Abstract
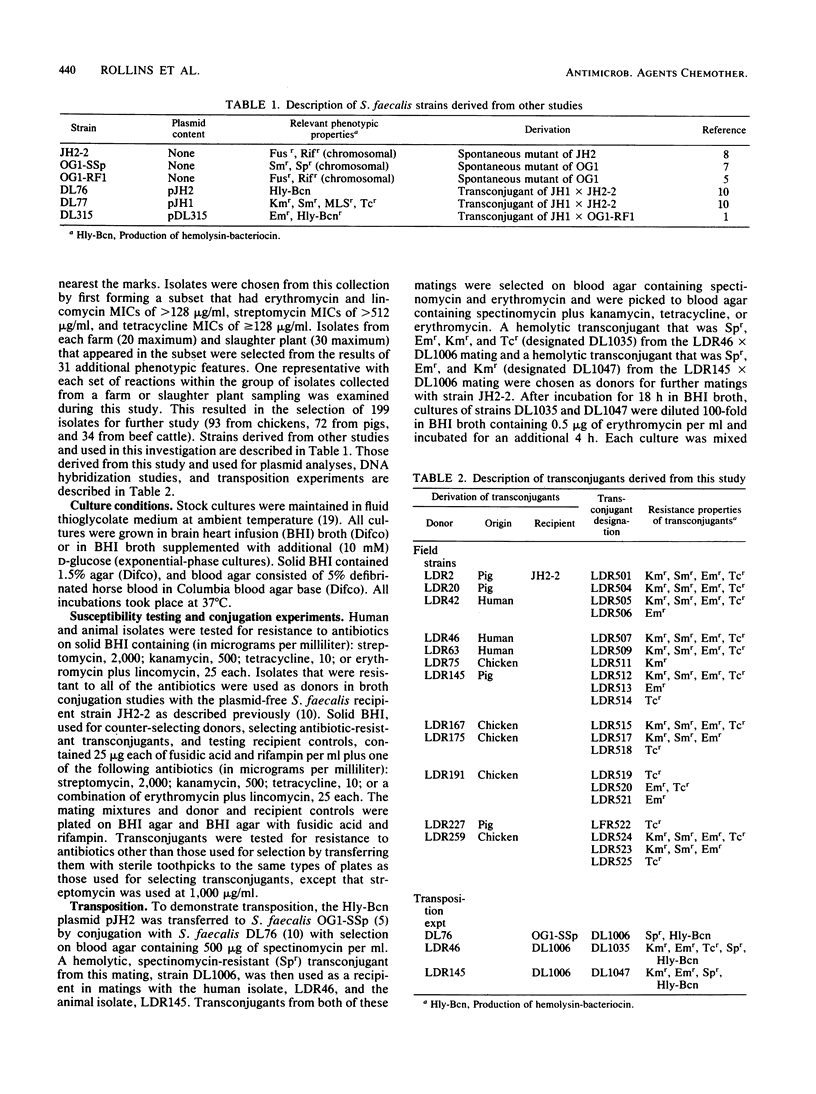
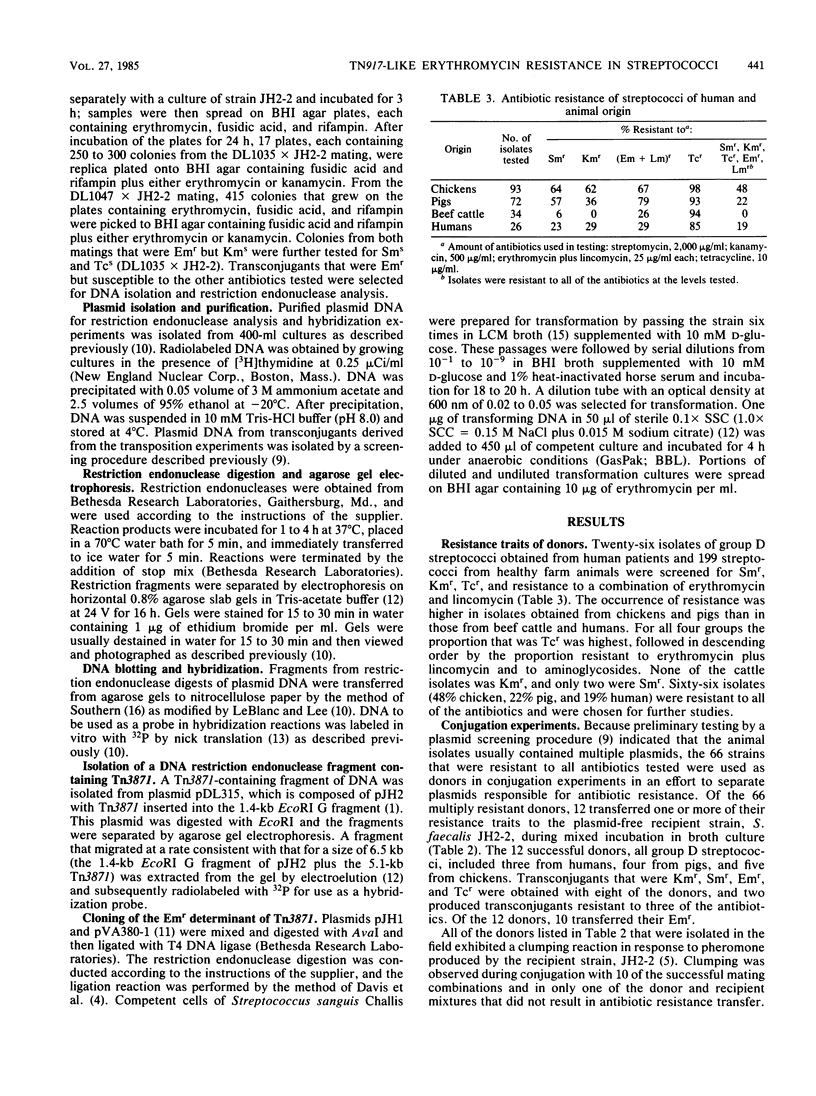
A total of 199 streptococci isolated from feces of healthy chickens, pigs, and beef cattle and 26 human clinical isolates were tested for resistance to kanamycin, streptomycin, tetracycline, erythromycin, and lincomycin. Of 66 isolates resistant to these antibiotics, 12 transferred one or more resistance traits by conjugation in broth. Erythromycin resistance (Emr) was transferred from 10 of the 12 successful donors. AvaI digests of plasmids isolated from Emr transconjugants derived from two human, two chicken, and one pig isolate contained three fragments similar in size to those produced from Tn3871, an Emr transposon. The three fragments from each of the five digests on Southern blots hybridized to radiolabeled Tn3871. Plasmid DNA from a transconjugant derived from a second pig isolate contained two of the three Tn3871-associated AvaI fragments. One of the AvaI fragments from each of the six plasmids hybridized with a radiolabeled probe containing a cloned AvaI fragment from Tn3871 that contained the Emr determinant. Transposition of the Emr trait was demonstrated for the plasmids derived from one human and one pig isolate. We concluded that extensive DNA homology existed between plasmids from streptococcal strains obtained from two human patients, two chickens, and two pigs and the Emr transposon Tn3871, which is very similar or identical to the well-characterized Emr transposon Tn917. The detection of Tn3871-like sequences in streptococcal isolates from Arkansas, Illinois, North Carolina, and Washington, D.C. indicates wide dissemination of Emr mediated by the same or closely related transposons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banai M., LeBlanc D. J. Streptococcus faecalis R plasmid pJH1 contains an erythromycin resistance transposon (Tn3871) similar to transposon Tn917. J Bacteriol. 1984 Jun;158(3):1172–1174. doi: 10.1128/jb.158.3.1172-1174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Davidson J. N., Novick R. P., Dunny G. M. Effects of tylosin feeding on the antibiotic resistance of selected gram-positive bacteria in pigs. Am J Vet Res. 1983 Jan;44(1):126–128. [PubMed] [Google Scholar]
- Clewell D. B., Tomich P. K., Gawron-Burke M. C., Franke A. E., Yagi Y., An F. Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982 Dec;152(3):1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N. Characterization of two tetracycline resistance determinants in Streptococcus faecalis JH1. J Bacteriol. 1982 May;150(2):835–843. doi: 10.1128/jb.150.2.835-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc D. J., Lee L. N. Rapid screening procedure for detection of plasmids in streptococci. J Bacteriol. 1979 Dec;140(3):1112–1115. doi: 10.1128/jb.140.3.1112-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Wood P. H., Jones K. R. Genetic transformation of Streptococcus sanguis (Challis) with cryptic plasmids from Streptococcus ferus. Infect Immun. 1980 Jun;28(3):692–699. doi: 10.1128/iai.28.3.692-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. A physical and functional analysis of Tn917, a Streptococcus transposon in the Tn3 family that functions in Bacillus. Plasmid. 1984 Sep;12(2):119–138. doi: 10.1016/0147-619x(84)90058-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Damle S. P., Clewell D. B. Plasmid-related transmissibility and multiple drug resistance in Streptococcus faecalis subsp. zymogenes strain DS16. Antimicrob Agents Chemother. 1979 Jun;15(6):828–830. doi: 10.1128/aac.15.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger C. L., Beaman A. J., Lee L. N. Tween 80 effect on glucosyltransferase synthesis by Streptococcus salivarius. J Bacteriol. 1978 Jan;133(1):231–239. doi: 10.1128/jb.133.1.231-239.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



