Abstract
Phosphoinositide-specific phospholipase C-γ1 (PLC-γ1) is a key enzyme that governs cellular functions such as gene transcription, secretion, proliferation, motility, and development. Here, we show that PLC-γ1 is regulated via a novel autoinhibitory mechanism involving its carboxy-terminal Src homology (SH2C) domain. Mutation of the SH2C domain tyrosine binding site led to constitutive PLC-γ1 activation. The amino-terminal split pleckstrin homology (sPHN) domain was found to regulate the accessibility of the SH2C domain. PLC-γ1 constructs with mutations in tyrosine 509 and phenylalanine 510 in the sPHN domain no longer required an intact amino-terminal Src homology (SH2N) domain or phosphorylation of tyrosine 775 or 783 for activation. These data are consistent with a model in which the SH2C domain is blocked by an intramolecular interaction(s) that is released upon cellular activation by occupancy of the SH2N domain.
Phosphoinositide-specific phospholipase C-γ1 (PLC-γ1) is activated in response to ligand binding by a variety of receptors (2). PLC-γ1 hydrolyzes phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], leading to the generation of the second messengers, inositol triphosphate (IP3) and diacylglycerol, which release Ca2+ from intracellular stores and activate Ras through the RasGRP/protein kinase C pathway, respectively (8, 13). PLC-γ1 has also been shown to play a role in the activation of NF-κB (7). These pathways in turn initiate signaling cascades that culminate in cytokine production, activation of effector function, and cell proliferation (2, 5). The central role of PLC-γ1 in cellular function is further illustrated by the fact that inactivation of the PLC-γ1 gene in mice is embryonic lethal (16).
Regulation of PLC-γ1 activity is complex. In quiescent cells, cytosolic sequestration of PLC-γ1 limits access to PI(4,5)P2 in the plasma membrane. Cell stimulation induces membrane recruitment of PLC-γ1 and its tyrosine phosphorylation, essential steps in PLC-γ1 activation (2, 23, 36). Key components of PLC-γ that are involved in these activation events are located within a region unique to PLC-γ between the X and Y portions of the catalytic domain. This area is composed of two Src homology 2 (SH2) domains and an Src homology 3 (SH3) domain. The N-terminal SH2 domain (SH2N) is critical for binding to either tyrosine-phosphorylated receptor kinases or adaptor molecules that mediate PLC-γ1 relocation to the plasma membrane (6, 14, 25). The function of the C-terminal SH2 domain (SH2C) is less clear but is also essential for PLC-γ1 activation (1, 6, 14, 33). Interestingly, the tyrosine residues that are essential for PLC-γ1 activity (Y775 and Y783 in PLC-γ1 and Y753 and Y759 in PLC-γ2) are also located in this region (29, 30).
Components of the SH region not only are essential for PLC-γ1 activation but also may participate in its autoregulation. The individual X or Y elements of the catalytic domain alone are inactive. When mixed together in vitro, the hydrolytic activity of the combined X and Y elements was found to be 20- to 100-fold greater than that of intact PLC-γ1 (12). Interestingly, a peptide composed of the PLC-γ1 SH2 and SH3 domains was shown to block the in vitro enzymatic activity of all members of the phosphoinositide-specific PLC family (11). An eight-amino-acid portion of this peptide sequence (termed the PLC catalytic inhibitory [PCI] peptide) located in the SH2C domain has been shown to provide most of this function. When tested as a myristoylated form, the PCI peptide suppressed both growth factor-activated IP3 generation and cell proliferation in Swiss 3T3 cells (10). These data were interpreted as an indication that the tertiary structure of PLC-γ positioned the PCI peptide in proximity to either the X or Y catalytic components, thereby blocking enzymatic activity.
Previously, we have reported evidence indicating PLC-γ1 autoregulation by its SH2C domain. Constitutive, tyrosine phosphorylation-independent in vitro PLC-γ1 catalytic activity was observed when the SH2C domain was functionally disrupted (6). The activity of this SH2C domain mutant was, in fact, greater than that of receptor-activated, wild-type PLC-γ1. These data suggest a role for both the PCI peptide and the phosphotyrosine binding function of the SH2C domain in PLC-γ1 autoinhibition.
Binding partners for the SH2C domain that are essential in PLC-γ1 activation remain undefined. The SH2C domain has been suggested to interact intramolecularly with phosphorylated tyrosine 783 (26) and intermolecularly with Grb2 (4) and to cooperate with the PLC-γ1 SH3 domain in an interaction with c-Cbl (28). However, in many cases, the in vitro interactions observed between the isolated SH2C domain and other phosphoproteins have not been shown to occur with intact PLC-γ1 (6, 33). When tested as glutathione S-transferase (GST) fusion proteins, both PLC-γ1 SH2 domains pulled down the key adaptor proteins, LAT and BLNK, from activated T- and B-cell lysates, respectively. However, the SH2N domain but not the SH2C domain interacted with these adapter proteins in intact cells (6, 33). The isolated PLC-γ1 SH2C domain was also shown by nuclear magnetic resonance to bind a phosphorylated peptide encompassing tyrosine 1021 of the platelet-derived growth factor (PDGF) receptor, but a role for this interaction in PDGF-activated cells was not identified (15, 25). Taken together, these studies suggest that access to the SH2C domain is tightly regulated by the overall structure of PLC-γ1.
The Src homology region is bracketed by a split pleckstrin homology (sPH) domain. Pleckstrin homology (PH) domains mediate protein-protein interactions as well as protein-lipid interactions and are characterized primarily by their fold structure rather than similarities in their amino acid sequences. The typical core PH domain fold consists of two β-sheets, composed of three and four β-strands, respectively, plus a C-terminal α-helix (41). While the N-terminal portion of the sPH domain contains a binding site for PI(4,5)P2 (3), it is not known whether a conformational rearrangement of PLC-γ to form a contiguous PH domain (34, 40) contributes to its activation.
In this study, we investigated the mechanism of PLC-γ1 autoregulation by its SH2C domain. For the first time, we provide in vivo evidence that intramolecular interactions involving both the PLC-γ1 SH2C domain and the amino-terminal portion of the split PH domain participate in maintaining PLC-γ1 in an inactive configuration in resting cells. Our data also indicate that in addition to its function in mediating membrane recruitment and phosphorylation of PLC-γ1, the SH2N domain is required to release PLC-γ1 from its autoinhibitory control mechanisms.
MATERIALS AND METHODS
Cell lines, antibodies, and GST fusion proteins.
The PLC-γ-negative chicken B-cell line P10-14, a derivative of DT-40 (35), was maintained as described in reference 6. The anti-influenza hemagglutinin (HA) antibody 12CA5 was a gift from Alan Weissman (National Cancer Institute, Bethesda, MD), and the high-affinity anti-HA antibody 3F10 was obtained from Roche Diagnostics (Indianapolis, IN). Goat anti-chicken immunoglobulin M (IgM) was obtained from Bethyl Laboratories (Montgomery, TX), rabbit anti-goat IgG and rabbit anti-mouse IgG were obtained from Cappel (MP Biomedicals, Irvine, CA), and sheep anti-mouse IgG-horseradish peroxidase (HRP) and donkey anti-rabbit IgG-HRP were obtained from Amersham BioSciences (GE Healthcare, Piscataway, NJ). Anti-PLC-γ1 mixed monoclonal antibody was purchased from UBI (Millipore, Bedford, MA). Anti-PLC-γ1 pY783 was purchased from Cell Signaling (Beverly, MA), and rabbit anti-PLC-γ1 pY775 was produced as described previously (30). Anti-GST was prepared in house by immunization of rabbits with recombinant GST. GST fusion proteins were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Plasmids.
The NFAT (nuclear factor of activated T cells) luciferase reporter plasmid was obtained from Gerald Crabtree (Stanford University, Stanford, CA). HA-tagged bovine PLC-γ1 in the expression vector pCI-neo (Promega, Madison, WI), the mutations of its SH2 domains and specific tyrosine motifs, and the addition of a palmitoylation signal sequence have previously been described (30, 33, 38). For the creation of PHΔ PLC-γ1, nucleic acids, including those encoding most of the amino-terminal portion of the split PH domain (amino acids 464 to 516), were excised from PLC-γ1-HA with BsaBI and BglII. The portion of the X catalytic domain removed by this procedure and the BglII site were reconstituted by ligation with a linker. Point mutations in the amino-terminal split pleckstrin homology (sPHN) domain were created using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). SH2 domain mutations and Y775F and Y783F substitutions were subcloned from pCI-neo PLC-γ1-HA constructs into pCI-neo PLC-γ1-HA displaying mutations in sPHN or PHΔ.
Transient transfection and establishment of stable transfectants.
P10-14 cells were grown to log phase, and 107 cells were transfected with the indicated plasmid DNA as previously described (33). For transient transfections, cells were electroporated and incubated overnight in complete medium before use in the indicated assay. For preparation of stable cell lines, P10-14 cells were transfected as described above with 20 μg of the indicated PLC-γ1 plasmid DNA along with 2 μg of the pBABE puromycin vector (22). Cells were subsequently selected with puromycin (Sigma, St. Louis, MO). Clones were chosen based on similarities in their levels of PLC-γ1-HA expression.
Peptides.
Two biotinylated peptides encompassing the reported PLC-γ1 binding site on human LAT (amino acids 129 to 140; one tyrosine phosphorylated [pY132 LAT] and one nonphosphorylated [Y132 LAT]) (42) were synthesized using a C6 biotin linker (CBER/FDA Core Facility, Bethesda, MD).
Peptide binding ELISA.
Lysates from P10-14 cells, either stably or transiently transfected with various PLC-γ1-HA constructs, were preevaluated for protein expression by enzyme-linked immunosorbent assay (ELISA). PLC-γ1-HA was captured on 3F10-biotin-coated streptavidin strip wells (Pierce, Rockford, IL) and quantified using anti-PLC-γ1, anti-mouse IgG-HRP, and TMB (Pierce). A single batch of lysate from a wild-type PLC-γ1 stable transfectant was employed as a reference standard. Based on these results, lysates were diluted with untransfected P10-14 lysates to equivalent concentrations of PLC-γ1-HA. For the peptide binding ELISA, biotinylated LAT peptide was bound to streptavidin strip wells. The lysates from transfectants, adjusted to contain equivalent amounts of PLC-γ1-HA, were incubated on peptide-coated wells. Bound PLC-γ1-HA was detected with HRP-conjugated anti-HA and TMB. For detection of GST-SH2 domain binding to LAT peptides, rabbit anti-GST, donkey anti-rabbit HRP, and TMB were utilized.
Precipitation and Western blotting.
P10-14 transfectants were stimulated with 10 μg of anti-chicken IgM for 1 min at 37°C and immediately lysed with buffer containing protease and phosphatase inhibitors. Lysis buffer contained either 1% Triton X-100 or, for raft-targeted PLC-γ1 constructs, 60 mM octyl-β-d-glucopyranoside (Sigma) (33, 38). Immunoprecipitations and Western blotting were carried out as described in reference 33.
Reporter gene assays.
P10-14 cells were transiently transfected with 5 μg of pCI-neo or the indicated PLC-γ1-HA construct and 5 μg of an NFAT luciferase reporter construct. After 16 h, cells were distributed into duplicate wells of a 24-well plate containing medium alone, prebound anti-goat IgG plus goat anti-chicken IgM, or 50 ng/ml phorbol myristate acetate plus 5 μM ionomycin to obtain maximum activity. After 6 h, cells were disrupted in lysis buffer (Promega) and assayed using luciferin (Promega).
Calcium mobilization.
P10-14 cells stably expressing the indicated PLC-γ1 constructs were resuspended in Hanks balanced salt solution supplemented with 1% fetal bovine serum and 10 mM HEPES. Cells were loaded with Indo-1/AM (Molecular Probes) for 30 min at 30°C. Calcium measurements were performed on a BD Biosciences LSR flow cytometer equipped with a helium-cadmium laser (325 UV). Cells were tested for loading using ionomycin treatment. Data were analyzed using FlowJo software (Tree Star).
RESULTS
Raft-targeted PLC-γ1 with a nonfunctional SH2C domain is constitutively active.
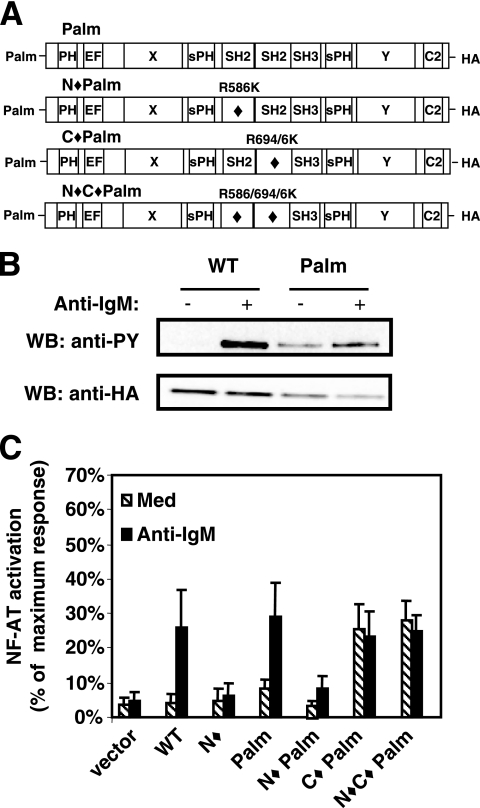
To investigate whether PLC-γ1 is negatively regulated in vivo by its SH2C domain (see Fig. 1 for PLC-γ1 structure), we employed a previously described dually acylated PLC-γ1 construct (Palm PLC-γ1) that is constitutively targeted to the plasma membrane lipid rafts (38). Either or both SH2 domains in Palm PLC-γ1 were mutated by substituting lysine for arginine (Arg) in the conserved tyrosine binding site (Fig. 2A). This represented Arg586 in the SH2N domain and Arg694 in the SH2C domain (6, 33). The SH2C domain contains a second arginine (Arg696), unique to the SH2C domain, that has previously been shown to have strong binding affinity for phosphorylated tyrosine 1021 in the PDGF receptor (24). Therefore, this arginine was also replaced with lysine to ensure complete disruption of the SH2C domain phosphotyrosine binding site (Fig. 2A) (6, 32). Constructs carrying these mutations were tested for their ability to induce transcription from a PLC-γ/calcium-dependent NFAT luciferase reporter construct. Palm PLC-γ1 expressed in the PLC-γ-deficient chicken B-cell line, P10-14, was constitutively tyrosine phosphorylated (Fig. 2B) but mediated a minimal level of NFAT transcriptional activity in resting cells (Fig. 2C). Stimulation of Palm PLC-γ1 transfectants with anti-IgM increased NFAT activity to a level similar to that observed when P10-14 cells were reconstituted with wild-type (WT) PLC-γ1. Therefore, as previously observed in Jurkat T cells (38), Palm PLC-γ1 was constitutively phosphorylated in P10-14 B cells but required receptor-mediated signals for activation.
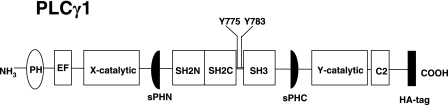
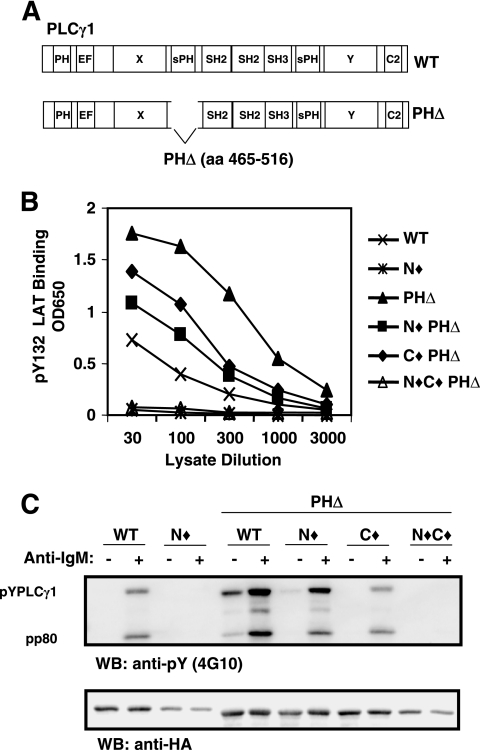
FIG. 1.
Schematic representation of PLC-γ1 structure. EF, EF hand domain.
FIG. 2.
Raft-targeted PLC-γ1 is constitutively active when the SH2C domain is mutated. (A) Representation of raft-targeted PLC-γ1 constructs bearing an N-terminal palmitoylation signal sequence (Palm PLC-γ1). Mutated domains are indicated by a diamond (⧫). The amino acid substitution(s) used to inactivate each SH2 domain is shown for each construct. (B) P10-14 cells transiently transfected with WT or Palm PLC-γ1 were treated with medium or anti-IgM. Lysates were immunoprecipitated with anti-HA antibody, Western blotted for pan-tyrosine phosphorylation with the 4G10 antibody, and reprobed with anti-HA antibody. PY, phosphotyrosine. (C) P10-14 cells were transiently cotransfected with the indicated PLC-γ1 construct and an NFAT luciferase reporter and stimulated with medium or anti-IgM as indicated. The measured luciferase activity was normalized to the activity obtained for treatment with phorbol myristate acetate plus ionomycin. The data presented are the means and standard errors of the means for three separate experiments, with each condition assayed in duplicate. WB, Western blot; Med, medium.
PLC-γ1 depends on both its SH2N domain and its SH2C domain for antigen receptor-induced NFAT activation (see Fig. 2C and 5C) (6, 33). When raft targeted, however, PLC-γ1 constructs with an inactive SH2C domain exhibited significant constitutive NFAT activity in either the presence (C⧫ Palm) or absence (N⧫C⧫ Palm) of a functional SH2N domain (Fig. 2C). B-cell receptor (BCR) stimulation did not further increase NFAT activity in cells expressing either of these Palm SH2C domain mutants. These data are consistent with our previous results demonstrating constitutive in vitro activity of SH2C domain-mutated PLC-γ1 and indicate that constitutive activation of the SH2C domain mutant can be detected in vivo if the requirement for membrane translocation is bypassed.
FIG. 5.
Amino acids Y509 and F510 in the sPHN domain participate in regulating SH2C domain availability. (A) Schematic diagram of PLC-γ1 displaying an expanded sPHN domain. Specifically noted are two amino acid residues, Y509 and F510 in the second β-sheet, that were mutated to alanine. Stars mark potential PI(4,5)P2 binding sites (18). (B) Serially diluted lysates from P10-14 cells transiently transfected with PLC-γ1 constructs bearing the indicated mutations in the sPHN domain were tested for binding to pY132 LAT by ELISA as described for Fig. 3B. (C) P10-14 cells were transiently transfected with the indicated PLC-γ1 construct and an NFAT luciferase reporter construct and then assayed for their response to treatment with anti-IgM as described for Fig. 2C. OD650, optical density at 650 nm; Med, medium.
Interestingly, P10-14 cells that stably expressed Palm PLC-γ1 with only the SH2N domain mutated (N⧫ Palm) did not exhibit either constitutive or BCR-induced NFAT activation (Fig. 2C). While membrane translocation is normally mediated by SH2N domain interaction with LAT (in T cells) and BLNK (in B cells) (6, 33), this function of the SH2N domain is not required in Palm PLC-γ1 because of its constitutive membrane raft localization (38). Since the activation of Palm PLC-γ1 still requires a functional SH2N domain when the SH2C domain is intact, these results suggest that engagement of the SH2N domain with its target phosphoprotein also promotes a structural change that is required to overcome SH2C domain-mediated autoinhibition.
The structure of PLC-γ1 regulates the ability of the SH2C domain to interact with phosphoproteins.
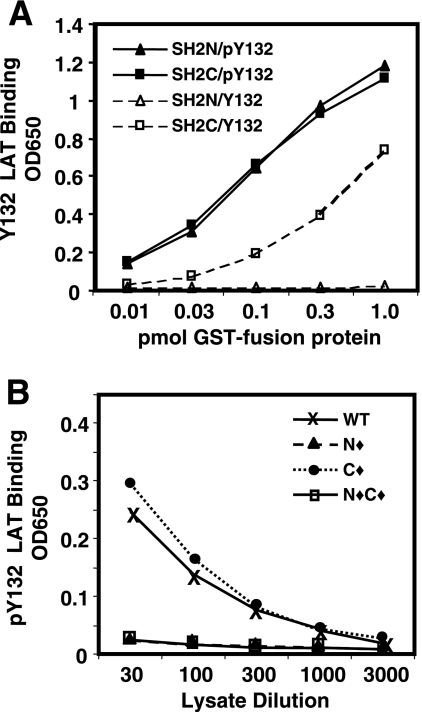
To test the hypothesis that accessibility of the SH2C domain is blocked by physical constraints within PLC-γ1, we utilized information gained from previous studies that showed that as single-domain fusion proteins, GST-SH2N as well as GST-SH2C fusion proteins pulled down LAT from anti-T-cell receptor (TCR)-activated Jurkat cell lysates (33). An ELISA was developed that detects binding to a 15-amino-acid phosphopeptide derived from the reported PLC-γ1 binding site on human LAT (pY132 LAT) (42). Both GST-SH2N and GST-SH2C fusion proteins tested in the ELISA bound pY132 LAT with nearly identical dose-dependent binding abilities (Fig. 3A), indicating that they were equally able to interact directly with the same phosphotyrosine binding site on LAT. Unexpectedly, GST-SH2C but not GST-SH2N also demonstrated significant binding to a nonphosphorylated Y132 LAT peptide (Fig. 3A). This binding is eliminated after mutation of the SH2C binding site (data not shown) but suggests that the SH2C fusion protein has promiscuous binding activity in vitro.
FIG. 3.
The structure of PLC-γ1 regulates the ability of the SH2C domain to interact with proteins. (A) Serially diluted GST-SH2N and GST-SH2C fusion proteins were placed in wells of a streptavidin plate that had been coated with either biotinylated nonphosphorylated Y132 LAT (Y132) or phosphorylated pY132 LAT (pY132) peptide as indicated. Binding was detected with anti-GST rabbit serum and HRP-conjugated second antibody. The data are representative of three separate experiments with samples tested in duplicate. (B) Serially diluted lysates from P10-14 cells stably transfected with the indicated PLC-γ1 constructs were assayed for their ability to bind to the pY132 LAT peptide as described for panel A, except that binding was detected with an anti-HA antibody. The data are presented as the average optical densities at 650 nm (OD650) for two replicate wells in a single experiment and are representative of at least three separate experiments.
WT PLC-γ1 in lysates from stable P10-14 transfectants also demonstrated dose-dependent binding to pY132 LAT (Fig. 3B). The binding of WT PLC-γ1 was mediated solely by its SH2N domain since no binding was observed with either SH2N domain-mutated PLC-γ1 (N⧫) or PLC-γ1 in which both SH2 domains were mutated (N⧫C⧫). Furthermore, SH2C domain-mutated PLC-γ1 (C⧫) bound as well as or better than WT PLC-γ1. This pattern of LAT binding was consistent with previous in vivo studies that showed phospho-LAT coprecipitation with PLC-γ1 only when the PLC-γ1 SH2N domain was intact (33) and demonstrates that the pY132 ELISA closely mimics the characteristics of in vivo binding of PLC-γ1 to phospho-LAT. The ability of the SH2C domain to interact with pY132 LAT when tested as an isolated GST fusion protein, but not when part of intact PLC-γ1, suggests that the SH2C domain is structurally restricted within the molecule.
The amino-terminal split pleckstrin homology domain regulates the binding activity of the Src homology domain region of PLC-γ1.
An examination of PLC-γ1 for regions that might regulate SH2C domain binding activity led us to focus on the sPH domain. Similar to the catalytic elements X and Y, the sPH domain is spatially divided by the three Src homology domains and likely assembles into a single functional domain which could affect the activity of the intervening Src homology region (12, 34, 40). We engineered a series of PLC-γ1 constructs in which most of the sPHN domain was deleted (PHΔ PLC-γ1) (Fig. 4A). Deletion of the sPHN domain alone (PHΔ) substantially increased the in vitro ability of PLC-γ1 to bind 132pY LAT compared to WT PLC-γ1, suggesting that the sPHN domain may regulate the accessibility of the Src homology region (Fig. 4B). Mutation of both SH2 domains in combination with the sPHN deletion (N⧫C⧫ PHΔ) abrogated 132pY LAT binding altogether, indicating that the interaction was still dependent on SH2 domain function. PHΔ PLC-γ1 with either the SH2N or the SH2C domain mutated individually (N⧫ PHΔ or C⧫ PHΔ, respectively) showed binding to pY132 LAT that was intermediate between that observed for WT PLC-γ1 and that for PHΔ PLC-γ1. Therefore, in the absence of the amino-terminal sPH domain, PLC-γ1 can bind to pY132 LAT via either SH2 domain while wild-type PLC-γ1 can interact with the LAT phosphopeptide only via the SH2N domain. These data suggest that the sPH domain blocks the SH2C domain from interaction with some target proteins and also limits the extent of SH2N domain function.
FIG. 4.
The amino-terminal split pleckstrin homology domain regulates the binding activity of the Src homology domain region of PLC-γ1. (A) Schematic diagram depicting the deletion of the amino-terminal split pleckstrin homology domain in PLC-γ1. (B) Lysates from P10-14 cells stably expressing PLC-γ1 PHΔ in combination with mutations of SH2N and/or SH2C were tested by ELISA for binding to pY132 LAT as described for Fig. 3B. (C) P10-14 cells stably expressing the indicated PLC-γ1 constructs were stimulated with medium or anti-IgM, lysed, and treated as described for Fig. 2B. EF, EF hand domain; aa, amino acids; OD650, optical density at 650 nm; WB, Western blot.
Further evidence that the sPHN domain regulates SH2C domain function was obtained by assessing BCR-induced PHΔ PLC-γ1 tyrosine phosphorylation and association with pp80 (BLNK) (6) in P10-14 transfectants. PHΔ PLC-γ1 demonstrated increased constitutive and BCR-stimulated pp80 association and tyrosine phosphorylation compared to WT PLC-γ1, in agreement with its enhanced binding to 132pY LAT (Fig. 4C). Normally, the association of PLC-γ1 with pp80 and receptor-induced phosphorylation of PLC-γ1 are significantly reduced when the SH2N domain is mutated (N⧫), indicating that the SH2C domain does not normally provide this function (Fig. 4C) (6). Upon deletion of the sPHN domain, however, SH2N-mutated PLC-γ1 (N⧫ PHΔ) was able to interact with pp80 and was significantly tyrosine phosphorylated when stimulated via the BCR, suggesting that when not blocked by the sPHN domain, the SH2C domain is available for binding phosphoproteins that enable PLC-γ1 tyrosine phosphorylation. C⧫ PHΔ PLC-γ1 exhibited a level of pp80 association and tyrosine phosphorylation that was similar to that exhibited by WT PLC-γ1, providing a further substantiation of a model in which the SH2N domain functions as the primary phosphoprotein binding region in PLC-γ1. Mutation of both SH2 domains in PHΔ PLC-γ1 (N⧫C⧫ PHΔ) abrogated the coprecipitation of pp80 and tyrosine phosphorylation of PLC-γ1. Together, these results suggest that in resting cells, the sPHN domain participates in hindering access to the Src homology-containing region, thereby limiting constitutive PLC-γ1 tyrosine phosphorylation. Once activation has been initiated via SH2N domain-mediated events, the SH2C domain plays a considerable role in augmenting PLC-γ1 phosphorylation.
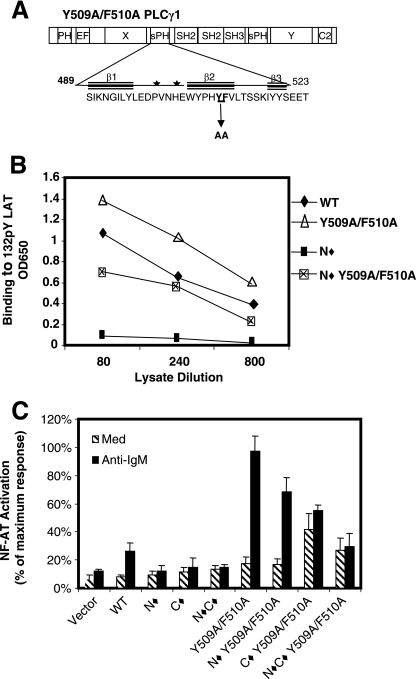
Tyrosine 509 and phenylalanine 510 in the sPHN domain regulate SH2C domain activity.
PHΔ PLC-γ1 constructs are not functional because the deleted sPHN domain contains the binding site for the substrate PI(4,5)P2 (3, 18). In an effort to maintain PI(4,5)P2 binding while disrupting sPHN-mediated regulation of the SH2C domain, a series of site-specific PLC-γ1 mutations were made and screened in the pY132 LAT ELISA for SH2N domain-independent binding activity. In the original series of mutants, each tyrosine residue of the sPHN domain was mutated to phenylalanine (F) alone or in combination as follows: Y495F, Y506F/Y509F and Y518F/Y519F. The in vitro pY132 LAT binding activity of these mutants was comparable to that of WT PLC-γ1, and none of these PLC-γ1 mutants showed SH2N-independent binding to pY132 LAT (data not shown). Because phenylalanine is structurally similar to tyrosine, the tyrosine-to-phenylalanine mutants may not have altered sPHN domain interactions sufficiently. Therefore, a second series of mutants, based on those of Chang et al. (3), were constructed in which various amino acids, including some of the tyrosine residues, were replaced with alanine. None of the sPHN mutations of PLC-γ1 prevented interaction with pY132 LAT, and while in some experiments the mutants demonstrated greater binding to pY132 LAT than WT PLC-γ1, this was not a consistent finding (data not shown). Significant SH2N domain-independent binding to pY132 LAT was observed, however, in an N⧫Y509A/F510A PLC-γ1 construct (Fig. 5B), suggesting that residues Y509 and F510 of the sPHN domain are required for regulation of SH2C domain availability.
The Y509A/F510A mutation does not affect PI(4,5)P2 binding by the sPHN domain (3, 18), and we found that PLC-γ1 bearing the Y509A and F510A dual substitution supported BCR-induced NFAT transcription, and in fact, this activity was substantially increased compared to that mediated by WT PLC-γ1 (Fig. 5C). Importantly, N⧫Y509A/F510A also reconstituted receptor-induced NFAT activation in P10-14 cells that, while less than that of Y509A/F510A, was greater than that of WT PLC-γ1. None of the other PLC-γ1 constructs bearing sPHN mutations in combination with a nonfunctional SH2N domain demonstrated receptor-induced NFAT activation (data not shown). Y509A/F510A constructs that had the SH2C domain mutated (C⧫ Y509A/F510A) demonstrated significant levels of constitutive NFAT activation that were not substantially increased upon BCR stimulation, supporting a role for the SH2C domain in the regulation of basal PLC-γ1 activity (Fig. 5C). When both SH2 domains together with Y509A/F510A were mutated (N⧫C⧫Y509A/F510A), there was some increased constitutive NFAT activity compared to that observed with WT PLC-γ1, but N⧫C⧫Y509A/F510A did not respond to stimulation via the BCR, suggesting that PLC-γ1-mediated, BCR-induced NFAT activation is dependent on SH2 domain function.
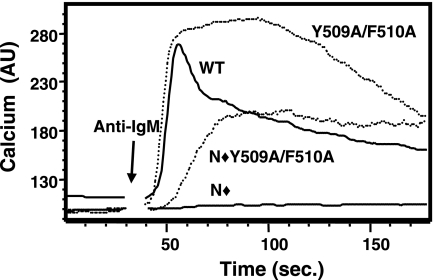
Mutation of Y509/F510 alters the kinetics of cellular calcium signaling.
NFAT gene transcription is driven by a sustained increase in intracellular calcium (27). The hydrolysis of PI(4,5)P2 by PLC-γ1 initiates this response via IP3-mediated release of stored calcium from the endoplasmic reticulum into the cytoplasm, but the mechanisms for maintaining increased calcium levels in the cell are less well understood. We compared the BCR-stimulated calcium responses in P10-14 cells that stably expressed the Y509A/F510A mutants to those in cells expressing WT PLC-γ1. The addition of anti-IgM to WT PLC-γ1-reconstituted cells stimulated a rapid rise in intracellular calcium, followed by a typical sustained but lower calcium level (Fig. 6). The Y509A/F510A mutant followed the same initial rise in intracellular calcium levels, but calcium was maintained at this level, or a slightly higher level, for a prolonged period. As expected, mutation of the SH2N domain in WT PLC-γ1 abrogated all responses to anti-IgM stimulation. In contrast, Y509A/F510A PLC-γ1 transfectants demonstrated an SH2N-independent calcium response to anti-IgM stimulation. The calcium flux mediated by N⧫Y509A/F510A PLC-γ1, while delayed compared to that mediated by WT PLC-γ1, reached a level similar to that of the WT-sustained response. The normal kinetics of the anti-IgM-stimulated calcium response of PLC-γ1 is therefore significantly altered when Y509 and F510 are mutated to alanine, but the signal is nevertheless sufficient to drive normal levels of NFAT transcriptional activity.
FIG. 6.
Mutation of Y509 and F510 alters the BCR-induced calcium response. P10-14 cells stably expressing the indicated PLC-γ1 constructs were loaded with Indo-1/AM and stimulated with anti-IgM. The ratio of fluorescence emission at 405 nm to that at 495 nm is plotted over a 3-minute time period and is shown as arbitrary calcium units (AU).
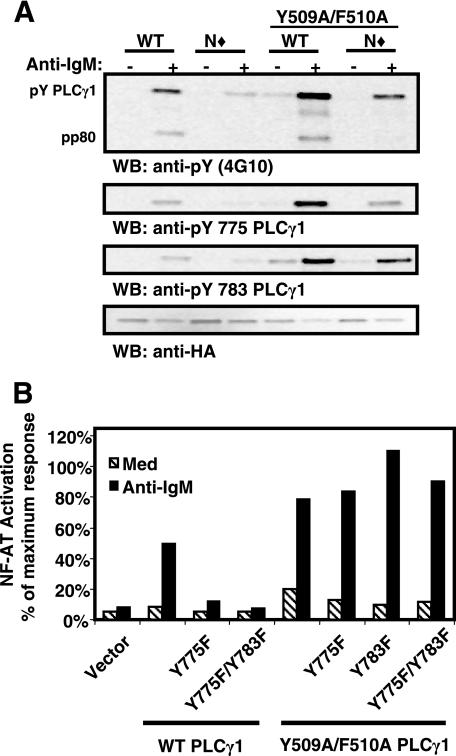
The requirement for PLC-γ1 tyrosine phosphorylation is circumvented by mutation of Y509 and F510.
To gain further insight into PLC-γ1 regulation by its sPHN domain, we assessed the role of tyrosine phosphorylation in various PLC-γ1 constructs. Western blot analysis of Y509A/F510A PLC-γ1 revealed that anti-IgM treatment stimulated an increase in Y509A/F510A PLC-γ1 tyrosine phosphorylation that was greater than that for WT PLC-γ1 on both Y775 and Y783 (Fig. 7A). N⧫Y509A/F510A PLC-γ1 also demonstrated BCR-induced tyrosine phosphorylation levels similar to those observed for WT PLC-γ1 (Fig. 7A). Mutation of Y775 and Y783 to phenylalanine either individually or together in Y509A/F510A PLC-γ1, however, did not prevent these constructs from mediating BCR-induced NFAT activity in P10-14 B cells (Fig. 7B). Therefore, mutation of Y509 and F510 obviates the need for both SH2N domain function and tyrosine phosphorylation in PLC-γ1 activation.
FIG. 7.
Mutation of Y509 and F510 alleviates the requirement for Y775 and Y783 phosphorylation in PLC-γ1 activation. (A) Stable P10-14 transfectants were treated with medium or anti-IgM, lysed, and immunoprecipitated with anti-HA antibody. Samples were divided for resolution on individual sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and immunoblotted with antiphosphotyrosine (4G10), anti-pY775 PLC-γ1, anti-pY783 PLC-γ1, and anti-HA antibodies. (B) P10-14 cells were transiently transfected with the indicated PLC-γ1 constructs plus an NFAT luciferase reporter construct and then assayed for their response to treatment with medium or anti-IgM antibody as described for Fig. 2C. The data are representative of at least three independent experiments. WB, Western blot; Med, medium.
DISCUSSION
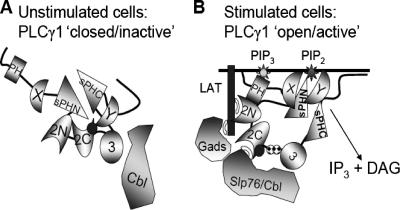
PLC-γ1 activation is necessary for many of the biological responses of normal and transformed cells, including proliferation, differentiation, motility, and cytokine production, and its activity is therefore tightly regulated. Previous studies have focused largely on its control via cellular localization or interactions with other molecules. Here, however, we present novel in vivo data that demonstrate that the basal activity of PLC-γ1 is regulated by complex intramolecular interactions (Fig. 8). These results indicate that the basal activity of PLC-γ1 is repressed by its SH2C domain and that the sPHN domain regulates SH2C domain availability. In addition, while the SH2N domain has long been appreciated for its requirement in mediating PLC-γ1 membrane translocation and receptor-induced phosphorylation, the data presented here indicate that ligand binding by the SH2N domain may participate in the “release” of PLC-γ1 from SH2C domain inhibition through a mechanism that is independent of tyrosine phosphorylation.
FIG. 8.
Intramolecular interactions regulate PLC-γ1 catalytic activity in unstimulated lymphocytes. (A) In unstimulated cells, PLC-γ1 is cytoplasmic. The carboxy-terminal SH2 (2C) domain and the sPHN domain cooperate to maintain PLC-γ1 in an inactive configuration. Intramolecular interactions between the SH2C domain and PLC-γ1 may position the PCI sequence (•) in proximity to the catalytic domain(s), inhibiting function. SH2C intramolecular binding also limits the ability of the SH2C domain to interact with phosphorylated target proteins. (B) Upon antigen receptor engagement, the amino-terminal SH2 (2N) domain interacts with its target protein (phospho-LAT in T cells or phospho-BLNK in B cells). This interaction is required to induce the release of the SH2C domain from its intramolecular interaction and free the catalytic domain(s) from inhibition by the PCI sequence. The SH2C domain is now able to interact with target proteins such as Slp76 and c-Cbl, and PLC-γ1 is phosphorylated on Y775 and Y783 ( ). Once released from intramolecular inhibition, the catalytic domains act in concert to hydrolyze PI(4,5)P2 (PIP2).
). Once released from intramolecular inhibition, the catalytic domains act in concert to hydrolyze PI(4,5)P2 (PIP2).
The SH2 domains of PLC-γ1 share 35% homology and prefer to bind a target phosphotyrosine that is followed by a hydrophobic amino acid at both the +1 and the +3 positions (32). While the PLC-γ1 binding site on LAT (YLVV) fits this description and the relative abilities of GST fusion proteins of the SH2N and SH2C domains to bind to pY132 LAT appear identical, differences in the fine specificities of binding of the two domains have been reported (32). For example, GST-SH2C, but not GST-SH2N, has previously been shown to bind a myriad of phosphoproteins, and as shown in this study, the SH2C domain interacts with nonphosphorylated Y132 LAT (Fig. 2A) (6, 33). The promiscuity of the isolated SH2C domain may be attributed to the unusual nature of its tyrosine binding pocket. Unlike the SH2N domain, the SH2C domain has three arginines in addition to the critical β5 arginine that may contribute to phosphotyrosine binding. There is also a long groove in which amino acids in the +4, +5, and +6 positions, relative to the phosphotyrosine, may bind (20, 24). These features may allow for a greater degree of variability in the binding capabilities of the SH2C domain than in those of the SH2N domain. Indeed, in order to ensure that we disabled the phosphotyrosine binding capacity of the SH2C domain, we used an SH2C mutant construct that contained a mutation in the βB5-arginine and the βB7-arginine that has been shown to have a strong association with a phosphotyrosine residue in the PDGF receptor (24). Our data indicate that the promiscuous binding potential is tightly regulated by the structure of PLC-γ1 and that alterations in the structure of PLC-γ1 that disrupt these normal control mechanisms can result in unregulated PLC-γ1 activity.
The Src homology region of PLC-γ1 has been thought to provide an autoinhibitory function since, when combined, the isolated catalytic domains have increased activity compared to the holoenzyme (12, 17). The SH2C domain appears to participate in blocking PLC-γ1 activity since PLC-γ1 with a mutated SH2C domain was constitutively active when tested in an in vitro miscellar PI(4,5)P2 hydrolysis assay (6). In vivo, however, this mutant was unable to reconstitute BCR-stimulated PI(4,5)P2 hydrolysis. The discrepancy between the activity of the SH2C domain in vitro and its in vivo activity is due to the requirement for SH2C domain function in antigen receptor-induced activation of PLC-γ1 (6, 14). The SH2C domain was recently shown to be necessary for stable membrane recruitment of PLC-γ1 after TCR stimulation (1), and it likely plays a similar role in B-cell activation. Braiman et al. (1) also reported that in addition to the SH2N domain, the SH2C domain was required for phosphorylation of Y775 and Y783 after TCR stimulation, a finding we have also observed in P10-14 chicken B cells (data not shown). However, because Palm PLC-γ1, which is constitutively raft localized and tyrosine phosphorylated, does not mediate significant NFAT activation in unstimulated cells, it appears that membrane recruitment and tyrosine phosphorylation alone are not sufficient for PLC-γ1 activation. Palm PLC-γ1 with a nonfunctional SH2C domain, however, does mediate significant NFAT activation in the absence of BCR stimulation. This suggests that while SH2C domain function is no longer necessary for PLC-γ1 activity when the enzyme is raft targeted, SH2C domain inhibition must still be overcome for full PLC-γ1 activation. Similarly, when sPHN domain inhibition was disrupted via mutation of Y509 and F510, mutation of the SH2C domain resulted in constitutive PLC-γ1 activity. These data indicate that the basal activity of PLC-γ1 is regulated by the SH2C domain and that receptor-induced activation of PLC-γ1 not only involves membrane translocation and tyrosine phosphorylation of PLC-γ1 but also requires that SH2C domain inhibition be overcome.
Distinct areas within the SH2C domain appear to have a role in PLC-γ1 regulation. An octomer sequence (PCI) located at the C-terminal end of the SH2C domain was previously found to inhibit the ability of four different phospholipase C isoforms to hydrolyze PI(4,5)P2 (11). Here, we demonstrate that disabling SH2C domain function by mutating two arginines (694 and 696) results in the activation of PLC-γ1 when it is targeted to the lipid rafts or released from sPHN domain constraints. We propose that the SH2C domain mediates an intramolecular interaction via its tyrosine binding site that either directly blocks the catalytic domain(s) or optimally positions the PCI sequence for this purpose.
A functional SH2N domain was necessary for the activation of raft-targeted Palm PLC-γ1 in which the SH2C domain was intact. The role of the SH2N domain in “releasing” PLC-γ1 from SH2C domain inhibition was not due to its ability to mediate phosphorylation since Palm PLC-γ1 is constitutively phosphorylated but not constitutively active. These data suggest that an interaction of the SH2N domain with its target phosphoprotein, in addition to mediating PLC-γ1 translocation and phosphorylation, forces a structural change that releases PLC-γ1 from SH2C domain inhibition and promotes full PLC-γ1 activation. This hypothesis is consistent with data from Koblan et al. (19) that showed an increase in the in vitro catalytic activity of PLC-γ1 upon the addition of phosphotyrosine-containing peptides corresponding to the reported PLC-γ1 binding sites on either the erythrocyte growth factor receptor or the fibroblast growth factor receptor. Furthermore, binding of the erythrocyte growth factor receptor phosphopeptide to PLC-γ1 was shown to alter the conformation of PLC-γ1 (9). Since both the SH2N and SH2C domains are required for Y775 and Y783 phosphorylation (Fig. 7A and data not shown) (1), we speculate that not only does SH2N domain occupancy release PLC-γ1 from SH2C domain-mediated inhibition, but it also promotes SH2C domain availability for interaction with target proteins that promote Y775 and Y783 phosphorylation and stable association with the membrane.
Results from experiments with PLC-γ1 in which the amino-terminal portion of the sPH domain was deleted or in which amino acids in the sPHN domain were mutated indicate that the PLC-γ1 sPHN domain regulates the ability of the SH2C domain to interact with phosphoproteins. The pY132 LAT ELISA data show that the isolated SH2C domain can interact with nonphosphorylated peptides and that this might involve hydrophobic interactions between sPHN aromatic amino acids and key amino acids in the SH2C tyrosine binding site (24). Alternatively, recent studies indicate that the sPH domains of PLC-γ1 fold into a canonical PH domain with high thermostability in the presence or absence of the Src homology domain region (34, 40). The assembly of a complete PH domain may place the SH2C domain into position for intramolecular binding and impose structural constraints on the Src homology region that regulate its accessibility. It is also interesting to speculate that the activation of PLC-γ1 modulates the association of the sPHN and C-terminal split pleckstrin homology (sPHC) domains to allow them to participate in intermolecular interactions. For instance, Y509 and F510 have been shown to be required for an interaction between PLC-γ1 and EF1-α, and this interaction appears to promote PLC-γ1 activation (3). Furthermore, the PLC-γ1 sPHC domain has been suggested to interact with an sPHN-like domain in the TRPC3 calcium channel to form a full PH domain capable of interacting with phosphoinositides (37). While our data strongly indicate that the sPHN and SH2C domains cooperate in their abilities to maintain PLC-γ1 in an inactive conformation, the precise mechanism responsible for this inhibition is still under investigation.
Delineation of the mechanism of PLC-γ1 activation is vital to understanding cellular responses. PLC-γ1 is a key regulator of signaling in many types of cells, and its activity is tightly controlled in order to maintain normal cell function. Unregulated PLC-γ1 activation has been suggested to be involved in the development of numerous cancers (21, 31, 39). Our results provide insight into the control of this complex molecule. The inhibitory mechanisms identified in this study are internal to the PLC-γ1 molecule itself. Therefore, in combination with various intermolecular interactions, they apply to the regulation of PLC-γ1 activity in multiple cell and receptor types and influence the signal transduction pathways that regulate biological responses.
Acknowledgments
We thank Wendy Weinberg, Ronald Wange, and Gibbes Johnson for critical review of the manuscript.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Braiman, A., M. Barda-Saad, C. L. Sommers, and L. E. Samelson. 2006. Recruitment and activation of PLCgamma1 in T cells: a new insight into old domains. EMBO J. 25:774-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter, G., and Q. Ji. 1999. Phospholipase C-gamma as a signal-transducing element. Exp. Cell Res. 253:15-24. [DOI] [PubMed] [Google Scholar]
- 3.Chang, J. S., H. Seok, T. K. Kwon, D. S. Min, B. H. Ahn, Y. H. Lee, J. W. Suh, J. W. Kim, S. Iwashita, A. Omori, S. Ichinose, O. Numata, J. K. Seo, Y. S. Oh, and P. G. Suh. 2002. Interaction of elongation factor-1alpha and pleckstrin homology domain of phospholipase C-gamma 1 with activating its activity. J. Biol. Chem. 277:19697-19702. [DOI] [PubMed] [Google Scholar]
- 4.Choi, J. H., W. P. Hong, S. Yun, H. S. Kim, J. R. Lee, J. B. Park, Y. S. Bae, S. H. Ryu, and P. G. Suh. 2005. Grb2 negatively regulates epidermal growth factor-induced phospholipase C-gamma1 activity through the direct interaction with tyrosine-phosphorylated phospholipase C-gamma1. Cell. Signal. 17:1289-1299. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree, G. R., and N. A. Clipstone. 1994. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu. Rev. Biochem. 63:1045-1083. [DOI] [PubMed] [Google Scholar]
- 6.DeBell, K. E., B. A. Stoica, M.-C. Verí, A. Di Baldassarre, S. Miscia, L. J. Graham, B. L. Rellahan, M. Ishiai, T. Kurosaki, and E. Bonvini. 1999. Functional independence and interdependence of the Src homology domains of phospholipase C-γ1 in B-cell receptor signal transduction. Mol. Cell. Biol. 19:7388-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dienz, O., A. Moller, A. Strecker, N. Stephan, P. H. Krammer, W. Droge, and M. L. Schmitz. 2003. Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa and phospholipase C gamma 1 are required for NF-kappa B activation and lipid raft recruitment of protein kinase C theta induced by T cell costimulation. J. Immunol. 170:365-372. [DOI] [PubMed] [Google Scholar]
- 8.Ebinu, J. O., S. L. Stang, C. Teixeira, D. A. Bottorff, J. Hooton, P. M. Blumberg, M. Barry, R. C. Bleakley, H. L. Ostergaard, and J. C. Stone. 2000. RasGRP links T-cell receptor signaling to Ras. Blood 95:3199-3203. [PubMed] [Google Scholar]
- 9.Gish, G., L. Larose, R. Shen, and T. Pawson. 1995. Biochemical analysis of SH2 domain-mediated protein interactions. Methods Enzymol. 254:503-523. [DOI] [PubMed] [Google Scholar]
- 10.Homma, M. K., M. Yamasaki, S. Ohmi, and Y. Homma. 1997. Inhibition of phosphoinositide hydrolysis and cell growth of Swiss 3T3 cells by myristoylated phospholipase C inhibitor peptides. J. Biochem. (Tokyo) 122:738-742. [DOI] [PubMed] [Google Scholar]
- 11.Homma, Y., and T. Takenawa. 1992. Inhibitory effect of Src homology (SH) 2/SH3 fragments of phospholipase C-gamma on the catalytic activity of phospholipase C isoforms. Identification of a novel phospholipase C inhibitor region. J. Biol. Chem. 267:21844-21849. [PubMed] [Google Scholar]
- 12.Horstman, D. A., K. DeStefano, and G. Carpenter. 1996. Enhanced phospholipase C-gamma1 activity produced by association of independently expressed X and Y domain polypeptides. Proc. Natl. Acad. Sci. USA 93:7518-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, Y., and R. L. Wange. 2004. T cell receptor signaling: beyond complex complexes. J. Biol. Chem. 279:28827-28830. [DOI] [PubMed] [Google Scholar]
- 14.Irvin, B. J., B. L. Williams, A. E. Nilson, H. O. Maynor, and R. T. Abraham. 2000. Pleiotropic contributions of phospholipase C-γ1 (PLC-γ1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-γ1-deficient Jurkat T-cell line. Mol. Cell. Biol. 20:9149-9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji, Q. S., A. Chattopadhyay, M. Vecchi, and G. Carpenter. 1999. Physiological requirement for both SH2 domains for phospholipase C-γ1 function and interaction with platelet-derived growth factor receptors. Mol. Cell. Biol. 19:4961-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji, Q. S., G. E. Winnier, K. D. Niswender, D. Horstman, R. Wisdom, M. A. Magnuson, and G. Carpenter. 1997. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc. Natl. Acad. Sci. USA 94:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamat, A., and G. Carpenter. 1997. Phospholipase C-gamma1: regulation of enzyme function and role in growth factor-dependent signal transduction. Cytokine Growth Factor Rev. 8:109-117. [DOI] [PubMed] [Google Scholar]
- 18.Kim, S. K., S. M. Wee, J. S. Chang, T. K. Kwon, D. S. Min, Y. H. Lee, and P. G. Suh. 2004. Point mutations in the split PLC-gamma1 PH domain modulate phosphoinositide binding. J. Biochem. Mol. Biol. 37:720-725. [DOI] [PubMed] [Google Scholar]
- 19.Koblan, K. S., M. D. Schaber, G. Edwards, J. B. Gibbs, and D. L. Pompliano. 1995. Src-homology 2 (SH2) domain ligation as an allosteric regulator: modulation of phosphoinositide-specific phospholipase C gamma 1 structure and activity. Biochem. J. 305:745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larose, L., G. Gish, and T. Pawson. 1995. Construction of an SH2 domain-binding site with mixed specificity. J. Biol. Chem. 270:3858-3862. [DOI] [PubMed] [Google Scholar]
- 21.Lee, S. J., S. D. Lee, J. G. Park, C. M. Kim, S. H. Ryu, and P. G. Suh. 1995. Overexpression of phospholipase C-gamma 1 in colorectal carcinomas is associated with overexpression of factors that bind its promoter. J. Biol. Chem. 270:16378-16384. [DOI] [PubMed] [Google Scholar]
- 22.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishibe, S., M. I. Wahl, S. G. Rhee, and G. Carpenter. 1989. Tyrosine phosphorylation of phospholipase C-II in vitro by the epidermal growth factor receptor. J. Biol. Chem. 264:10335-10338. [PubMed] [Google Scholar]
- 24.Pascal, S. M., A. U. Singer, G. Gish, T. Yamazaka, S. E. Shoelson, T. Pawson, L. E. Kay, and J. D. Forman-Kay. 1994. Nuclear magnetic resonance structure of an SH2 domain of phospholipase C-gamma1 complexed with a high affinity binding peptide. Cell 77:461-472. [DOI] [PubMed] [Google Scholar]
- 25.Poulin, B., F. Sekiya, and S. G. Rhee. 2000. Differential roles of the Src homology 2 domains of phospholipase C-gamma1 (PLC-gamma1) in platelet-derived growth factor-induced activation of PLC-gamma1 in intact cells. J. Biol. Chem. 275:6411-6416. [DOI] [PubMed] [Google Scholar]
- 26.Poulin, B., F. Sekiya, and S. G. Rhee. 2005. Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-gamma1. Proc. Natl. Acad. Sci. USA 102:4276-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 28.Rellahan, B. L., L. J. Graham, A. Y. Tysgankov, K. E. DeBell, M. C. Veri, C. Noviello, and E. Bonvini. 2003. A dynamic constitutive and inducible binding of c-Cbl by PLCgamma1 SH3 and SH2 domains (negatively) regulates antigen receptor-induced PLCgamma1 activation in lymphocytes. Exp. Cell Res. 289:184-194. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez, R., M. Matsuda, O. Perisic, J. Bravo, A. Paul, N. P. Jones, Y. Light, K. Swann, R. L. Williams, and M. Katan. 2001. Tyrosine residues in phospholipase Cgamma 2 essential for the enzyme function in B-cell signaling. J. Biol. Chem. 276:47982-47992. [DOI] [PubMed] [Google Scholar]
- 30.Serrano, C. J., L. Graham, K. DeBell, R. Rawat, M. C. Veri, E. Bonvini, B. L. Rellahan, and I. G. Reischl. 2005. A new tyrosine phosphorylation site in PLC gamma 1: the role of tyrosine 775 in immune receptor signaling. J. Immunol. 174:6233-6237. [DOI] [PubMed] [Google Scholar]
- 31.Sewell, J. M., J. F. Smyth, and S. P. Langdon. 2005. Role of TGF alpha stimulation of the ERK, PI3 kinase and PLC gamma pathways in ovarian cancer growth and migration. Exp. Cell Res. 304:305-316. [DOI] [PubMed] [Google Scholar]
- 32.Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson, W. G. Haser, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, et al. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72:767-778. [DOI] [PubMed] [Google Scholar]
- 33.Stoica, B., K. E. DeBell, L. Graham, B. L. Rellahan, M. A. Alava, J. Laborda, and E. Bonvini. 1998. The amino-terminal Src homology 2 domain of phospholipase C gamma 1 is essential for TCR-induced tyrosine phosphorylation of phospholipase C gamma 1. J. Immunol. 160:1059-1066. [PubMed] [Google Scholar]
- 34.Sugimoto, K., Y. Mori, K. Makino, K. Ohkubo, and T. Morii. 2003. Functional reassembly of a split PH domain. J. Am. Chem. Soc. 125:5000-5004. [DOI] [PubMed] [Google Scholar]
- 35.Takata, M., Y. Homma, and T. Kurosaki. 1995. Requirement of phospholipase C-gamma 2 activation in surface immunoglobulin M-induced B cell apoptosis. J. Exp. Med. 182:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todderud, G., M. I. Wahl, S. G. Rhee, and G. Carpenter. 1990. Stimulation of phospholipase C-gamma 1 membrane association by epidermal growth factor. Science 249:296-298. [DOI] [PubMed] [Google Scholar]
- 37.van Rossum, D. B., R. L. Patterson, S. Sharma, R. K. Barrow, M. Kornberg, D. L. Gill, and S. H. Snyder. 2005. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature 434:99-104. [DOI] [PubMed] [Google Scholar]
- 38.Verí, M.-C., K. E. DeBell, M.-C. Seminario, A. DiBaldassarre, I. Reischl, R. Rawat, L. Graham, C. Noviello, B. L. Rellahan, S. Miscia, R. L. Wange, and E. Bonvini. 2001. Membrane raft-dependent regulation of phospholipase Cγ-1 activation in T lymphocytes. Mol. Cell. Biol. 21:6939-6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells, A., and J. R. Grandis. 2003. Phospholipase C-gamma1 in tumor progression. Clin. Exp. Metastasis 20:285-290. [DOI] [PubMed] [Google Scholar]
- 40.Wen, W., J. Yan, and M. Zhang. 2006. Structural characterization of the split PH domain in phospholipase C-gamma 1 and its interaction with TRPC3. J. Biol. Chem. 281:12060-12068. [DOI] [PubMed] [Google Scholar]
- 41.Yu, J. W., J. M. Mendrola, A. Audhya, S. Singh, D. Keleti, D. B. DeWald, D. Murray, S. D. Emr, and M. A. Lemmon. 2004. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol. Cell 13:677-688. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, W., R. P. Trible, M. Zhu, S. K. Liu, C. J. McGlade, and L. E. Samelson. 2000. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J. Biol. Chem. 275:23355-23361. [DOI] [PubMed] [Google Scholar]