Abstract
NirA, the specific transcription factor of the nitrate assimilation pathway of Aspergillus nidulans, accumulates in the nucleus upon induction by nitrate. NirA interacts with the nuclear export factor KapK, which bridges an interaction with a protein of the nucleoporin-like family (NplA). Nitrate induction disrupts the NirA-KapK interaction in vivo, whereas KapK associates with NirA when this protein is exported from the nucleus. A KpaK leptomycin-sensitive mutation leads to inducer-independent NirA nuclear accumulation in the presence of the drug. However, this does not lead to constitutive expression of the genes controlled by NirA. A nirAc1 mutation leads to constitutive nuclear localization and activity, remodeling of chromatin, and in vivo binding to a NirA upstream activation sequence. The nirAc1 mutation maps in the nuclear export signal (NES) of the NirA protein. The NirA-KapK interaction is nearly abolished in NirAc1 and NirA proteins mutated in canonical leucine residues in the NirA NES. The latter do not result in constitutively active NirA protein, which implies that nuclear retention is necessary but not sufficient for NirA activity. The results are consistent with a model in which activation of NirA by nitrate disrupts the interaction of NirA with the NplA/KapK nuclear export complex, thus resulting in nuclear retention, leading to AreA-facilitated DNA binding of the NirA protein and subsequent chromatin remodeling and transcriptional activation.
Nitrate, one of the most abundant nitrogen compounds in soil, is metabolized by microorganisms and plants. In the saprophytic fungus Aspergillus nidulans, nitrate utilization involves transcription of the nitrate transporter and nitrate- and nitrite-reductase genes (42). Transcriptional activation of these genes occurs upon induction by nitrate in the absence of repressing nitrogen sources (ammonium, glutamine), and it is strictly dependent on the synergistic, positive action of the pathway-specific Cys6Zn2 binuclear cluster factor NirA and the GATA factor AreA (3, 33, 35, 50). niaD and niiA, encoding nitrate and nitrite reductases, respectively, are transcribed from a bidirectional promoter containing four NirA and ten AreA-binding sites of varying functional importance (see Fig. 4B for the most relevant NirA and AreA binding sites) (33, 46, 55). This promoter undergoes a drastic chromatin rearrangement upon induction. AreA, necessary for both transcriptional activation and chromatin rearrangement, is inactivated by multiple mechanisms elicited by the presence of preferred nitrogen sources leading to repression (2, 32). At least one of the GATA sites (GATA site 5) is permanently occupied by AreA (33). In contrast, NirA binding site 2 becomes occupied shortly after nitrate induction, but this occupancy is lost again when cultures are repressed by ammonium. NirA occupancy at promoter sites is AreA dependent (5). Protein-protein interactions, as shown for these nitrate regulators in A. nidulans (34) and in Neurospora crassa (16), may underlie the involvement of AreA in NirA binding to site 2.
FIG. 4.
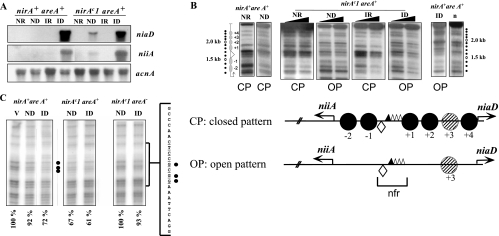
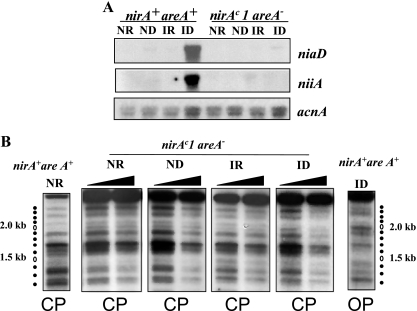
nirAc1 phenotype. (A) Northern blots of niiA and niaD mRNAs in the nirA+ areA+ and the nirAc1 areA+ strains grown under ND (urea as the sole nitrogen source), ID (nitrate as the sole nitrogen source), IR (nitrate plus ammonium as the nitrogen sources), or NR (ammonium as the sole nitrogen source) conditions. The acnA (actin) hybridization serves as loading control. (B) Nucleosome positioning in the bidirectional niiA-niaD promoter region comparing nirA+ and nirAc1 strains in an areA+ background. Samples from the same mycelia as in panel A were incubated with increasing amounts of MNase (black triangles). The top panel shows an MNase digestion pattern of a nirAc1 mutant under all of the conditions described in panel A. Patterns of MNase digestion for the wild-type strain (nirA+, areA+) grown under the same conditions have been published previously; here we show two extreme (NR and ID) conditions as controls. A naked DNA control, also published previously, is also shown (lane n). The position of the DNA fragments is shown by dots to the side of the autoradiogram. A 100-bp DNA ladder run in these gels, used to calculate the position of the MNase cuts, is not shown for the sake of clarity. The positions of the nucleosomes are shown to the left of the autoradiograms and are numbered divergently from the nucleosome-free region (nfr) as referred to in reference 33. The DNA bands correspond exactly to those described in previous publications (33-35). These experiments were repeated several times with identical results. The bottom panel shows the two patterns of nucleosome organization of the niiA-niaD intergenic region, a closed pattern (CP) that is seen in the wild-type strain (nirA+ areA+) under NR, ND, and IR conditions and an open pattern (OP) that is seen only under ID conditions (33, 34). For the nirAc1 mutant the OP is seen also under ND conditions. The corresponding patterns are also indicated under all MNase digestion tracks in the upper panel. Positioned nucleosomes (CP) are depicted as closed circles and numbered divergently from the nucleosome-free region (nfr). In the open pattern there are no positioned nucleosomes in the intergenic region. The putative positioning of nucleosome +3 cannot be determined by MNase digestion and hence is depicted as a hatched circle. Transcription factor binding sites discussed in this study are shown as a lozenge (NirA binding site 2) and triangles (AreA binding sites 5 to 8). AreA binding site 5, which was shown to be occupied by the protein under all nitrogen conditions, is represented as a filled triangle. Transcription start points are indicated by arrows. (C) In vivo methylation protection of NirA site 2 in the wild-type strain (nirA+ areA+) and in nirAc1 strains with functional (nirAc1 areA+) and nonfunctional (nirAc1 areA−) AreA. Strains were grown under the conditions described above. V, in vitro-methylated control DNA. Filled circles indicate protected guanines within the NirA binding sequence that is shown to the side of the autoradiographs (site 2, underlined). The results of quantitative phosphorimager analysis are presented as the percent intensity of the relevant guanines within NirA binding site 2 compared to nonprotected guanines in the same lane. The relationship of the intensities between the three relevant guanines within the NirA binding sequence and the three nonprotected guanines in the in vitro-methylated DNA (V) was set to 100%. All panels are from the same experiment and were run in parallel in the same gel but are shown separately for the sake of clarity. Values under the panels indicate percentage of protection of the three relevant guanines.
Considerable experimental (11, 30, 59, 62) and genomic evidence (http://www.broad.mit.edu, and http://www.fgsc.net) suggest that this regulatory mechanism is conserved in the filamentous ascomycetes, although clustering of the structural genes is not always conserved (31) and functionally redundant nitrate transporters have only been identified in A. nidulans thus far (60).
The eukaryotic nuclear membrane allows regulatory signals to modulate the subcellular distribution of transcription factors. Some Cys6Zn2 binuclear cluster proteins such as Saccharomyces cerevisiae Gal4p, Put3p, or Leu3p (4, 7, 51) are constitutively bound to their cognate UASs, and induction occurs by unmasking of their activation domains (28, 29, 61). In contrast, PrnA is constitutively located in the nucleus but necessitates induction to bind DNA (21, 44), whereas Hap1p enters the nucleus and binds DNA in response to its specific ligand (23, 24, 27). NirA is located in the cytoplasm in the absence of nitrate (the inducer) and accumulates in the nucleus upon induction. Replacement of inducer by neutral (noninducing and nonrepressing, e.g., urea or arginine) or repressing (ammonium) nitrogen sources leads to dramatically rapid redistribution of NirA to the cytosol (5).
Karyopherins are essential mediators of inward and outward trafficking of macromolecules through the nuclear pore complex (49, 54). Thus, the interaction of transcription factors with cognate karyopherins is a possible regulatory checkpoint both at the level of nuclear import and export. Activation of the S. cerevisiae anti-oxidant response upregulator Yap1p occurs directly by masking a nuclear export signal (NES) through the formation of an intramolecular disulfide bridge (12, 64). This process prevents the interaction with Crm1p, the main yeast exportin, thus resulting in accumulation of Yap1p in the nucleus (12).
The identification of NirA interacting proteins and the characterization of the constitutive mutant nirAc1 (10, 42) presented below implies that nitrate acts on NirA by disrupting its interaction with the nuclear export machinery, thus resulting in their nuclear retention and subsequent transcriptional activation and that the constitutive mutation mimics this effect.
MATERIALS AND METHODS
Strains, plasmids, and genetic techniques.
The A. nidulans and S. cerevisiae strains and transformation plasmids are listed in Tables S1, S2, and S3 in the supplemental material. Escherichia coli strain JM109 was used for routine plasmid propagation. The nirAc1 mutation was mapped according to the procedure described by Burger et al. (8) except that strain areA18 nirAd106 was used as the recipient strain for transformation of the overlapping PCR fragments derived from the nirAc1 strain. Plasmid and strain constructions are described in in the supplemental material.
S. cerevisiae growth conditions.
All strains were grown at 30°C. Media and genetic methods used to construct and screen the two-hybrid libraries and to test for protein-protein interactions followed the instruction manual of the kit provider (HybriZAP-2.1 XR library construction kit instruction manual; Stratagene). Yeast cells were transformed with DNA by using the lithium acetate transformation method (20).
A. nidulans growth conditions and techniques.
A. nidulans growth conditions and techniques were as published previously (33, 35). Transformation was as described by Tilburn et al. (56).
Preparation of cDNA library and yeast two-hybrid screen.
Nitrate-induced cultures of A. nidulans have been used to prepare mRNA as a template for the cDNA prey library, which was prepared by using the HybriZAP-2.1 XR library system. For construction and quality control of the library, the instructions of the manufacturer were followed. Titers of the amplified HybriZAP-2.1 library were in the range of 1010 PFU/μl. Transformation of the NirA bait fragments (see supplemental material) with the A. nidulans cDNA library was performed by using a high-efficiency lithium acetate transformation protocol (20). Further analysis of the potential interaction partners and elimination of false-positive interactions were done according to the instructions in the same publication. Selected clones positive for HIS3 and ADE2 expression were further tested for β-galactosidase activity. The plasmids encoding the preys were finally rescued from yeast by E. coli transformation and sequenced. The kapK cDNA was used in fusions to either the GAL4p-binding domain (BD; bait fusion) or the GAL4p activation domain (AD; prey fusion). Interaction with NplA (amino acids 80 to 512) was tested with the BD-KapK fusion since an autoactivation sequence is present in NplA. Interactions with NirA (NirA bait 1, positions 1 to 229) was tested by using both the AD-KapK fusion (BD-NirA/AD-KapK) and the BD-KapK fusion (AD-NirA/BD-KapK). In these tests, KapK overexpression resulted in toxicity to the yeast strains. The toxicity of the expressed proteins affects the plasmid copy number, making quantitative β-galactosidase determination unreliable (52). We thus assessed all interactions involving KapK by complementation of the ade2 mutation.
β-Galactosidase assay in yeast extracts.
The yeast strains were grown in triplicate assays overnight at 30°C in appropriate selective medium. The cells were washed once with sterile water and collected by centrifugation, and the pellet was resuspended in YPAD medium to an optical density at 600 nm (OD600) of 0.2. These cultures were grown at 30°C to an OD600 of 0.8 before being harvested. Pelleted cells were washed and finally resuspended in 500 μl of sodium phosphate buffer (50 mM Na3PO4, 1 mM EDTA [pH 8.0]). Then, 500-μl glass beads (0.75 to 1 mm in diameter) were added to the tubes, and the cells were broken in a RiboLyser (Hybaid, Heidelberg, Germany). Cell debris was removed by centrifugation steps, and the protein concentration in the crude protein extracts was determined (BCA assay; Pierce); the liquid β-galactosidase assay, using ONPG (o-nitrophenyl-β-d-galactopyranoside) as the substrate, was done as described for yeast protein extracts (48). The specific β-galactosidase activities (Miller units) were calculated as described previously (48).
Recombinant protein expression, purification, and column binding assay.
Purification of glutathione S-transferase (GST) fusion proteins. Expression of GST fusion proteins in E. coli BL21 cells harboring vector pGEX4T1 was achieved according to our published protocol (55). Due to a lower yield of the NplA fusion protein, the scale of this purification was increased fivefold (1.25 liter). Crude cell preparations were loaded onto GST-agarose columns (Sigma Europe), washed extensively, and subsequently incubated with the in vitro transcription-translation products (see below). KapK was in vitro translated using the coupled transcription-translation reticulocyte lysate system provided by Promega (Mannheim, Germany). The reaction was performed with vector pGAD-THKAP as a template for expression and 35S-labeled methionine (GE Healthcare, Buckinghamshire, United Kingdom) for protein labeling.
To detect recombinant protein-protein interactions, 500 μl of gel slurry packed in columns was equilibrated with column buffer (150 mM NaCl, 100 mM Tris [pH 7.5]) and subsequently loaded with crude extracts containing GST fusion proteins. Columns were washed extensively and then incubated with 50 ng of [35S]KapK for 1 h in binding buffer (50 mM Tris [pH 7.5], 2 mM dithiothreitol). Columns were washed with washing buffer containing increasing salt concentrations from 5 to 150 mM NaCl. Proteins still bound to glutathione-agarose after all of the washing steps were eluted with 5 mM reduced glutathione-50 mM Tris (pH 8), and fractions of 300 μl were collected. Wash and elution fractions were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Coomassie blue staining and autoradiography, respectively.
Fluorescence microscopy.
Fluorescence microscopy was performed as described by Berger et al. (5). Green fluorescent protein (GFP) fusion proteins were induced by 100 pM diethylstilbestrol (DES; Sigma). The media and nitrogen sources used were the same as those used to investigate transcriptional activation, except that 3 mM arginine was used for the noninduced, derepressed (ND) conditions due to the high level of mycelial vacuolization observed on 5 mM urea in areA600 strains.
Transcription analysis, in vivo footprinting, and investigation of nucleosome positioning.
Strains were precultured on 1.25 mM ammonium D(+) tartrate as the sole nitrogen source for 7 h at 37°C as described previously (33, 35). After washing and resuspension on fresh minimal medium, 10 mM ammonium D(+) tartrate (noninducing, repressing [NR] conditions) or 5 mM urea (ND conditions) or 10 mM NaNO3 (inducing, derepressing [ID] conditions) or 10 mM ammonium D(+) tartrate plus 10 mM NaNO3 (inducing, repressing [IR] conditions) were added. The cultures were grown for a further 2 h before harvesting. Northern analysis was carried out according to published procedures (33). The relative intensities of the signals were calculated using the ImageQuant software (Molecular Dynamics) from phosphorimages (Storm; Molecular Dynamics, Inc.). Normalized niiA and niaD signal intensities were obtained by dividing the intensity of the niiA or niaD signal by the intensity of the actin gene signal, respectively. Reverse transcription quantitative real-time PCR used the primers 5′-GCGACGACGACAACGGCAAATACT and 5′-CATACGCCTCAAACGGGTCCACAG for amplification of niiA and the primers 5′-TGGAAACCGGCTAGAGGAAGACAT and 5′-CAGGCCCGCAAACCAAAACCAT for niaD. All signals were normalized to the constitutively transcribed actin gene (acnA) by using the primers 5′-CGAGCGCGGATACACCTTC and 5′-TACGGACGTCGACATCACAC. The Bio-Rad (Hercules, CA) MyiQ cycler was used as device and the platinum SYBR green qPCR SuperMix-UDG (Invitrogen) for cDNA synthesis and consecutive PCR. The niiA and niaD signal intensities were normalized by dividing these values by the values obtained for the acnA (actin) signal. Micrococcal nuclease (MNase) digestion and indirect end labeling was performed as described previously (33). Methylation protection in vivo footprinting of the NirA binding site 2 in the niiA-niaD intergenic region was carried out as described by Wolschek et al. (63).
Construction of S-tagged kapK and chromatin immunoprecipitation (ChIP).
The S-tagged KapK-expressing strain was constructed by using the epitope-tagging techniques described by Yang et al. (66). Genomic fragments corresponding to 2.5 kb of the 3′ end of the kapK coding region and 1.5 kb of the 3′ untranslated region and a fragment containing the S-tag fused to the selectable marker pyrG gene from Aspergillus fumigatus were amplified with specific primers. These three fragments were fused to yield a PCR fragment that was used in transformation in a pyrG89 A. nidulans strain. Transformants were selected as uracil prototrophs and purified, and the integration of the construct was analyzed by Southern blotting. The plasmid containing the S-tag expressing fragment and was provided by Steven Osmani. The strain used as a recipient, TNO2A3 (pyroA4 pyrG89 ΔnkuA), which carries the nkuA deletion allele that increases the homologous recombination (36), was provided by Berl Oakley.
ChIP was carried out as described previously (40), with the following modifications. Conidia (108) were inoculated in Aspergillus minimal medium with arginine as the sole nitrogen source and then grown for 14 h at 37°C. Cultures were induced by 10 mM nitrate for 30 min, followed by a shift to repressing conditions (10 mM ammonium). Immediately before the medium shift the induced sample was withdrawn (inducing conditions and 0-min repression), and incubation under repressing conditions proceeded for 10 min (sample 10 min) or 30 min (sample 30 min). Mycelia were treated with a 1% final concentration of formaldehyde for 15 min. Fixation was stopped with 125 mM glycin, and the cultures were filtered and immediately frozen in liquid nitrogen. Then, 100 mg of frozen mycelium was sonicated in 1 ml of 50 mM HEPES KOH (pH 7.5)-140 mM NaCl-1 mM EDTA (pH 7.5)-1% Triton X-100-0.1% sodium deoxycholate-1× fungal protease inhibitor mix (Promega) for two pulses of 30 s with 1 min of resting. The insoluble debris was pelleted by centrifugation, and the soluble fraction was stored at −80°C. The equivalent of 200 μg of protein was used for immunoprecipitation with S-tag antibody (ab19321; Abcam), and 20 μg of protein was used to determine the concentration of DNA (referred to as the input DNA). DNA was eluted by using a PCR purification kit (QIAGEN) column with 100 μl of 1 mM Tris-HCl (pH 8.5). A total of 5 μl of the elution was used for quantitative real-time PCR (myiQ cycler; Bio-Rad) using platinum SYBR green qPCR SuperMix-UDG (Invitrogen).
The primers used for quantitative PCR were niiA_nuc-1_F (5′-GGAAATTCAGGCAGTGCATC-3′) and niiA_nuc-1_R (5′-TAGGATCTGGAGTGTGGCTAGAG-3′).
To detect the background signal of the immunoprecipitation reaction (control reactions), two different strains carrying no S-tagged version of KapK (pabaA1 and biA1 strains, respectively) were analyzed in parallel with the S-tagged kapK strain. Each ChIP experiment was repeated twice. The relative amounts of DNA were calculated by dividing the immunoprecipitated DNA by the input DNA. The standard deviation was calculated from at least two biological repetitions, and significance (P < 0.01) was determined by analysis of variance.
RESULTS
NirA interacts with a nucleoporin.
Using overlapping fragments of NirA fused to the GAL4 DNA-binding domain as baits (see the scheme in Fig. S3 in the supplemental material), we searched for NirA interaction partners encoded by a mixed A. nidulans cDNA-GAL4 activation domain prey library in a yeast two-hybrid screen. Bait 4 of NirA spanning amino acids 612 to 892 contains a putative activation domain, leads to activation of the reporter gene in the absence of a prey plasmid, and hence could not be used for further screens (not shown). In the NirA bait 1 to bait 3 screen we isolated two overlapping prey clones (codons 80 to 512 and codons 127 to 512, respectively) corresponding to auto-called gene AN4595.3 (http://www.broad.mit.edu) interacting with bait 1 and bait 2. The interacting protein was designated Nup42 by De Souza et al. (13). To comply with A. nidulans gene nomenclature (9), we denoted the gene nplA, encoding a 512-residue NplA protein (named for nucleoporin-like protein A). The domain structure of NplA is similar to the vertebrate NLP1 nucleoporin-like protein (15) and hRIP/Rab (6, 19). An NplA-GFP fusion driven from the strong gpdA promoter (45) is localized in the nucleus (not shown), and De Souza et al. (13) have shown that NplA, when driven by its own promoter, localizes perinuclearly. NplA contains a 15-fold repeated Phe-Gly motif characteristic of nucleoporins (see Fig. S1A in the supplemental material), which occurs in pentamers with iterations such as FGQPV, FGQPA, FGQSS, FGQAS, FGKPS, and FGVPS. A putative N-terminal C3H1 zinc finger is predicted for NplA, and this feature is conserved in many putative nucleoporin-like proteins of fungi and metazoans (see Fig. S1B in the supplemental material). Consistent with the predicted redundancy of nucleoporins in the A. nidulans genome, deletion of the complete nplA open reading frame results in moderately impaired growth on complete medium and on all minimal media tested using different carbon and nitrogen sources, including ammonium and glutamine. There were no specific effects on nitrate or nitrite, and the nplA deletion does not affect either niiA and niaD expression (as monitored by Northern blotting) or NirA localization under standard growth conditions (not shown).
The interaction of NirA and NplA is mediated by the Crm1p homlogue KapK.
We failed to show any direct in vitro interaction between NirA (positions 1 to 229) and NplA by coimmunoprecipitation of epitope-tagged proteins either directly from crude yeast extracts or of purified epitope-tagged proteins produced in a reticulocyte cell-free system. This suggested that the NirA/NplA two-hybrid interaction is indirect and mediated through yet another protein. In a screen using bait 5 (full NirA sequence lacking the C-terminal 133 amino acids of the activation region), we isolated a prey plasmid coding for the C-terminal portion of a putative exportin (AN1401.2), suggesting that this could be the bridging protein (data not shown). This protein has recently been described to be involved in the nuclear export of the wide-domain nitrogen regulator AreA (57). The S. cerevisiae Crm1p factor and its homologues (CRM1/exportin-1 in higher eukaryotes) have been shown to interact with Leu-rich NESs (18). Inspection of the NirA 1 and NirA 2 baits revealed the presence of a putative NES, and the Crm1p homologue KapK (encoded by kapK/AN1401.2) was thus a likely candidate to mediate the NirA-NplA interaction in the yeast assay. A complete cDNA of kapK, which encodes a 1,072-residue protein (GenBank accession no. AY555733), was cloned and used in two-hybrid assays with either the NplA region identified in the screen (amino acids 80 to 512) or with NirA bait 1 (amino acids 1 to 229). KapK interacts with both NplA and NirA. The yeast two-hybrid interactions between NirA, KapK, and NplA are summarized in Table 1.
TABLE 1.
Summary of yeast two-hybrid interactions between NirA, KapK, and NplAa
| Vector | Interactionb
|
|||
|---|---|---|---|---|
| AD-Tag | AD-NplA | AD-KapK | AD-NirA | |
| BD-p53 | + | − | − | − |
| BD-NirA | − | + | + | + |
| BD-KapK | − | + | ND | + |
Yeast two-hybrid interactions were monitored by complementation of the ade2 mutation. Bait vectors containing the GAL4 DNA-binding domain (BD) were constructed to result in a fusion with a control protein (BD-p53), with NirA1-229 (BD-NirA), or with KapK (BD-KapK). Prey vectors containing the GAL4 AD were constructed to result in a fusion with a control protein (T-antigen, AD-Tag, interacting with p53), with NplA (AD-NplA), with KapK (AD-KapK), or with NirA (AD-NirA). The BD-NirA-AD-NirA interaction serves as an additional positive control due to the homodimerization element in the NirA protein (55).
+, Interaction; −, no interaction; ND, not determined.
NirA-KapK and KapK-NplA interact in vitro.
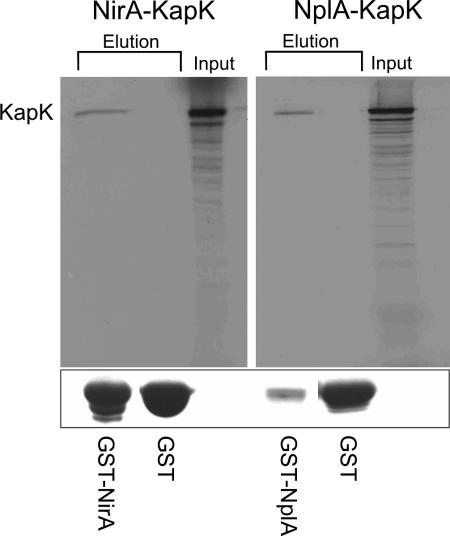
We confirmed the interactions found in the yeast two-hybrid system by in vitro assays. In these experiments the NirA bait 1 and NplA were expressed as GST fusion proteins in E. coli and KapK was expressed as [35S]methionine-labeled protein in a coupled transcription-translation assay. The GST control proteins, GST-NirA bait1 fusions or GST-NplA fusions present in crude E. coli extracts were loaded onto glutathione columns and incubated with [35S]KapK. Columns were extensively washed and subsequently eluted using reduced glutathione. In SDS-PAGE, GST proteins were revealed by Coomassie blue staining, and [35S]KapK was detected by radiography. In both cases, [35S]KapK specifically interacted with the fusion proteins but not with the GST control, providing additional biochemical evidence for the interactions identified in the yeast two-hybrid screen (Fig. 1).
FIG. 1.
KapK interacts in vitro with NirA and NplA. Glutathione-agarose columns containing GST-NirA, GST-NplA, or GST were loaded with [35S]methionine-labeled KapK (input), which was prepared in a coupled in vitro transcription-translation system. Columns were washed (see Materials and Methods for details), and fractions were eluted using 5 mM reduced glutathione. The autoradiographs show KapK coeluted with fractions containing GST-NirA (NirA-KapK, left panel) or GST-NplA (NplA-KapK, right panel) fusion proteins. Columns containing only GST did not show KapK in the eluted fractions. KapK showed the expected mass of ∼125 kDa, as determined with a molecular mass marker (not shown). Below the autoradiographs GST and GST fusion proteins of the same elution fractions are visualized by using SDS-PAGE and Coomassie blue staining.
NirA export from the nucleus is abolished in vivo in a leptomycin B (LMB)-sensitive kapK1 mutant.
We then investigated whether KapK is directly involved in NirA nuclear export in A. nidulans. Since CRM1 is essential for Schizosaccharomyces pombe (1) and S. cerevisiae (53), we constructed a conditional kapK mutant. In higher eukaryotes and in S. pombe, CRM1/exportin-1 is specifically inhibited by LMB through its covalent modification of a cysteine residue (26) present in the central conserved region. In S. cerevisiae Crm1p and A. nidulans KapK, a threonine substitutes for the target cysteine, resulting in LMB resistance (37). We constructed by allelic substitution a strain of A. nidulans sensitive to LMB, carrying the kapK1 T525C mutation, equivalent to the T539C mutation of S. cerevisiae (37) and of CrmA in A. nidulans (57). We first tested the nuclear retention phenotype of the kapK1 T525C mutation using a GFP chimera, denoted cNLS::NES::GFP, to which the simian virus 40 nuclear localization signal (NLS) and the PKI-α inhibitor NES had been attached, using as a control a second GFP fusion protein (cNLS::GFP) carrying the simian virus 40 NLS (cNLS::GFP) but not the NES. As predicted, LMB treatment results, in the kapK1 mutant but not in the wild type, in nuclear retention of the cNLS::NES::GFP but not of cNLS::GFP (see Fig. S2 in the supplemental material).
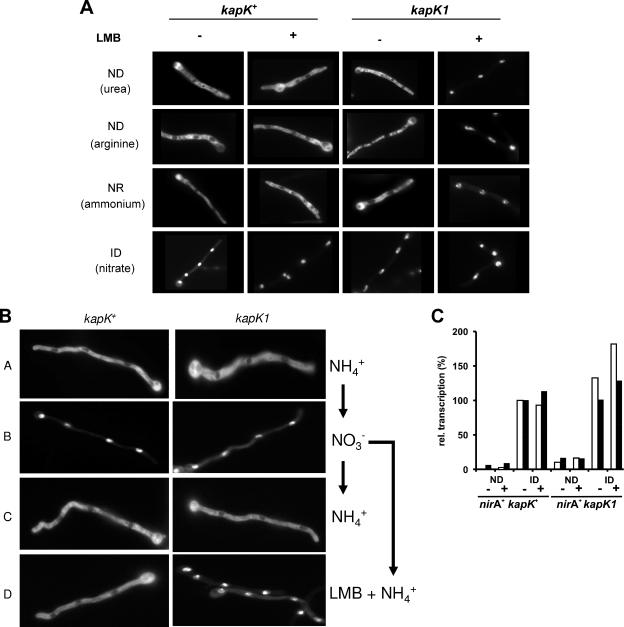
The effect of kapK1 in NirA-GFP localization was tested. In a kapK+, leptomycin-insensitive background, NirA-GFP is excluded from the nuclei when nitrate is absent. In the kapK1 mutant background, however, leptomycin treatment resulted in exclusive nuclear localization under all tested conditions. Notably, these results show that nitrate is not required for NirA nuclear accumulation (Fig. 2A). In the wild type, nuclear NirA-GFP rapidly relocalizes to the cytosol upon shifting cells to ammonium. In the kapK1 strain, LMB fully prevented NirA-GFP relocalization after transfer to ammonium (Fig. 2B). These results imply that KapK is the only exportin protein able to translocate NirA from the nucleus to the cytoplasm in response to nitrate depletion. Figure 2C shows that nuclear retention in a kapK1 strain does not result in constitutive expression of either niiA or niaD. The slightly elevated transcriptional level in strain nirA+ kapK1 under ND conditions is not significant because inactivation of KapK1 by LMB has no additional effect. These results imply that the nuclear localization of NirA is required, but not sufficient, for transcriptional activation of NirA target genes.
FIG. 2.
Nuclear export of NirA-GFP is mediated by KapK. (A) Mycelia from the wild-type (kapK+) and the kapK1 mutant strains were grown for 16 h at 25°C on coverslips in Aspergillus minimal medium under ND conditions (urea, arginine), NR conditions (ammonium), and ID conditions (nitrate). The effect of LMB on the subcellular localization of NirA was tested in the presence (+) or absence (−) of 10 ng of LMB/ml. (B) Analysis of the kapK-dependent nuclear export of NirA-GFP. Wild-type (kapK+) or kapK mutant (kapK1) cells were grown on coverslips in Aspergillus minimal medium containing ammonium (NH4+) as the sole nitrogen source for 16 h at 25°C (A). Nitrate (NO3−), was added to these cells and photographed after 2 min of incubation (B). Subsequently, the medium was withdrawn from the cultures and replaced again by ammonium-containing medium (NH4+) (C) or by medium containing ammonium and 10 ng of LMB/ml (LMB + NH4+) (D). Incubation proceeded for 10 min before the NirA-GFP localization was recorded. (C) Nuclear retention of NirA is not sufficient for transcriptional activation. Quantitative reverse transcription-PCR analysis of niiA (□) and niaD (▪) transcription in a nirA+ kapK+ strain was performed and compared to the LMB-sensitive nirA+ kapK1 mutant. Cultures were pregrown for 10 h on a noninducing-derepressing nitrogen source (urea), harvested, and shifted onto media containing urea (ND) or nitrate (ID) as the sole nitrogen source either in the absence (−) or in the presence (+) of 10 ng of LMB/ml. Incubation proceeded for 2 h before the cultures were harvested and analyzed. The actin gene (acnA) was used to normalize niiA and niaD expression. The transcriptional level of niiA and niaD in the nitrate-induced (ID) wild-type cultures in the absence of LMB (nirA+ kapK+, ID −) was set to 100%.
In vivo, nitrate disrupts the NirA-KapK interaction.
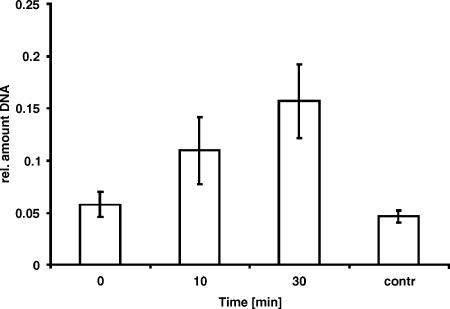
We have shown above that inactivation of the exportin recognizing a putative NES sequence in NirA results in a permanent, nitrate-independent, nuclear localization of NirA. These results strongly suggest that nuclear accumulation of NirA in response to nitrate induction results from the inhibition of export rather than from import activation. If this is the case, we should be able to detect NirA-KapK interactions under conditions in which NirA is exported (e.g., shifting the cultures from nitrate-induced to ammonium-repressed conditions; compare Fig. 2B), whereas we should not detect such interactions under conditions in which NirA accumulates in the nucleus (e.g., nitrate-induced conditions). To probe for this in vivo interaction in A. nidulans, we used ChIP analysis and monitored the presence of an S-tagged version of KapK at the NirA recognition site in the niiA-niaD promoter based on our previous observations that NirA binds immediately after nitrate induction and remains bound to its promoter sequence until at least 30 min of repression (35). The results presented in Fig. 3 show that under nitrate-induced conditions (0 min corresponds to samples taken immediately before the shift to repressing conditions) the amount of NirA binding site precipitated with S-tagged KapK is at the background level (control using a strain without S-tagged KapK). In contrast, when cultures are shifted to repressing conditions (for 10 and 30 min), the amount of precipitated NirA-binding site significantly increases. These results suggest that KapK is not interacting with DNA-bound NirA under conditions of nitrate induction but associates with NirA upon ammonium repression, and this association results in the translocation of NirA to the cytosol.
FIG. 3.
KapK-NirA in vivo interaction at the NirA binding site in the niiA-niaD promoter. ChIP using anti-S-tag antibody directed against S-tagged KapK and amplifying precipitated DNA at the NirA binding site 2 in the niiA-niaD intergenic region. Nitrate-induced cultures were submitted to repression by a shift to ammonium-containing medium, and samples were taken immediately before the shift (0 min) and after 10 min (10) and 30 min (30) of incubation, respectively. Relative amounts of precipitated DNA compared to input DNA (for details see Materials and Methods) at two time points of repression are shown. ChIP reactions with the anti-S-tag antibody in a strain lacking the S-tag epitope at the kapK locus (contr) were used as controls for background amplification. Error bars indicate the standard deviations of at least three biological replicates.
A nirA-constitutive mutation (nirAc1) leads to inducer-independent activity and maps in the putative NES.
In genetic screens searching for mutants affected in the nitrate assimilation genes of A. nidulans, rare mutations have been selected that lead to an inducer-independent activation of the nitrate cluster and that map in the nirA gene (42, 47). We have determined the sequence of the nirAc1 allele and found a single alteration, i.e., a G2926T transversion resulting in a Gly167Val substitution. Gly167 lies within an amino acid sequence motif strongly conserved among NirA orthologues (see Fig. S3 in the supplemental material). This motif conforms to a canonical nuclear export signal (NES), but Gly167 is not one of the residues defining the consensus sequence.
A nirAc1 mutation results in partial constitutivity (Fig. 4A, ND) of both niiA and niaD transcription and in an open chromatin pattern in the niiA-niaD intergenic region under ND (i.e., the absence of nitrate and ammonium) conditions (Fig. 4B). This open pattern is obtained in the wild-type strain (nirA+, areA+) only in the presence of nitrate (Fig. 4B, ID). nirAc1 also results in occupancy of the NirA binding site 2 in the absence of inducer (Fig. 4C). As expected (47, 58), nirAc1 does not affect transcriptional repression of niiA and niaD by ammonium (Fig. 4A, IR). Ammonium also results in the positioning of all nucleosomes (i.e., a “closed” nucleosomal pattern, Fig. 1B, IR and NR; marked CP) and in the loss of NirA site 2 occupancy (not shown) in the nirAc1 background. areA loss-of-function mutations are epistatic to nirAc1 as assessed by growth tests and enzyme assays (47, 58). This epistasis holds (Fig. 5A) at the level of the niiA and niaD mRNA steady states, at the level of protection of NirA binding site 2 (Fig. 4C, right panel), and at the level of chromatin rearrangements (Fig. 5B), confirming the essential role of AreA in these processes (33, 35).
FIG. 5.
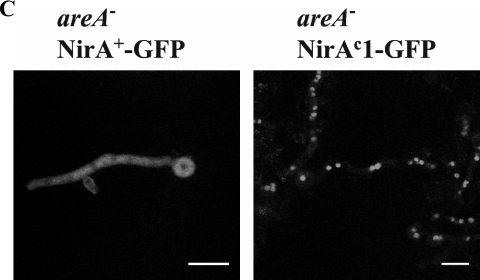
Dependence of the nirAc1 phenotype on areA function. (A) Northern blots of niiA and niaD mRNAs in the nirA+ areA+ and nirAc1 areA600 strains grown under ND (urea as the sole nitrogen source), ID (nitrate as the sole nitrogen source), IR (nitrate plus ammonium as the nitrogen sources), or NR (ammonium as the sole nitrogen source) conditions. The acnA (actin) hybridization serves as a loading control. (B) Nucleosome positioning in the bidirectional niiA-niaD promoter region comparing nirA+ areA+ and nirAc1 areA600 strains. Samples from the mycelia shown in panel A were subjected to increasing amounts of MNase (black triangles). To the sides of the autoradiographs, the position of DNA fragments calculated from a 100-bp ladder is shown. Independently obtained control patterns from the wild-type nirA+ areA+ strain under NR and ID conditions are shown for comparison. Molecular mass markers were run in the same gel. The naked DNA control is shown in Fig. 1B. CP, closed pattern; OP, open pattern (see Fig. 1B and the legend to this figure). (C) Subcellular localization of NirA as a GFP fusion protein in an areA mutant background. areA mutant strains ERE-nirA+-GFP (left) and ERE-nirAc1-GFP (right) were grown as described in Materials and Methods for ∼14 h under ND conditions with arginine as the sole nitrogen source (identical nuclear localization patterns were obtained with other ND nitrogen sources such as urea, proline, or hypoxanthine). Induction of the GFP fusion proteins was obtained by the addition of 100 pM DES 5 h prior to the microscopic observations. GFP localization was examined by confocal laser scanning microscopy, and the positions of the nuclei were determined by DAPI (4′,6′-diamidino-2-phenylindole) staining (not shown). Scale bar, 10 μm. Urea, used as a standard ND nitrogen source, resulted in unexpected high vacuolization of mycelia in areA strains and, consequently, observations on arginine rather than on urea are shown in this figure.
Mutations in the NES abolish NirA interactions in yeast two-hybrid systems and nuclear export in A. nidulans.
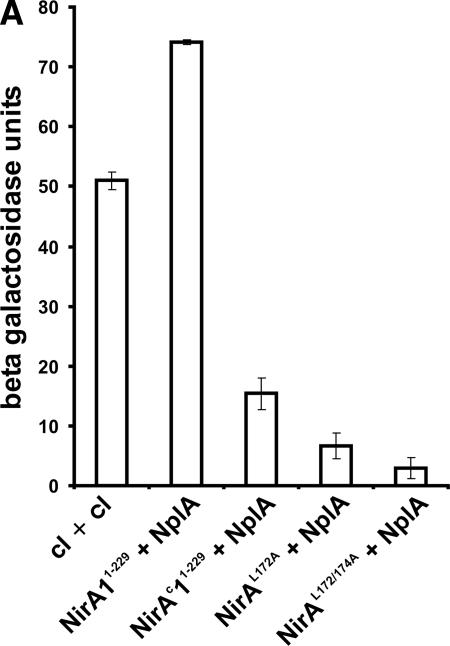
We have introduced alternative mutations in the region of the nirAc1 mutation replacing either one (Leu172Ala) or two (Leu172Ala and Leu174Ala) canonical leucines of the NES consensus sequences by alanines. We then quantified by lacZ reporter enzyme measurements the effect of these mutations on NirA-NplA in comparison to the nirA wild-type and nirAc1 sequences in the yeast two-hybrid system. While the nirAc1 Gly167Val substitution within the NES strongly reduced NplA interaction, single Leu172Ala or double Leu172Ala Leu174Ala substitutions completely abolished the two- hybrid interaction of NirA with NplA (Fig. 6A). Because KapK bridges the NirA-NplA interaction, the same effect of the NES mutations should also be evident in NirA-KapK interactions. This is shown in Fig. 6B. The NirA-KapK interaction is strongly impaired in NirAc1Gly167Val and completely abolished in NirAL172A and NirAL172/174A.
FIG. 6.
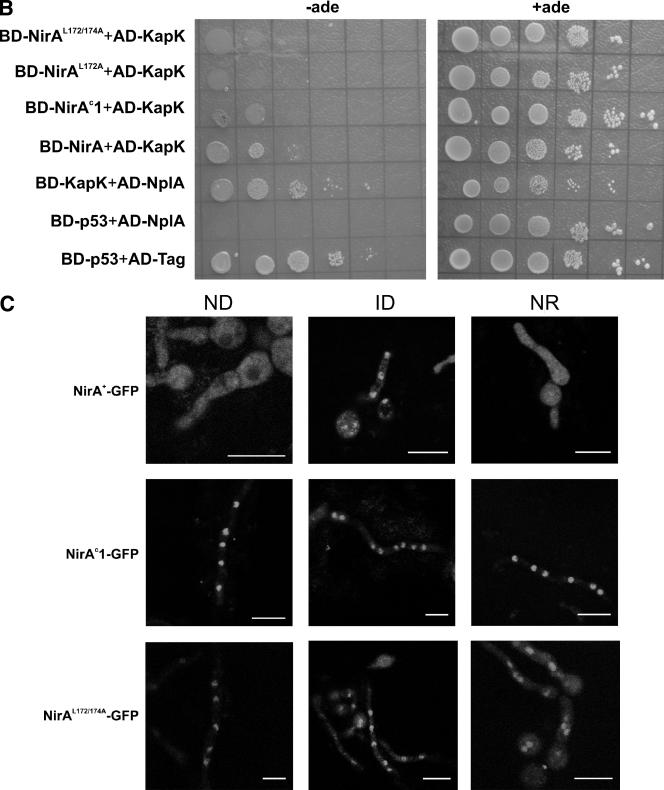
Mutations in the NirA NES disrupt the interaction with nuclear export factors and lead to nuclear accumulation without nitrate. (A) Quantitative measure of the NirA-NplA interaction. Amino acids 1 to 229 of wild-type and mutant NirA proteins were expressed as fusions with the GAL4p DNA-binding domain (BD-NirA). The NplA protein (amino acids 80 to 512) was expressed as a fusion with the GAL4p activation domain (AD-NplA). BD-cI+AD-cI indicates the results obtained using the dimerization domain of the phage λ cI protein as a positive control in both bait and prey fusion proteins. BD-NirA+AD-NplA, BD-NirAc1+AD-NplA, BD-NirAL172A+AD-NplA, and BD-NirAL172/174A+AD-NplA indicate the results obtained using as prey the AD-NplA80−512 fragment and as bait the fragment 1-229 of NirA, carrying, respectively, no mutations, the nirAc1 mutation, or in vitro-constructed mutations in the NES as described in the text. The β-galactosidase activity of each bait-prey combination was determined according to the procedures described in Materials and Methods. (B) Yeast two-hybrid interactions of NirA NES mutants (amino acids 1 to 229) with KapK (amino acids 1 to 1072) and NplA (amino acids 80 to 512). Protein-protein interactions were monitored by complementation of the ade2 mutation in the yeast two-hybrid strain PJ69-4A. Tenfold serial dilutions of liquid cultures grown to an OD600 of 0.3 were spotted onto minimal medium (−leu, −trp) in the presence (+ade) or in the absence (−ade) of supplementing adenine. Photographs were taken after 4 to 5 days of incubation at 28°C. NirA wild-type protein (BD-NirA), with the constitutive mutation (BD-NirAc1) and with the in vitro-constructed single NES mutation (BD-NirAL172A) or the double NES mutation (BD-NirAL172/174A), were expressed as a fusion with the Gal4p-binding domain. KapK was expressed either as a fusion with the GAL4p activation domain (AD-KapK) for interaction tests with NirA or with the GAL4p-binding domain (BD-KapK) for interaction tests with NplA. The latter protein was expressed as a fusion with the GAL4p activation domain (AD-NplA). Control proteins p53 and the large fragment of the T-antigen (see Materials and Methods) were expressed as a fusion with the GAL4p-binding domain (BD-p53) or with the GAL4p activation domain (AD-Tag), respectively. (C) Subcellular localization of NirA as a GFP fusion protein. Strains ERE-nirA+-GFP (top), ERE-nirAc1-GFP (middle), and ERE-nirAL172/174A (bottom) were grown as detailed in Materials and Methods for ∼14 h under ND conditions (with arginine as the sole nitrogen source; identical nuclear localization patterns were obtained with other ND nitrogen sources such as urea, proline, or hypoxanthine), under ID conditions (with nitrate as the sole nitrogen source), or under NR conditions (with ammonium as the sole nitrogen source). Induction of the GFP fusion proteins was obtained by the addition of 100 pM DES 5 h prior to the microscopic observations. GFP localization was examined by confocal laser scanning microscopy, and the position of the nuclei was determined by DAPI staining (not shown). Scale bar, 10 μm. Urea, the standard ND nitrogen source in transcriptional analysis, chromatin studies, and in vivo footprinting, resulted in an unexpected high vacuolization of mycelia in areA mutant strains and, consequently, observations on arginine rather than on urea are shown in this figure and in Fig. 5C.
A NirA-GFP driven from the very weak nirA promoter complements a nirA mutation, but no fluorescence is visible (5). We investigated the localization of NirA-GFP fusion proteins in strains expressing the human estrogen receptor α (hER) from a single-copy transgene in which the expression of NirA-GFP or NirAc1-GFP is driven by a promoter containing an estrogen responsive element (ERE) and thus can be modulated to avoid gross overexpression (5, 41). NirAc1-GFP localizes to the nucleus equally well in the presence and in the absence of nitrate and even in the presence of ammonium as the sole nitrogen source, whereas localization of NirA+-GFP in the nucleus is strictly nitrate dependent (Fig. 6C). In accordance with the yeast two-hybrid results, the substitutions of the “canonical” Leu172 and Leu174 within this motif also led to constitutive nuclear localization, confirming the functionality of the NES. Interestingly, these sequence changes result in a complete loss-of-function phenotype as assessed by both growth tests (no growth on nitrate and nitrite as sole nitrogen sources) and niiA and niaD Northern blots (results not shown). This implies that this region of NirA has an additional, probably structural, function in addition to promoting NirA nuclear export and that the nirAc1 mutation results in a nirA gain of function both at the level of nuclear retention and at the level of transcriptional activation. Notably, whereas AreA is essential for NirA constitutivity, it is not required for the nuclear retention of NirAc1 (Fig. 5C). This supports our previous proposal that AreA is not directly involved NirA nuclear localization (5).
DISCUSSION
We reported previously that nitrate induces rapid NirA accumulation in the nucleus (5). Three lines of evidence presented here demonstrate that this accumulation results from a blockage in nuclear export rather than from permitting nuclear import. First, in a hunt for NirA-interacting proteins we identified the exportin KapK and a member of the nuclear pore family of proteins, NplA. We showed by yeast two-hybrid analysis and in vitro assays that NirA directly binds KapK, and this protein bridges an interaction between NirA and the nuclear pore-like protein, NplA. The second line of evidence establishes that mutations in the NirA NES disrupt the NirA-KapK and subsequently the NirA-NplA interactions in the yeast two-hybrid system. In A. nidulans, NES mutations lead to a constitutive, nitrate-independent nuclear accumulation of NirA. Importantly, inactivation of KapK by LMB in an LMB-sensitive kapK1 strain also leads to the same phenotype (permanent NirA nuclear accumulation) showing that nuclear import of NirA is nitrate independent. Consequently, we hypothesize that NirA nuclear import and export is a permanently active process under all nitrogen conditions but that the export process is disrupted when nitrate becomes available. Under these NirA-activating conditions, nuclear export would be blocked and NirA accumulates in the nucleus, eventually leading to transcriptional activation of the target genes. This hypothesis is supported by our third line of evidence, i.e., the finding that the exportin KapK can be precipitated in ChIP experiments from the NirA binding site in a nirA+ strain under conditions of NirA nuclear export (ammonium-repressing conditions) but cannot be detected in a significant amount at this binding site under conditions of nuclear accumulation (nitrate-inducing conditions). We propose that in the absence of nitrate NirA is a cargo for KapK and thus prevents its nuclear accumulation. Nuclear export would involve interactions with nucleoporins, including NplA. Nitrate would disrupt the interaction between KapK and the NES of NirA, thus preventing nuclear export. In light of these findings, it appears highly unlikely that nitrate induction specifically triggers the nuclear accumulation of NirA, an independent effect of induction on nuclear import rate is not, however, excluded.
In the major nuclear export mechanism, a Crm1p/CRM1/exportin 1 homologue recognizes a hydrophobic NES of a given nuclear protein and forms a ternary complex with Ran/GTP (see reference 43 for a review). This tripartite complex would then interact with nucleoporins. Interaction of CRM1/exportin-1 homologues with a number of nucleoporins has been reported in a number of different systems (see, for example, references 17, 18, 22, and 68). Our results suggest a role for a conserved putative nucleoporin-like protein, NplA. Exported cargoes do not interact directly with nucleoporins in agreement with our failure to detect any in vitro interactions between NirA and NplA and with the finding that KapK bridges this interaction. The conserved nature of such interactions is certainly of interest. Crm1p, the yeast homologue of KapK, serves as a bridging factor between the human NES-containing protein REV and several human and yeast nucleoporins (38). The yeast Crm1p and human CRM-1, orthologues of KapK, interact with NLP-1. This accounts for two-hybrid assays revealing an interaction between the NES of REV and the human NPL-1, followed by the failure to demonstrate a direct interaction between these proteins (15). The analogy with our results suggests a striking conservation of nuclear export pathways among eukaryotes. The deletion of the nplA gene has no specific phenotype, and this is in line with the multiplicity of putative nucleoporins found in the genome of A. nidulans (13). We must conclude that NplA is one of several nucleoporins able to interact with KapK. Inactivation of KapK, on the other hand, leads to nuclear accumulation of NirA, and these results argue against overlapping functions of exportins in NirA nuclear export and support an exclusive role for KapK in this process.
We were surprised by the finding that nuclear retention is necessary, but not sufficient, for the activation of NirA. While the inactivation of KapK by LMB in the kapK1 strain grown in the absence of nitrate results in NirA nuclear accumulation, it does not lead to transcriptional activation of either niiA or niaD (Fig. 2C). This suggests an additional role for nitrate in transforming NirA to its transcriptionally active form. The results from the nirAc1 gain-of-function mutation, which mimics nitrate induction not only for nuclear accumulation but also for transcriptional activation, shed some light into this additional role of nitrate. NirAc1 constitutively localizes to the nucleus and occupies the crucial NirA binding site 2, leading to partial constitutivity of niiA and niaD expression and complete constitutivity of all of the chromatin rearrangements extant in the niiA-niaD bidirectional promoter. With the exception of nuclear localization, all of these phenotypes are dependent on the GATA factor AreA. This is in line with previous work which revealed a synergy of AreA and NirA in eliciting transcription and strongly suggested an in vivo interaction between these two proteins (5, 16, 33-35). Particularly challenging is the observation that complete chromatin remodeling in the intergenic region occurs in nirAc1 strains in the absence of nitrate because previous work has shown that gross chromatin remodeling determined by MNase is independent of NirA (33). However, recent results (5) suggest that NirA has a role in the kinetics of loss-of-positioning of nucleosome −1. The nirAc1 mutation, besides disrupting the interaction of NirA with the nuclear export machinery, must also result in interactions with the transcriptional and chromatin restructuring machinery in the absence of nitrate. The nirAc1 mutation maps in the NES and exchanges a neutral amino acid (glycine) for a hydrophobic residue (valine) in a region proposed to form hydrophobic interactions with the nuclear export machinery. The G167V substitution may lead to a densely packed hydrophobic pocket restricting the access of KapK. Substitutions in the canonical leucines of the NES by alanines, which result in nuclear retention, result also in nirA loss-of-function phenotypes, which implies a role of this region for the protein to be active as a transcriptional activator. The molecular basis of nitrate-mediated inhibition of NirA export remains unknown. Posttranslational modification of proteins such as phosphorylation (14, 25, 39, 65, 67) or as shown for Yap1p (see the introduction), through the formation of an intramolecular disulfide bridge, could mask (or unmask) an NES (12, 64). It is tempting to speculate that the NirA NES is involved in intramolecular interactions with the NirA activation domain and that nitrate or the nirAc1 mutation acts by disrupting these interactions, making the transcriptional activation domain accessible while masking the NES. If this were the case, it should be possible to isolate additional constitutive, gain-of-function mutations mapping in these proposed interaction domains. Studies that support this view are under way. Additional constitutive mutations and suppressor mutations of thermosensitive nirA mutations have been identified in the central region and the C terminus of NirA (Andreas Bernreiter, Lisa Olsson, Herb N. Arst, Jr., and Joseph Strauss, unpublished results).
Supplementary Material
Acknowledgments
Work at Vienna was supported by the Austrian Science Fund FWF grants START-Y114 and P-17018 to J.S. Work at Orsay was supported by the Université Paris-Sud, CNRS, the Institut Universitaire de France, and EC contract PL-96-0535. A.R. was partially supported by the above-mentioned contract and a studentship of the Fondation de la Recherche Médicale. Work at Madrid was supported by Ministerio de Educación y Ciencia grant BMC2003-00874 to E.A.E., and J.F.-M. and L.A.-B. held PFPI and FPU positions, respectively.
We thank Steve Osmani for plasmids and Berl Oakley for ΔnkuA strains.
Footnotes
Published ahead of print on 20 November 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adachi, Y., and M. Yanagida. 1989. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kDa protein preferentially localized in the nucleus and its periphery. J. Cell Biol. 108:1195-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrianopoulos, A., S. Kourambas, J. A. Sharp, M. A. Davis, and M. J. Hynes. 1998. Characterization of the Aspergillus nidulans nmrA gene involved in nitrogen metabolite repression. J. Bacteriol. 180:1973-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arst, H. N., Jr., and D. J. Cove. 1973. Nitrogen metabolite repression in Aspergillus nidulans. Mol. Gen. Genet. 126:111-141. [DOI] [PubMed] [Google Scholar]
- 4.Axelrod, J. D., J. Majors, and M. C. Brandriss. 1991. Proline-independent binding of PUT3 transcriptional activator protein detected by footprinting in vivo. Mol. Cell. Biol. 11:564-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger, H., R. Pachlinger, I. Morozov, S. Goller, F. Narendja, M. Caddick, and J. Strauss. 2006. The GATA factor AreA regulates localization and in vivo binding site occupancy of the nitrate activator NirA. Mol. Microbiol. 59:433-446. [DOI] [PubMed] [Google Scholar]
- 6.Bogerd, H. P., R. A. Fridell, S. Madore, and B. R. Cullen. 1995. Identification of a novel cellular cofactor for the Rev/Rex class of retroviral regulatory proteins. Cell 82:485-494. [DOI] [PubMed] [Google Scholar]
- 7.Brisco, P. R., and G. B. Kohlhaw. 1990. Regulation of yeast LEU2: total deletion of regulatory gene LEU3 unmasks GCN4-dependent basal level expression of LEU2. J. Biol. Chem. 265:11667-11675. [PubMed] [Google Scholar]
- 8.Burger, G., J. Strauss, C. Scazzocchio, and B. F. Lang. 1991. nirA, the pathway-specific regulatory gene of nitrate assimilation in Aspergillus nidulans, encodes a putative GAL4-type zinc finger protein and contains four introns in highly conserved regions. Mol. Cell. Biol. 11:5746-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clutterbuck, A. J., and H. Arst. 1995. Genetic nomenclature guide. Aspergillus nidulans. Trends Genet.: 13-14. [PubMed]
- 10.Cove, D. J. 1979. Genetic studies of nitrate assimilation in Aspergillus nidulans. Biol. Rev. Camb. Philos. Soc. 54:291-327. [DOI] [PubMed] [Google Scholar]
- 11.Daboussi, M. J., T. Langin, F. Deschamps, Y. Brygoo, C. Scazzocchio, and G. Burger. 1991. Heterologous expression of the Aspergillus nidulans regulatory gene nirA in Fusarium oxysporum. Gene 109:155-160. [DOI] [PubMed] [Google Scholar]
- 12.Delaunay, A., A. D. Isnard, and M. B. Toledano. 2000. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 19:5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Souza, C. P., A. H. Osmani, S. B. Hashmi, and S. A. Osmani. 2004. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr. Biol. 14:1973-1984. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez, D., B. Montserrat-Sentis, A. Virgos-Soler, S. Guaita, J. Grueso, M. Porta, I. Puig, J. Baulida, C. Franci, and A. Garcia de Herreros. 2003. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol. Cell. Biol. 23:5078-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farjot, G., A. Sergeant, and I. Mikaelian. 1999. A new nucleoporin-like protein interacts with both HIV-1 Rev nuclear export signal and CRM-1. J. Biol. Chem. 274:17309-17317. [DOI] [PubMed] [Google Scholar]
- 16.Feng, B., and G. A. Marzluf. 1998. Interaction between major nitrogen regulatory protein NIT2 and pathway-specific regulatory factor NIT4 is required for their synergistic activation of gene expression in Neurospora crassa. Mol. Cell. Biol. 18:3983-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Floer, M., and G. Blobel. 1999. Putative reaction intermediates in Crm1-mediated nuclear protein export. J. Biol. Chem. 274:16279-16286. [DOI] [PubMed] [Google Scholar]
- 18.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 19.Fritz, C. C., M. L. Zapp, and M. R. Green. 1995. A human nucleoporin-like protein that specifically interacts with HIV Rev. Nature 376:530-533. [DOI] [PubMed] [Google Scholar]
- 20.Gietz, R. D., B. Triggs-Raine, A. Robbins, K. C. Graham, and R. A. Woods. 1997. Identification of proteins that interact with a protein of interest: applications of the yeast two-hybrid system. Mol. Cell. Biochem. 172:67-79. [PubMed] [Google Scholar]
- 21.Gomez, D., B. Cubero, G. Cecchetto, and C. Scazzocchio. 2002. PrnA, a Zn2Cys6 activator with a unique DNA recognition mode, requires inducer for in vivo binding. Mol. Microbiol. 44:585-597. [DOI] [PubMed] [Google Scholar]
- 22.Guan, T., R. H. Kehlenbach, E. C. Schirmer, A. Kehlenbach, F. Fan, B. E. Clurman, N. Arnheim, and L. Gerace. 2000. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol. Cell. Biol. 20:5619-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hon, T., A. Hach, H. C. Lee, T. Cheng, and L. Zhang. 2000. Functional analysis of heme regulatory elements of the transcriptional activator Hap1. Biochem. Biophys. Res. Commun. 273:584-591. [DOI] [PubMed] [Google Scholar]
- 24.Hon, T., H. C. Lee, A. Hach, J. L. Johnson, E. A. Craig, H. Erdjument-Bromage, P. Tempst, and L. Zhang. 2001. The Hsp70-Ydj1 molecular chaperone represses the activity of the heme activator protein Hap1 in the absence of heme. Mol. Cell. Biol. 21:7923-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida, N., T. Hara, T. Kamura, M. Yoshida, K. Nakayama, and K. I. Nakayama. 2002. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277:14355-14358. [DOI] [PubMed] [Google Scholar]
- 26.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96:9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, H. C., T. Hon, and L. Zhang. 2002. The molecular chaperone Hsp90 mediates heme activation of the yeast transcriptional activator Hap1. J. Biol. Chem. 277:7430-7437. [DOI] [PubMed] [Google Scholar]
- 28.Leuther, K. K., and S. A. Johnston. 1992. Nondissociation of GAL4 and GAL80 in vivo after galactose induction. Science 256:1333-1335. [DOI] [PubMed] [Google Scholar]
- 29.Leuther, K. K., J. M. Salmeron, and S. A. Johnston. 1993. Genetic evidence that an activation domain of GAL4 does not require acidity and may form a beta sheet. Cell 72:575-585. [DOI] [PubMed] [Google Scholar]
- 30.Malardier, L., M. J. Daboussi, J. Julien, F. Roussel, C. Scazzocchio, and Y. Brygoo. 1989. Cloning of the nitrate reductase gene (niaD) of Aspergillus nidulans and its use for transformation of Fusarium oxysporum. Gene 78:147-156. [DOI] [PubMed] [Google Scholar]
- 31.Marzluf, G. A. 1997. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 61:17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morozov, I. Y., M. Galbis-Martinez, M. G. Jones, and M. X. Caddick. 2001. Characterization of nitrogen metabolite signalling in Aspergillus via the regulated degradation of areA mRNA. Mol. Microbiol. 42:269-277. [DOI] [PubMed] [Google Scholar]
- 33.Muro-Pastor, M. I., R. Gonzalez, J. Strauss, F. Narendja, and C. Scazzocchio. 1999. The GATA factor AreA is essential for chromatin remodeling in a eukaryotic bidirectional promoter. EMBO J. 18:1584-1597. (Erratum, 18:2670.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muro-Pastor, M. I., J. Strauss, A. Ramon, and C. Scazzocchio. 2004. A paradoxical mutant GATA factor. Eukaryot. Cell 3:393-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narendja, F., S. P. Goller, M. Wolschek, and J. Strauss. 2002. Nitrate and the GATA factor AreA are necessary for in vivo binding of NirA, the pathway-specific transcriptional activator of Aspergillus nidulans. Mol. Microbiol. 44:573-583. [DOI] [PubMed] [Google Scholar]
- 36.Nayak, T., E. Szewczyk, C. E. Oakley, A. Osmani, L. Ukil, S. L. Murray, M. J. Hynes, S. A. Osmani, and B. R. Oakley. 2006. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172:1557-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neville, M., and M. Rosbash. 1999. The NES-Crm1p export pathway is not a major mRNA export route in Saccharomyces cerevisiae. EMBO J. 18:3746-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neville, M., F. Stutz, L. Lee, L. I. Davis, and M. Rosbash. 1997. The importin-beta family member Crm1p bridges the interaction between Rev. and the nuclear pore complex during nuclear export. Curr. Biol. 7:767-775. [DOI] [PubMed] [Google Scholar]
- 39.Okamura, H., J. Aramburu, C. Garcia-Rodriguez, J. P. Viola, A. Raghavan, M. Tahiliani, X. Zhang, J. Qin, P. G. Hogan, and A. Rao. 2000. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol. Cell 6:539-550. [DOI] [PubMed] [Google Scholar]
- 40.Orlando, V., H. Strutt, and R. Paro. 1997. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods 11:205-214. [DOI] [PubMed] [Google Scholar]
- 41.Pachlinger, R., R. Mitterbauer, G. Adam, and J. Strauss. 2005. Metabolically independent and accurately adjustable Aspergillus sp. expression system. Appl. Environ. Microbiol. 71:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pateman, J. A., and D. J. Cove. 1967. Regulation of nitrate reduction in Aspergillus nidulans. Nature 215:1234-1237. [DOI] [PubMed] [Google Scholar]
- 43.Pemberton, L. F., and B. M. Paschal. 2005. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 6:187-198. [DOI] [PubMed] [Google Scholar]
- 44.Pokorska, A., C. Drevet, and C. Scazzocchio. 2000. The analysis of the transcriptional activator PrnA reveals a tripartite nuclear localization sequence. J. Mol. Biol. 298:585-596. [DOI] [PubMed] [Google Scholar]
- 45.Punt, P. J., M. A. Dingemanse, A. Kuyvenhoven, R. D. Soede, P. H. Pouwels, and C. A. van den Hondel. 1990. Functional elements in the promoter region of the Aspergillus nidulans gpdA gene encoding glyceraldehyde-3-phosphate dehydrogenase. Gene 93:101-109. [DOI] [PubMed] [Google Scholar]
- 46.Punt, P. J., J. Strauss, R. Smit, J. R. Kinghorn, C. A. van den Hondel, and C. Scazzocchio. 1995. The intergenic region between the divergently transcribed niiA and niaD genes of Aspergillus nidulans contains multiple NirA binding sites which act bidirectionally. Mol. Cell. Biol. 15:5688-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rand, K. N., and H. N. Arst, Jr. 1978. Mutations in nirA gene of Aspergillus nidulans and nitrogen metabolism. Nature 272:732-734. [DOI] [PubMed] [Google Scholar]
- 48.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 49.Ryan, K. J., and S. R. Wente. 2000. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr. Opin. Cell Biol. 12:361-371. [DOI] [PubMed] [Google Scholar]
- 50.Scazzocchio, C. 2000. The fungal GATA factors. Curr. Opin. Microbiol. 3:126-131. [DOI] [PubMed] [Google Scholar]
- 51.Selleck, S. B., and J. E. Majors. 1987. In vivo DNA-binding properties of a yeast transcription activator protein. Mol. Cell. Biol. 7:3260-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serebriiskii, I. G., and E. A. Golemis. 2001. Two-hybrid system and false positives: approaches to detection and elimination. Methods Mol. Biol. 177:123-134. [DOI] [PubMed] [Google Scholar]
- 53.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 54.Stoffler, D., B. Fahrenkrog, and U. Aebi. 1999. The nuclear pore complex: from molecular architecture to functional dynamics. Curr. Opin. Cell Biol. 11:391-401. [DOI] [PubMed] [Google Scholar]
- 55.Strauss, J., M. I. Muro-Pastor, and C. Scazzocchio. 1998. The regulator of nitrate assimilation in ascomycetes is a dimer which binds a nonrepeated, asymmetrical sequence. Mol. Cell. Biol. 18:1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tilburn, J., C. Scazzocchio, G. G. Taylor, J. H. Zabicky-Zissman, R. A. Lockington, and R. W. Davies. 1983. Transformation by integration in Aspergillus nidulans. Gene 26:205-221. [DOI] [PubMed] [Google Scholar]
- 57.Todd, R. B., J. A. Fraser, K. H. Wong, M. A. Davis, and M. J. Hynes. 2005. Nuclear accumulation of the GATA factor AreA in response to complete nitrogen starvation by regulation of nuclear export. Eukaryot. Cell 4:1646-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tollervey, D., and H. N. Arst, Jr. 1981. Mutations to constitutivity and derepression are separate and separable in a regulatory gene of Aspergillus nidulans. Curr. Genet. 4:63-68. [DOI] [PubMed] [Google Scholar]
- 59.Unkles, S. E., K. L. Hawker, C. Grieve, E. I. Campbell, P. Montague, and J. R. Kinghorn. 1991. crnA encodes a nitrate transporter in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 88:204-208. (Errata, 88:4564, 1991, and 92:3076, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unkles, S. E., D. Zhou, M. Y. Siddiqi, J. R. Kinghorn, and A. D. Glass. 2001. Apparent genetic redundancy facilitates ecological plasticity for nitrate transport. EMBO J. 20:6246-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, R., M. Okamoto, X. Xing, and N. M. Crawford. 2003. Microarray analysis of the nitrate response in Arabidopsis Roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 132:556-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitehead, M. P., S. J. Gurr, C. Grieve, S. E. Unkles, D. Spence, M. Ramsden, and J. R. Kinghorn. 1990. Homologous transformation of Cephalosporium acremonium with the nitrate reductase-encoding gene (niaD). Gene 90:193-198. [DOI] [PubMed] [Google Scholar]
- 63.Wolschek, M. F., F. Narendja, J. Karlseder, C. P. Kubicek, C. Scazzocchio, and J. Strauss. 1998. In situ detection of protein-DNA interactions in filamentous fungi by in vivo footprinting. Nucleic Acids Res. 26:3862-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood, M. J., G. Storz, and N. Tjandra. 2004. Structural basis for redox regulation of Yap1 transcription factor localization. Nature 430:917-921. [DOI] [PubMed] [Google Scholar]
- 65.Yagita, K., S. Yamaguchi, F. Tamanini, G. T. van Der Horst, J. H. Hoeijmakers, A. Yasui, J. J. Loros, J. C. Dunlap, and H. Okamura. 2000. Dimerization and nuclear entry of mPER proteins in mammalian cells. Genes Dev. 14:1353-1363. [PMC free article] [PubMed] [Google Scholar]
- 66.Yang, L., L. Ukil, A. Osmani, F. Nahm, J. Davies, C. P. De Souza, X. Dou, A. Perez-Balaguer, and S. A. Osmani. 2004. Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans. Eukaryot. Cell 3:1359-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, Y., and Y. Xiong. 2001. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science 292:1910-1915. [DOI] [PubMed] [Google Scholar]
- 68.Zhu, J., R. J. Hill, P. J. Heid, M. Fukuyama, A. Sugimoto, J. R. Priess, and J. H. Rothman. 1997. end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev. 11:2883-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.