Abstract
Elastic fibers contribute to the structural support of tissues and to the regulation of cellular behavior. Mice deficient for the fibulin-5 gene (fbln5−/−) were used to further elucidate the molecular mechanism of elastic fiber assembly. Major elastic fiber components were present in the skin of fbln5−/− mice despite a dramatic reduction of mature elastic fibers. We found that fibulin-5 preferentially bound the monomeric form of elastin through N-terminal and C-terminal elastin-binding regions and to a preexisting matrix scaffold through calcium-binding epidermal growth factor (EGF)-like (CB-EGF) domains. We further showed that adenovirus-mediated gene transfer of fbln5 was sufficient to regenerate elastic fibers and increase elastic fiber-cell connections in vivo. A mutant fibulin-5 lacking the first 28 amino acids of the first CB-EGF domain, however, was unable to rescue elastic fiber defects. Fibulin-5 thus serves as an adaptor molecule between monomeric elastin and the matrix scaffold to aid in elastic fiber assembly. These results also support the potential use of fibulin-5 as a therapeutic agent for the treatment of elastinopathies.
The tissue property of elasticity and recoil is provided by elastic fibers comprised of an amorphous elastin core surrounded by a peripheral mantle of fibrillin-rich microfibrils. Failure to assemble elastic fibers or loss of elastic fibers due to aging or local disruption leads to loose skin, stiff vessels, and pulmonary emphysema. Once a tropoelastin monomer is secreted, it is rapidly cross-linked with other monomers via the enzymatic activity of lysyl oxidase(s) to form elastin dimers and subsequently multimers by further polymerization (20). Although self-aggregation of tropoelastin, a property known as coacervation, takes place in vitro and is sufficient to form a fibrillar structure (5, 34), in vivo evidence of this phenomenon and its role in elastic fiber assembly has yet to be established.
Microfibrils have historically been regarded as serving as a scaffold for elastic fiber assembly (20). Recently, this notion was supported by gene inactivation studies of mouse fibrillin-1 and fibrillin-2, which are the major components of microfibrils (4). Impaired elastic fiber formation in the double-knockout mouse compared to that in the single knockouts provided genetic evidence that two fibrillin proteins function redundantly to aid in the initial assembly of elastic fibers during embryonic development. In addition to fibrillins, a myriad of extracellular matrix (ECM) proteins are associated with elastic fibers (13). Some of these proteins colocalize with elastin or are found at the elastin-microfibril interface (7), whereas others have been identified as microfibril components (11, 35). Based on the accumulating knowledge of biochemical binding profiles of elastic fiber-associated proteins, a sequential interaction between elastin polymers and elastic fiber-associated proteins must take place in a temporally and spatially regulated manner to assemble and form functional elastic fibers in vivo.
Our group and others previously established a mouse model of elastic fiber assembly disorder by inactivating the mouse fibulin-5 gene (fbln5−/−) (22, 36). Elastic fiber defects in fbln5−/− mice were evident at birth and progressively worsened, suggesting an underlying impairment of elastic fiber formation during embryogenesis and postnatal development. The importance of fibulin-5 for elastic fiber assembly has also been supported by genetic mutations found in three cutis laxa patients, including a homozygous point mutation of S227P (8, 16) and a tandem duplication of exons 4 to 9 (17).
Fibulin-5 belongs to the fibulin ECM family, which consists of six members characterized by tandem repeats of calcium binding epidermal growth factor (EGF)-like (CB-EGF) domains and a C-terminal fibulin module (2, 33). Fibulin-1, -2, -4, and -5 colocalize with elastic fibers and bind tropoelastin (19, 27, 36), whereas the elastic fiber binding properties of fibulin-3 and -6 have not been determined. In addition to tropoelastin, fibulin-5 was shown to bind N-terminal fragments of fibrillin-1 without interfering with either the homophilic interaction of fibrillin-1 or the heterotypic interaction of fibrillin-1 with tropoelastin (9). Binding of fibulin-5 to the integrin receptors αVβ3, αVβ5, and α9β1 in adhesion assays (22, 23) suggests that fibulin-5 may serve to anchor tropoelastin to surrounding cells during assembly and/or final organization of functional elastic fibers. Fibulin-5 is also capable of tethering extracellular enzymes to elastic fibers. For example, fibulin-5 appears to be required for the correct localization of lysyl oxidase like-1 (LOXL-1), an elastin cross-linking enzyme critical for maintenance of elastic fiber homeostasis in adult mice (15).
Ultimately, an understanding of the general mechanism of elastic fiber assembly, as well as the tissue-specific organization of elastic fibers, will be critical to build a basis for therapeutic regeneration of elastic fibers. In the present study, we focused on the skin of fbln5−/− mice and performed a systematic characterization of the dermal phenotype. Structure-function analyses were also performed to determine the domain of fibulin-5 that binds elastin and to characterize other functional domains of fibulin-5. Finally, we provided genetic evidence that fibulin-5 is sufficient to facilitate de novo synthesis of elastic fibers.
MATERIALS AND METHODS
Mice.
Fbln5−/− mice were maintained with a C57BL6 × 129/SvEv hybrid background and kept on a 12-h/12-h light/dark cycle under specific-pathogen-free conditions (36). Wild-type or heterozygous littermates were used as a control. All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committees.
Plasmid constructs and generation of stable cell lines.
Full-length rat fbln5 cDNA was used as a template to generate deletion mutants by PCR. PCR-amplified products were ligated into the pcDNA5.0/FRT/V5-His TOPO plasmid vector (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Expression constructs and pOG44 encoding the Flp recombinase were cotransfected into Flp-In-CHO cells (Invitrogen) using Fugene 6 (Roche, Germany), and stable transformants were selected with 200 μg/ml of hygromycin B (Roche) for 10 to 14 days. Expression of mutant proteins and their secretion from cells were examined by Western blotting using acetone-precipitated conditioned media collected from stably transformed cells after serum starvation overnight. Primer sequences used to generate deletion constructs are available upon request.
Semiquantitative RT-PCR.
Dorsal skin was harvested from wild-type mice aged from 1 day after birth (P1) and P360. Total RNA was isolated using Trizol (Invitrogen) according to the manufacturer's protocol and treated with DNase I (DNA free; Ambion, Austin, TX). Random hexamer-primed first-strand cDNAs were synthesized from 1 μg of total RNA by using Superscript II (Invitrogen). PCR was performed with 2 μl of the cDNA and 0.1 μCi of [32P]dCTP, using gene-specific primers within the linear range for each condition. Primer sequences for reverse transcription-PCR (RT-PCR) are available upon request.
Histology and immunostaining.
Hematoxylin-and-eosin staining was used for routine histological observation and modified Hart's and Masson-Trichrome staining for visualization of elastic fibers and collagen fibers, respectively. Immunostaining with rabbit polyclonal anti-fibulin-5 (BSYN 1923; 1:200) and antitropoelastin (1:200; generous gift from Robert P. Mecham) were performed on paraffin-embedded skin sections fixed in 4% paraformaldehyde. Briefly, deparaffinized sections were blocked in 3% bovine serum albumin (BSA) for 1 h at room temperature. Primary antibodies were diluted in 3% BSA and incubated on sections for 2 h at room temperature. After washing five times for 5 min (each) in phosphate-buffered saline (PBS), sections were incubated with biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories, CA) for 30 min at room temperature. Immunoreactivity was detected by using the Vectastain ABC kit (Vector Laboratories), using diaminobenzidine as a substrate.
In situ hybridization analysis.
[35S]UTP-labeled antisense riboprobes for fbln5 and eln were transcribed and hybridized as previously described (30) and exposed for 28 days.
Desmosine and hydroxyproline analysis.
Dorsal skin was harvested from 3-month-old mice, and desmosine and hydroxyproline measurements were performed as previously described (31).
Isolation of MEF cells.
Mouse embryonic fibroblast (MEF) cells were isolated from E13.5 embryos. After the embryos were harvested, the head and internal organs were removed and remaining tissues were passed through a sterile 18-gauge hypodermic needle. Cells were grown in 10-cm tissue culture dishes in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (FBS), 100 units/ml of penicillin G, and 100 μg/ml streptomycin. Cells with passage numbers from 3 to 5 were used for the experiments.
Immunoprecipitation (IP) assays.
COS cells were plated in a 6-well dish at 105 cells per well. Flag- or V5-tagged wild-type fibulin-5 was separately transfected into COS cells by Fugene 6, following the manufacturer's instructions. Cells were switched to serum-free medium 24 h posttransfection and starved for an additional 24 h. Conditioned media were collected and centrifuged at 14,000 rpm for 5 min to remove cell debris. Conditioned media containing V5- or Flag-tagged fibulin-5 (500 μl each) were either mixed together directly or mixed after incubation with 10 mM EGTA and 10 mM EDTA for 5 min at room temperature. Conditioned media were then incubated with 1 μg/ml anti-Flag antibody (Sigma, St. Louis, MO) at 4°C for 1 h with agitation, followed by the addition of 20 μl of resuspended protein-A beads (Zymed) and incubated overnight with agitation. Beads were washed three times with PBS and incubated in 2× sodium dodecyl sulfate (SDS) gel sample buffer for 5 min at 90°C. Ten microliters of each sample was subjected to Western blot analysis using anti-V5 (1:5,000; Invitrogen) or anti-FLAG (1:4,000; Sigma, St. Louis, MO).
Preparation of conditioned media or cell lysates containing wild-type or mutant fibulin-5 proteins.
Flp-In-CHO cells expressing various constructs of fibulin-5 were grown in 10-cm dishes to semiconfluence and serum starved in 7 ml of F12 medium (Invitrogen) overnight. Conditioned media were harvested and quickly spun down to remove cell debris. For cell lysate preparation, cells were washed twice in PBS and scraped up in 2 ml of NP-40 buffer (1% NP-40, 50 mM Tris, pH 8.0, 150 mM NaCl) supplemented with a Complete Mini Tablets protease inhibitor cocktail (EDTA-free; Roche, Germany). Cell debris was removed by centrifugation at 14,000 rpm for 10 min, and supernatants were collected and dialyzed against TBS buffer (25 mM Tris, pH 7.5, 100 mM NaCl) at 4°C overnight to remove detergent.
Solid-phase binding assays.
Histidine-tagged bovine tropoelastin (exons 2 to 36) in pQE30 (a generous gift from R. P. Mecham) was expressed in the M15 bacterial strain and purified using a Ni2+ affinity column (Probond; Invitrogen). Bovine alpha-elastin was purchased from Elastin Products Company (Owensville, MO). Solid-phase binding assays were carried out as previously described (24) with modifications. Briefly, 96-well MaxiSorp plates (Nunc) were coated with 1 μg/well recombinant bovine tropoelastin or bovine alpha-elastin in bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.2) overnight at 4°C. Plates were washed three times with PBS and then blocked with 5% nonfat milk in TBS for 1 h at room temperature. Conditioned media or cell lysates containing V5-tagged recombinant proteins were added in triplicate in a serial dilution in TBS-2% milk with 2 mM CaCl2 and incubated at 37°C for 2 h. Plates were washed five times with TBS-Tween 20 (0.025%) containing 2 mM CaCl2. Anti-V5 was diluted in TBS-2% milk (1:5,000) and incubated for 1.5 h at room temperature. Goat anti-mouse antibody conjugated with horseradish peroxidase (Bio-Rad, Hercules, CA) diluted 1:3,000 in TBS-2% milk was incubated for 1 h at room temperature. Plates were washed and subjected to a colorimetric reaction using a horseradish peroxidase substrate reagent kit (BD Biosciences). Absorption at 470 nm was measured using a FluoroStar automatic plate reader (BMG, Germany).
Matrix binding assay.
Fetal bovine chondrocytes (FBCs) (a generous gift from R. P. Mecham) were plated at 2 × 105 cells on a coverglass in a six-well dish in Dulbecco's modified Eagle medium containing 10% FBS, penicillin G, and streptomycin and were allowed to grow for 7 days to ensure abundant ECM deposition. The medium was changed to serum-free conditioned medium containing various fibulin-5 proteins and incubated for 16 to 24 h unless otherwise indicated. Cells were washed three times with PBS and fixed in 4% paraformaldehyde for 15 min at 4°C. After washing with PBS three times, cells were blocked with 1% BSA and 1% normal goat serum for 1 h at room temperature. Primary antibodies for elastin (BA4; a generous gift of R. P. Mecham; 1:100) (PR396; Elastin Products Company, 1:100), fibrillin-1 (a generous gift of R. P. Mecham; 1:200), and anti-V5 antibody (1:500) in 1% BSA were incubated 2 h at room temperature. Cells were washed four times in PBS before incubation with fluorescein isothiocyanate- or Texas red-conjugated secondary antibody (1:200; Vector Laboratories) in 1% BSA for 30 min at room temperature in the dark. After washing with PBS, coverslips were mounted with Vectashield (Vector Laboratories) containing 4′,6′-diamidino-2-phenylindole (DAPI). Slides were viewed by fluorescence microscopy (Leica). Fibulin-5 protein expression in conditioned medium was confirmed before and after incubation with FBCs by Western blotting.
In vivo rescue by adenovirus-mediated gene transfer.
V5 epitope-tagged wild-type fibulin-5 and the Δ1 mutant (lacking residues 41 to 68) were cloned into the pACCMVpLpA (−) loxP-SSP vector and prepared as previously described (1). Empty vector or adenovirus encoding β-galactosidase (β-Gal) was used as a negative control. For high-titer injection, viruses were propagated on 911 cells and purified using discontinuous cesium chloride gradients as previously described (10). A total of 50 μl of PBS containing adenovirus was subcutaneously injected into three midline injection points in the dorsal skin of P2 pups. Dorsal skin was harvested after 19 days (P21 pups), fixed in 10% formalin, and processed for hematoxylin-and-eosin and Hart's staining. Regeneration of elastic fibers was evaluated in a blinded manner. Four pictures were captured from each skin sample, and Photoshop grids were overlaid as an arbitrary unit for quantification. Fibers with lengths greater than 30 pixels (2 grids) were counted.
Statistical analysis.
Statistical analysis was performed using the unpaired Student's t test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Fibulin-5 and elastin are synthesized during neonatal period in the murine dermis.
To systematically examine postnatal changes in the expression of elastic-fiber-associated genes, we performed semiquantitative RT-PCR analysis using RNA extracted from the dorsal skin of P1 to P360 mice. As Fig. 1A shows, expression of the elastin gene (eln) and fbln5 were highest at birth and sharply declined by P10. Fbln3 and fbln4, the elastin cross-linking enzymes, LOX and LOXL-1, and the microfibril components, fibrillin-1, fibrillin-2, LTBP-1, and LTBP-2, showed a similar expression pattern (data not shown). In contrast, fbln1 and fbln2 showed a mild increase after birth and reached a plateau after P45.
FIG. 1.
Expression analysis of elastic fiber-associated molecules in the skin. A. Semiquantitative RT-PCR for elastin (eln) and elastic fiber-associated genes during postnatal development. Expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase expression (G3pdh). Each time point represents at least five animals. B, C. In situ hybridization of P8 skin with eln (B) and fbln5 (C) riboprobes. (B) Eln is expressed in the upper dermis around hair follicles (double arrows) and in the blood vessel wall (arrow). A single layer of elastin-expressing cells is seen at the base of dermis (arrowhead in B). (C) Fbln5 shows a similar expression pattern but is less intense in the upper dermis (double arrows). Strong expression is seen in dermal vessels (arrow). D, E. Immunohistochemistry of P8 skin. Elastin (D) and fibulin-5 (E) colocalized in the upper dermis (ud), dermal vessels (V), and in a layer adjacent to the panniculus carnosus muscle (arrowheads). Bars indicate 60 μm.
To identify the specific locations of eln and fbln5 expression, in situ hybridization was performed on P8 skin. P8 is an age when elastic fibers are visible by Hart's staining (data not shown). Strong expression of eln was detected in the upper dermis, around dermal vessels, and in a layer below the hypodermis near the panniculus carnosus muscle (Fig. 1B). Although fbln5 expression was weaker than that of eln, colocalization of gene expression was observed (Fig. 1C). Immunohistochemistry showed a corresponding distribution of both proteins in the skin (Fig. 1D and E). These data indicate that proteins required for elastic fiber assembly are synthesized during early postnatal development and that fibulin-5 and elastin are synthesized at the same site and colocalize in the dermis.
Dual role of fibulin-5 in assembly and maintenance of elastic fibers in the skin.
Dermal elastic fibers located between hair follicles and at the epidermal-dermal junction are severely disrupted in fbln5−/− mice (36). Consistent with our histological findings, the elastin-specific cross-link desmosine was markedly decreased in fbln5−/− skin (Fig. 2A). This finding is distinct from the aorta, where only a mild decrease in the desmosine content was observed (36). Collagen fiber ultrastructure was indistinguishable between wild-type and fbln5−/− skin (data not shown), and hydroxyproline, a measure of collagen content, was unchanged (Fig. 2B). To rule out the possibility that the absence of fibulin-5 in the dermis altered the expression of eln and elastic fiber-associated genes, semiquantitative RT-PCR was performed using P1 skin RNA. No difference in eln expression was observed between wild-type and fbln5−/− mice (Fig. 2C), and other elastic fiber-associated genes, including fbln1-4, fibrillin-1 and -2, LOX, and LOXL-1 showed the same result (data not shown). In addition, no difference was observed at the protein level for tropoelastin, LOX, and LOXL-1 by Western blot analysis (Fig. 2D). Consistent with these data, robust elastin immunoreactivity was found in the reticular dermis of P8 wild-type (Fig. 2E, panel a) and fbln5−/− (Fig. 2E, panel b) skin, whereas no staining was observed in the P2 eln−/− skin (Fig. 2E, panel c). These data indicate that major components of elastic fibers are present at the time of elastic fiber assembly in the fbln5−/− skin.
FIG. 2.
Defective dermal elastic fiber assembly in fbln5−/− mice. A, B. Desmosine and hydroxyproline measurements. Dorsal skin from 3-month-old wild-type (WT) (n = 11), heterozygous (Het) (n = 7), or fbln5−/− (KO) (n = 16) mice was used for desmosine analysis, and that from wild-type (n = 8) or fbln5−/− (n = 8) mice for hydroxyproline. Desmosine content was significantly reduced in fbln5−/− skin, whereas no difference was seen in hydroxyproline content between the genotypes. Data are expressed as means ± standard errors. n.s., not significant. C. RT-PCR showing eln expression in P1 skin. D. Western blots showing tropoelastin (TE), lysyl oxidase (LOX), and lysyl oxidase-like 1 (LOXL-1) content in P1 skin. Alpha-tubulin (α-tbin) is shown as a loading control. E. Elastin immunostaining of P8 skin harvested from wild-type (panel a) or fbln5−/− (panel b) mice. Robust immunoreactivity to elastin was seen for both genotypes. Elastin immunostaining in P2 eln−/− skin serves as a negative control (panel c). Fibulin-5 immunostaining of wild-type (panels d and e) or fbln5−/− (panel f) skin at 3 months. Intense immunoreactivity was observed around hair follicles, whereas no staining was observed in the fbln5−/− skin. Bars, 40 μm.
In adult skin, the diffuse fibulin-5 immunoreactivity observed in the P8 reticular dermis became more focused (Fig. 2E, panel d), and strong fibulin-5 staining in distinct fibrillar structure was observed around hair follicles (Fig. 2E, panel e). No staining was observed in adult fbln5−/− skin (Fig. 2E, panel f). We also observed that LOXL-1 staining was not significantly different between wild-type and fbn5−/− skin at P8 but was reduced in the adult fbln5−/− skin (data not shown). Interestingly, LOXL-1 staining seen in the epidermis of newborn mice becomes predominantly perifollicular in old mice (12). The redistribution of fibulin-5 in adult skin, from diffuse to specifically localized, together with progressive worsening of the dermal phenotype in fbln5−/− mice, suggests that fibulin-5 plays a dual role both in the assembly of elastin into elastic fibers during development and in the maintenance of the structural integrity of dermal elastic fibers throughout adulthood.
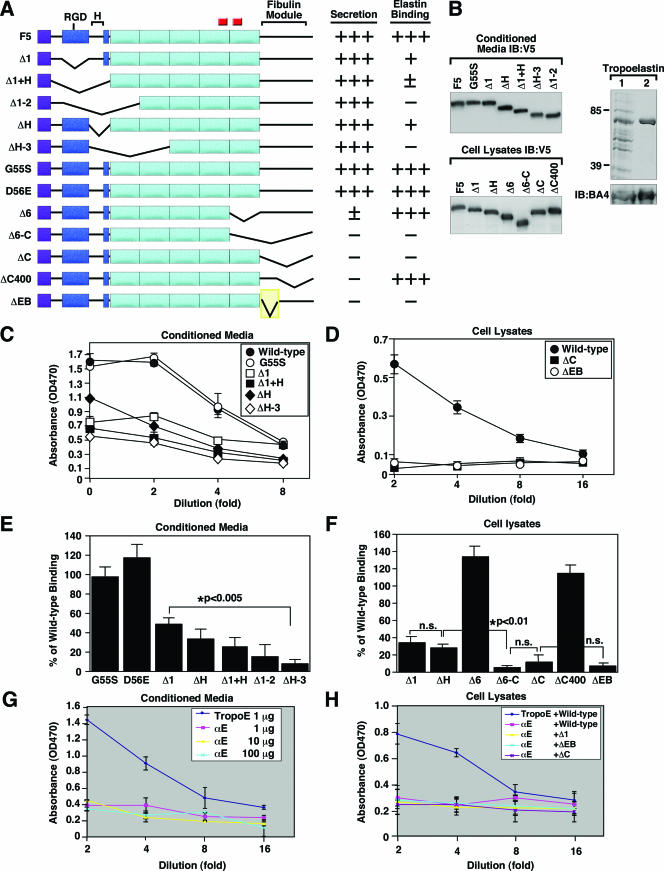
Identification of the elastin-binding regions on fibulin-5.
To gain insight into the molecular mechanism of fibulin-5 in the process of elastic fiber assembly, structure-function analyses were carried out to determine the elastin-binding domain of fibulin-5. Stable CHO cells expressing various forms of fibulin-5 deletion mutants were established (Fig. 3A). Deletion of the C-terminal portion of fibulin-5 (ΔC) resulted in retention of protein inside the cell, whereas deletions or point mutations of the N-terminal region did not affect secretion (data not shown; summarized in Fig. 3A). Based on these findings, conditioned media from N-terminal mutants and cell lysates from C-terminal mutants were used in solid-phase binding assays. Expression of each mutant protein was examined by Western blot analysis using anti-V5 antibody (Fig. 3B, left; D56E not shown). Bacterium-derived bovine tropoelastin, confirmed by SDS-polyacrylamide gel electrophoresis and Western blotting (Fig. 3B, right), was coated on a 96-well plate and used as the solid phase. Wild-type (F5) and mutant fibulin-5 proteins were used in serial dilution as soluble ligands. Wild-type fibulin-5 from either conditioned media (Fig. 3C) or cell lysates (Fig. 3D) exhibited strong binding to tropoelastin in a dose-dependent manner.
FIG. 3.
Identification of the elastin-binding region of fibulin-5. A. Schematic representation of fibulin-5 mutants. C-terminal elastin-binding region (shaded yellow) is located within the fibulin module. The purple square indicates the signal peptides. The blue box indicates the first CB-EGF domain, separated from the remaining CB-EGF domain (aqua boxes) by a hinge region (H). An RGD motif is located in the first CB-EGF domain, and small red squares indicate glycosylation sites. B. Verification of various fibulin-5 mutant proteins and recombinant tropoelastin. Left panel: Western blot analysis of conditioned media (upper blot) or cell lysates (lower panel) containing recombinant fibulin-5 proteins detected by anti-V5 antibody. Right panel: bacterium-derived tropoelastin (lane 1) was purified through a nickel affinity column (lane 2) and stained with Coomassie blue. Western blots of these samples using antielastin antibody (BA4) is shown below. C, D. Solid-phase binding assays using tropoelastin (1 μg/well) as the solid phase and a serial dilution of conditioned medium (C) or cell lysates (D) as the soluble ligand. Note that wild-type fibulin-5 exhibits strong binding to tropoelastin in a dose-dependent manner. E, F. Binding of fibulin-5 mutants to tropoelastin expressed as a percentage of wild-type fibulin-5 binding. Data were analyzed within a linear range at a 1:4 dilution using conditioned media (E) or cell lysates (F). Note that point mutations of the integrin binding motif (G55S and D56E) did not affect tropoelastin binding; however, the binding was significantly reduced when the N-terminal CB-EGF motifs were sequentially deleted (E). Deletion of the C-terminal EB region (ΔEB) abolished tropoelastin binding. Data from five independent experiments using conditioned media and three independent experiments using cell lysates, performed in triplicate, are shown. G. Solid-phase binding assays using tropoelastin (TropoE) (1 μg/well) or alpha-elastin (αE) (1, 10, or 100 μg/well) as the solid phase and a serial dilution of conditioned medium containing wild-type fibulin-5 as the soluble ligand. Data from two independent experiments performed in triplicate are shown. H. Solid-phase binding assays using tropoelastin (1 μg/well) or alpha-elastin (100 μg/well) as the solid phase and wild-type fibulin-5 or deletion mutants as soluble ligands. Data from two independent experiments performed in triplicate are shown. Data are expressed as means ± standard errors.
To quantify the abilities of various mutant fibulin-5 proteins to bind tropoelastin, absorbance obtained at a 1:4 dilution was expressed as a percentage of that of wild-type fibulin-5. In the N-terminal mutation series, fibulin-5 lacking the first 28 amino acids of the first CB-EGF domain (residues 41 to 68 [Δ1]) or lacking the hinge region (residues 69 to 125 [ΔH]) showed approximately a 50 to 60% reduction of tropoelastin binding compared to the wild-type protein (Fig. 3E). Further deletion of additional CB-EGF domains (ΔH-3) severely impaired tropoelastin binding, indicating that CB-EGF domains are important for binding to tropoelastin. This is consistent with our previous observation that tropoelastin binding of fibulin-5 was inhibited by the absence of Ca2+ (36). In contrast, fibulin-5 with mutated RGD motifs (G55S and D56E) did not affect binding to tropoelastin, indicating that the RGD integrin-binding motif was not required for tropoelastin binding in vitro.
Solid-phase binding assays with the C-terminal mutants were performed using cell lysates (Fig. 3F). Cell lysates from Δ1 and ΔH mutants, included as internal controls, showed tropoelastin binding comparable to that of Δ1 and ΔH conditioned media. Deletion of the sixth CB-EGF domain alone (residues 287 to 341 [Δ6]) or the last 48 amino acids (residues 400 to 448 [CΔ400]) did not affect tropoelastin binding. In contrast, deletion of the sixth CB-EGF domain through the entire C-terminal fibulin module (residues 287 to 448 [Δ6-C]) or deletion of the C-terminal fibulin domain alone (residues 342 to 448 [ΔC]; also shown in Fig. 3D) abolished binding activity, indicating that a specific elastin-binding domain was located between residues 342 and 399. To confirm that this domain is required for tropoelastin binding, a mutant fibulin-5 lacking the putative elastin-binding domain was generated (ΔEB) (shown in Fig. 3A) and used for solid-phase binding. As Fig. 3D and 3F show, the ΔEB mutant showed a marked reduction in tropoelastin-binding activity compared to the wild-type fibulin-5. However, a mutant protein consisting of the C-terminal fibulin module alone was not sufficient to bind tropoelastin (data not shown). These results indicate that the C-terminal elastin-binding region cooperates with CB-EGF domains to bind tropoelastin in vitro. Although the nature of the functional association between the elastin-binding region and CB-EGF domains remains to be addressed, the rigidity of the CB-EGF domains may impart a necessary structural stability to the elastin-binding domain to allow for binding.
Finally, we asked whether fibulin-5 was able to bind mature cross-linked elastin. We performed solid-phase binding assays using alpha-elastin, a solubilized form of insoluble elastin, as the solid phase and wild-type and three deletion mutants (Δ1, ΔC, and ΔEB) as soluble ligands. As Fig. 3G shows, wild-type fibulin-5 binding to alpha-elastin was markedly decreased compared to binding to the tropoelastin monomer, and none of the fibulin-5 deletion mutants bound alpha-elastin (Fig. 3H). These data suggest that fibulin-5 preferentially binds the monomeric form of elastin and thus may function as an adaptor protein at the cell surface.
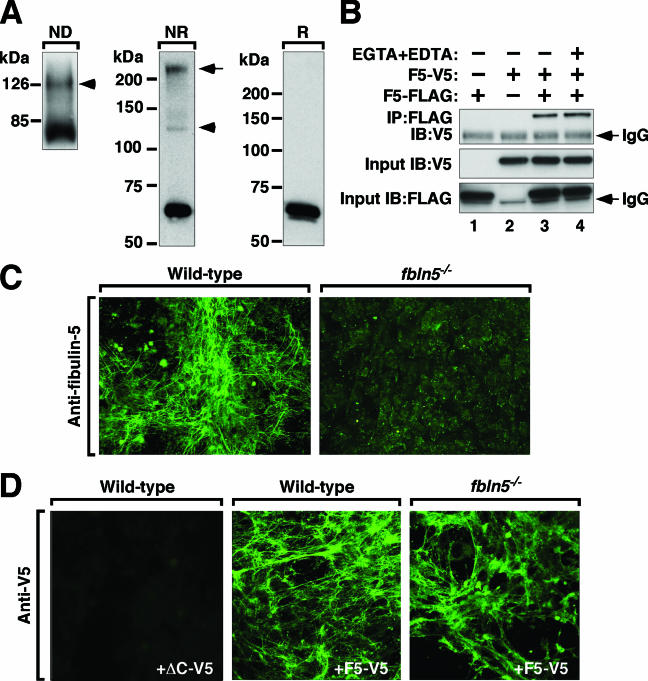
Soluble fibulin-5 is incorporated into a matrix scaffold.
The molecular organization of fibulin-5 in the ECM is unknown. In vivo, fibulin-5 staining shows a fibrillar pattern (Fig. 2E, panel e), suggesting that the protein assembles together with other fibrillar ECM proteins. Alternatively, fibulin-5 may self-assemble into higher-order structures with the potential of forming an independent fibrillar assembly. To better understand the nature of fibulin-5 in the matrix, we first tested whether fibulin-5 can undergo a homophilic interaction, as was suggested for fibulin-2, where dimerization facilitates macromolecular interactions (28). Acetone-precipitated, conditioned medium containing V5-tagged fibulin-5 (F5-V5) showed two bands at approximately 66 kDa and 130 kDa in a nondenaturing gel corresponding to a monomer and dimer form of fibulin-5 (Fig. 4A, ND). SDS-polyacrylamide gel electrophoresis under nonreduced conditions (Fig. 4A, NR) showed two higher-molecular-weight bands corresponding to the dimer and an oligomer which disappeared under reducing conditions (Fig. 4A, R). These results suggest that dimerization/oligomerization of fibulin-5 is dependent on disulfide bonds.
FIG. 4.
Homophilic interaction of fibulin-5. A. Monomeric and dimeric (arrowhead) forms of fibulin-5 were detected on a nondenaturing gel, followed by Western blotting using anti-V5 antibody (ND). Under nonreducing conditions (NR) on a denatured gel, the dimer (arrowhead) and a higher-molecular-weight band (arrow) were detected. These bands were undetectable in reducing condition (R), and only monomeric fibulin-5 was seen. B. IP assays using a full-length fibulin-5 tagged with V5 (F5-V5) or Flag (F5-Flag). Results indicate that F5-V5 and F5-Flag can form a dimer. Dimer formation was not affected by the presence of 10 mM EGTA and EDTA, indicating that Ca2+ is not required for dimer formation. A nonspecific immunoglobulin G chain can be seen in some blots (IgG). C. MEF cells were prepared from E13.5 wild-type (left) or fbln5−/− (right) embryos, grown to postconfluency, and stained for fibulin-5. D. Binding of exogenous recombinant V5-tagged fibulin-5 to MEF cell matrices. Wild-type and fbln5−/− MEF cells were grown to confluence and incubated with conditioned medium containing F5-V5. Exogenous fibulin-5 binding does not require predeposition of fibulin-5. Incubation with ΔC-V5 serves as a negative control for other proteins present in the conditioned medium, since the ΔC mutant fibulin-5 is not secreted.
We next performed coimmunoprecipitation assays to confirm homophilic binding of fibulin-5 using conditioned medium containing F5-V5, FLAG-tagged fibulin-5 (F5-FLAG), or both (Fig. 4B). F5-FLAG was not detected by anti-V5 (lane 1), and F5-V5 was not pulled down by anti-FLAG (lane 2). Coincubation with F5-V5 and F5-FLAG, followed by IP with anti-FLAG and immunoblotting with anti-V5, showed a band indicating that F5-V5 and F5-FLAG formed dimers (lane 3). Reverse coimmunoprecipitation with anti-V5 antibody followed by anti-FLAG immunoblotting showed the same result (data not shown). The presence of 10 mM EGTA and EDTA did not inhibit dimer formation (lane 4), indicating that Ca2+ was not required for homophilic interaction.
To determine the ability of fibulin-5 to form a homophilic interaction in the context of a preexisting intact ECM, MEF cells from wild-type and fbln5−/− embryos were prepared. To establish the appearance of endogenous fibulin-5 in the ECM, wild-type MEF cells were stained with anti-fibulin-5 antibody. A mesh-like fibulin-5-positive matrix was detected in the wild-type but not in fbln5−/− MEF cells (Fig. 4C). We next determined whether exogenous fibulin-5 could assemble on this preexisting matrix by incubating conditioned medium containing F5-V5 with a confluent monolayer of wild-type and fbln5−/− MEF cells and staining for V5 (Fig. 4D). No staining was detected in wild-type MEF cultures incubated with control medium from the fibulin-5 ΔC mutant, a mutant that is not secreted into the medium (Fig. 4D, +ΔC-V5). In contrast, both wild-type and fbln5−/− cells incubated with F5-V5 showed a fibrillar matrix pattern (Fig. 4D, +F5-V5). Since these results indicate that exogenous fibulin-5 is able to bind a fibrillar matrix in the absence of preassembled fibulin-5, other ECM proteins must mediate binding of fibulin-5 to the matrix.
Role of CB-EGF domains in matrix binding.
To further investigate the matrix binding ability of fibulin-5, we performed in vitro matrix binding assays using fetal bovine chondrocytes (FBCs). FBCs deposit large amounts of elastic fiber-associated ECM proteins, and their assembly of elastic fibers has been well characterized (25). Since CB-EGF domains are known to be involved in matrix protein-protein interaction (18), we focused on the N-terminal mutants of fibulin-5 to assess matrix binding ability. A confluent monolayer of FBCs was grown for 7 days in the presence of 10% FBS, followed by incubation for 24 h with serum-free conditioned medium containing F5-V5. As was shown for the MEF cells, FBCs incubated with F5-V5 exhibited a strong matrix staining pattern for fibulin-5, and this staining partially colocalized with endogenous elastin fibers (Fig. 5A). We also detected partial colocalization of fibulin-5 and fibrillin-1 fibers (Fig. 5B, +F5-V5, anti-V5, and anti-fibrillin-1).
FIG. 5.
Matrix binding domain of fibulin-5. A. Cell-based matrix binding assays. Confluent FBCs were incubated with conditioned media containing F5-V5 or ΔC-V5 in the absence of serum for 24 h, followed by immunostaining with anti-V5 or antielastin (PR396) antibodies. Double staining of the FBC matrix shows partial colocalization of fibulin-5 and elastin. Incubation with ΔC-V5 and staining for V5 served as a negative control. B. FBCs were incubated with F5-V5 or Δ1-V5 and stained with DAPI, anti-V5, anti-fibrillin-1, and antielastin antibodies. Δ1-V5 showed a severe reduction in matrix binding but did not affect endogenous elastin or fibrillin-1 matrix formation. Protein content in the media after 24 h of incubation with FBC cells was evaluated by Western blot analysis (right). C. FBCs were incubated with ΔC-V5, F5-V5, ΔH-V5, or a 1:1 ratio of F5-V5 and Δ1-V5 in the absence of serum for 24 h and stained with DAPI and anti-V5 antibody. The ΔH mutant showed a fibrillar matrix pattern. Coincubation of F5-V5 with Δ1-V5 did not affect matrix binding of the wild-type fibulin-5.
Next, we used N-terminal deletion mutants, which are fully secreted into media, to test if CB-EGF domains are critical for matrix binding in vitro. The Δ1 mutant protein showed markedly reduced matrix binding without affecting the formation of the endogenous elastin or fibrillin-1 matrix (Fig. 5B). Western blot analysis using the media harvested after 24 h of incubation with FBCs showed comparable amounts of the wild-type and Δ1 mutant proteins (Fig. 5B), indicating that the compromised matrix binding was not due to increased protein degradation. Similar results were obtained with the Δ1+H and ΔH-3 mutants (data not shown). In contrast, the ΔH mutant lacking only the hinge region (residues 69 to 125 [ΔH]) showed matrix binding comparable to that of F5-V5 (Fig. 5C, ΔH). Matrix binding of F5-V5 was not affected by coincubation with the Δ1 mutant, indicating that the Δ1 mutant does not act in a dominant-negative manner (Fig. 5C, F5-V5:Δ1-V5).
De novo synthesis of elastic fibers by adenovirus-mediated gene transfer of fibulin-5 in vivo.
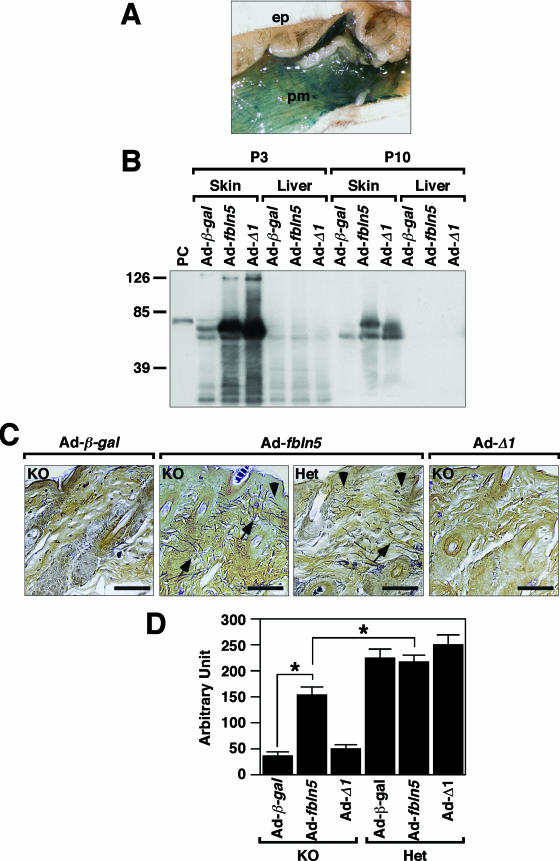
Because exogenous fibulin-5 could become incorporated into a preexisting ECM, we were interested in testing whether exogenous expression of fibulin-5 would be sufficient to induce elastic fiber assembly in vivo by adenovirus-mediated gene transfer. Since the ΔEB mutant protein is retained inside the cell (data not shown), we used the Δ1 mutant construct for the adenovirus assays (Ad-Δ1). As shown above, the Δ1 mutant contains an intact C-terminal elastin-binding region and is secreted into media but exhibits a marked reduction in matrix binding activity in vitro. Using this mutant, we could test whether the C-terminal elastin-binding region was sufficient to assemble elastic fibers or whether the N-terminal matrix binding domain was required for de novo, in vivo assembly of elastic fibers.
Preliminary experiments using adenovirus encoding β-Gal (Ad-β-gal) showed intense LacZ staining in the skin 19 days after injection, with LacZ-positive staining detected in adipocytes and panniculus carnosus muscle cells below the hypodermis (Fig. 6A; also data not shown). No staining was observed in the epidermis or dermal fibroblasts, nor in remote organs, such as the liver and lungs (data not shown). Two titers of adenovirus (low, 5 × 108 PFU/mouse; high, 1 × 1011 virus particles/mouse) encoding wild-type fbln5 (Ad-fbln5) or the Δ1 mutant (Ad-Δ1) or Ad-β-gal were injected into the dorsal skin of P2 fbln5−/− and fbln5+/− pups (summarized in Table1). Western blot analysis using anti-V5 antibody revealed strong production of fibulin-5 in Ad-fbln5- and Ad-Δ1-infected skin on P3; however, protein production was significantly reduced by P10 (Fig. 6B) and became undetectable by P21 (data not shown). No fibulin-5 protein was detected in the liver, indicating that the exogenous fibulin-5 did not enter systemic circulation (Fig. 6B).
FIG. 6.
Adenovirus-mediated gene transfer of fbln5 in the skin. A. LacZ staining of Ad-β-gal-injected skin at P21; epidermis, ep; panniculus carnosus muscle, pm. B. Protein expression of adenovirus-derived fibulin-5 in the skin and liver. Dorsal skin injected with high-titer adenovirus was harvested on P3 or P10 and subjected to Western blot analysis using anti-V5 antibody. Robust production of full-length fibulin-5 (from Ad-fbln5 injection) or Δ1 mutant fibulin-5 (from Ad-Δ1 injection) was observed in the skin on P3; however, protein content was significantly decreased by P10. C. Hart's elastic fiber staining of fbln5+/− and fbln5−/− skin injected with Ad-fbln5, Ad-Δ1, or Ad-β-gal on P2 and harvested on P21. Long bundles of elastic fibers were regenerated in Ad-fbln5-injected skin (arrows) but not Ad-Δ1-injected skin. Arrowhead indicates elastic fibers linking to epidermis. Bars, 40 μm. D. Quantification of regenerated elastic fibers. Bars are means ± standard errors. Asterisks indicate P values of <0.05 in comparison between fbln5−/− (KO) skin injected with Ad-β-gal and Ad-fbln5- or Ad-fbln5-injected KO and heterozygous (Het) skin.
TABLE 1.
Adenovirus-mediated gene transfer in the skin
| Injection size and mouse genotypea | No. of mice injected with:
|
||
|---|---|---|---|
| Ad-Control | Ad-fbln5 | Ad-fbln5 Δ1 | |
| Low titerb (5 × 108 PFU/mouse) | |||
| KO | 5 | 8 | 5 |
| Het | 2 | 0 | 4 |
| High titerc (1011 particles/mouse) | |||
| KO | 6 | 5 | 4 |
| Het | 2 | 3 | 3 |
KO, fbln5−/−; Het, heterozygous.
Empty adenovirus (Ad-RR5) was used as a control in low-titer experiments.
Adenovirus encoding β-Gal (Ad-β-gal) was used as a control in high-titer experiments.
To determine the effect of exogenous expression of fibulin-5 on the assembly and organization of elastic fibers in the dermis, dorsal skin harvested between the scapula and iliac crest was examined by Hart's staining for elastin. In the high-titer group, Ad-β-gal-injected fbln5−/− skin showed severely disrupted elastic fibers in the upper dermis (Fig. 6C, Ad-β-gal). In contrast, Ad-fbln5-injected fbln5−/− skin showed focal accumulations of long, nondisrupted elastic fibers interposed between hair follicles and linking to the epidermis (Fig. 6C, KO/Ad-fbln5). These fibers resemble those detected in fbln5+/− mouse (Fig. 6C, HET/Ad-fbln5). Although the extent of rescue was partial at the whole-tissue level, the assembly defect and regeneration of elastic fibers were significantly rescued at the molecular and local level. The low-titer group showed similar results but with a less improved elastic fiber assembly than that of the high-titer group (data not shown). Of interest, Ad-Δ1-injected fbln5−/− skin was indistinguishable from Ad-β-gal-injected fbln5−/− skin, with no areas of intact elastic fibers observed (Fig. 6C, KO/Ad-Δ1).
Quantification of the fibers revealed a statistically significant increase of elastic fibers in Ad-fbln5-injected fbln5−/− skin compared with Ad-β-gal- or Ad-Δ1-injected fbln5−/− skin; however, the quantity was still less than that of fbln5+/− mice (Fig. 6D). No enhancement of elastic fiber formation was seen in Ad-fbln5-injected fbln5+/− skin compared to results with Ad-β-gal-injected skin. This suggests that the amount of fibulin-5 present in fbln5+/− skin is functionally saturated with respect to its role in elastic fiber assembly. Of note, injecting the Ad-Δ1 mutant did not disturb elastic fiber formation in fbln5+/− skin, supporting our in vitro observation that the Δ1 protein does not act in a dominant-negative manner. Overall, our results indicated the following: (i) transient expression of fbln5 at the time of assembly can promote elastic fiber formation in vivo, (ii) Δ1 mutant fibulin-5 fails to rescue the skin phenotype even with an intact elastin-binding region in the C terminus, and (iii) the matrix binding and/or a cell attachment domain of fibulin-5 may be necessary for elastic fiber assembly.
DISCUSSION
Initial formation of elastic fibers and maintenance of assembled fibers are critical for proper functioning of elastogenic tissues. In fbln5−/− mice, an elastic fiber-specific defect is evident as early as P8 at the microscopic level (Q. Zheng et al., unpublished observation), and this time point coincides with initiation of dermal elastic fiber assembly that normally occurs in wild-type mice. The absence of fibulin-5 does not affect the expression of elastic fiber-associated genes, indicating that the primary defect is in the assembly of elastic fibers in fbln5−/− skin. In addition, we found that fibulin-5 is also involved in the subsequent organization of elastic fibers, since (i) fibulin-5 localizes in the upper dermis at the time of elastic fiber assembly but becomes more intense at the follicular-dermal junction in later development, and (ii) fbln5−/− MEF cells assemble elastin-positive fibers in vitro, but fbln5−/− skin lacks intact elastic fibers (22; also our unpublished observation). These results indicate that fibulin-5 is required for a higher order of organization of mature elastic fibers in vivo.
Interaction between fibulin-5 and elastin.
We showed that fibulin-5 preferentially bound the monomeric form of elastin, and both N-terminal and C-terminal regions of the protein were critical for elastin binding. Although deletion of 58 amino acid residues located in the C-terminal fibulin-type module abolished the ability to bind elastin, this region was not sufficient for elastin binding in vitro. Serial deletions of the N-terminal CB-EGF domains showed a significant reduction in elastin binding, and Ad-Δ1 failed to regenerate elastic fibers in an in vivo rescue experiment. These results indicate that the presence of the C-terminal elastin-binding region alone is not sufficient to mediate assembly of elastic fibers, but rather it requires CB-EGF domains to potentiate tropoelastin binding and/or to bind to the matrix scaffold in vivo. An interaction between fibulin-4 and tropoelastin has been established in vitro, and fbln4−/− mice develop severe elastic fiber defects that result in aortic aneurysms and perinatal death (19). Thus, identification of elastin-binding domain(s) of fibulin-4 and sequence comparison with fibulin-5 may provide further insight into the interaction between fibulins and tropoelastin, as well as identifying potential differential roles of fibulin-4 and fibulin-5 in elastic fiber assembly.
Alignment of cells, matrix scaffold, elastin, and cross-linking enzymes by fibulin-5.
Involvement of cell surface binding of elastin following its secretion into media has been previously demonstrated by time-lapse imaging studies using a bovine tropoelastin timer reporter fusion protein in vitro (6, 14). In these studies, newly synthesized tropoelastin was shown to form small aggregates called “globules” on the cell surface, which were transferred to preexisting elastic fibers to form larger aggregates and ultimately fibers. Since fibulin-5 has been shown to promote cell attachment in an RGD-dependent manner (23), fibulin-5 may mediate this cell surface tethering of elastin in concert with molecules previously shown to be involved in elastin binding, such as glycosaminoglycans (3), integrins (26) and elastin binding protein (21). Characterization of a mouse strain expressing a mutant form of fibulin-5 in which the integrin binding RGD motif is mutated to RGE is under way and will elucidate the role of cell surface binding of fibulin-5 during the initial elastin assembly (Yanagisawa et al., unpublished data).
In the present study, we demonstrated that the first CB-EGF domain is involved in matrix binding and that fibulin-5 partially colocalizes with fibrillin-1. In contrast, our previous data using solid-phase binding showed that fibulin-5 does not display a strong binding affinity for either the C-terminal or N-terminal half of fibrillin-1 (36). This difference suggests that a fibulin-5 binding site on fibrillin-1, mediated by the first CB-EGF domain of fibulin-5, may be more efficiently exposed in a cell-based assay. We cannot formally exclude the possibility, however, that other microfibrillar proteins produced by FBCs may have mediated the binding of fibulin-5 to fibrillin-1. The proenzyme regions of LOX and LOXL-1 bind to a C-terminal fragment of tropoelastin, and this binding is required for deposition of both enzymes onto elastic fibers in vitro (32). In addition, our previous report indicated that LOXL-1 binds the C terminus of fibulin-5, and this binding is required for tethering of LOXL-1 to elastic fibers in adult skin (15). Therefore, we speculate that fibulin-5 may act as an adapter protein that binds to the secreted tropoelastin and to the fibrillin-containing matrix scaffold, thus facilitating cross-linking of LOXL-1 to tropoelastin during the initial fiber assembly.
Fibulin-5 as potential therapeutic target for cutis laxa syndrome.
Adenovirus-mediated gene transfer by subcutaneous injection was previously shown to induce strong expression of exogenous genes in various dermal cells (29). Using a similar strategy, we have demonstrated that a single injection of Ad-fbln5 provided transient but sufficient production of fibulin-5 during the early neonatal period to regenerate elastic fibers in fbln5−/− mice. These findings clearly indicate that despite preferential adenovirus transduction to adipocytes and pannicular muscle cells in our animals, fibulin-5 is secreted and redistributed to the reticular dermis, where it initiates the assembly of elastic fibers. Our in vitro data showing that exogenously administered soluble fibulin-5 can become incorporated into a fibulin-5-null matrix scaffold supports the use of soluble fibulin-5 as a potential therapeutic reagent. A complete rescue of elastic fibers, however, may require a higher efficiency of gene delivery, sustained expression of virus-derived fibulin-5, and/or coexpression of elastin and fibulin-5 in the same cell type during the assembly period.
Acknowledgments
We thank members of the Histology Core Lab at University of Texas Southwestern Medical Center for excellent histological preparations, Robert Mecham, Dean Li, and Thomas Broekelmann for reagents, and Shelby Chapman, Andrew Hall, and Seiji Yokoyama for technical assistance. We also thank Alisha Tizenor for graphic assistance and Eric Olson for a critical reading of the manuscript.
This work was supported by grants from the NIH (HL071157), Skin Disease Research Center at the University of Texas Southwestern Medical Center, and SHISEIDO Grants for Scientific Research. E.C. Davis is supported as the Canada Research Chair.
Footnotes
Published ahead of print on 27 November 2006.
REFERENCES
- 1.Aoki, K., C. Barker, X. Danthinne, M. J. Imperiale, and G. J. Nabel. 1999. Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol. Med. 5:224-231. [PMC free article] [PubMed] [Google Scholar]
- 2.Argraves, W. S., L. M. Greene, M. A. Cooley, and W. M. Gallagher. 2003. Fibulins: physiological and disease perspectives. EMBO Rep. 4:1127-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broekelmann, T. J., B. A. Kozel, H. Ishibashi, C. C. Werneck, F. W. Keeley, L. Zhang, and R. P. Mecham. 2005. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J. Biol. Chem. 280:40939-40947. [DOI] [PubMed] [Google Scholar]
- 4.Carta, L., L. Pereira, E. Arteaga-Solis, S. Y. Lee-Arteaga, B. Lenart, B. Starcher, C. A. Merkel, M. Sukoyan, A. Kerkis, N. Hazeki, D. R. Keene, L. Y. Sakai, and F. Ramirez. 2006. Fibrillins 1 and 2 p1erform partially overlapping functions during aortic development. J. Biol. Chem. 281:8016-8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox, B. A., B. C. Starcher, and D. W. Urry. 1974. Communication: coacervation of tropoelastin results in fiber formation. J. Biol. Chem. 249:997-998. [PubMed] [Google Scholar]
- 6.Czirok, A., J. Zach, B. A. Kozel, R. P. Mecham, E. C. Davis, and B. J. Rongish. 2006. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. J. Cell Physiol. 207:97-106. [DOI] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Doliana, R., M. Mongiat, F. Bucciotti, E. Giacomello, R. Deutzmann, D. Volpin, G. M. Bressan, and A. Colombatti. 1999. EMILIN, a component of the elastic fiber and a new member of the C1q/tumor necrosis factor superfamily of proteins. J. Biol. Chem. 274:16773-16781. [DOI] [PubMed] [Google Scholar]
- 9.Elahi, E., R. Kalhor, S. S. Banihosseini, N. Torabi, H. Pour-Jafari, M. Houshmand, S. S. Amini, A. Ramezani, and B. Loeys. 2006. Homozygous missense mutation in fibulin-5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. J. Investig. Dermatol. 126:1506-1509. [DOI] [PubMed] [Google Scholar]
- 10.Freeman, L. J., A. Lomas, N. Hodson, M. J. Sherratt, K. T. Mellody, A. S. Weiss, A. Shuttleworth, and C. M. Kielty. 2005. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem. J. 388:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerard, R. D., and R. S. Meidell. 1995. Adenovirus vectors, p. 285-307. In B. D. Hames and D. Glover (ed.), DNA cloning—a practical approach: mammalian systems. Oxford University Press, Oxford, United Kingdom.
- 12.Hanssen, E., F. H. Hew, E. Moore, and M. A. Gibson. 2004. MAGP-2 has multiple binding regions on fibrillins and has covalent periodic association with fibrillin-containing microfibrils. J. Biol. Chem. 279:29185-29194. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, K., K. S. Fong, F. Mercier, C. D. Boyd, K. Csiszar, and M. Hayashi. 2004. Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. J. Mol. Histol. 35:845-855. [DOI] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Reference deleted.
- 16.Kielty, C. M., M. J. Sherratt, and C. A. Shuttleworth. 2002. Elastic fibres. J. Cell Sci. 115:2817-2828. [DOI] [PubMed] [Google Scholar]
- 17.Kozel, B. A., B. J. Rongish, A. Czirok, J. Zach, C. D. Little, E. C. Davis, R. H. Knutsen, J. E. Wagenseil, M. A. Levy, and R. P. Mecham. 2006. Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. J. Cell Physiol. 207:87-96. [DOI] [PubMed] [Google Scholar]
- 18.Liu, X., Y. Zhao, J. Gao, B. Pawlyk, B. Starcher, J. A. Spencer, H. Yanagisawa, J. Zuo, and T. Li. 2004. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 36:178-182. [DOI] [PubMed] [Google Scholar]
- 19.Loeys, B., L. Van Maldergem, G. Mortier, P. Coucke, S. Gerniers, J. M. Naeyaert, and A. De Paepe. 2002. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet. 11:2113-2118. [DOI] [PubMed] [Google Scholar]
- 20.Markova, D., Y. Zou, F. Ringpfeil, T. Sasaki, G. Kostka, R. Timpl, J. Uitto, and M. L. Chu. 2003. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am. J. Hum. Genet. 72:998-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurer, P., and E. Hohenester. 1997. Structural and functional aspects of calcium binding in extracellular matrix proteins. Matrix Biol. 15:569-580; discussion, 581. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin, P. J., Q. Chen, M. Horiguchi, B. C. Starcher, J. B. Stanton, T. J. Broekelmann, A. D. Marmorstein, B. McKay, R. Mecham, T. Nakamura, and L. Y. Marmorstein. 2006. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol. Cell. Biol. 26:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mecham, R. P., and E. C. Davis. 1994. Elastic fiber structure and assembly, p. 281-314. In P. D. Yurchenco, D. E. Birk, and R. P. Mecham (ed.), Extracellular matrix assembly and structure. Academic Press, New York, NY.
- 24.Mochizuki, S., B. Brassart, and A. Hinek. 2002. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J. Biol. Chem. 277:44854-44863. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura, T., P. R. Lozano, Y. Ikeda, Y. Iwanaga, A. Hinek, S. Minamisawa, C. F. Cheng, K. Kobuke, N. Dalton, Y. Takada, K. Tashiro, J. Ross, Jr., T. Honjo, and K. R. Chien. 2002. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415:171-175. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura, T., P. Ruiz-Lozano, V. Lindner, D. Yabe, M. Taniwaki, Y. Furukawa, K. Kobuke, K. Tashiro, Z. Lu, N. L. Andon, R. Schaub, A. Matsumori, S. Sasayama, K. R. Chien, and T. Honjo. 1999. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J. Biol. Chem. 274:22476-22483. [DOI] [PubMed] [Google Scholar]
- 27.Reinhardt, D. P., D. R. Keene, G. M. Corson, E. Poschl, H. P. Bachinger, J. E. Gambee, and L. Y. Sakai. 1996. Fibrillin-1: organization in microfibrils and structural properties. J. Mol. Biol. 258:104-116. [DOI] [PubMed] [Google Scholar]
- 28.Robb, B. W., H. Wachi, T. Schaub, R. P. Mecham, and E. C. Davis. 1999. Characterization of an in vitro model of elastic fiber assembly. Mol. Biol. Cell 10:3595-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodgers, U. R., and A. S. Weiss. 2004. Integrin alpha v beta 3 binds a unique non-RGD site near the C-terminus of human tropoelastin. Biochimie 86:173-178. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki, T., W. Gohring, N. Miosge, W. R. Abrams, J. Rosenbloom, and R. Timpl. 1999. Tropoelastin binding to fibulins, nidogen-2 and other extracellular matrix proteins. FEBS Lett. 460:280-284. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki, T., K. Mann, H. Wiedemann, W. Gohring, A. Lustig, J. Engel, M. L. Chu, and R. Timpl. 1997. Dimer model for the microfibrillar protein fibulin-2 and identification of the connecting disulfide bridge. EMBO J. 16:3035-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setoguchi, Y., H. A. Jaffe, C. Danel, and R. G. Crystal. 1994. Ex vivo and in vivo gene transfer to the skin using replication-deficient recombinant adenovirus vectors. J. Investig. Dermatol. 102:415-421. [DOI] [PubMed] [Google Scholar]
- 33.Spencer, J. A., S. L. Hacker, E. C. Davis, R. P. Mecham, R. H. Knutsen, D. Y. Li, R. D. Gerard, J. A. Richardson, E. N. Olson, and H. Yanagisawa. 2005. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc. Natl. Acad. Sci. USA 102:2946-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starcher, B., and M. Conrad. 1995. A role for neutrophil elastase in the progression of solar elastosis. Connect. Tissue Res. 31:133-140. [DOI] [PubMed] [Google Scholar]
- 35.Thomassin, L., C. C. Werneck, T. J. Broekelmann, C. Gleyzal, I. K. Hornstra, R. P. Mecham, and P. Sommer. 2005. The Pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers. J. Biol. Chem. 280:42848-42855. [DOI] [PubMed] [Google Scholar]
- 36.Timpl, R., T. Sasaki, G. Kostka, and M. L. Chu. 2003. Fibulins: a versatile family of extracellular matrix proteins. Nat. Rev. Mol. Cell Biol. 4:479-489. [DOI] [PubMed] [Google Scholar]
- 37.Vrhovski, B., S. Jensen, and A. S. Weiss. 1997. Coacervation characteristics of recombinant human tropoelastin. Eur. J. Biochem. 250:92-98. [DOI] [PubMed] [Google Scholar]
- 38.Werneck, C. C., B. C. Trask, T. J. Broekelmann, T. M. Trask, T. M. Ritty, F. Segade, and R. P. Mecham. 2004. Identification of a major microfibril-associated glycoprotein-1-binding domain in fibrillin-2. J. Biol. Chem. 279:23045-23051. [DOI] [PubMed] [Google Scholar]
- 39.Yanagisawa, H., E. C. Davis, B. C. Starcher, T. Ouchi, M. Yanagisawa, J. A. Richardson, and E. N. Olson. 2002. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415:168-171. [DOI] [PubMed] [Google Scholar]