15, 19). In this model, the higher decay rate observed in the rrp6Δ strain would be due to faster release of the transcripts from the locus, resulting in faster nuclear export and cytoplasmic decay. To explain the increased levels of the SRG1-l and SRG1-rt RNAs (Fig. 2), nuclear retention of SRG1 would need to be hypothesized to have a direct or feedback effect on some aspect of transcription, possibly elongation, which normally would limit production of the SRG1-l and SRG1-rt transcripts. Thus, in this model, the loss of Rrp6p or Trf4p relieves a transcriptional block, allowing the production of more SRG1-l and SRG1-rt RNAs. However, a requisite of this model is that any such transcriptional inhibition would have to act after initiation and function only after the RNA polymerase passes the 3′-end site of the SRG1-s RNA, as the level of this transcript is not affected by loss of Rrp6p (Fig. 2).
A second model is that Rrp6p and Trf4p recognize and rapidly degrade a significant percentage of nascent SRG1-l and SRG1-rt transcripts. This model requires several additional features. First, this nuclear degradation must be extremely fast such that we cannot measure its rate at steady state, since the vast majority of the RNAs present in the population at steady state would be escapees from this nuclear decay. Second, this model stipulates that the SRG1-rt transcript is also a substrate for Rrp6p-dependent nuclear decay, despite its having a normal 3′-end processing site. Third, because the decay rates of SRG1 transcripts are accelerated in the rrp6Δ strain, Rrp6p must still limit the ability of at least some RNAs, SRG1-s transcripts included, to reach the cytoplasm. An alternative possibility is a hybrid model wherein Rrp6p functions to retain RNAs with aberrant 3′ ends, which has feedback effects on transcription and targets some of the molecules for nuclear decay, as this could explain all of the observations.
Intergenic RNAs can enter translation: implications for the evolution of new ORFs.
Two lines of evidence now suggest that at least some intergenic transcripts can enter translation. First, we observed that a subpopulation of intergenic transcripts accumulated in strains defective in NMD (Fig. 4). Since NMD requires translation, this implies that these intergenic RNAs are entering translation. More directly, we observed that the NMR026w RNA was associated with polyribosomes (Fig. 6). However, because the ORF in NMR026w is not conserved, we do not think that this RNA encodes a genuine protein. Instead, the simplest model is that NMR026w represents a class of intergenic transcripts that, because they are produced by RNA Pol II and are capped and polyadenylated, can fortuitously enter translation at some rate. Thus, some intergenic RNAs can both be exported to the cytoplasm and enter translation, even if they do not encode a functional protein.
The ability of “noncoding” intergenic transcripts to enter translation suggests a possible mechanism whereby new ORFs encoded by bona fide mRNAs might arise (Fig. 7). First, a noncoding intergenic transcript would be produced by RNA Pol II. Note that in many cases fortuitous intergenic transcription might arise simply due to the bidirectional nature of eukaryotic transcriptional enhancers. For example, NEL025c lies head to head with the gene DLD3. Between them are two palindromic transcription factor binding sites, which mediate a response to available nitrogen sources (6, 23). We have shown that NEL025c levels respond to nitrogen source in a manner similar to that of DLD3 (data not shown) (23). Second, these intergenic transcripts would gain a 3′ end, either by normal polyadenylation or potentially by other methods for 3′-end generation. Not only would intergenic transcripts that gained a normal poly(A) tail be good substrates for nuclear export, but once exported, the capped and adenylated RNA would be expected to engage the cytoplasmic translation machinery. Intergenic transcripts producing an advantageous polypeptide would then be subjected to natural selection, allowing further evolution into a bona fide mRNA with a functional ORF. Thus, intergenic transcripts may not simply be genomic noise but may also be a factor of adaptive mechanisms requiring variation and selection, thus providing fodder for the evolution of new ORFs.
FIG. 7.
Model for new ORF evolution from intergenic transcripts. Shown is the potential mechanism for the evolution of new protein-encoding ORFs from intergenic transcripts. The process begins with adventitious transcription, followed by gain of a proper 3′ end to allow for nuclear export, evolution of a start codon and engagement of the translation machinery, and natural selection of a desirable polypeptide. IT, intergenic transcript.
Acknowledgments
We thank Carla Beckham, Carolyn Decker, and Tracy Nissan for critical review of the manuscript and helpful discussions, the members of the Parker laboratory for support and discussions while this project was under way, Manny Ares for comments on the manuscript, and George Watts for assistance with array analysis.
This work was supported by the Howard Hughes Medical Institute and grant GM45443 from the National Institutes of Health.
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Albers, E., V. Laize, A. Blomberg, S. Hohmann, and L. Gustafsson. 2003. Ser3p (Yer081wp) and Ser33p (Yil074cp) are phosphoglycerate dehydrogenases in Saccharomyces cerevisiae. J. Biol. Chem. 278:10264-10272. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. S., and R. P. Parker. 1998. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 17:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, K. E., and R. Parker. 2004. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr. Opin. Cell Biol. 16:293-299. [DOI] [PubMed] [Google Scholar]
- 4.Brengues, M., D. Teixeira, and R. Parker. 2005. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science 310:486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caponigro, G., D. Muhlrad, and R. Parker. 1993. A small segment of the MATα1 transcript promotes mRNA decay in Saccharomyces cerevisiae: a stimulatory role for rare codons. Mol. Cell. Biol. 13:5141-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelstowska, A., Z. Liu, Y. Jia, D. Amberg, and R. A. Butow. 1999. Signalling between mitochondria and the nucleus regulates the expression of a new D-lactate dehydrogenase activity in yeast. Yeast. 15:1377-1391. [DOI] [PubMed] [Google Scholar]
- 7.Coller, J. M., M. Tucker, U. Sheth, M. A. Valencia-Sanchez, and R. Parker. 2001. The DEAD box helicase, Dhh1p, functions in mRNA decapping and interacts with both the decapping and deadenylase complexes. RNA 7:1717-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, C. A., and M. Ares, Jr. 2006. Accumulation of unstable promoter-associated transcripts upon loss of the nuclear exosome subunit Rrp6p in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 103:3262-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decker, C. J., and R. Parker. 1993. A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev. 7:1632-1643. [DOI] [PubMed] [Google Scholar]
- 10.Dunckley, T., and R. Parker. 1999. The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J. 18:5411-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He, F., X. Li, P. Spatrick, R. Casillo, S. Dong, and A. Jacobson. 2003. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast. Mol. Cell 12:1439-1452. [DOI] [PubMed] [Google Scholar]
- 12.Hilleren, P., T. McCarthy, M. Rosbash, R. Parker, and T. H. Jensen. 2001. Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature 413:538-542. [DOI] [PubMed] [Google Scholar]
- 13.Hilleren, P. J., and R. Parker. 2003. Cytoplasmic degradation of splice-defective pre-mRNAs and intermediates. Mol. Cell 12:1453-1465. [DOI] [PubMed] [Google Scholar]
- 14.Huh, W. K., J. V. Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson, J. S. Weissman, and E. K. O'Shea. 2003. Global analysis of protein localization in budding yeast. Nature 425:686-691. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, T. H., K. Patricio, T. McCarthy, and M. Rosbash. 2001. A block to mRNA nuclear export in S. cerevisiae leads to hyperadenylation of transcripts that accumulate at the site of transcription. Mol. Cell 7:887-898. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J. M., S. Edwards, D. Shoemaker, and E. E. Schadt. 2005. Dark matter in the genome: evidence of widespread transcription detected by microarray tiling experiments. Trends Genet. 21:93-102. [DOI] [PubMed] [Google Scholar]
- 17.Kadaba, S., A. Krueger, T. Trice, A. M. Krecic, A. G. Hinnebusch, and J. Anderson. 2004. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 18:1227-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaCava, J., J. Houseley, C. Saveanu, E. Petfalski, E. Thompson, A. Jacquier, and D. Tollervey. 2005. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121:713-724. [DOI] [PubMed] [Google Scholar]
- 19.Libri, D., K. Dower, J. Boulay, R. Thomsen, M. Rosbash, and T. H. Jensen. 2002. Interactions between mRNA export commitment, 3′-end quality control, and nuclear degradation. Mol. Cell. Biol. 22:8254-8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martens, J. A., L. Laprade, and F. Winston. 2004. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429:571-574. [DOI] [PubMed] [Google Scholar]
- 21.Martens, J. A., P. Y. Wu, and F. Winston. 2005. Regulation of an intergenic transcript controls adjacent gene transcription in Saccharomyces cerevisiae. Genes Dev. 19:2695-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muhlrad, D., and R. Parker. 2005. The yeast EDC1 mRNA undergoes deadenylation-independent decapping stimulated by Not2p, Not4p, and Not5p. EMBO J. 24:1033-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tate, J. J., K. H. Cox, R. Rai, and T. G. Cooper. 2002. Mks1p is required for negative regulation of retrograde gene expression in Saccharomyces cerevisiae but does not affect nitrogen catabolite repression-sensitive gene expression. J. Biol. Chem. 277:20477-20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanacova, S., J. Wolf, G. Martin, D. Blank, S. Dettwiler, A. Friedlein, H. Langen, G. Keith, and W. Keller. 2005. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 3:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Hoof, A., R. R. Staples, R. E. Baker, and R. Parker. 2000. Function of the Ski4p (Csl4p) and Ski7p proteins in 3′-to-5′ degradation of mRNA. Mol. Cell. Biol. 20:8230-8243.11027292 [Google Scholar]
- 26.Velculescu, V. E., L. Zhang, W. Zhou, J. Vogelstein, M. A. Basrai, D. E. Bassett, Jr., P. Hieter, B. Vogelstein, and K. W. Kinzler. 1997. Characterization of the yeast transcriptome. Cell 88:243-251. [DOI] [PubMed] [Google Scholar]
- 27.Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson, B. Andre, R. Bangham, R. Benito, J. D. Boeke, H. Bussey, A. M. Chu, C. Connelly, K. Davis, F. Dietrich, S. W. Dow, M. El Bakkoury, F. Foury, S. H. Friend, E. Gentalen, G. Giaever, J. H. Hegemann, T. Jones, M. Laub, H. Liao, N. Liebundguth, D. J. Lockhart, A. Lucau-Danila, M. Lussier, N. M'Rabet, P. Menard, M. Mittmann, C. Pai, C. Rebischung, J. L. Revuelta, L. Riles, C. J. Roberts, P. Ross-MacDonald, B. Scherens, M. Snyder, S. Sookhai-Mahadeo, R. K. Storms, S. Veronneau, M. Voet, G. Volckaert, T. R. Ward, R. Wysocki, G. S. Yen, K. Yu, K. Zimmermann, P. Philippsen, M. Johnston, and R. W. Davis. 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901-906. [DOI] [PubMed] [Google Scholar]
- 28.Wyers, F., M. Rougemaille, G. Badis, J. C. Rousselle, M. E. Dufour, J. Boulay, B. Regnault, F. Devaux, A. Namane, B. Seraphin, D. Libri, and A. Jacquier. 2005. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121:725-737. [DOI] [PubMed] [Google Scholar]