Abstract
Dendritic cells (DCs) have long been recognized as key regulators of immune responses. However, the process of their recruitment to peripheral tissues and turnover during homeostasis remains largely unknown. The chemokine CXCL14 (BRAK) is constitutively expressed in skin and other epithelial tissues. Recently, the human chemokine was proposed to play a role in the homeostatic recruitment of macrophage and/or DC precursors toward the periphery, such as skin. Although so far no physiological function could be demonstrated for the murine CXCL14, it shows a remarkable homology to the human chemokine. In order to elucidate the in vivo role of CXCL14, we generated a mouse defective for this chemokine. We studied various components of the immune system with emphasis on monocytes/macrophages and DC/Langerhans cell (LC) populations in different tissues during steady state but did not find a significant difference between knockout (CXCL14−/−) and control mice. Functionally, LCs were able to become activated, to migrate out of skin, and to elicit a delayed type of hypersensitivity reaction. Overall, our data indicate that murine CXCL14 is dispensable for the homeostatic recruitment of antigen-presenting cells toward the periphery and for LC functionality.
Antigen-presenting cells (APCs) are unique in their capacity to initiate adaptive immunity in naïve T cells. The vast majority of dendritic cells (DCs) are present at extravascular sites including epithelial tissues such as skin and gut (2, 3, 45). In an “immature” state, characterized by their ability to take up and process antigen and by their responsiveness to inflammatory chemokines, DCs screen the environment and act as essential sentinel cells. “Mature” DCs home to lymph nodes, where they present antigen to T cells and provide expert costimulatory signals. Besides this proinflammatory role, DCs are also important in the induction of tolerance (48). During steady state, also described as a condition lacking “danger” signals, DCs take up and present self-antigen for the elimination of self-reactive T cells and/or the induction of regulatory T cells (23, 48).
The process of DC localization and relocation in the course of inflammation has been widely studied (39). At the site of injury or infection, inflammatory chemokines such as CCL2, CCL5, and CXCL8 are produced to attract immature DCs that express CCR1, CCR2, CCR5, and CXCR1. After antigen uptake, DCs downregulate their inflammatory chemokine receptors and upregulate the lymph node homing receptor CCR7. Maturing CCR7+ DCs then enter CCL21-expressing lymphatic vessels and travel to the draining lymph nodes where CCL19 and CCL21, the ligands of CCR7, are produced (39). On the other hand, little is known about the constitutive recruitment of DC and also macrophage precursors into peripheral tissues such as skin, intestine, and lung in the absence of inflammation.
CXCL14 is a chemokine with unknown function and receptor selectivity (4, 9, 15). Human CXCL14, alternatively called BRAK and MIP-2γ, is constitutively produced in healthy epithelial tissues such as skin and gut. CXCL14 is also present in various tumors of epithelial origin, but the level of expression is heterogeneous, with the majority showing a downregulation with respect to healthy tissue (4, 9, 15, 19, 43, 44, 46). Recently, we have shown that human CXCL14 is selective for blood monocytes and CD14+ DC precursors, either derived from CD34+ hematopoietic progenitor cells or isolated from blood (19, 42). The prominent expression in normal epidermis as well as on blood vessels in the superficial dermal plexus led us to propose the hypothesis that human CXCL14 might contribute to the recruitment of cutaneous DCs under steady-state conditions. Additionally, several recent reports provide evidence for a role in antitumor immunity (32, 43, 46). Much less is known about murine CXCL14, originally termed BMAC or KS1 (47). Murine CXCL14 has 94% sequence similarity at the protein level with its human orthologue and shows a similar expression profile with high and constitutive expression in normal peripheral tissues such as ovary, lung, and brain (47) and also skin, intestine, and kidney (unpublished observation). The function of murine CXCL14 remains largely unknown.
To explore the role of murine CXCL14 in vivo, we generated C57BL/6 mice with a targeted disruption of the CXCL14 gene. We made a comprehensive analysis of the CXCL14−/− mice, including gross anatomy, histology, and hematology examinations. In addition, as a consequence of our findings with human CXCL14 (19, 42), we examined in detail the macrophage and DC populations during steady state in several peripheral organs, with emphasis on the skin. Finally, we tested different Langerhans cell (LC) functions during inflammation in vivo. Surprisingly, the absence of murine CXCL14 did not produce an obvious phenotype. Murine CXCL14 seems to be dispensable, or at least redundant, for DC and macrophage homeostasis and various LC functions.
MATERIALS AND METHODS
Antibodies.
The following antibodies were used: rat antibodies to CD8a-fluorescein isothiocyanate ([FITC] 53-6.7), CD11b-biotin (M1/70), CD115-phycoerythrin ([PE] AFS98), F4/80-allophycocyanin ([APC] BM8), B220 (RA3-6B2), hamster anti-CD11c-PE, and anti-CD11c-biotin (N418; all from eBioscience). Hamster anti-CD3 (48-2B; Santa Cruz), rat anti-F4/80 (A3-1; Serotec), and mouse anti-I-Ab-biotin (25-9-17; BioLegend) were only used for immunohistochemistry. Donkey anti-rat immunoglobulin G (IgG)-PE (Jackson ImmunoResearch), mouse anti-rat IgG2a-FITC (BD Biosciences), goat anti-hamster IgG-biotin (Jackson ImmunoResearch), and donkey anti-rat IgG-biotin (Jackson ImmunoResearch) were used as secondary antibodies. Biotinylated antibodies were detected by streptavidin-APC (BD Biosciences). Isotype-matched control antibodies were purchased from BD Biosciences.
Targeted disruption of CXCL14.
A mouse 129/Sv genomic library was screened with a mouse CXCL14 cDNA probe to isolate a pPAC4 clone containing an EcoRI/EcoRI fragment (18.3 kb) of CXCL14 genomic DNA, which was subcloned into the pBS/KS vector. To disrupt the CXCL14 gene, a 5′ fragment including the 5′ part of exon 1 and a 3′ fragment containing exon 4 were subcloned into the pWH9 vector (from R. Fässler, MPI, Martinsried, Germany) at either end of a neomycin resistance gene (neo), under the control of the phosphoglycerate kinase promoter. The 4.7-kb 5′ fragment was cloned into the HindIII-NotI sites of the pWH9 vector, and the 5.4-kb 3′ fragment was cloned into the XhoI-EcoRI sites to generate the targeting vector pWH9-mCXCL14.
Generation of CXCL14−/− mice.
The linearized construct was electroporated into the 129/Sv embryonic stem (ES) cell line, and neomycin-resistant clones were selected. The colonies were screened by Southern blotting of EcoRI-digested DNA using a 405-bp 5′ external probe to identify homologous recombination. The probe hybridizes either to a 18.3-kb wild-type fragment or to a 7.9-kb recombinant fragment. Positive ES clones were further validated with a second 3′ internal probe. Mutant ES clones were injected into C57BL/6 blastocysts, and male chimeras were bred to C57BL/6 females. Offspring were tested by Southern blotting and, routinely, by PCR of genomic tail DNA using the following oligonucleotides that cover the 5′ recombinant starting region: 5′ CXCL14, 5′-AGAGAGGCGTGCTTGAAACC-3′; 3′ CXCL14 wild type, 5′-TCGCCGCCGTACAGGTCT-3′; and 3′ CXCL14−/−, 5′-CGAGAGTACTTCTAGAGCGG-3′. The expected sizes for the endogenous and targeting alleles were 326 bp and 149 bp, respectively. A second primer set spanning the 3′ end was used to verify the results. To confirm the absence of CXCL14 message in homozygous mutant mice, reverse transcription-PCR analysis was performed on RNA prepared from lung using the following oligonucleotides that span the whole CXCL14 message: 5′ CXCL14, 5′-TTGAAACCGAGAACCAAGCCG-3′, and 3′CXCL14, 5′-CTGCTGAAGTCTCTCAACTGG-3′, resulting in a 453-bp fragment. β-Actin was used to control RNA quality.
CXCL14−/− mice were backcrossed onto the C57BL/6 background for at least five generations. Mice were used at 8 to 12 weeks of age, and C57BL/6 mice with the same mixed background were used as wild-type controls. All mice were housed in a germ-free barrier facility.
Immunohistochemistry.
Normal abdominal skin samples were frozen at −80°C in Tissue Tek OCT (22-oxyacalcitriol) Compound (Sakura, Zoeterwoude, The Netherlands) or, alternatively, formaldehyde fixed and embedded in paraffin; samples were cut into 7-μm sections. Frozen sections were fixed for 30 min in 4% formaldehyde, while deparaffinized sections were digested for 10 min with protease from Streptomyces giseus (Sigma) at 37°C before a blocking step with 10% donkey serum (Jackson ImmunoResearch), 5 mg/ml casein sodium salt, 0.01% Tween 20, and 0.01% sodium azide in Tris-buffered saline solution (25 mM Tris, 136 mM NaCl, 2.7 mM KCl, pH 7.5). Cryosections were stained with hamster anti-mouse CD11c-biotin, and paraffin sections were stained with rat anti-mouse F4/80 and isotype control antibodies overnight at 4°C. F4/80 was detected by biotinylated donkey anti-rat IgG. Subsequent detection was performed by the avidin-biotin complex technique (DakoCytomation) and New Fuchsin solution (New Fuchsin Kit; DakoCytomation). The slides were counterstained with Mayer's hematoxylin (Sigma) and mounted using Aquamount (BDH Laboratory Supplies).
Epidermal and dermal sheets.
Fresh ears were split with the aid of fine forceps and incubated for 40 min at 37°C on 0.5 M ammonium thiocyanate. The epidermis was separated from the dermis using forceps and washed in phosphate-buffered saline (PBS; pH 7.2). Epidermal sheets were fixed in acetone for 20 min at −20°C, washed in PBS, and incubated at room temperature for 30 min with PBS-10% donkey serum. Following this blocking step, the sheets were stained overnight with hamster anti-mouse CD3 or biotinylated mouse anti-mouse I-Ab at 4°C in PBS-5% donkey serum. The sheets were washed for 10 min in PBS and, for the CD3 staining, incubated for a further 90 min with biotinylated goat anti-hamster IgG at 37°C in PBS-5% donkey serum. After another washing step, the sheets were incubated for 90 min with streptavidin-AF488 (Molecular Probes) at 37°C in PBS-5% donkey serum. Finally, the sheets were washed in PBS and mounted on microscope slides using a SlowFade Antifade Kit (Molecular Probes) and sealed with nail varnish. Samples were examined by fluorescent microscopy, and the frequency of stained cells was assessed using an eyepiece with a calibrated grid at a magnification of ×20. Eight randomly selected fields were counted per sheet, and results are expressed as the mean number of cells/mm2 (± standard error of the mean [SEM]) derived from five to seven animals in two independent experiments. The statistical significance of differences between experimental groups was calculated using a Student's t test. For the staining of activated LCs and dermal cords, the whole ear skin was first cultured for 3 days in complete RPMI medium as described below, before separation into epidermal and dermal sheets and staining for LCs as described above.
Ear skin explants.
The protocol used was that described by Larsen et al. and Ortner et al. (21, 31). In brief, the ears were dissected, rinsed in 70% ethanol, and allowed to dry for 10 min. The ventral and dorsal sheets were separated with a pair of fine forceps. The two halves were transferred dermal side down in 24-well tissue culture plates in 1.5 ml of RPMI 1640 medium supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 50 μg/ml penicillin-streptomycin, 0.5 μM β-mercaptoethanol (all Invitrogen), and 10% heat-inactivated fetal calf serum (Biological Ind.); cells were allowed to migrate out for 7 days. The ear halves were transferred on new culture wells with fresh medium every day, and the cells that had migrated into the medium were collected daily. The cells from the two ear halves were pooled and evaluated by fluorescence-activated cell sorting (FACS). LCs/DCs were identified by I-Ab and CD11c.
Flow cytometry.
Single-cell suspensions were prepared from spleen, femoral bone marrow, and blood and from axillary, brachial, and inguinal lymph nodes. The spleen was disrupted by mechanical dissociation, while the lymph nodes were incubated for 40 min at 37°C with 1 mg/ml collagenase D (Roche), 20 U/ml DNase I (Roche) in serum-free RPMI medium. Cells were separated by passing them through a 40-μm-pore-size cell strainer and washed in PBS-5 mM EDTA-2% fetal calf serum. Prior to staining, blood, splenocytes, and bone marrow cells were subjected to red blood cell lysis using 5 to 10 ml of lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 M EDTA). Cells were routinely incubated with 2.4G2 (BD Biosciences) or, if a secondary anti-rat antibody was used, with 600 μg/ml mouse IgG (Sigma) to block Fc receptors; cells were subsequently labeled with antibodies diluted in PBS-1% bovine serum albumin-0.05% azide for 30 min on ice. In some cases, a secondary incubation step using FITC- or PE-labeled secondary antibody or streptavidin-APCs was added. Cells were stained with propidium iodide to exclude dead cells and analyzed using a FACSCalibur (Becton Dickinson) instrument and CellQuest software.
Delayed-type hypersensitivity (DTH).
Mice were sensitized on the shaved back skin with 25 μl of 0.5% dinitrofluorobenzene (DNFB) in acetone 4 and 5 days prior to challenge with 10 μl of 0.25% DNFB in acetone on each side of the ear. Ear thickness was measured for the next consecutive 8 days.
Wound healing.
Mice were anesthetized with a single intraperitoneal injection of avertin (0.5 μg per 10 g of body weight), and four full-thickness wounds were created on the back of each mouse by using a 6-mm biopsy punch. Wound closure was monitored daily for 17 days in at least eight mice for each time point and genotype, and wound area measurements were performed as described previously (50).
Peritonitis.
Peritoneal inflammation was induced in 6- to 8-week old female wild-type or CXCL14−/− mice by intraperitoneal injection of 1 ml of sterile 3% Brewer's thioglycolate solution (Sigma). After 5 days, total peritoneal cells were collected by washing the peritoneum with 40 ml of PBS and analyzed by flow cytometry.
RESULTS
Generation of CXCL14−/− mice.
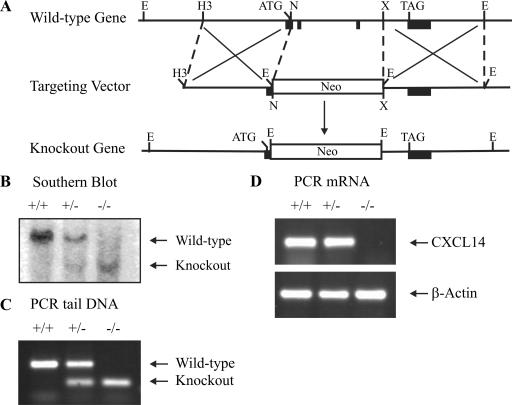
To completely eliminate the expression of CXCL14, a targeting vector was constructed for deletion of the 3′ part of exon 1 and full-length exons 2 and 3 (Fig. 1A). After electroporation into a 129/Sv ES cell line, ES cells were screened by Southern blotting with a 5′ external probe (data not shown). Positive clones were confirmed with a 3′ internal probe, and one clone was injected into C57BL/6 blastocysts to generate chimeric mice. Germ line transmission of the mutant allele yielded heterozygous mice, which were intercrossed to generate CXCL14−/− mice, as determined by Southern blotting (Fig. 1B) and, routinely, by PCR (Fig. 1C) of genomic tail DNA. PCR of lung RNA (Fig. 1D) confirmed the lack of CXCL14 mRNA.
FIG. 1.
Generation of the CXCL14−/− mouse. (A) Schematic diagram of the wild-type mouse CXCL14 gene, the targeting vector, and the CXCL14−/− allele. Filled black boxes, exons; empty box, neomycin (Neo) expression cassette. Restriction enzymes: E, EcoRI; H3, HindIII; N, Not; X, Xho. (B) Southern blot analysis of EcoRI-digested genomic tail DNA. PCR of genomic tail DNA (C) and PCR of lung RNA (D) from wild-type (+/+), heterozygous (+/−) and knockout (−/−) mice.
Mice heterozygous or homozygous for the CXCL14 allele appeared healthy and developed normally. Both heterozygous × heterozygous and homozygous × homozygous breeding pairs produced litters; however, litter size of the homozygous pairs seemed smaller, with occasional postnatal death between days 2 and 3. Whole-body analysis of either living or dying newborns did not show an obvious defect. In addition, intercrossing of heterozygous mice did not yield the predicted Mendelian frequency of 25% but only 10.3% CXCL14−/− mice (Table 1). Further experiments are under way to investigate this unexpected and unusual finding.
TABLE 1.
Breeding pattern
| Breeding paira | Genotype (%)
|
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| +/+ × +/− (n = 207) | 50.2 | 49.8 | |
| +/− × +/− (n = 390) | 24.6 | 65.1 | 10.3 |
+/+, wild-type; +/−, heterozygous; −/−, knockout.
The surviving CXCL14−/− mice were significantly lighter than their wild-type littermates (females, 12 to 16%; males, 15 to 20%) as measured over a time period of 6 weeks. However, they exhibited no macroscopic or histological alteration in all organs examined by hematoxylin-eosin staining (spleen, thymus, lung, liver, heart, intestine, kidney, brain, muscle, ovary, and skin) (data not shown).
Normal distribution of leukocyte populations in blood and lymphoid tissues.
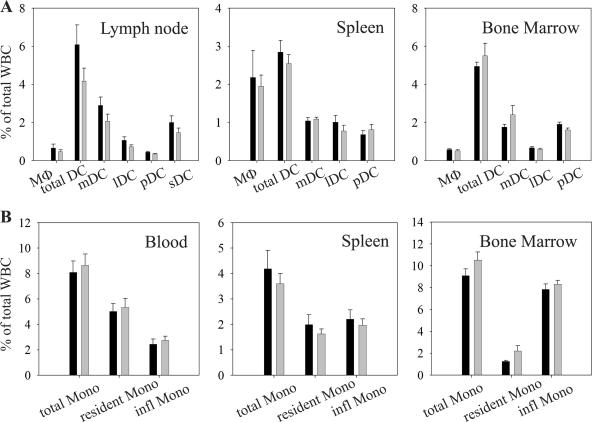
As human CXCL14 was shown to be involved in monocyte and DC recruitment (19, 42, 44, 46), these subsets were analyzed in more detail in blood, spleen, bone marrow, and peripheral lymph nodes (Fig. 2A and B). At least four main DC subsets have been identified in mice, which are distinguished by their expression of CD11c, CD8, CD11b, B220, and CD205 (1, 12). Three of these populations, known as myeloid (CD11chigh CD11b+ CD8− B220−), lymphoid (CD11chigh CD8+ CD11b− B220−), and plasmacytoid (CD11clow CD11b− CD8− B220+) (28) DCs, are found in all lymphoid tissues, including the spleen. Because the spleen lacks afferent lymphatics, the precursors of these subsets probably are blood derived. Skin-draining lymph nodes contain a fourth population of CD11chigh CD11blow CD8low B220− CD205+ cells that contain both dermal DCs and epidermal LCs (1, 12, 17). Macrophages were identified by F4/80 and high autofluorescence, while monocytes (CD115+ SSClow) were divided into CD11c+ and CD11c− subsets to distinguish between “resident” and “inflammatory” monocytes, respectively (11, 51). No significant differences could be found in any of these subsets between the CXCL14−/− and the wild-type animals. No qualitative and quantitative differences were observed with respect to other cell types in blood and lymphoid organs, e.g., B cells (B220, IgM, and IgD), T cells (CD4, CD8, and T-cell receptor γδ [TCR-γδ]), NK cells (NK1.1), NKT cells (NK1.1 and CD3), neutrophils (Gr1high), or eosinophils (F4/80 and SSChigh) (data not shown).
FIG. 2.
Normal distribution of monocytes and related leukocytes in lymphoid tissues of CXCL14−/− mice. Cell suspensions from blood and lymphoid tissues of normal (black bars) and CXCL14−/− (gray bars) mice were examined by flow cytometry for the presence of DC subsets and macrophages (A) and monocyte subsets (B). Data are given as mean percentage of total living leukocytes ± SEM from 5 to 7 mice in three independent experiments. A Student's t test was used to calculate significance. WBC, white blood cells; MΦ, macrophages, mDC, myeloid DC; lDC, lymphoid DC; pDC, plasmacytoid DC; sDC, skin-derived DC; Mono, monocytes; infl Mono, inflammatory monocytes.
To analyze DC homing to lymphoid tissues in vivo, carboxyfluorescein succinimidyl ester-labeled myeloid DCs were injected intravenously, and 18 h later, thymus, spleen, lymph nodes, and bone marrow were checked for the presence of labeled DCs by flow cytometry. Again, no significant difference in homing frequency could be observed between the CXCL14−/− and control mice (data not shown).
Therefore, mice deficient in CXCL14 were devoid of any obvious alteration in their leukocyte distribution in blood, bone marrow, and secondary lymphoid organs, such as spleen and peripheral lymph nodes.
Macrophages and DCs are present at normal numbers in epithelial tissues of CXCL14−/− mice.
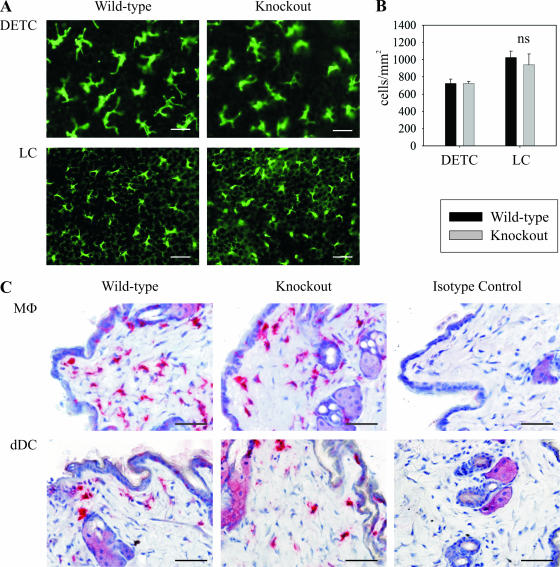
To address the question whether murine CXCL14 could be involved in the homeostatic recruitment of monocytes and/or DC precursors toward the periphery, various tissues were examined for the presence of macrophages and DCs. Due to the prominent expression of CXCL14 in the human epidermis (19, 42), emphasis was placed on normal (uninflamed) murine skin. Epidermal sheets from CXCL14−/− mice stained with an anti-major histocompatibility complex class II (MHC-II) antibody showed normal numbers of LCs. They displayed a DC morphology and expression of MHC-II similar to control mice (Fig. 3A and B). Dendritic epidermal γδ-T cells (DETCs), as detected by CD3 expression, were also present in normal numbers in the epidermis of CXCL14−/− mice (Fig. 3A and B). Macrophages and dermal DCs, analyzed by immunohistochemistry with antibodies to F4/80 and CD11c, respectively, showed no alteration in location and numbers in CXCL14−/− mice (Fig. 3C). Other immune cell populations in normal skin, such as T cells and mast cells, were not altered either (data not shown). Similar distribution of macrophages and DCs could also be found in other tissues such as lung, intestine, kidney, liver, thymus, ovary, and heart (data not shown).
FIG. 3.
CXCL14−/− mice have no significant difference in the resident immune cell populations in normal skin. (A) Epidermal sheets from wild-type and CXCL14−/− animals were stained for LCs (MHC-II) and DETCs (CD3). Scale bar, 25 μm. (B) Quantification of total DETC and LC cell numbers in the epidermis of control (black bars) and CXCL14−/− (gray bars) mice. Results show mean number of cells/mm2 (± SEM) from 5 to 7 separate animals in two independent experiments. Significance was calculated using a Student's t test. ns, not significant. (C) Immunohistochemical staining for macrophages (F4/80) and dermal DCs (CD11c) in normal skin of wild-type and CXCL14−/− animals; isotype-matched antibodies were used in control stainings (Isotype Control). MΦ, macrophages; dDC, dermal DCs. Scale bar, 50 μm.
Thus, macrophages and DCs are present in normal numbers in peripheral tissues of CXCL14−/− mice and feature distribution and morphological characteristics similar to those in control mice.
LCs of CXCL14−/− mice display normal activation and skin emigration properties.
Due to their prototypical location, LCs play a pivotal role in the induction of cutaneous immunity in response to a multitude of external threats, such as bacteria, viruses, toxins, and chemicals (18, 36). LCs capture and process exogenous antigens, upregulate MHC-II and costimulatory molecules, and leave the epidermis by migrating through lymphatic vessels into skin-draining lymph nodes, where they present antigens to naïve T cells.
The skin explant culture established by Larsen et al. and Ortner et al. has become a widely used model to study the morphology and migration pathway of LCs and dermal DCs in vitro (21, 31, 54). At the onset of the cultures, LCs round up, enlarge in size, and upregulate MHC-II before they start to leave the epidermis and migrate through the dermis into the culture medium. In the dermis, DCs and LCs accumulate in characteristic string-like lymphatic vessels called dermal cords that were also observed upon stimulation in vivo but not in normal, uninflamed skin (35, 54).
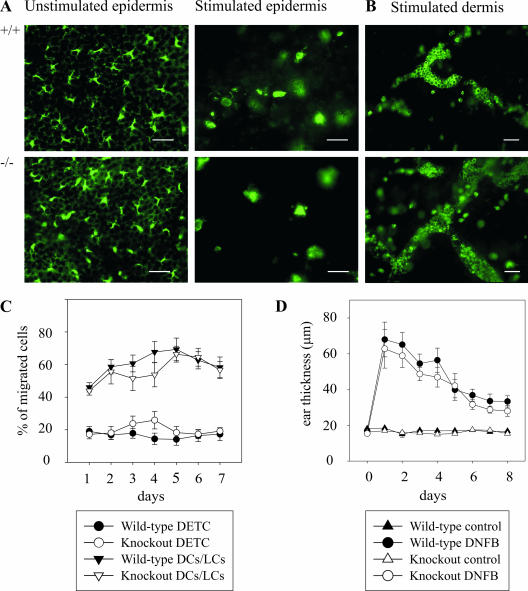
We therefore studied whether the absence of CXCL14 could influence these early steps in LC activation and migration. Epidermal sheets were prepared from either fresh skin (Fig. 4A, left frames) or after culturing of skin tissue for 3 days, a situation known to mimic inflammation (Fig. 4A, right frames), and then were stained for LCs. As described previously, after 3 days of culture, many cells have already left the epidermis, and the LCs remaining within the epidermal sheets showed an activated phenotype (33, 49, 54). In the dermis, DCs and LCs, identified by MHC-II, have accumulated in dermal cords (Fig. 4B). No significant difference could be found in any of these steps between the CXCL14−/− and wild-type animals. The culture medium of the skin explants was analyzed daily by FACS for the presence of emigrant cells that consisted mainly of activated DCs/LCs (MHC-IIhigh CD11c+) and DETCs (TCR-γδ+) (Fig. 4C). Also, the addition of the CCR7 ligand, CCL21, enhanced the accumulation of CD11c+ cells but not DETCs, indicating that the culturing of skin explants induced DC maturation (data not shown) (38). Again, DCs/LCs and DETCs from CXCL14−/− mice were indistinguishable from control mice with regard to both absolute numbers of emigrant cells and cellular composition (percentage of total emigrant cells). The addition of CXCL14 into the culture medium did not influence the migration of any of these cells out of skin explants, suggesting that these cells did not respond to this chemokine (data not shown).
FIG. 4.
Unchanged migration and function of LCs/DCs and DETCs in CXCL14−/− mice. (A) Immunofluorescence staining of LCs by MHC-II of fresh epidermis (left frames) and epidermal sheets prepared after a culture period of 3 days (right frames) in wild-type (+/+) and CXCL14−/− (−/−) animals. Scale bar, 25 μm. (B) MHC-II staining of dermal sheets after 3 days of culture. Scale bar, 50 μm. (C) Whole-ear skin explants were cultured over a period of 7 days in complete medium and analyzed daily by flow cytometry. DCs/LCs were identified by staining for CD11c and MHC-II, and DETCs were identified by staining for γδ-TCR. Data are presented as mean percentage of total migrating cells ± SEM in 6 CXCL14−/− (open symbols) and 11 wild-type (filled symbols) mice in three independent experiments. (D) DNFB-induced DTH reaction is unchanged in CXCL14−/− mice. Mice were DNFB sensitized on the back skin 5 and 4 days before ears were challenged with either solvent alone (control) or DNFB in acetone. Ear thickness was measured daily from days 1 to 8. Error bars indicate SEM of 10 CXCL14−/− (open symbols) and 10 wild-type (filled symbols) animals. A Student's t test was used to calculate significance.
Taken together, these data indicate that murine CXCL14 is not involved in the early steps of LC activation and migration.
Normal skin DC function in CXCL14−/− mice in response to inflammation.
Tissue inflammation as a result of a DTH reaction is dependent on tissue-resident DCs, which mature upon capture of antigens and migrate to tissue-draining lymph nodes in order to prime antigen-specific T cells. To assess whether the lack of CXCL14 affects inflammatory DC function, we elicited a DNFB-dependent DTH reaction. Ear thickness as a result of tissue inflammation was measured in DNFB-sensitized wild-type and CXCL14−/− mice after a single challenge with DNFB. No difference in ear swelling was found after 1 day and for the next consecutive 7 days, indicating that lack of CXCL14 does not affect the DC-dependent initiation and resolution of the inflammatory response (Fig. 4D).
Normal peritoneal recruitment of inflammatory macrophages in CXCL14−/− mice.
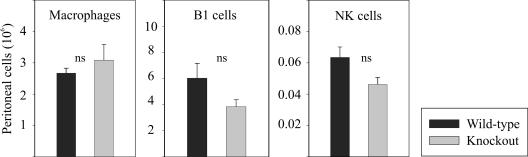
We have recently shown that blood monocytes, at least in humans, are attracted by CXCL14. Therefore, CXCL14 may promote the accumulation of macrophages (the progeny of monocytes) at the site of inflammation. In order to examine this hypothesis, we induced sterile peritonitis by thioglycolate injection and determined the numbers of inflammatory leukocytes after 5 days. At that time, macrophages are a prominent cell type. However, recruitment of macrophages, B1 B cells, NK cells, neutrophils, and DCs was similar in CXCL14−/− and wild-type mice (Fig. 5 and data not shown), indicating that CXCL14 is not needed for inflammatory macrophage recruitment.
FIG. 5.
Recruitment of leukocytes during peritoneal inflammation is unchanged in CXCL14−/− mice. Inflammatory leukocytes were harvested by peritoneal lavage after 5 days of peritonitis induction by thioglycolate. The three most prominent cell types (B1 B cells, macrophages, and NK cells) were not significantly different in cell numbers in wild-type (black bars) and CXCL14−/− mice (gray bars) as analyzed by quantitative flow cytometry. Results show absolute peritoneal cell numbers (± SEM) from six animals per genotype. A Student's t test was used to calculate significance. ns, not significant.
CXCL14−/− mice display unchanged skin wound healing.
After skin injury, a dynamic process of tissue repair is initiated that consists of an inflammatory response followed by reepithelialization of the wound area and establishment of the granulation tissue with accompanying angiogenesis and wound contraction (6). Besides their role in migration, certain chemokines are involved in angiogenesis, hematopoiesis, and organogenesis (14, 22, 37). As CXCL14 was shown to be absent from a variety of tumors, Shellenberger et al. hypothesized that CXCL14 could be involved in the tumor-associated angiogenesis and could indeed demonstrate inhibition of angiogenesis by CXCL14 (44). Furthermore, activated macrophages release growth factors and cytokines, namely, platelet-derived growth factor, fibroblast growth factor 2, and transforming growth factor β1, as well as tumor necrosis factor α and interleukin-1, that modulate tissue repair and angiogenesis (29). The critical role of macrophages in angiogenesis and tissue remodeling together with their responsiveness to CXCL14 led us to examine whether a lack of CXCL14 affects wound healing. Wound closure as a measure of wound healing was measured on the back skin of CXCL14−/− and control mice that initially carried a full-thickness wound. Surprisingly, the absence of CXCL14 did not affect the overall wound healing process since skin wounds closed at similar rates in CXCL14−/− and wild-type mice, i.e., within 2 weeks (data not shown).
DISCUSSION
Characterization of target cells for CXCL14 is hampered by the fact that its receptor has not been identified. Still, the summary of locations at which CXCL14 is being produced narrows the spectrum of potential target cells. Strong expression of CXCL14 in healthy human skin (19, 42), which is not further upregulated in skin diseases, such as atopic dermatitis (19) and psoriasis (data not shown), indicates a homeostatic as opposed to inflammatory role for this chemokine (25, 26). We have shown previously that human CXCL14 attracts blood monocytes and CD14+ DC precursors generated during in vitro culture of CD34+ hematopoietic progenitor cells (19, 42). We were unable to detect alternative CXCL14-responsive cell types, such as LCs and DCs, or other cell lineages like T, B, and NK cells. Other laboratories reported a role for CXCL14 in the recruitment of DCs by showing chemotactic activity for immature DCs (44, 46). At present we have no explanation for this obvious discrepancy between our findings and those of other groups. Nevertheless, the abundant expression of CXCL14 in the epidermis and on blood vessels in the dermis provided further evidence for our hypothesis that human CXCL14 contributes to the recruitment of DC/LC precursors toward the skin under steady-state conditions.
Similar to T cells (41), the process of APC precursor recruitment toward peripheral tissues during homeostasis remains largely unknown. Most of the studies so far have concentrated on the recruitment of LCs into the epidermis (5, 8, 20, 52). The chemokine CCL20, the single ligand for CCR6, was originally proposed to regulate LC recruitment during steady state (5); however, the expression of CCL20 in uninflamed skin could not be confirmed by others (8, 13). The prominent upregulation of CCL20 during inflammation may suggest an inflammatory role for this chemokine (8, 13, 52). In addition, although the CCR6 knockout mice show altered localization of intestinal DCs, a defect in LCs within the epidermis was not apparent (7, 53).
Another provocative study supports the view that during inflammation, LCs are replaced by circulating bone marrow precursors or monocytes, via inflammatory chemokine receptors, while during steady state, LCs are long-lived and either are replaced by a skin resident precursor or proliferate locally to sustain the low number of migratory LCs (10, 20, 24). Whether this model turns out to be true in vivo remains to be shown, and how it might relate to other DC subsets is unknown. In fact, in most tissues, DCs are turning over rapidly even in the steady state and therefore cannot be compared directly with LCs.
To get further insight into the role of CXCL14 and to test whether it could indeed be involved in any of the processes described above, we generated a mouse defective for CXCL14. Murine CXCL14 and human CXCL14 are highly conserved at the protein level and resemble each other with regard to the tissue distribution and control of CXCL14 expression. Surprisingly, the CXCL14−/− mouse developed normally and displayed no obvious phenotype, with the exception of a significant weight reduction and a disturbed Mendelian breeding pattern and occasional postnatal death of newborns at day 2 to 3. Whole-body analysis of these newborns did not reveal a striking abnormality, and further experiments aimed at identifying the potential role of CXCL14 in embryogenesis are under way.
The series of organs from CXCL14−/− mice that we examined did not exhibit macroscopic or morphological alterations and did not display abnormal cell numbers of leukocytes, including monocytes/macrophages, DCs/LCs, and lymphocytes. Moreover, to test whether murine CXCL14 could be involved in the homeostatic recruitment of APC precursors toward the periphery, various organs were screened for the presence of macrophages and DCs by immunohistochemistry. No difference could be found in any of the major CXCL14-producing tissues such as intestine, kidney, and lung. CXCL14 was shown to be prominently expressed in the epidermis and, therefore, emphasis was laid on the analysis of different immune cell populations present in the skin. The epidermal MHC-II-positive dendritic network within CXCL14−/− mice was found to be indistinguishable in morphology or absolute numbers from wild-type mice. Other leukocytes, such as DETCs, dermal DCs, macrophages, T cells, and mast cells were also present. Thus, right now we have no evidence for a role of murine CXCL14 in the recruitment of leukocyte subsets with known functions within normal murine skin.
To test whether the absence of CXCL14 could alter LC function, an ex vivo ear explant model was used to analyze early steps in LC activation and migration, and, additionally, a DTH reaction was induced to investigate the in vivo relevance. Again, no difference was observed between the CXCL14−/− and control mice, indicating that neither the migratory nor the APC functions of LCs are compromised in the absence of CXCL14.
Chemokines not only control immune cell recruitment but also mediate functions unrelated to migration but related to hematopoiesis and embryogenesis (14, 22). As CXCL14 is very strongly and constitutively expressed in epithelia throughout the body, it was surprising to find no obvious phenotype in any of these events. Angiogenesis is essential for a number of physiological and pathophysiological events, such as embryogenesis, wound healing, chronic inflammation, and tumor growth and is also known to be influenced by chemokines (14, 37). CXCL14 was once even suggested to negatively regulate angiogenesis (44), and, therefore, we examined the potential role of CXCL14 in wound healing. However, we show here that CXCL14 is also dispensable for this process.
The lack of phenotype is unexpected, particularly with regard to the strong and ubiquitous expression pattern of CXCL14. Especially, the undisturbed APC homeostasis is surprising and might be explained with species differences between mice and humans. Of course, we also cannot rule out a more subtle role of CXCL14 that we have not addressed so far. The chemokine receptor knockout of CX3CR1, for example, initially revealed no phenotype (16). However, in a later study it was discovered that lamina propria DCs were unable to develop transepithelial dendrites, leading to impaired access to the intestinal lumen and bacterial clearance (30).
Another possible explanation for the unexpected lack of phenotype could be the redundancy or compensation by other chemokines. The whole chemokine system, composed of approximately 50 ligands and 20 receptors, is known for its considerable functional redundancy. In particular, single deletions of inflammatory chemokines or their receptors often had little or no discernible effect upon immune function (34). Homeostatic chemokines, on the other hand, often developed a more profound defect upon their removal, as shown by the lethality of CXCL12/CXCR4 knockout mice (34) or the severe defects in mice lacking CCL19/CCL21/CCR7 or CXCL13/CXCR5 (27).
Candidates with potentially synergistic activity with CXCL14 are the ligands for CCR2 and CCR6. Both have been proposed to be involved in APC recruitment toward the skin, but single knockouts did not clarify this hypothesis (40, 52, 53). Possibly, mice with multiple defects in chemokine production may prove to be more useful. Our CXCL14−/− mouse may represent a valuable tool for further investigations on APC recruitment and function and may provide a critical first step in elucidating the in vivo role of CXCL14.
Acknowledgments
We thank R. Fässler at the Max-Planck-Institute for Biochemistry (Martinsried, Germany) and C. Müller and his team at the Institute of Pathology (University of Bern) for their initial help in the handling of mice, and K. Willimann and A. Blaser at the Institute of Cell Biology (University of Bern) for advice in immunostaining of mouse tissue.
Our work is supported by grant 31-106583 from the Swiss National Science Foundation, grant 03.0441-2 from the Staatssekretariat für Bildung und Forschung, and grant 518167 from the European Framework Programme 6. P.S. is supported by the Roche Research Foundation.
Footnotes
Published ahead of print on 27 November 2006.
REFERENCES
- 1.Anjuere, F., P. Martin, I. Ferrero, M. L. Fraga, G. M. Del Hoyo, N. Wright, and C. Ardavin. 1999. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood 93:590-598. [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Cao, X., W. Zhang, T. Wan, L. He, T. Chen, Z. Yuan, S. Ma, Y. Yu, and G. Chen. 2000. Molecular cloning and characterization of a novel CXC chemokine macrophage inflammatory protein-2 gamma chemoattractant for human neutrophils and dendritic cells. J. Immunol. 165:2588-2595. [DOI] [PubMed] [Google Scholar]
- 5.Charbonnier, A. S., N. Kohrgruber, E. Kriehuber, G. Stingl, A. Rot, and D. Maurer. 1999. Macrophage inflammatory protein 3α is involved in the constitutive trafficking of epidermal Langerhans cells. J. Exp. Med. 190:1755-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, R. A. 1993. Biology of dermal wound repair. Dermatol. Clin. 11:647-666. [PubMed] [Google Scholar]
- 7.Cook, D. N., D. M. Prosser, R. Forster, J. Zhang, N. A. Kuklin, S. J. Abbondanzo, X. D. Niu, S. C. Chen, D. J. Manfra, M. T. Wiekowski, L. M. Sullivan, S. R. Smith, H. B. Greenberg, S. K. Narula, M. Lipp, and S. A. Lira. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12:495-503. [DOI] [PubMed] [Google Scholar]
- 8.Dieu-Nosjean, M. C., C. Massacrier, B. Homey, B. Vanbervliet, J. J. Pin, A. Vicari, S. Lebecque, C. Dezutter-Dambuyant, D. Schmitt, A. Zlotnik, and C. Caux. 2000. Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 192:705-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frederick, M. J., Y. Henderson, X. Xu, M. T. Deavers, A. A. Sahin, H. Wu, D. E. Lewis, A. K. El Naggar, and G. L. Clayman. 2000. In vivo expression of the novel CXC chemokine BRAK in normal and cancerous human tissue. Am. J. Pathol. 156:1937-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginhoux, F., F. Tacke, V. Angeli, M. Bogunovic, M. Loubeau, X. M. Dai, E. R. Stanley, G. J. Randolph, and M. Merad. 2006. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7:265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon, S., and P. R. Taylor. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953-964. [DOI] [PubMed] [Google Scholar]
- 12.Henri, S., D. Vremec, A. Kamath, J. Waithman, S. Williams, C. Benoist, K. Burnham, S. Saeland, E. Handman, and K. Shortman. 2001. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167:741-748. [DOI] [PubMed] [Google Scholar]
- 13.Homey, B., M. C. Dieu-Nosjean, A. Wiesenborn, C. Massacrier, J. J. Pin, E. Oldham, D. Catron, M. E. Buchanan, A. Muller, M. R. deWaal, G. Deng, R. Orozco, T. Ruzicka, P. Lehmann, S. Lebecque, C. Caux, and A. Zlotnik. 2000. Up-regulation of macrophage inflammatory protein-3α/CCL20 and CC chemokine receptor 6 in psoriasis. J. Immunol. 164:6621-6632. [DOI] [PubMed] [Google Scholar]
- 14.Homey, B., A. Muller, and A. Zlotnik. 2002. Chemokines: agents for the immunotherapy of cancer? Nat. Rev. Immunol. 2:175-184. [DOI] [PubMed] [Google Scholar]
- 15.Hromas, R., H. E. Broxmeyer, C. Kim, H. Nakshatri, K. Christopherson, I. I., M. Azam, and Y. H. Hou. 1999. Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus malignant cells. Biochem. Biophys. Res. Commun. 255:703-706. [DOI] [PubMed] [Google Scholar]
- 16.Jung, S., J. Aliberti, P. Graemmel, M. J. Sunshine, G. W. Kreutzberg, A. Sher, and D. R. Littman. 2000. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20:4106-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kissenpfennig, A., S. Henri, B. Dubois, C. Laplace-Builhe, P. Perrin, N. Romani, C. H. Tripp, P. Douillard, L. Leserman, D. Kaiserlian, S. Saeland, J. Davoust, and B. Malissen. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22:643-654. [DOI] [PubMed] [Google Scholar]
- 18.Kissenpfennig, A., and B. Malissen. 2006. Langerhans cells—revisiting the paradigm using genetically engineered mice. Trends Immunol. 27:132-139. [DOI] [PubMed] [Google Scholar]
- 19.Kurth, I., K. Willimann, P. Schaerli, T. Hunziker, I. Clark-Lewis, and B. Moser. 2001. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J. Exp. Med. 194:855-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larregina, A. T., A. E. Morelli, L. A. Spencer, A. J. Logar, S. C. Watkins, A. W. Thomson, and L. D. Falo, Jr. 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151-1158. [DOI] [PubMed] [Google Scholar]
- 21.Larsen, C. P., R. M. Steinman, M. Witmer-Pack, D. F. Hankins, P. J. Morris, and J. M. Austyn. 1990. Migration and maturation of Langerhans cells in skin transplants and explants. J. Exp. Med. 172:1483-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le, Y., Y. Zhou, P. Iribarren, and J. Wang. 2004. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol. Immunol. 1:95-104. [PubMed] [Google Scholar]
- 23.Mahnke, K., and A. H. Enk. 2005. Dendritic cells: key cells for the induction of regulatory T cells? Curr. Top. Microbiol. Immunol. 293:133-150. [DOI] [PubMed] [Google Scholar]
- 24.Merad, M., M. G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I. L. Weissman, J. G. Cyster, and E. G. Engleman. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moser, B., and P. Loetscher. 2001. Lymphocyte traffic control by chemokines. Nat. Immunol. 2:123-128. [DOI] [PubMed] [Google Scholar]
- 26.Moser, B., M. Wolf, A. Walz, and P. Loetscher. 2004. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 25:75-84. [DOI] [PubMed] [Google Scholar]
- 27.Müller, G., U. E. Höpken, H. Stein, and M. Lipp. 2002. Systemic immunoregulatory and pathogenic functions of homeostatic chemokine receptors. J. Leukoc. Biol. 72:1-8. [PubMed] [Google Scholar]
- 28.Nakano, H., M. Yanagita, and M. D. Gunn. 2001. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naldini, A., and F. Carraro. 2005. Role of inflammatory mediators in angiogenesis. Curr. Drug Targets Inflamm. Allergy 4:3-8. [DOI] [PubMed] [Google Scholar]
- 30.Niess, J. H., S. Brand, X. Gu, L. Landsman, S. Jung, B. A. McCormick, J. M. Vyas, M. Boes, H. L. Ploegh, J. G. Fox, D. R. Littman, and H. C. Reinecker. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254-258. [DOI] [PubMed] [Google Scholar]
- 31.Ortner, U., K. Inaba, F. Koch, M. Heine, M. Miwa, G. Schuler, and N. Romani. 1996. An improved isolation method for murine migratory cutaneous dendritic cells. J. Immunol. Methods 193:71-79. [DOI] [PubMed] [Google Scholar]
- 32.Ozawa, S., Y. Kato, R. Komori, Y. Maehata, E. Kubota, and R. I. Hata. 2006. BRAK/CXCL14 expression suppresses tumor growth in vivo in human oral carcinoma cells. Biochem. Biophys. Res. Commun. 348:406-412. [DOI] [PubMed] [Google Scholar]
- 33.Price, A. A., M. Cumberbatch, I. Kimber, and A. Ager. 1997. Alpha 6 integrins are required for Langerhans cell migration from the epidermis. J. Exp. Med. 186:1725-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proudfoot, A. E. 2002. Chemokine receptors: multifaceted therapeutic targets. Nat. Rev. Immunol. 2:106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roake, J. A., A. S. Rao, P. J. Morris, C. P. Larsen, D. F. Hankins, and J. M. Austyn. 1995. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 181:2237-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romani, N., S. Holzmann, C. H. Tripp, F. Koch, and P. Stoitzner. 2003. Langerhans cells—dendritic cells of the epidermis. APMIS 111:725-740. [DOI] [PubMed] [Google Scholar]
- 37.Rosenkilde, M. M., and T. W. Schwartz. 2004. The chemokine system—a major regulator of angiogenesis in health and disease. APMIS 112:481-495. [DOI] [PubMed] [Google Scholar]
- 38.Saeki, H., A. M. Moore, M. J. Brown, and S. T. Hwang. 1999. Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162:2472-2475. [PubMed] [Google Scholar]
- 39.Sallusto, F., and A. Lanzavecchia. 2000. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177:134-140. [DOI] [PubMed] [Google Scholar]
- 40.Sato, N., S. K. Ahuja, M. Quinones, V. Kostecki, R. L. Reddick, P. C. Melby, W. A. Kuziel, and S. S. Ahuja. 2000. CC chemokine receptor (CCR)2 is required for Langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaerli, P., and B. Moser. 2005. Chemokines: control of primary and memory T-cell traffic. Immunol. Res. 31:57-74. [DOI] [PubMed] [Google Scholar]
- 42.Schaerli, P., K. Willimann, L. M. Ebert, A. Walz, and B. Moser. 2005. Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation. Immunity 23:331-342. [DOI] [PubMed] [Google Scholar]
- 43.Schwarze, S. R., J. Luo, W. B. Isaacs, and D. F. Jarrard. 2005. Modulation of CXCL14 (BRAK) expression in prostate cancer. Prostate 64:67-74. [DOI] [PubMed] [Google Scholar]
- 44.Shellenberger, T. D., M. Wang, M. Gujrati, A. Jayakumar, R. M. Strieter, M. D. Burdick, C. G. Ioannides, C. L. Efferson, A. K. El Naggar, D. Roberts, G. L. Clayman, and M. J. Frederick. 2004. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 64:8262-8270. [DOI] [PubMed] [Google Scholar]
- 45.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 46.Shurin, G. V., R. L. Ferris, I. L. Tourkova, L. Perez, A. Lokshin, L. Balkir, B. Collins, G. S. Chatta, and M. R. Shurin. 2005. Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo. J. Immunol. 174:5490-5498. [DOI] [PubMed] [Google Scholar]
- 47.Sleeman, M. A., J. K. Fraser, J. G. Murison, S. L. Kelly, R. L. Prestidge, D. J. Palmer, J. D. Watson, and K. D. Kumble. 2000. B cell- and monocyte-activating chemokine (BMAC), a novel non-ELR alpha-chemokine. Int. Immunol. 12:677-689. [DOI] [PubMed] [Google Scholar]
- 48.Steinman, R. M., D. Hawiger, and M. C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685-711. [DOI] [PubMed] [Google Scholar]
- 49.Stoitzner, P., M. Zanella, U. Ortner, M. Lukas, A. Tagwerker, K. Janke, M. B. Lutz, G. Schuler, B. Echtenacher, B. Ryffel, F. Koch, and N. Romani. 1999. Migration of Langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-α and IL-1β. J. Leukoc. Biol. 66:462-470. [PubMed] [Google Scholar]
- 50.Streit, M., P. Velasco, L. Riccardi, L. Spencer, L. F. Brown, L. Janes, B. Lange-Asschenfeldt, K. Yano, T. Hawighorst, L. Iruela-Arispe, and M. Detmar. 2000. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 19:3272-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunderkotter, C., T. Nikolic, M. J. Dillon, N. Van Rooijen, M. Stehling, D. A. Drevets, and P. J. Leenen. 2004. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172:4410-4417. [DOI] [PubMed] [Google Scholar]
- 52.Vanbervliet, B., B. Homey, I. Durand, C. Massacrier, S. Ait-Yahia, O. de Bouteiller, A. Vicari, and C. Caux. 2002. Sequential involvement of CCR2 and CCR6 ligands for immature dendritic cell recruitment: possible role at inflamed epithelial surfaces. Eur. J. Immunol. 32:231-242. [DOI] [PubMed] [Google Scholar]
- 53.Varona, R., R. Villares, L. Carramolino, I. Goya, A. Zaballos, J. Gutierrez, M. Torres, A. Martinez, and G. Marquez. 2001. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J. Clin. Investig. 107:R37-R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinlich, G., M. Heine, H. Stossel, M. Zanella, P. Stoitzner, U. Ortner, J. Smolle, F. Koch, N. T. Sepp, G. Schuler, and N. Romani. 1998. Entry into afferent lymphatics and maturation in situ of migrating murine cutaneous dendritic cells. J. Invest Dermatol. 110:441-448. [DOI] [PubMed] [Google Scholar]