Abstract
RANK and RANKL, the key regulators of osteoclast differentiation and activation, also play an important role in the control of proliferation and differentiation of mammary epithelial cells during pregnancy. Here, we show that RANK protein expression is strictly regulated in a spatial and temporal manner during mammary gland development. RANK overexpression under the control of the mouse mammary tumor virus (MMTV) promoter in a transgenic mouse model results in increased mammary epithelial cell proliferation during pregnancy, impaired differentiation of lobulo-alveolar structures, decreased expression of the milk proteins β-casein and whey acidic protein, and deficient lactation. We also show that treatment of three-dimensional in vitro cultures of primary mammary cells from MMTV-RANK mice with RANKL results in increased proliferation and decreased apoptosis in the luminal area, resulting in bigger acini with filled lumens. Taken together, these results suggest that signaling through RANK not only promotes proliferation but also inhibits the terminal differentiation of mammary epithelial cells. Moreover, the increased proliferation and survival observed in a three-dimensional culture system suggests a role for aberrant RANK signaling during breast tumorigenesis.
The tumor necrosis factor (TNF) family member RANKL and its receptor RANK are key regulators of osteoclast differentiation and activation (16, 30). The balance between RANKL, RANK, and osteoprotegerin (OPG), a soluble decoy receptor that competes with RANK for binding to RANKL (32), regulates osteoclast differentiation and activation and therefore bone remodeling and mobilization of calcium from the skeleton (27). In mice, RANK and RANKL are also essential for the development of the mammary gland during pregnancy (18), in particular, for the formation of lobulo-alveolar structures after pregnancy day 14 (P14) which are required for a functional lactating mammary gland.
Deregulation in the RANKL/OPG system has been reported in malignant bone disease including bone metastasis and humoral hypercalcemia of malignancy (13, 21, 23). Numerous lines of evidence in animal models indicate that blocking RANK/RANKL interaction effectively prevents tumor-induced hypercalcemia or reduces tumor-induced bone lesions, due to its critical role in osteoclast differentiation and activation (reviewed in references 38 and 41). While RANK expression has been detected in breast cancer lines and in primary tumors including breast tumors (45) and may play a functional role in tumor cell migration and metastasis to the bone (24), it is not clear whether activation of the RANK/RANKL pathway may also contribute to primary tumor growth.
The mammary gland defects observed in RANK- and RANKL-deficient mice support the notion that the RANK/RANKL pathway plays an active role in mammary cell proliferation and survival; however, the direct effect of increased RANK signaling in mammary cells is unknown. To determine if activation of the RANK/RANKL pathway can directly increase proliferation or survival of primary mammary epithelial cells, we have generated transgenic mice that overexpress RANK under the control of the mouse mammary tumor virus (MMTV) promoter. Analysis of the mammary gland of the MMTV-RANK transgenic mice revealed higher levels of proliferation during pregnancy and defective differentiation to lobulo-alveolar structures. Moreover, RANKL treatment of three-dimensional (3D) cultures of primary mammary acini overexpressing RANK results in a significant increase in size and occlusion of the lumen, providing additional evidence that activation of the RANK/RANKL pathway not only enhances proliferation but also inhibits apoptosis. These results indicate that RANK/RANKL interaction during mammary gland development sends a proliferative and survival signal that impairs differentiation and that activation of the RANK/RANKL pathway disrupts the morphology of mammary epithelial acini.
MATERIALS AND METHODS
Generation of MMTV-RANK mice.
The mouse 1,878-base pair RANK cDNA open reading frame was amplified by PCR using the following oligonucleotides: 4036-17 (ATAAATAAGCTTGCCACCATGGCCCCGCGCGCC), a sense primer containing a HindIII site, a consensus Kozak translation initiation site, and the beginning of the open reading frame; and 4036-18 (GCCCGCGAATTCTCATTCTGCACATTGTCCGGA), an antisense primer containing an EcoRI site downstream of the stop codon. The HindIII/EcoRI fragment was inserted into the MMTV-BSSK vector (36) downstream of the MMTV promoter and was sequenced to confirm that it contained no errors generated by PCR. Expression of RANK is driven by a 2.4-kb fragment of the MMTV long terminal repeat (LTR). Downstream of the MMTV LTR, the rat VL30 retrotransposon internal ribosome entry site improves translation of genes placed downstream of it. The simian virus 40 (SV40) cassette provides the proper polyadenylation of the transgenic transcript as well as the splicing of the SV40 small T intron (Fig. 1A). The construct was linearized by digestion with SpeI and SalI and injected into the fertilized eggs of BDF2 (C57BL/6 × DBA/2 F2 hybrid) mice. To identify founder animals, ear punch DNA was screened by PCR to amplify a 368-bp SV40-cassette-specific fragment. Founders were tested further for their ability to transmit the transgene to progeny. RNA was isolated on partial mammary biopsy tissue from offspring, and quantitative reverse transcription-PCR (RT-PCR) using RANK-specific primers was performed to assess mRNA levels. The two highest expressor lines, MMTV-RANK 1 and 2, were established by repeated backcrossing to C57BL/6 mice (Taconic) and maintained in the hemizygous state. To estimate the number of copies in the two transgenic lines, a quantitative PCR approach (TaqMan) was used. For murine RANK we used a primer/probe set that spans an exon junction so that they detect the transgenic RANK but not the endogenous RANK gene. Two genes, mCCR1 and mCCR8, were used as housekeeping genes; both have two copies in the genome. For these housekeeping genes we chose primer/probe sets designed within an exon. Genomic tail DNA was prepared from wild-type (WT) and MMTV-RANK mice using a DNeasy kit (QIAGEN) and treated with RNase (Sigma). Quantitative real-time PCR was performed on an ABI 7700 Sequence Detector using the TaqMan universal PCR Master Mix (ABI). Three different amounts of genomic DNA (20, 10, and 5 nanograms) were used to ensure that we were quantifying DNA levels within the linear range. Primer/probe sets were obtained from the Applied Biosystems Assay-on-Demand. As expected, no significant signal was detected for RANK in the WT samples, whereas the housekeeping genes were detected similarly in WT and MMTV-RANK samples. Using this approach, we determined that MMTV-RANK transgenic line 1 had 12.15 ± 1.50 copies, and MMTV-RANK transgenic line 2 had 20.79 ± 3.78 copies (results normalized to the two copies of the housekeeping genes). We can estimate that MMTV-RANK mice from line 1 carry 10 to 14 copies and that MMTV-RANK mice from line 2 carry 17 to 24 copies. Each of the results presented here was obtained using MMTV-RANK line 1 mice but was also observed in MMTV-RANK line 2 mice (data not shown). For the subsequent experiments, virgin (10 to 15 weeks old) or pregnant WT and MMTV-RANK littermates were sacrificed at P14.5, P15.5, P16.5, P17.5, P18.5, lactation day 1 (L1), and involution day 3 (I3), and mammary glands were collected for histology or RNA extraction. Mice were maintained at Amgen, Inc. (Seattle, WA), under specific-pathogen-free conditions. All animal procedures were approved by and performed under the guidelines of Amgen's Institutional Animal Care and Use Committee.
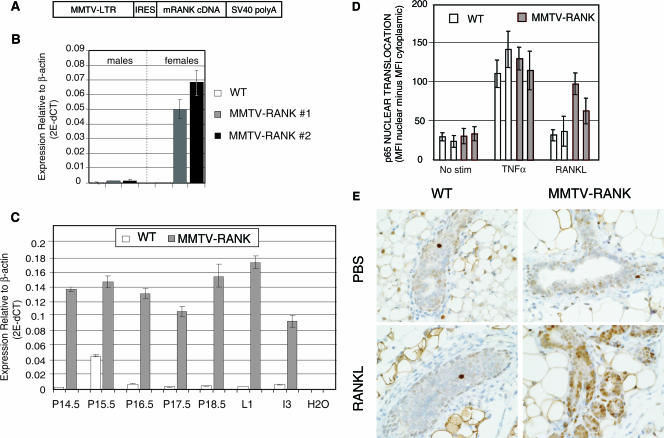
FIG. 1.
Overexpression of RANK in MMTV-RANK mice and increased response to RANKL in MMTV-RANK mammary epithelial cells. (A) Schematic diagram of the MMTV-RANK transgene construct including MMTV-LTR, the rat VL30 retrotransposon internal ribosome entry site (IRES), mouse RANK CDS gene, and the SV40 polyadenylation sequences. (B) mRANK mRNA expression levels in the mammary glands of WT and MMTV-RANK (males and females) relative to β-actin in two transgenic lines generated were measured by quantitative RT-PCR. Each bar represents the average of data for 3 to 9 mice and the standard errors are indicated. We have estimated (see Materials and Methods) that MMTV-RANK mice from line 1 carry 10 to 14 copies and MMTV-RANK mice from line 2 carry 17 to 24 copies of the transgene construct. (C) mRANK expression levels in the mammary glands of WT and MMTV-RANK females relative to β-actin at different time points during pregnancy were measured by RT-PCR. Each bar represents the values for one representative mouse. Amplifications were performed in triplicate, and the standard deviation is shown. (D) Quantitative immunostaining to measure p65 nuclear translocation in primary mammary epithelial cells from WT and MMTV-RANK mice after 30 min of treatment with TNF-α (40 ng/ml) or murine RANKL (200 ng/ml). Each bar represents cells from a single mouse. Translocation is quantified using the Cellomics software as the difference between the MFI of the nuclear area minus the MFI of the cytoplasmic area. The experiment shown is representative of three experiments. (E) Phospho-p65 IHC in WT and MMTV-RANK virgin mammary glands after 30 min of stimulation with murine RANKL. No significant increase in the phospho-p65 signal is detected in luminal WT cells after RANKL stimulation. In MMTV-RANK mice a dose of 250 μg of murine RANKL results in phospho-p65 detection in most of the mammary epithelial cells. Scale bar, 100 μm.
Quantitative real-time RT-PCR.
Total RNA was isolated from WT and MMTV-RANK mammary glands or acinar structures using an RNeasy kit (QIAGEN). mRNA was pretreated with DNase I (Ambion) and reverse transcribed using Applied Biosystems TaqMan reverse transcriptase reagents and random hexamer priming. Twenty nanograms of the cDNA obtained was subjected to quantitative real-time amplification. Primer/probe sets were obtained from the Applied Biosystems Assay-on-Demand (β-casein, whey acidic protein [WAP], cytokeratin 18 [CK18]) or generated in-house (RANK, RANKL, and β-actin). For each sample, amplifications were done in triplicate. Analysis was performed using ABI Prism Sequence Detector system software. Reactions lacking reverse transcriptase were performed to control for genomic DNA amplification. The relative concentrations were normalized to β-actin or CK18 as indicated.
Histology and immunostaining.
For histological analysis mammary tissues were fixed in formalin or zinc-Tris solution and embedded in paraffin. Acinar structures were extracted from the matrigel with phosphate-buffered saline (PBS)-0.5 M EDTA containing protease inhibitors, mixed in histogel (Richard Allan Scientific), and fixed in Clarke's fixative (methanol absolute:glacial acetic acid, 8:2). Sections (4 μm) were cut and stained with hematoxylin and eosin. For immunostaining, paraffin-embedded sections were incubated with antibodies against phospho-p65 (Cell Signaling), RANKL (R & D Systems), RANK (R & D Systems), bromodeoxyuridine (BrdU) (Accurate), or activated caspase 3 (Cell Signaling) and detected using biotin-conjugated secondary antibodies (Vector Laboratories). The antigen-antibody complexes were conjugated with streptavidin horseradish peroxidase (Perkin Elmer) and visualized with diaminobenzidine (Biogenex). Sections were counterstained with hematoxylin.
For evaluation of p65 activation in the virgin mammary gland, nulliparous 5-month-old females were injected intraperitoneally with TNF-α (25 μg/kg; R & D Systems) or murine RANKL-LZ (5 μg or 250 μg; Amgen Inc). Recombinant murine RANKL-LZ is an N-terminal fusion of a leucine zipper trimerization domain with residues 134 to 316 of murine RANKL (1). Mice were sacrificed 30 min later. Mammary glands and liver were collected and fixed in formalin and processed as described above.
Pregnant females were injected intraperitoneally with BrdU (1 mg/100 μl) 2 h before euthanasia. For BrdU or phospho-p65 quantification the number of BrdU or phospho-p65 positive cells relative to the total number of epithelial cells was determined in five separate fields at a magnification of ×200.
In situ hybridization.
In order to localize RANKL by in situ hybridization, a 236-bp DNA fragment of the mouse RANKL gene (corresponding to nucleotides 515 to 750 from AF053713) was amplified by reverse transcriptase PCR and cloned into the pBluescript II vector (Stratagene). An antisense 33P-labeled RNA probe was synthesized by in vitro transcription of the template using T7 RNA polymerase after linearization of the vector with EcoRI restriction enzyme. Tissue sections were hybridized overnight at 60°C in hybridization solution containing 1 × 106 cpm of 33P-labeled antisense riboprobe per slide. Following hybridization, sections were treated with RNase A to digest unhybridized probe and rinsed in a series of SSC washes with the highest stringency in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 55°C for 30 min. After the sections were quickly dehydrated through graded ethanol series, the slides were then dipped in Kodak nitroblue tetrazolium emulsion, air dried, and allowed to expose for 3 weeks in the dark at 4°C. Subsequently, the slides were developed in Kodak D19 and counterstained with hematoxylin and eosin.
Isolation of primary mammary epithelial cells and 3D cultures.
Mammary glands were dissected from day 16.5 pregnant mice as previously described (14, 34). Briefly, tissues were minced in Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco) containing antibiotics and incubated with 0.3% collagenase (Roche) and 2.5 U/ml dispase (Invitrogen) at 37°C for 90 min with trituration every 30 min. Crude digests were filtered through 500 AM Nitex mesh (Technicon) to remove undigested clumps and centrifuged at 100 × g to collect epithelial spheroids. Pellets were washed five times in DMEM/F12 with antibiotics and 5% fetal bovine serum (FBS) and collected each time with a low-speed spin. Spheroids were plated on 10-cm plates in growth medium (DMEM/F12 with antibiotics, 10 μg/ml insulin, 10 ng/ml epidermal growth factor, and 5% FBS), for 2 to 5 days before being harvested for immunofluorescent staining (Cellomics) or for 3D culture. The culture medium was changed every other day. For 3D cultures, first passage primary mammary epithelial cells (MECs) were harvested by trypsinization. Cells were counted and replated in MatrigelR (Collaborative Research) coated onto eight-well chamber slides (LabTek), and growth medium was added. After 24 h, the medium was changed to differentiation medium (DMEM/F12 with antibiotics, liquid medium supplement ITS [Sigma], 3 μg/ml prolactin [Sigma], 1 μg/ml hydrocortisone [Sigma],) with or without murine RANKL-LZ (200 ng/ml); medium was changed every day.
Immunofluorescence analysis.
Acinar structures were stained as previously described (12). MEC organoids were cultured in matrigel in eight-well chamber slides. Medium was removed, and acini were fixed in 2% paraformaldehyde and permeabilized using 0.5% Triton X-100 before blocking. Cells were incubated with the primary antibodies against cleaved caspase 3 (Cell Signaling) or Ki-67 (Zymed) overnight at room temperature and then with Alexa-conjugated secondary antibodies (Molecular Probes) for 45 min at room temperature and DRAQ5 (Alexis) for nuclear staining. Slides were mounted with Prolong Gold Antifade Reagent (Molecular Probes). Confocal analysis was performed using a Zeiss confocal microscopy system equipped with argon and HeNe lasers. Images were captured using LSM version 5 software (Zeiss).
To evaluate p65 activation in vitro, second passage primary MECs obtained from midgestation WT and MMTV-RANK females and kept in growth medium were plated in plastic and stimulated for 30 min with TNF-α (40 ng/ml; R & D Systems) or murine RANKL-LZ (200 ng/ml; Amgen Inc.) after starvation in 0.1% FBS and no epidermal growth factor. After stimulation, cells were fixed in 3% paraformaldehyde, permeabilized with 0.5% Triton X-100, blocked with 5% normal goat serum, and incubated for 1 h using reagents from the NF-κB “Hit-Kit” (Cellomics). Relative nuclear translocation of p65 was quantified using Cellomics software. The final quantification is the difference between the mean fluorescence intensity (MFI) of the nuclear area minus the MFI of the cytoplasmic area.
RESULTS
Generation of MMTV-RANK transgenic mice and increased response to RANKL in MMTV-RANK mammary epithelial cells.
To clarify the role of RANK/RANKL in the mammary gland, we generated transgenic mice that overexpress murine RANK under the control of the MMTV promoter (Fig. 1A). This promoter is active in virgin mice and is regulated during pregnancy in ductal and alveolar mammary epithelial cells (22). Five different founders of MMTV-RANK transgenic mice were identified. Overexpression of RANK mRNA in the founders was confirmed (data not shown), and two transgenic lines with similar levels of expression (MMTV-RANK line 1 and line 2) were studied (Fig. 1B). We can estimate that MMTV-RANK mice from line 1 carry 10 to 14 copies of the transgene and MMTV-RANK mice from line 2 carry 17 to 24 copies (see Materials and Methods). In transgenic males the levels of RANK mRNA in the mammary glands are three- to fourfold higher than in WT males. In glands of virgin transgenic females, the levels of RANK mRNA are 50- to 80-fold higher than those found in WT (Fig. 1B). During pregnancy there is an increase in the levels of RANK RNA in both WT and MMTV-RANK mice compared with virgin females, and the expression of RANK in the transgenic animals remains approximately 50-fold higher than the levels detected in WT at each pregnancy point analyzed (e.g., P14.5, P15.5, P16.5, P17.5, P18.5, L1, and I3) (Fig. 1C). These data show a significant and sustained increase in RANK mRNA expression in virgin and pregnant MMTV-RANK females.
We next determined the functionality of the RANK overexpressed in the MMTV-RANK transgenic mice by measuring its ability to respond to RANKL stimulation. Binding of RANKL to its receptor RANK initiates a signaling cascade that efficiently activates NF-κB, resulting in p65 phosphorylation and nuclear translocation (1). We determined the ability of RANKL to induce p65 nuclear translocation in vitro using primary MECs isolated from WT and MMTV-RANK midgestation females. TNF-α induced an efficient and comparable response in both WT and MMTV-RANK cells whereas the response to RANKL occurs more prominently in the MMTV-RANK MECs than in the WT MECs (Fig. 1D). To confirm the functionality of the transgenic RANK construct in vivo, we measured the capability of RANKL to increase phospho-p65 levels in the mammary glands of virgin WT and MMTV-RANK females. Phosphorylation of p65 in the liver induced by TNF-α was included as a control for p65 staining (9) (data not shown). Thirty minutes after injection with 250 μg of RANKL, a significant increase in phospho-p65 signal was detected in the nuclei of luminal epithelial cells of MMTV-RANK mammary glands compared with animals treated with PBS (Fig. 1E). Even when a dose of as little as 5 μg of RANKL was used, more phospho-p65-positive cells were observed in the MMTV-RANK glands compared to PBS controls (data not shown). No clear response to RANKL was observed in the WT luminal cells at any of the RANKL doses used. Occasionally, a minimal response to RANKL treatment was observed in the basal layer of cells of some ducts in WT and MMTV-RANK glands (data not shown). These results demonstrate the functionality of the transgenic construct.
Impaired differentiation of lobuloalveolar structures in MMTV-RANK females.
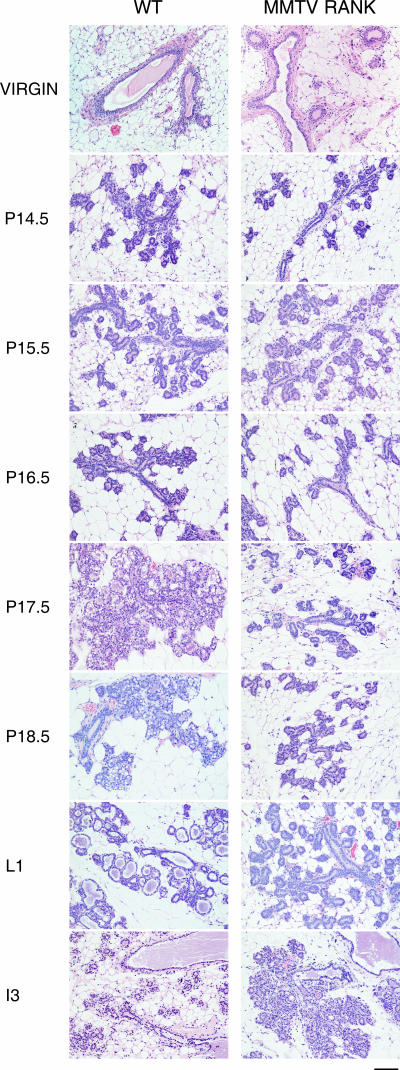
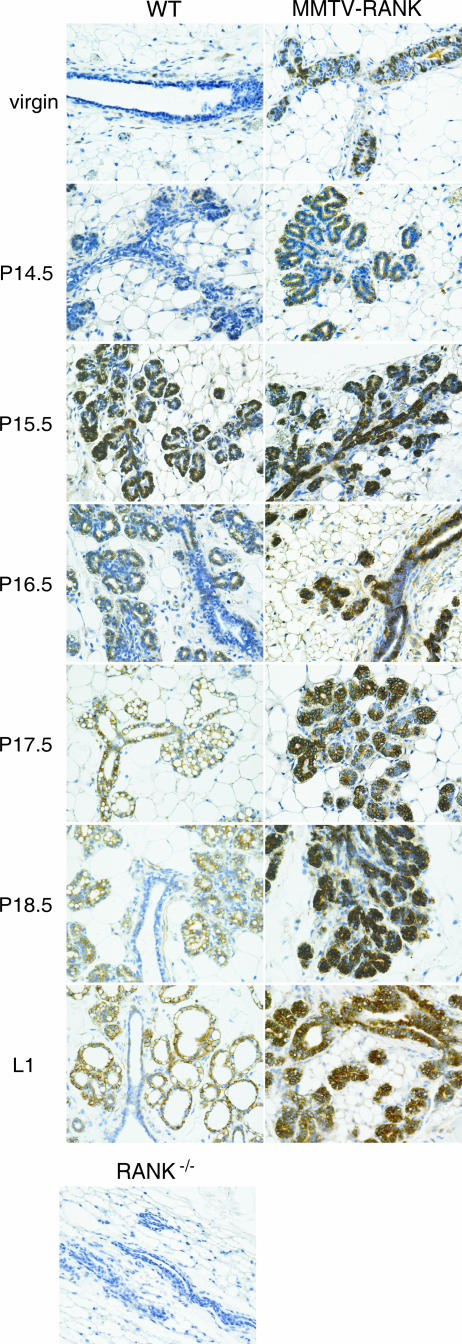
Transgenic MMTV-RANK mice are healthy and fertile, and the females give birth to litters of normal size. The pups are morphologically normal but die within 48 h of birth despite normal nursing. Milk was not detected in the stomach of pups, suggesting that pregnant MMTV-RANK females have a lactation defect. This defect was observed even after multiple pregnancies (up to 10); thus, the lactation defect is not rescued by enhanced exposure to pregnancy hormones (data not shown). To specifically address what stage in mammary gland development is altered in these mice, we compared mammary glands from WT and MMTV-RANK females at different time points (virgin, P14.5, P15.5, P16.5, P17.5, P18.5, L1, and I3) by histology. Nulliparous MMTV-RANK females show no defects in the mammary gland structure, and no clear differences in morphology are observed between WT and both transgenic lines up to P15.5 (Fig. 2). The elongation and extension of the ductal tree and ductal side branching, including proliferation of the ductal epithelium, occur normally in the MMTV-RANK females. However, the transition to the alveolar phenotype is impaired in the MMTV-RANK females. In the WT glands extensive alveolar differentiation, vacuolation, and secretion of milk proteins that accumulate in the dilated lumen occur during later stages of gestation (P16.5 to L1), but the MMTV-RANK glands show dramatically reduced alveolar development after P15.5 and remain arrested at this stage from P15.5 through lactation except for a slightly more extensive ductal development (Fig. 2). During involution, the ductal epithelial cells in the transgenic glands undergo apoptosis and degenerate in a manner similar to the involution process that occurs in differentiated secretory mammary glands. Therefore, we conclude that MMTV-RANK females show no alterations in the ductal branching system, but the lobuloalveolar differentiation and maturation is impaired and a functional lactating mammary gland is not formed.
FIG. 2.
Impaired alveolar differentiation in MMTV-RANK mice: morphology of mammary gland development in WT and MMTV-RANK transgenic mice. Glands from virgin mice and mice at different time points during pregnancy were stained with hematoxylin and eosin. Scale bar, 100 μm.
Significant reduction in the levels of β-casein and WAP in MMTV-RANK glands.
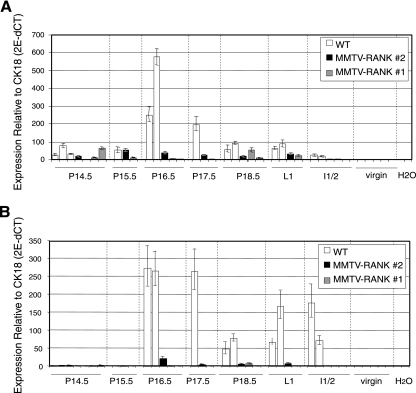
To confirm whether the absence of differentiation to lobulo-alveolar structures observed by histology correlates with alterations in molecular markers of mammary differentiation, we measured the mRNA levels of the milk proteins β-casein and WAP in WT and MMTV-RANK females at different time points during pregnancy (virgin and P14.5 to I3). β-Casein is absent in virgin animals but present in WT glands at P14.5, as expected. In both MMTV-RANK transgenic lines, β-casein mRNA was detectable in the mammary gland, but the levels were dramatically reduced through gestation (Fig. 3A, P16.5). WAP, a late differentiation marker, is present at high levels at P16.5 in WT glands and remains high until involution, whereas it is nearly undetectable in the glands of all MMTV-RANK mice analyzed throughout P14.5 to I3 (Fig. 3B). These results confirm that the arrest in lobular differentiation observed in the MMTV-RANK mice occurs at a stage prior to P16.5.
FIG. 3.
Reduction in β-casein and WAP mRNA levels in MMTV-RANK mice. β-Casein (A) and WAP (B) mRNA expression levels relative to CK18 in the mammary glands of WT and both lines of MMTV-RANK transgenic at different time points during pregnancy are shown. Each bar represents one mouse. Amplifications were performed in triplicate, and the standard deviation is shown.
RANKL expression pattern is not altered in the MMTV-RANK glands.
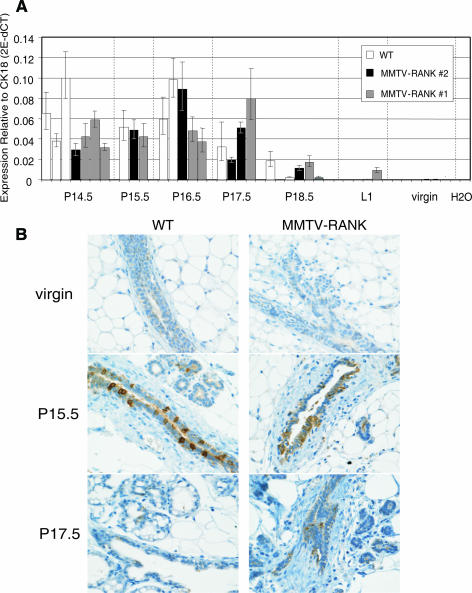
RANKL expression is strictly regulated during pregnancy, and it is under the influence of sex and reproductive hormones (18). RANKL expression is absent in virgin glands, then gradually increases during pregnancy, and decreases again to an undetectable level at P18.5 (43). To determine if the overexpression of RANK in the mammary gland could influence the expression pattern of RANKL, we quantified the expression of RANKL mRNA in the mammary glands at different time points during pregnancy using quantitative RT-PCR. As shown by RT-PCR analysis (Fig. 4A), the kinetics and magnitude of RANKL mRNA expression are comparable between WT and MMTV-RANK females. RANKL protein expression is mostly localized in the luminal area of ducts and in the developing alveolar buds, but it is greatly attenuated in differentiated, secretory alveoli in both WT and MMTV-RANK mice (Fig. 4B shows RANKL immunohistochemistry [IHC] from virgin, P15.5, and P17; for the complete time course, see Fig. S1A in the supplemental material). In situ hybridization results confirmed that the ductal cells lining the lumen are predominantly the source of RANKL in the mammary gland (see Fig. S1B in the supplemental material). Thus, RANKL mRNA and protein expression are absent in virgin glands but strongly upregulated during pregnancy from P10.5 to P17.5 in both WT and MMTV-RANK mice. OPG mRNA levels in the mammary gland are also comparable between WT and MMTV-RANK mice (data not shown), indicating that neither RANKL nor OPG was affected locally by the expression of the MMTV-RANK transgene.
FIG. 4.
RANKL expression is comparable between WT and MMTV-RANK mammary glands. (A) RANKL mRNA expression levels relative to CK18 in the mammary glands of WT and both lines of MMTV-RANK females at different time points during pregnancy measured by quantitative RT-PCR. Each bar represents one mouse. Amplifications were performed in triplicate, and the standard deviation is shown. (B) RANKL IHC in WT and MMTV-RANK mammary glands from virgin and pregnant mice at P15.5 and P17.5. Scale bar, 100 μm.
RANK protein expression is strictly regulated in a spatial and temporal manner during mammary gland development.
In contrast to RANKL, RANK mRNA is detectable in virgin WT mammary glands, and its expression levels are maintained at a low but constant level during pregnancy (18, 43). However, the expression of RANK mRNA and protein does not always correlate (1), and the latter has not been characterized in the mammary gland during gestation. We analyzed expression of RANK protein in mammary glands in both virgin and pregnant WT and MMTV-RANK females. Mammary glands from RANK-deficient mice are included as a negative control. RANK expression is weak in the epithelial cells of virgin WT ductules (Fig. 5). Interestingly, a faint staining is detected in the abluminal area of some WT ducts, correlating with the phospho-p65 signal previously detected in basal cells of virgin WT glands after RANKL injection (data not shown). RANK protein becomes more prominently expressed at P14.5 within the alveolar buds, is strongly upregulated at P15.5, and decreases as the alveoli become vacuolated and secretory. Expression is mainly restricted to the lobuloalveolar structures, and generally RANK is detected only within the ducts in areas where lobular cells are branching (Fig. 5, P16.5 WT). In contrast to the WT glands, strong expression of RANK protein is detected in the glands of MMTV-RANK virgin females. During pregnancy a significant increase of RANK expression is observed in MMTV-RANK glands, reaching a maximum level at P15.5, similarly to WT glands. RANK expression is localized predominantly in the alveoli but also includes most of the ductules in both luminal and basal cells. High levels of RANK protein are sustained throughout the course of pregnancy in MMTV-RANK mice compared to WT (Fig. 5). These data show that, similar to RANKL, the spatial and temporal distribution of RANK protein levels is strictly regulated during pregnancy in WT mice, but this precise regulation is lost in the MMTV-RANK mice.
FIG. 5.
RANK protein expression is strictly regulated in the mammary gland during gestation. Images show IHC of RANK in WT and MMTV-RANK mammary glands at different time points during pregnancy. Mammary gland from a RANK-null virgin female is included as a negative control. Scale bar, 100 μm.
Sustained proliferation and p65 activation at later stages of pregnancy in MMTV-RANK mammary glands.
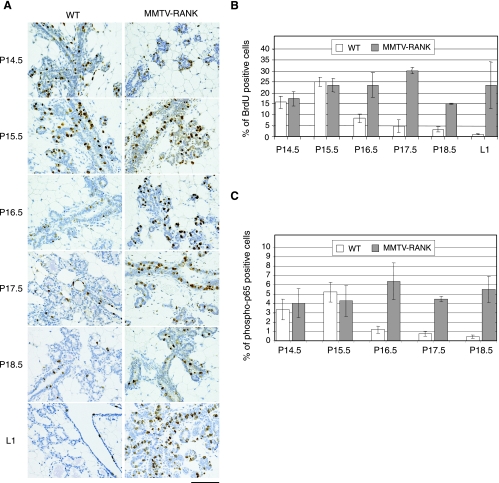
The defect in mammary gland development that occurs in the RANK and RANKL null mice is associated with increased apoptosis and decreased proliferation of the mammary epithelial cells (18). We determined whether RANK overexpression led to changes in survival and/or proliferation during gestation (P14.5 to L1) in WT and MMTV-RANK mice. The number of cells in which activated caspase 3 was detected was minimal in WT and MMTV-RANK mammary glands between P14.5 to L1 (data not shown). However, differences in the degree of proliferation (as detected by BrdU labeling) were observed between WT and MMTV-RANK glands at later stages in pregnancy (Fig. 6A and B). At P14.5 and P15.5 when the phenotype of the MMTV-RANK mice is not yet histologically evident, the percentage of cells positive for BrdU is similar: 25.2% ± 2.1% in WT and 23.5% ± 3.1% in MMTV-RANK mice at P15.5. At later time points proliferation in WT glands gradually decreases to 8.4% ± 2% at P16.5 and reaches levels of 1.1% ± 0.2% at L1, whereas in the transgenic mammary epithelia a much greater percentage of proliferating epithelial cells (23.6% ± 3.1% at P16.5 and 23.6% ± 10.7% of cells at L1) is observed throughout pregnancy (Fig. 6B).
FIG. 6.
Sustained proliferation and p65 activation in MMTV-RANK glands at later stages of pregnancy. (A) Proliferation in the mammary glands of WT and MMTV-RANK mice at different time points during pregnancy detected by BrdU immunostaining. Note the impaired differentiation to mature alveoli in the MMTV-RANK glands. Scale bar, 100 μm. (B) Quantification of the percentage of BrdU-positive cells versus the total number of epithelial cells through pregnancy. Each bar represents the average of two to three mice, and standard errors are shown. For each mouse five fields at a magnification of ×200 were counted. (C) Quantification of the percentage of phospho-p65-positive cells versus the total number of epithelial cells throughout pregnancy is shown. For each mouse five fields at a magnification of ×200 were counted. Each bar represents one to three mice, and standard deviations or errors are shown.
RANKL and RANK can activate NF-κB during pregnancy (7), and we have shown that virgin MMTV-RANK mice have increased p65 nuclear translocation in the mammary cells after stimulation with RANKL. To determine if NF-κB participates in the MMTV-RANK phenotype, we compared the levels of phospho-p65 during pregnancy in WT and MMTV-RANK mice. The highest levels of phospho-p65 in WT glands are detected at P15.5, and a substantial decrease occurs through P18.5 (Fig. 6C). Phospho-p65 is particularly high in the new-formed alveoli; much lower levels are detected in the highly vacuolated, secretory alveoli (data not shown). In MMTV-RANK glands, phospho-p65 levels are also high at P15.5, but in contrast to the decrease shown in WT glands, the levels are maintained during the consecutive days. Thus, RANK overexpression during mammary gland development results in sustained and higher rates of mammary epithelial proliferation and persistent activation of NF-κB through pregnancy.
RANKL directly induces proliferation and aberrant survival in MMTV-RANK acini.
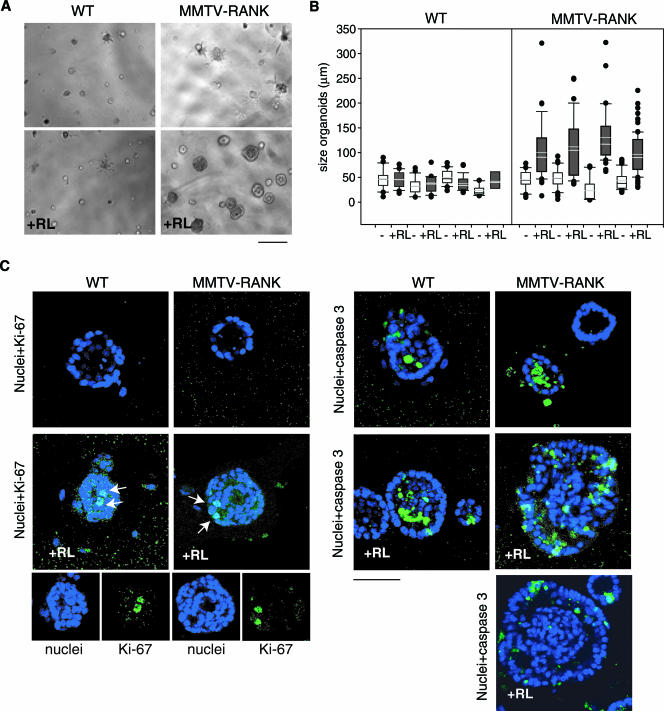
To determine if RANKL directly mediates the increased levels of proliferation observed in the mammary epithelial cells of MMTV-RANK mice, we used primary acinar cultures of mammary epithelial cells established from pregnant WT and MMTV-RANK transgenic females (4, 17). Acini are spherical monolayers of epithelial cells that enclose a central lumen. Maturation of acini occurs within 4 days and is comprised of three consecutive steps: polarization of apicobasal cells, death of luminal cells, and suppression of proliferation and accumulation of secretory material. These acinar cultures allow us to model the architecture of the MMTV-RANK epithelium in vitro and to address the oncogenic potential of the RANK/RANKL pathway in mammary epithelia. In the absence of RANKL no apparent differences were observed between WT and MMTV-RANK acini during the first 5 days of culture. However, RANKL presence drastically changes the MMTV-RANK acini morphology (Fig. 7A). The average size of the acini cultured without RANKL is 38 ± 12 μm in diameter in WT and 40 ± 11 μm in MMTV-RANK. When RANKL is present in the WT cultures, the WT acini remain at a similar size (41 ± 4 μm) compared with the untreated cultures; the majority of the acini were smaller than 50 μm and no acini greater than 90 μm in diameter were observed. In contrast, a dramatic increase in size is observed in the MMTV-RANK acini (110 ± 15 μm) treated with RANKL. Untreated MMTV-RANK cultures have a similar percentage of acini smaller than 50 μm as WT cultures, but when RANKL is present, 50 to 85% of the spheroids are greater than 90 μm. Some of these RANKL-induced MMTV-RANK acini reach sizes of 320 μm in diameter at day 5 of culture (Fig. 7B).
FIG. 7.
RANKL directly induces proliferation and lumen repopulation in MMTV-RANK acini. (A) Phase-contrast microscopy of WT and MMTV-RANK primary MEC 3D cultures, without or with RANKL (RL) at day 4. Scale bar, 100 μm. The images are representative of five experiments. Note the significant change in morphology of the MMTV-RANK acini in the presence of RANKL. (B) Size of WT and MMTV-RANK acini after 5 days in culture in the absence (−) or presence (+) of RANKL (RL). The upper and lower bars indicate the 90th and 10th percentiles of the data. The data points greater than the 90th or less than the 10th percentiles are plotted as individual dots. For each treatment 12 to 92 spheroids were measured. (C) Confocal images at day 5 of WT and MMTV-RANK mammary acini cultured without or with RANKL (+RL) as indicated. Nuclei were stained with DRAQ5 (blue) and Ki-67 or activated caspase 3 (green). Arrows indicate Ki-67-positive cells. One or more Ki-67-positive cells was detected in 30 to 50% of the WT acini and in 100% of the MMTV-RANK acini cultured with RANKL. Representative images are shown. Scale bar, 50 μm. Note the mislocalized or sparse apoptotic cells detected in MMTV-RANK acini cultured with RANKL and the presence of a filled lumen.
To evaluate whether RANKL would affect the proliferative levels of mammary acinar cultures, we analyzed the expression of proliferating-cell antigen Ki-67 by immunostaining. RANKL treatment resulted in Ki-67-positive cells in 30 to 50% of the WT acini and in 100% of the MMTV-RANK acini at day 5 of culture (Fig. 7C). By this time proliferation has ceased, and Ki-67 expression is not detected in WT or MMTV-RANK acini in the absence of RANKL. Moreover, in both WT and MMTV-RANK cultures, the number of acini with secretory material is severely reduced when RANKL is present (see Fig. S2 in the supplemental material). This observation is in agreement with our hypothesis that RANK/RANKL signaling drives proliferation in mammary epithelial cells and impairs differentiation. MMTV-RANK MECs cultured in the absence of RANKL for more than 1 week remain sensitive to the mitogenic and morphogenic effects of RANKL upon subsequent exposure, as suggested by the presence of proliferative cells (Ki-67-positive cells) in all the MMTV-RANK acini 48 h after the addition of RANKL (data not shown). These results suggest that RANKL directly reinitiates proliferation of arrested acini. MMTV-RANK acini are dependent on the presence of RANKL to maintain a high proliferative rate, as demonstrated by the regression that occurs if RANKL is removed from the medium once the abnormally large spheroids are formed (data not shown).
WT and MMTV-RANK acini cultured in the absence of RANKL become cavitated as a result of the removal of centrally localized cells by apoptosis. Apoptotic cells (indicated by activated caspase 3 staining in green) are localized to the lumen of WT and MMTV-RANK acini untreated with RANKL (Fig. 7C). RANKL treatment of WT spheroids does not impair the cavitation process. In contrast, MMTV-RANK acini treated with RANKL no longer retain a hollow lumen. Moreover, RANKL treatment induces lumen repopulation in MMTV-RANK acini cultured in the absence of RANKL for up to 1 week. In the presence of RANKL, all MMTV-RANK acini larger than 90 μm and 50% of acini between 50 to 90 μm show a filled lumen. Apoptosis is not completely impaired, as evidenced by the presence of activated caspase 3-positive cells, but these apoptotic cells are preferentially localized in the outer layer of cells and not in the luminal area, as is observed in WT acini. In fact, in some of the spheroids, predominantly the larger ones, the number of apoptotic cells is very low, suggesting that apoptosis is progressively diminished (Fig. 7C).
We evaluated RANK protein expression in WT and MMTV-RANK acini during their evolution in the presence or absence of RANKL every day along the 3D culture: days 1, 2, 3, 4, and 5. As expected, RANK expression levels are much higher in MMTV-RANK acini than in WT acini although, with longer incubation times (overnight incubation), RANK protein expression is clearly detected in the WT acini (see Fig S2 in the supplemental material). In the absence of RANKL no significant differences in the expression levels of RANK protein are noted in either WT or MMTV-RANK acini during the course of the culture (see Fig S2 in the supplemental material). By quantitative RT-PCR a slight increase in RANK expression is observed in the WT cultures (see Fig. S3 in the supplemental material). Interestingly, when RANKL is present, RANK protein and mRNA levels decrease (see Fig. S2 and S3 in the supplemental material), and no secretory acini are observed in either WT or MMTV-RANK cultures. The lack of differentiation to the secretory phenotype is consistent with the increase proliferation observed in WT and MMTV-RANK acini treated with RANKL (Fig. 7C) and with the results we obtained in vivo.
All together, these results demonstrate that in acinar cultures RANKL drives a direct and potent proliferative response in the MMTV-RANK MECs that impairs differentiation, as well as an antiapoptotic effect, and suggest that the phenotype observed in MMTV-RANK mice is dependent on RANKL.
DISCUSSION
RANK and RANKL have been defined as the key regulators of osteoclast survival and differentiation and are consequentially required for bone remodeling. RANK and RANKL knockout mice have also revealed a role for these factors in mouse mammary gland development during pregnancy (18). RANK and RANKL null females have a complete block in the formation of the lobuloalveolar compartment due to enhanced cell death and defective proliferation of the alveolar epithelial cells. One limitation of these studies is that it can only provide information about the initial requirement for RANK and RANKL in the mammary gland; because the alveolar compartment failed to develop at a relatively early stage, one cannot determine a function for RANK or RANKL at later stages in mammary development.
In the current study, we have used a transgenic model to demonstrate that activation of the RANK/RANKL pathway (via RANK overexpression in the mammary gland by the MMTV promoter) results in a marked increase in cellular proliferation from P16.5 until lactation. This increase in proliferation interferes with the differentiation of the lobuloalveolar structures. Alterations in the transcripts for the milk proteins β-casein and WAP confirm that alveolar development is blocked at a stage prior to P16.5 in MMTV-RANK transgenic animals. Importantly, RANK overexpression had no discernible effect on mammary glands from virgin females, in accordance with the lack of mammary phenotype in the virgin RANK- or RANKL-deficient mice (18).
The observation that either RANK overexpression (this study) or the complete absence of RANK (18) results in a strikingly similar defect in alveolar development during pregnancy may seem like a paradox. However, these apparently conflicting observations can be explained by the strict temporal regulation of RANK and RANKL expression that occurs during mammary development and the stage-specific function that the RANK/RANKL pathway plays during this process. We have confirmed that RANKL expression is absent in virgin glands, upregulated during pregnancy, and decreases after day P18.5 (43). Using immunohistochemistry, we demonstrate that RANK protein expression, like RANKL, is also strictly regulated during mammary development, and the highest levels of RANK expression correlate with the peak of mammary epithelial proliferation at P15.5. Decreased RANK expression is detected as the maturation of the alveolar compartment progresses. Importantly, the mammary defect observed in the RANK knockout becomes evident at P14 when mammary epithelial proliferation is high (18), whereas MMTV-RANK mammary glands develop normally until P15.5. Thus, the observations made using the MMTV-RANK transgenic mice reveal a critical function for RANK at later points in mammary gland development. Taken together, these data suggest that the proper development of the lobulo-alveolar structures requires an initial wave of proliferation signaled through RANK that allows the expansion of the alveolar bud epithelium, followed by a decrease in RANK signal that is critical for the lobulo-alveolar structures to differentiate to highly vacuolated and secretory alveoli.
Progesterone, prolactin, parathyroid hormone-related peptide, and the transcription factor STAT 5 play a role in mammary development and, in fact, regulate RANKL expression in the mammary gland (10, 18). However, the factors responsible for the temporal RANK regulation in mammary epithelial cells that we observe in this study have not yet been elucidated. In osteoclasts RANK expression is regulated by macrophage colony-stimulating factor 1 (3), transforming growth factor β (26), and the transcription factor PU.1 (31). Both colony-stimulating factor 1 and transforming growth factor β have important roles in mammary gland development (29, 35, 47); additional experiments are required to determine if these same factors regulate RANK expression in the mammary gland.
Clearly the RANK/RANKL pathway promotes both survival and proliferation of mammary epithelia during pregnancy (reference 18 and this study). We utilized 3D primary mammary epithelial cultures that closely resemble the acinar development and differentiation that occur in vivo, and we have shown that RANKL directly induces proliferation and impairs accumulation of secretory material in both WT and MMTV-RANK epithelial cells. In WT acini, the enhanced proliferation induced by RANKL is presumably balanced by increased apoptosis, thus preventing a net change in size. Indeed, the selective apoptosis of luminal cells is not altered in WT acini, thereby maintaining an open luminal space and a properly polarized structure. In contrast, an imbalanced response occurs in the MMTV-RANK acini after RANKL treatment, resulting in dramatically increased organoid size and an inability to cavitate and form a normal lumen. Moreover, RANKL is able to reinitiate proliferation in already preformed growth-arrested acini, thus promoting the repopulation of the luminal space. These 3D culture systems of nontransformed MECs have been extensively used to assess the biological effects of activation of specific oncogenes. Notably, neither inhibiting apoptosis (by exogenously expressing antiapoptotic family members) nor enhancing proliferation (via cyclin D1 or human papillomavirus E7 overexpression) results in luminal filling, and only when both activities are combined (for instance by ErbB2 activation) does the lumen become filled (11, 12, 37). Cyclin D1 is described as a downstream target of RANK/RANKL pathway in mammary cells (7) and may transmit proliferative signals from RANK. Recently, it has been reported that RANKL induces proliferation of primary mammary epithelial cells via nuclear translocation of the inhibitor of helix-loop-helix protein Id2 (28). Id2 translocation to the nuclei allows cell cycle progression and down-modulation of p21 expression. While Id2 or cyclin D1 is a likely candidate for RANKL-induced proliferation, our results suggest that additional antiapoptotic signals must be driven by RANK/RANKL to result in lumen repopulation. NF-κB is a good candidate as a downstream intermediate for the RANK/RANKL pathway in mammary epithelia (7), and it is generally understood that NF-κB expression and activity are tightly regulated during mammary gland development (6). This strict regulation appears to be critical for mammary gland development and tumorigenesis as genetically modified mice carrying alterations in proteins of the NF-κB pathway show marked defects at different stages of pregnancy, and elevated NF-κB DNA-binding activity has been documented in both mammary cell lines and primary human breast cancer tissues (8, 15). NF-κB has also been shown to promote proliferation and block apoptosis in different cells types including breast cancer cells (5, 25). Our results corroborate the idea that RANK/RANKL activates NF-κB in mammary epithelial cells as we have observed correlations between RANK expression levels, p65 activation, and cellular proliferation in both WT and MMTV-RANK glands. Recently, the proapoptotic BH3-only protein Bim has been identified as a significant contributor to luminal apoptosis (39) and may also be a downstream target of RANK. Using 3D cultures, it may be possible to determine whether alterations in NF-κB and/or in factors such as Bim are responsible for the impaired luminal cavitation phenotype observed in the MMTV-RANK organoids treated with RANKL and whether cyclin D1 and Id2 are functionally responsible for the proliferation observed in RANKL-treated organoids.
Due to the combined survival and proliferative role that RANK/RANKL plays in the mammary epithelia, we would hypothesize that overexpression of RANK and/or RANKL in the mammary epithelial cells might result in a hyperplasic mammary gland or even enhance mammary tumorigenesis. Of note, we have not observed a significant increase in the spontaneous incidence of mammary carcinomas in virgin MMTV-RANK mice. We show that RANK overexpression results in an increased proliferation and block of differentiation of the lobular compartment during pregnancy. Similar phenotypes are observed in MMTV transgenic models of several oncogenes, including c-myc (2, 44), ras (42), int3 (19), or erbB2 (20, 33), but in these cases mammary tumors are observed. Overexpression of cyclin D1 or c-Rel, both downstream effectors of the RANK/RANKL pathway (7), results in increased mammary gland tumorigenesis (40, 46). One plausible explanation for the absence of spontaneous tumor incidence in the MMTV-RANK mice may be that the levels of RANKL limit the activation of the RANK pathway. Several facts support the idea that the proliferative effect observed in MMTV-RANK mice is dependent on RANKL: (i) the phenotype of the MMTV-RANK mice is observed only at midgestation when RANKL levels are high, (ii) the effective regression of the MMTV-RANK gland at involution occurs as RANKL levels decline; (iii) the MMTV-RANK acinar phenotype is not observed in the absence of RANKL, and (iv) RANKL induces a direct proliferative response in the RANK-overexpressing primary mammary cells and alters their polarization and apoptosis in vitro. These observations indicate that the phenotype observed in the RANK-overexpressing mice is dependent on RANKL and that both RANK and RANKL levels must be high to achieve constitutive mammary cell proliferation that might increase tumorigenesis. Other factors such as the C57BL/6 background, which is significantly resistant to mammary tumorigenesis, or the developmental state at which the oncogene is expressed can contribute to the lack of tumor phenotype.
In summary, RANK overexpression in the presence of RANKL stimulates proliferation, impairs differentiation, and decreases apoptosis, but further alterations are needed to accomplish tumorigenesis. Thus, RANK/RANKL overexpression might be involved in breast tumor progression rather than tumor initiation. Further studies are aimed to address whether RANK/RANKL activation cooperates with other oncogene alterations (MMTV-neu or MMTV-ras background) or chemical carcinogenesis to increase tumorigenesis.
Supplementary Material
Acknowledgments
We thank Ken Schooley for technical support with the confocal microscopy and the Cellomics software; Jim Petrorius for in situ hybridization analysis; Michael Wiley, William Lawrence, Moira Glaccum, the Animal Facility and the Pathology Department, including Kathy Rohrbach, Larry Wood, Angie Warren, Deanna Hill, Julie Hahn, Michelle Pace and Brenda Heron, and Phenopath Laboratories for their technical assistance; Helen Hathaway (University of New Mexico School of Medicine) for her support and advice; and Michelle Chaisson for helpful discussions and critical comments on the manuscript.
Footnotes
Published ahead of print on 4 December 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Anderson, D. M., E. Maraskovsky, W. L. Billingsley, W. C. Dougall, M. E. Tometsko, E. R. Roux, M. C. Teepe, R. F. DuBose, D. Cosman, and L. Galibert. 1997. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175-179. [DOI] [PubMed] [Google Scholar]
- 2.Andres, A. C., M. A. van der Valk, C. A. Schonenberger, F. Fluckiger, M. LeMeur, P. Gerlinger, and B. Groner. 1988. Ha-ras and c-myc oncogene expression interferes with morphological and functional differentiation of mammary epithelial cells in single and double transgenic mice. Genes Dev. 2:1486-1495. [DOI] [PubMed] [Google Scholar]
- 3.Arai, F., T. Miyamoto, O. Ohneda, T. Inada, T. Sudo, K. Brasel, T. Miyata, D. M. Anderson, and T. Suda. 1999. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 190:1741-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barcellos-Hoff, M. H., J. Aggeler, T. G. Ram, and M. J. Bissell. 1989. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas, D. K., Q. Shi, S. Baily, I. Strickland, S. Ghosh, A. B. Pardee, and J. D. Iglehart. 2004. NF-κB activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc. Natl. Acad. Sci. USA 101:10137-10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brantley, D. M., F. E. Yull, R. S. Muraoka, D. J. Hicks, C. M. Cook, and L. D. Kerr. 2000. Dynamic expression and activity of NF-κB during post-natal mammary gland morphogenesis. Mech. Dev. 97:149-155. [DOI] [PubMed] [Google Scholar]
- 7.Cao, Y., G. Bonizzi, T. N. Seagroves, F. R. Greten, R. Johnson, E. V. Schmidt, and M. Karin. 2001. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107:763-775. [DOI] [PubMed] [Google Scholar]
- 8.Cao, Y., and M. Karin. 2003. NF-κB in mammary gland development and breast cancer. J. Mammary Gland Biol. Neoplasia 8:215-223. [DOI] [PubMed] [Google Scholar]
- 9.Chaisson, M. L., J. T. Brooling, W. Ladiges, S. Tsai, and N. Fausto. 2002. Hepatocyte-specific inhibition of NF-κB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J. Clin. Investig. 110:193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui, Y., G. Riedlinger, K. Miyoshi, W. Tang, C. Li, C. X. Deng, G. W. Robinson, and L. Hennighausen. 2004. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 24:8037-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debnath, J., K. R. Mills, N. L. Collins, M. J. Reginato, S. K. Muthuswamy, and J. S. Brugge. 2002. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111:29-40. [DOI] [PubMed] [Google Scholar]
- 12.Debnath, J., S. K. Muthuswamy, and J. S. Brugge. 2003. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30:256-268. [DOI] [PubMed] [Google Scholar]
- 13.Deftos, L. J. 2000. Prostate carcinoma: production of bioactive factors. Cancer 88:3002-3008. [DOI] [PubMed] [Google Scholar]
- 14.de la Cruz, L., K. Steffgen, A. Martin, C. McGee, and H. Hathaway. 2004. Apoptosis and involution in the mammary gland are altered in mice lacking a novel receptor, beta 1,4-galactosyltransferase I. Dev. Biol. 272:286-309. [DOI] [PubMed] [Google Scholar]
- 15.Demicco, E. G., K. T. Kavanagh, R. Romieu-Mourez, X. Wang, S. R. Shin, E. Landesman-Bollag, D. C. Seldin, and G. E. Sonenshein. 2005. RelB/p52 NF-κB complexes rescue an early delay in mammary gland development in transgenic mice with targeted superrepressor IκB-α expression and promote carcinogenesis of the mammary gland. Mol. Cell. Biol. 25:10136-10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougall, W. C., M. Glaccum, K. Charrier, K. Rohrbach, K. Brasel, T. De Smedt, E. Daro, J. Smith, M. E. Tometsko, C. R. Maliszewski, A. Armstrong, V. Shen, S. Bain, D. Cosman, D. Anderson, P. J. Morrissey, J. J. Peschon, and J. Schuh. 1999. RANK is essential for osteoclast and lymph node development. Genes Dev. 13:2412-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emerman, J. T., and D. R. Pitelka. 1977. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 13:316-328. [DOI] [PubMed] [Google Scholar]
- 18.Fata, J. E., Y. Y. Kong, J. Li, T. Sasaki, J. Irie-Sasaki, R. A. Moorehead, R. Elliott, S. Scully, E. B. Voura, D. L. Lacey, W. J. Boyle, R. Khokha, and J. M. Penninger. 2000. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103:41-50. [DOI] [PubMed] [Google Scholar]
- 19.Gallahan, D., C. Jhappan, G. Robinson, L. Hennighausen, R. Sharp, E. Kordon, R. Callahan, G. Merlino, and G. H. Smith. 1996. Expression of a truncated Int3 gene in developing secretory mammary epithelium specifically retards lobular differentiation resulting in tumorigenesis. Cancer Res. 56:1775-1785. [PubMed] [Google Scholar]
- 20.Guy, C. T., M. A. Webster, M. Schaller, T. J. Parsons, R. D. Cardiff, and W. J. Muller. 1992. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 89:10578-10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen, M. F., M. J. Nellissery, and P. Bhatia. 1999. Common mechanisms of osteosarcoma and Paget's disease. J. Bone Miner. Res. 14(Suppl. 2):39-44. [DOI] [PubMed] [Google Scholar]
- 22.Hennighausen, L. 2000. Mouse models for breast cancer. Breast Cancer Res. 2:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, L., J. Xu, D. J. Wood, and M. H. Zheng. 2000. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am. J. Pathol. 156:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, D. H., T. Nakashima, O. H. Sanchez, I. Kozieradzki, S. V. Komarova, I. Sarosi, S. Morony, E. Rubin, R. Sarao, C. V. Hojilla, V. Komnenovic, Y. Y. Kong, M. Schreiber, S. J. Dixon, S. M. Sims, R. Khokha, T. Wada, and J. M. Penninger. 2006. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440:692-696. [DOI] [PubMed] [Google Scholar]
- 25.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 26.Karsdal, M. A., P. Hjorth, K. Henriksen, T. Kirkegaard, K. L. Nielsen, H. Lou, J. M. Delaisse, and N. T. Foged. 2003. Transforming growth factor-beta controls human osteoclastogenesis through the p38 MAPK and regulation of RANK expression. J. Biol. Chem. 278:44975-44987. [DOI] [PubMed] [Google Scholar]
- 27.Karsenty, G. 1999. The genetic transformation of bone biology. Genes Dev. 13:3037-3051. [DOI] [PubMed] [Google Scholar]
- 28.Kim, N. S., H. J. Kim, B. K. Koo, M. C. Kwon, Y. W. Kim, Y. Cho, Y. Yokota, J. M. Penninger, and Y. Y. Kong. 2006. Receptor activator of NF-κB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol. Cell. Biol. 26:1002-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirma, N., R. Luthra, J. Jones, Y. G. Liu, H. B. Nair, U. Mandava, and R. R. Tekmal. 2004. Overexpression of the colony-stimulating factor (CSF-1) and/or its receptor c-fms in mammary glands of transgenic mice results in hyperplasia and tumor formation. Cancer Res. 64:4162-4170. [DOI] [PubMed] [Google Scholar]
- 30.Kong, Y. Y., H. Yoshida, I. Sarosi, H. L. Tan, E. Timms, C. Capparelli, S. Morony, A. J. Oliveira-dos-Santos, G. Van, A. Itie, W. Khoo, A. Wakeham, C. R. Dunstan, D. L. Lacey, T. W. Mak, W. J. Boyle, and J. M. Penninger. 1999. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315-323. [DOI] [PubMed] [Google Scholar]
- 31.Kwon, O. H., C. K. Lee, Y. I. Lee, S. G. Paik, and H. J. Lee. 2005. The hematopoietic transcription factor PU. 1 regulates RANK gene expression in myeloid progenitors. Biochem. Biophys. Res. Commun. 335:437-446. [DOI] [PubMed] [Google Scholar]
- 32.Lacey, D. L., E. Timms, H. L. Tan, M. J. Kelley, C. R. Dunstan, T. Burgess, R. Elliott, A. Colombero, G. Elliott, S. Scully, H. Hsu, J. Sullivan, N. Hawkins, E. Davy, C. Capparelli, A. Eli, Y. X. Qian, S. Kaufman, I. Sarosi, V. Shalhoub, G. Senaldi, J. Guo, J. Delaney, and W. J. Boyle. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165-176. [DOI] [PubMed] [Google Scholar]
- 33.Lazar, H., A. Baltzer, C. Gimmi, A. Marti, and R. Jaggi. 2000. Over-expression of erbB-2/neu is paralleled by inhibition of mouse-mammary-epithelial-cell differentiation and developmental apoptosis. Int. J. Cancer. 85:578-583. [PubMed] [Google Scholar]
- 34.Lee, E. Y., G. Parry, and M. J. Bissell. 1984. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J. Cell Biol. 98:146-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin, E. Y., V. Gouon-Evans, A. V. Nguyen, and J. W. Pollard. 2002. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J. Mammary Gland Biol. Neoplasia 7:147-162. [DOI] [PubMed] [Google Scholar]
- 36.Muller, W. J., E. Sinn, P. K. Pattengale, R. Wallace, and P. Leder. 1988. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54:105-115. [DOI] [PubMed] [Google Scholar]
- 37.Muthuswamy, S. K., D. Li, S. Lelievre, M. J. Bissell, and J. S. Brugge. 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oyajobi, B. O., D. M. Anderson, K. Traianedes, P. J. Williams, T. Yoneda, and G. R. Mundy. 2001. Therapeutic efficacy of a soluble receptor activator of nuclear factor κB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 61:2572-2578. [PubMed] [Google Scholar]
- 39.Reginato, M. J., K. R. Mills, E. B. Becker, D. K. Lynch, A. Bonni, S. K. Muthuswamy, and J. S. Brugge. 2005. Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol. Cell. Biol. 25:4591-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romieu-Mourez, R., D. W. Kim, S. M. Shin, E. G. Demicco, E. Landesman-Bollag, D. C. Seldin, R. D. Cardiff, and G. E. Sonenshein. 2003. Mouse mammary tumor virus c-rel transgenic mice develop mammary tumors. Mol. Cell. Biol. 23:5738-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roodman, G. D. 2004. Mechanisms of bone metastasis. N. Engl. J. Med. 350:1655-1664. [DOI] [PubMed] [Google Scholar]
- 42.Sinn, E., W. Muller, P. Pattengale, I. Tepler, R. Wallace, and P. Leder. 1987. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell 49:465-475. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava, S., M. Matsuda, Z. Hou, J. P. Bailey, R. Kitazawa, M. P. Herbst, and N. D. Horseman. 2003. Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J. Biol. Chem. 278:46171-46178. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, T. A., P. K. Pattengale, and P. Leder. 1984. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell 38:627-637. [DOI] [PubMed] [Google Scholar]
- 45.Thomas, R. J., T. A. Guise, J. J. Yin, J. Elliott, N. J. Horwood, T. J. Martin, and M. T. Gillespie. 1999. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140:4451-4458. [DOI] [PubMed] [Google Scholar]
- 46.Wang, T. C., R. D. Cardiff, L. Zukerberg, E. Lees, A. Arnold, and E. V. Schmidt. 1994. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369:669-671. [DOI] [PubMed] [Google Scholar]
- 47.Wrobel, C. N., J. Debnath, E. Lin, S. Beausoleil, M. F. Roussel, and J. S. Brugge. 2004. Autocrine CSF-1R activation promotes Src-dependent disruption of mammary epithelial architecture. J. Cell Biol. 165:263-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.