Abstract
SHP has been implicated as a pleiotropic regulator of diverse biological functions by its ability to inhibit numerous nuclear receptors. Recently, we reported that SHP inhibits transcription of CYP7A1, a key gene in bile acid biosynthesis, by recruiting histone deacetylases (HDACs) and a Swi/Snf-Brm complex. To further delineate the mechanism of this inhibition, we have examined whether methylation of histones is also involved and whether a functional interplay between chromatin-modifying enzymes occurs. The histone methyltransferase G9a, but not SUV39, was colocalized with SHP in the nucleus and directly interacted with SHP in vitro. G9a, which was coimmunoprecipitated with hepatic SHP, methylated Lys-9 of histone 3 (H3K9) in vitro. Expression of G9a enhanced inhibition of CYP7A1 transcription by SHP, while a catalytically inactive G9a dominant negative (DN) mutant reversed the SHP inhibition. G9a was recruited to and H3K9 was methylated at the CYP7A1 promoter in a SHP-dependent manner in bile acid-treated HepG2 cells. Expression of the G9a-DN mutant inhibited H3K9 methylation, blocked the recruitment of the Brm complex, and partially reversed CYP7A1 inhibition by bile acids. Inhibition of HDAC activity with trichostatin A blocked deacetylation and methylation of H3K9 at the promoter, and, conversely, inhibition of H3K9 methylation by G9a-DN partially blocked deacetylation. Hepatic expression of G9a-DN in mice fed cholic acid disrupted bile acid homeostasis, resulting in increased bile acid pools and partial de-repression of Cyp7a1 and Cyp8b1. Our studies establish a critical role for G9a methyltransferase, histone deacetylases, and the Swi/Snf-Brm complex in the SHP-mediated inhibition of hepatic bile acid synthesis via coordinated chromatin modification at target genes.
The small heterodimer partner (SHP, or NR0B2), an unusual orphan nuclear receptor that lacks a DNA binding domain but contains a putative ligand binding domain, interacts with a number of nuclear receptors, including liver receptor homologue 1, hepatic nuclear factor 4 (HNF-4), estrogen-related receptor, constitutive androstane receptor, liver X receptor, glucocorticoid receptor, and estrogen receptor, and inhibits their transcriptional activities (1, 4, 7, 30, 39, 40). Thus, SHP acts as a pleiotropic transcriptional repressor affecting diverse biological functions, including cholesterol and glucose metabolic pathways, energy homeostasis, and reproductive biology (2). SHP has been shown to be a key regulator in the negative feedback regulation of bile acid biosynthesis from cholesterol in the liver (15, 25, 31, 48). Bile acid-activated farnesoid X receptor (FXR), upon heterodimerization with retinoid X receptor, binds to the promoter of the SHP gene and increases transcription (15, 31). The bile acid-induced SHP then suppresses transcription of CYP7A1 and CYP8B1, key hepatic genes involved in the neutral pathway of bile acid biosynthesis, by interacting with liver receptor homologue 1 and/or HNF-4, which are bound to the promoters of the genes. In addition to regulation of bile acid biosynthesis by SHP, recent studies show that bile acid-induced SHP also inhibits expression of hepatic bile acid transporters, such as NTCP (Na+ taurocholate cotransport peptide) and bile salt export pump (5, 11, 13). Bile acid homeostasis was disrupted in SHP-null mice, establishing an in vivo physiological role of SHP in the regulation of cholesterol and bile acid metabolism (25, 48).
Previous studies showed that SHP interacts with the AF-2 domain of nuclear receptors through the LXXLL motifs in SHP and subsequently inhibits the activity of nuclear receptors by competing with coactivators (20, 21, 29). In addition to this coactivator competition model, it was also proposed that SHP could directly repress the activities of nuclear receptors via its C-terminal intrinsic repression domain, presumably by recruiting corepressors (29, 30). However, the molecular mechanisms underlying the direct suppression of transcription by SHP have not been well defined, and the mechanisms of SHP action at target genes in a natural chromatin context are largely unexplored. Recently, we reported that SHP mediates repression by recruiting chromatin-modifying enzymes including the mSin3A/histone deacetylase 1 and 2 (HDAC-1/2) corepressor and Swi/Snf-Brm chromatin remodeling complexes to the CYP7A1 promoter in HepG2 cells after bile acid treatment, which results in histone deacetylation and chromatin remodeling (24).
Chromatin structure plays a fundamental role in the regulation of eukaryotic gene activity (23, 26, 50). Primary structural units of chromatin are nucleosomes, which suppress transcription by imposing a barrier for access of transcription factors and basal transcriptional machinery to DNA. ATP-dependent chromatin remodeling complexes, such as Swi/Snf, weaken DNA-histone contacts in the nucleosome core, which may disrupt or alter nucleosome conformation (23, 50). The Swi/Snf complexes contain either of two ATPases, Brm or Brg-1, and variable subunits of Brm or Brg-1 associated factors (BAFs). Brm and Brg-1 preferentially interact with different types of transcription factors and target promoters (22). Recent studies, including ours, show that these Swi/Snf complexes are involved in gene repression as well as activation (24, 51). A second class of chromatin-modifying complexes includes enzymes that are involved in covalent modification of core histone tails, such as acetylation and methylation of lysine (19, 44). While histone acetylation generally correlates with transcriptional activation (19), histone methylation has either negative or positive effects on transcription, depending on the residue methylated and the type of modification (19, 27, 38). Methylation at Lys-9 of histone 3 (H3K9) has been correlated with transcriptional silencing in most cases, whereas methylation at Lys-4 of histone 3 (H3K4) is associated with gene activation.
The mammalian histone methyltransferase, G9a, is responsible for mono- and dimethylation of H3K9 in euchromatin (45). Studies in G9a-null mice showed that euchromatic H3K9 methylation by G9a is essential in early embryogenesis and is critically involved in transcriptional silencing of developmentally regulated genes (46). In contrast, SUV39H1, a heterochromatic histone lysine methyltransferase, directs trimethylation of H3K9 in pericentric heterochromatin, and this modification creates a binding motif for the HP1 protein that is directly associated with DNA methylation and gene silencing (27, 43).
Our previous study showed that SHP interacts with HDAC-1, HDAC-2, and mSin3A corepressors and recruits these factors to the CYP7A1 promoter in response to bile acid treatment (24). We further observed that acetylation of histones H3 and H4 in the CYP7A1 promoter was markedly decreased in a SHP-dependent manner after bile acid treatment. Consistent with our observations are recent reports that SHP interacts with HDACs and that its repressive effects are mediated through recruitment of HDACs (14). It was also shown from a recent interesting study that SHP functionally interacts with HDAC-1 and with G9a in cells (6), but a role for G9a in SHP-mediated suppression of genes involved in hepatic bile acid metabolism has not been demonstrated. Since CYP7A1 and CYP8B1, two key genes in hepatic bile acid biosynthesis, are well-known SHP target genes, we explored whether H3K9 methylation catalyzed by G9a at the promoters of the endogenous CYP7A1 and CYP8B1 genes is critical for SHP-mediated feedback inhibition of the expression of these genes by bile acids. Since HDACs and Brm have been shown to be involved in gene repression by SHP (24), another important question is whether coordinated enzymatic modification of histones by G9a and HDACs and ATP-dependent remodeling by the Swi/Snf-Brm complex at the CYP7A1 promoter are critical for transcriptional silencing mediated by bile acid-induced SHP.
We found that H3K9 methylation by G9a and H3K9 deacetylation by HDACs are interdependent and necessary for the recruitment of the Swi/Snf-Brm complex, which is essential for SHP-mediated transcriptional silencing of the CYP7A1 gene. Interestingly, inhibition of hepatic G9a activity in mice by expression of a dominant negative (DN) mutant of G9a partially derepressed Cyp7a1 and Cyp8b1 and resulted in elevated bile acid pools in mice fed cholic acid. Our studies establish a critical role for these chromatin-modifying enzymes in the suppression of SHP target genes involved in hepatic bile acid metabolism.
MATERIALS AND METHODS
Cell culture and HepG2 stable cells.
Human hepatoma HepG2 cells (ATCC HB8065) were grown in phenol red-free Dulbecco's modified Eagle's medium/F12 (1:1). Cos-1 and human embryonic cell-derived Ad-293 cells (Cell Biolabs, Inc.) were maintained in Dulbecco's modified Eagle's medium. Medium was supplemented with 100 units/ml penicillin G-streptomycin sulfate and 10% heat inactivated fetal bovine serum. For chromatin immunoprecipitation (ChIP) and reverse transcription-PCR (RT-PCR) experiments, HepG2 cells were seeded, incubated, and treated with 25 to 50 μM chenodeoxycholic acid (CDCA; Sigma) for 6 h to 18 h in serum-free medium. Because we observed that the response of the HepG2 cells to bile acids was often diminished after long-term culture, new cultures were established every 6 to 8 weeks from frozen aliquots of our original HepG2 cell culture. In addition, the effect of CDCA on CYP7A1 and SHP mRNA levels was regularly assessed to ensure that the response of the cells to CDCA had not been changed.
To construct a HepG2 cell line with the CYP7A1-luc reporter stably incorporated into the genome, a DNA fragment containing human −1887CYP7A1-luc (8) was inserted into pcDNA3 in which the cytomegalovirus (CMV) promoter had been removed. HepG2 cells were transfected with the pcDNA3-CYP7A1-luc plasmid and selected with 800 μg/ml of GM418 for 2 to 3 weeks. Drug-resistant colonies were pooled and expanded and subjected to luciferase assays to ensure that the CYP7A1-luc plasmid was stably incorporated into genome.
Transfection reporter assays.
For transient transfections, HepG2 or Cos-1 cells were cotransfected with DNA of the −1887 human CYP7A1-luc reporter (8), Gal4-TATA-luc reporter (29), Gal4DBD (G4), Gal4DBD plus residues 130 to 368 of HNF-4 [Gal4DBD-HNF-4(130-368)] (28), pcDNA3-PGC-1α (3), pcDNA3-SHP (24), pCMV-HNF-4, pcDNA3.1-G9a (16), pEGFP-G9a (36), pcDNA3.1 G9a-DN (16), or pcDNA3.1-HA-SUV39H1 (where HA is hemagglutinin) (34) as indicated in the figure legends. Empty vector DNA was added as needed so that the same amounts of CMV expression vector DNA were present in each transfection. Transfection was carried out using Lipofectamine 2000 in 24-well plates. Twenty-four hours after transfection, cells were collected, and luciferase and β-galactosidase activities were determined. Firefly luciferase activities were divided by β-galactosidase activities to normalize for transfection efficiency. Consistent results were observed in two to four independent triplicate transfection assays in each experiment.
Construction of plasmids and adenoviral vectors.
Plasmid pGEX4T1-SHP region 1 (amino acids 1 to 92) was constructed by PCR amplification of mouse SHP using forward and reverse primers that contained BamH1 and Xho1 sites, respectively. The PCR product was digested with BamH1 and Xho1 and inserted into pGEX4T-1 digested with the same enzymes. Recombinant adenoviruses, Ad-human SHP small interfering RNA (Ad-siSHP) and Ad-G9a-DN, were constructed with the Ad-Easy system (18) in which the recombinant adenoviruses also express green fluorescent protein (GFP). For the construction of Ad-G9a-DN virus, a Hind III/EcoRV fragment encoding G9a-DN from pcDNA3.1G9a-DN (16) was inserted into Ad-Track CMV shuttle vector. For the construction of Ad-human SHP small interfering RNA (siRNA), the pSUPER vector containing the siRNA for human SHP (residues +76 to +94) (24) was digested with EcoR1, filled in with the Klenow fragment of Escherichia coli DNA polymerase, further digested with Hind III, and cloned between the EcoRV and HindIII sites of the Ad-Track vector (18). E. coli BJ5183 cells containing the AdEasy backbone vector were transformed with the Ad-Track vector DNA that was linearized by Pme1 digestion. Homologous recombination via adenoviral sequences in the shuttle vector and backbone vector in these cells yielded the recombinant adenoviral vectors. The recombinant viral vectors were characterized by Pac1 digestion to ensure that no rearrangement had occurred. Ad-293 cells were transfected with the recombinant adenoviral DNA, and the recombinant virus was then amplified by several rounds of infection of the Ad-293 cells. The viruses were isolated by CsCl step gradient centrifugation as described previously (18) and dialyzed in phosphate-buffered saline (PBS)-10% glycerol. Total viral particles were determined by absorbance at A260 (1 A260 unit is approximately 1 × 1012 particles), and the number of active viral particles was determined by measurement of cells expressing GFP by confocal microscopy.
In vivo experiments.
BALB/c male mice (8 to 12 weeks old) were maintained on a cycle of 12 h of light and 12 h of dark. For ChIP assays, mice were randomly divided into groups that were fed normal chow or normal chow supplemented with 0.5% cholic acid for the times (5 h to 24 h) indicated in the figure legends. For in vivo G9a-DN experiments, mice were injected with about 1 × 109 active viral particles in 500 μl of PBS via the tail vein, and 3 days after infection, the mice were briefly fasted for 5 h to synchronize feeding and then fed normal or the 0.5% cholic acid-containing chow for 5 h or 24 h. Feeding with cholic acid-containing diets was always started at 5:00 p.m. The liver, gall bladder, and entire small intestine were collected for further analyses. All the animal use and adenoviral protocols were approved by the Institutional Use and Care of Animals and Bio-safety Committees at University of Illinois and were in accordance with National Institutes of Health guidelines.
Measurement of total bile acid pool size.
Bile acid pools were colorimetrically measured using a diagnostic bile acid analysis kit (Trinity Biotech) as previously described (48). Briefly, gall bladder, parts of liver directly surrounding the gall bladder, and the entire small intestine were collected for measuring the total pool of bile acids. Samples were homogenized, and bile acids were extracted in 75% ethanol at 50°C for 2 h, followed by centrifugation. The bile acid pool size was calculated as micromoles of bile acid per gram of body weight.
Real-time RT-PCR.
Total RNA was isolated from mouse liver or HepG2 cells using Trizol reagent, and cDNA was synthesized using a reverse transcriptase kit (Promega Inc.). Real-time RT-PCR was performed with an iCycler iQ (Bio-Rad, Inc.) instrument following the manufacturer's instructions. The amount of PCR product for each mRNA was normalized by dividing by the amount of β-actin or 36B4 PCR product. Each real-time RT-PCR was repeated at least three times with similar reproducible results (data not shown).
Colocalization study.
Cos-1 cells were seeded on coverslips in six-well plates and cotransfected with expression plasmid DNA for GFP-SHP, G9a, or SUV39H1. Twenty-four hours after transfection, cells were fixed with 2.5% paraformaldehyde and permeabilized with 0.1% Triton X-100. Samples were blocked with 3% bovine serum albumin for 30 min, incubated with rabbit G9a antibody (1:50) or HA rat monoclonal antibody (1:25; Roche Inc.) for 30 min, washed, and then incubated with rhodamine-conjugated secondary antibody (1:200) for 30 min. Mounting medium was added, and green fluorescence and red immunofluorescence were detected with a Zeiss LSM confocal microscope as before (3, 33).
Coimmunoprecipitation assay.
Coimmunoprecipitation assays were performed as previously described with minor modifications (3, 24). Cell extracts or nuclear extracts from HepG2 cells or mouse liver were incubated in lysis buffer (20 mM KOH-HEPES, pH 8.0, 0.2 mM EDTA, 5% glycerol, 250 mM NaCl, 0.5% NP-40, 0.25% sodium deoxycholate, 1 mM dithiothreitol, and protease inhibitors) with antibodies against G9a or SHP or rabbit immunoglobulin G (IgG) at 4°C for 4 h to overnight, and the immune complex was collected by incubation with 30 μl of a 25% slurry of protein A or G agarose for 2 h. Immunoprecipitates were washed four times with lysis buffer supplemented with NaCl to 400 mM and subjected to Western blotting or used in in vitro histone methyltransferase (HMT) assays.
GST pull down assay.
Glutathione S-transferase (GST)-SHP fusion proteins (7) and GST-G9a containing the region of residues 621 to 1000 of G9a [GST-G9a(621-1000)] (45) were expressed in E. coli BL21/DE3/RIL (Stratagene, Inc.) and purified by binding to glutathione-Sepharose (Pharmacia, Inc.). 35S-labeled SHP or G9a was synthesized by the in vitro TNT system (Promega, Inc.). GST pull-down assays were performed as previously described (3, 24). Briefly, 1 μg of the GST fusion proteins was incubated with 35S-labeled proteins in incubation buffer (20 mM KOH-HEPES, pH 7.8, 0.1 mM EDTA, 10% glycerol, 100 mM KCl, 0.5% NP-40, protease inhibitors, 1 mM dithiothreitol) at 4°C for 2 h. After being washed five times with incubation buffer, proteins associated with GST fusion proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Five micrograms of GST-SHP or GST was incubated with 0.5 mg or 1.4 mg protein of HepG2 and mouse liver nuclear extracts, respectively, in incubation buffer at 4°C for 2 h. After being washed four times with incubation buffer (supplemented to 400 mM NaCl and 0.5% NP-40), proteins associated with GST-SHP or GST were subjected to Western blotting or HMT assays.
HMT assays.
HMT assays were performed as previously described with minor modifications (12, 16, 36, 43). Briefly, anti-SHP immunoprecipitates or GST-SHP protein complexes were washed with 1× HMT buffer (50 mM Tris-HCl, pH 8.0) twice and incubated with 2 μg of core histones isolated from HeLa cells or 2 μg of bacterially expressed and purified GST-H3(1-84) or GST-H3R9 as histone substrates in HMT buffer containing 0.25 μCi of S-adenosyl-[methyl-3H]methionine at 30°C for 1 h with occasional gentle mixing. Proteins were separated by SDS-PAGE and visualized by Coomassie blue staining, and radioactivity was detected by autoradiography.
ChIP assays in mouse liver and HepG2 cells.
ChIP assays in HepG2 cells and in mouse liver were carried out essentially as previously described (3, 24, 32). Briefly, livers were finely minced and incubated in PBS containing 1% formaldehyde at room temperature for 10 min, and glycine was added to stop the reaction. Cells were resuspended in hypotonic buffer (10 mM KOH-HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.2% NP-40, 0.15 mM spermine, 0.5 mM spermidine, 1 mM EDTA, 5% sucrose) and lysed by homogenization. Nuclei were pelleted and resuspended in sonication buffer (50 mM Tris-HCl, pH 8.0, 2 mM EDTA, 0.85% SDS). The samples were sonicated to reduce DNA length to between 200 to 1,000 bp. After centrifugation, the chromatin sample was precleared and further immunoprecipitated with 1 to 3 μg of antisera at 4°C for overnight. G9a antibody was kindly provided by Y. Nakatani (37, 41), and SHP antibody was described previously (7, 21). Antibodies against acetylated histone H3, diacetylated H3K9/K14, dimethylated H3K9, and HDAC-1 were purchased from Upstate Biotech, and antibodies against Brm, BAF170, mSin3A, HDAC-2, HDAC-3, and NcoR1, were purchased from Santa Cruz Biotechnology. The immune complex was collected, the beads were extensively washed, and the bound chromatin was eluted as described previously (3, 24, 32). Genomic DNA was purified by organic extraction and used as a template for semiquantitative PCR. The linearity of the PCRs was established using different numbers of cycles and different amounts of template. Each ChIP experiment was repeated at least three times with similar reproducible results.
For cells infected with adenoviral vectors before ChIP assays in HepG2 cells, confluent cells in 15-cm plates (2 × 107) were infected at a multiplicity of infection of 10 with adenoviruses, and 24 h after infection, cells were treated with CDCA or vehicle for the times indicated in the figure legends before collection of cells for ChIP assays. For trichostatin A (TSA) experiments, HepG2 cells were pretreated with 100 nM TSA for 1 h before 25 μM CDCA treatment for 7 h, and cells were then collected for either real-time RT-PCR or ChIP assay. Sequences of the primers used for ChIP assays in mouse liver and in HepG2 cells are available upon request.
RESULTS
G9a is associated with SHP in mouse liver and HepG2 cells.
SHP recruited HDAC-containing corepressor complexes to the native CYP7A1 promoter, which resulted in histone deacetylation and transcriptional repression after bile acid treatment (24). Since histone methylation as well as deacetylation are functionally correlated with gene repression (19, 27, 44), we examined whether G9a and SUV39H1, euchromatic and heterochromatic histone lysine methyltransferases, respectively (27), colocalize with SHP in the nucleus. Cos-1 cells were cotransfected with expression plasmid DNA for either G9a or SUV39H1 and GFP-SHP. SHP was detected by GFP fluorescence, and G9a or SUV39H1 was detected by immunofluorescence in cells imaged by confocal microscopy. Staining of G9a (Fig. 1A) and SUV39H1 (Fig. 1B) was localized diffusely in the nucleus with patches of higher intensity. SHP was present in both the cytoplasm and nucleus with bright punctuated fluorescence as well as more diffuse patches of increased fluorescence. In 80% of the cells observed, the positions of the brighter patches of fluorescence of GFP-SHP overlapped patches of increased staining of G9a but not that of SUV39H1, which is consistent with colocalization of SHP and G9a in the nucleus.
FIG. 1.
G9a associates with SHP in mouse liver and HepG2 cells. (A and B) SHP and G9a (A), but not SUV39H1 (B), colocalize in the nucleus. Cos-1 cells were cotransfected with expression plasmid DNA for GFP-SHP and either G9a or HA-SUV39H1. SHP was detected by GFP fluorescence (green), and G9a and SUV39H1 were detected by immunofluorescence using rhodamine-conjugated secondary antibody (red) as described in Materials and Methods. Representative confocal microscopic images for G9a or SUV39H1 immunofluorescence, green fluorescent images for GFP-SHP, and merged images are shown. The yellow regions in the merged images indicate colocalization of G9a and SHP. (C) Nuclear extracts (NE) were prepared from mouse liver or HepG2 cells and incubated with GST or GST-SHP. The GST proteins were bound to glutathione-Sepharose, and after a washing step, proteins associated with the GST proteins were detected by SDS-PAGE and Western blotting using G9a antibody. The position of G9a is indicated, and positions of molecular weight markers are indicated at left. The input contains 10% of the amount of extract used in the binding reactions. (D) Nuclear extracts prepared from HepG2 cells were immunoprecipitated using IgG as a control or with antibody (Ab) against SHP or G9a, and association of endogenous G9a or SHP in the immunoprecipitates was detected by SDS-PAGE and Western blotting using G9a or SHP antibodies, respectively. The diffuse bands for G9a compared to those in panel C result from the large amount of protein from the precipitating antibody in the sample, which reduces the resolution of the bands. (E) Schematic diagrams of SHP and full-length (FL) GST-SHP and GST-SHP deletion mutants. RID and RD refer to receptor interacting and intrinsic repression domains, respectively. (F) 35S-G9a was synthesized in vitro, and GST pull-down assays were performed as described in Materials and Methods. The input represents 20% of the 35S-labeled G9a used in the binding reactions. The position of G9a is indicated. (G) A schematic diagram of the GST-G9a deletion mutant (residues 621 to 1000). SET indicates a domain common to Su(var), Enhancer of Zeste, and Trithorax. (H) 35S-SHP was synthesized, and GST pull-down assays were performed as described in panel F. The input represents 20% of the 35S-labeled SHP used in the reactions.
Next, we examined if G9a associates with SHP in mouse hepatocytes in vivo and human hepatoma HepG2 cells. Nuclear extracts from mouse liver and HepG2 cells were incubated with either GST-SHP or GST bound to glutathione-Sepharose. After the beads were washed, the bound proteins were analyzed by Western blotting. G9a was pulled down from both extracts by GST-SHP but not by GST (Fig. 1C). In addition, interaction between endogenous SHP and G9a in HepG2 cells was examined by coimmunoprecipitation. HepG2 nuclear extracts were immunoprecipitated with antisera against SHP, G9a, or control IgG, and the immunoprecipitates were analyzed by Western blotting. G9a was detected in the anti-SHP immunoprecipitates and, conversely, SHP was detected in the anti-G9a immunoprecipitates (Fig. 1D). These results indicate that endogenous SHP interacts directly, or indirectly within a complex, with endogenous G9a in hepatic cells.
To test if G9a can directly interact with SHP, GST fusions with SHP or fragments of SHP (Fig. 1E) that had been bound to glutathione-Sepharose were incubated with 35S-labeled G9a. G9a bound to SHP or SHP mutants containing the N-terminal domain (amino acids 1 to 92), whereas no binding was detected with SHP mutants lacking this region (Fig. 1F). In parallel experiments (not shown), the C-terminal domain (amino acids 160 to 260) of SHP was required for its interaction with mSin3A and Brm as previously reported (24). To examine if the C-terminal catalytic SET [Su(var), Enhancer of Zeste, and Trithorax] domain of G9a was sufficient for interaction with SHP, a GST-G9a C-terminal fragment containing amino acids 621 to 1000 (Fig. 1G) was bound to the glutathione-Sepharose and incubated with 35S-labeled SHP. The GST-G9a C-terminal fragment efficiently interacted with SHP (Fig. 1H). These results indicate that the N terminus of SHP is required for direct interaction with G9a and that the C-terminal SET domain of G9a is sufficient for direct interaction with SHP in vitro.
G9a coimmunoprecipitated with SHP methylates H3K9 in vitro.
If the G9a associated with SHP is functionally active, then HMT activity should be detected in the protein complex immunoprecipitated by SHP antisera. SHP-containing protein complexes from mouse liver or HepG2 nuclear extracts were obtained by immunoprecipitation with SHP antibody or by binding to GST-SHP and used in in vitro HMT assays. Histone H3 was methylated in reactions containing proteins pulled down by GST-SHP from nuclear extracts of mouse liver or HepG2 cells while proteins pulled down by GST did not exhibit HMT activity (Fig. 2A). Further, Western blotting with antibody specific for dimethyl H3K9 revealed that the methylation of histone H3 in the HMT reaction included methylation at K9 (Fig. 2B). Histone H3 was also methylated in reactions containing anti-SHP immunoprecipitates from HepG2 nuclear extracts, but not in reactions containing immunoprecipitates with control IgG (Fig. 2C). Since G9a is pulled down by GST-SHP (Fig. 1) and specifically catalyzes K9 methylation of histone H3 (45), these results are consistent with the conclusion that the H3K9 methylation was catalyzed by G9a.
FIG. 2.
G9a in SHP-containing complexes methylates H3K9 in vitro. (A). Nuclear extracts (NE) from HepG2 cells or mouse liver were incubated with bacterially expressed, purified GST-SHP or GST that had been bound to glutathione-Sepharose. The proteins associated with GST-SHP or GST were used as the source of enzyme in in vitro HMT assays. Proteins were separated by SDS-PAGE, followed by Coomassie staining (lower panel) of the gels and autoradiography (upper panel). The positions of histones H3 and H4 are indicated. (B) Mouse liver nuclear extracts (NE) were incubated with bacterially expressed, purified GST-SHP or GST that had been bound to glutathione-Sepharose. The proteins associated with the Sepharose beads were used as the source of enzyme in HMT assays. After the reaction, proteins were separated by SDS-PAGE, and dimethylated H3K9 histones were detected by Western blotting using dimethylated H3K9 antibody. The position of a molecular mass marker is shown at left. The position of H3 (dimethyl H3K9) is indicated at right. (C) HepG2 nuclear extracts were immunoprecipitated with either SHP antibody (Ab) or control IgG, and the immunoprecipitates were used as the source of enzyme in HMT assays. The proteins were separated by SDS-PAGE and then by Coomassie staining of the gels to detect the amounts of core histone substrate used in the assay (lower panel), followed by autoradiography to detect 3H-labeled histones (upper panel). The positions of histones H3 and H4 are indicated. (D) Experimental outline. HepG2 cells were infected with control empty adenoviral vector (Ad-empty) or adenoviral vector encoding G9a-DN (Ad-G9a-DN), and 36 h later, the infected cells were subjected to semiquantitative RT-PCR, Western blotting, or immunoprecipitation followed by in vitro HMT assays. (E) Cell extracts were subjected to Western blotting using G9a antibody and HNF-4 antibody as a control. (F) Cell extracts were immunoprecipitated with SHP antibody (Ab) or control IgG, and the immunoprecipitates were used as the source of enzyme in in vitro HMT assays. Bacterially expressed and purified GST-H3(1-84) (WT) or a GST-H3 mutant (H3R9) in which K9 is mutated to Arg as shown was used as histone substrates. The HMT reaction samples were analyzed as described in panel A.
To directly determine whether the G9a in the SHP complex catalyzes H3K9 methylation, we utilized a catalytically inactive G9a dominant negative mutant (G9a-DN) in HepG2 cells. The G9a-DN contains two amino acid substitutions within the catalytic SET domain and efficiently blocked activity of wild-type G9a in the PRD-BF1-mediated inhibition of the beta interferon gene (16). The G9a-DN interacted as efficiently as wild type with the N terminus of GST-SHP in GST pull-down assays (not shown). Furthermore, overexpression of the G9a-DN enhanced SHP-mediated inhibition of HNF-4 transactivation in transfection reporter assays (data not shown). HepG2 cells were infected with adenoviral vector encoding G9a-DN (Ad-G9a-DN) or control empty vector (Ad-empty), and the cells were further subjected to real-time RT-PCR, Western blotting, or coimmunoprecipitation followed by in vitro HMT assays (Fig. 2D). The adenoviral vectors express GFP, and GFP fluorescence was detected in over 90% of cells by confocal microscopy (not shown).
RT-PCR and Western blotting revealed that mRNA and protein levels of total G9a (endogenous G9a and G9a-DN) were substantially increased in HepG2 cells infected with Ad-G9a-DN (Fig. 2E). HMT activity in anti-SHP or control IgG immunoprecipitates of extracts from the HepG2 cells infected with the Ad-empty or Ad-G9a-DN virus was analyzed using purified GST-H3(1-84) as the substrate. Methylation of GST-H3 was observed with the anti-SHP immunoprecipitates of extracts from cells infected with the Ad-empty virus. However, methylation of GST-H3 was not observed in the anti-SHP immunoprecipitates from cells infected with Ad-G9a-DN (Fig. 2F), which indicates that G9a is responsible for the observed methylation of GST-H3. To provide further evidence that the residue methylated was Lys-9, purified GST-H3R9, in which Lys-9 is mutated to Arg, was added as substrate in the reactions. GST-H3R9 was not methylated in reactions containing anti-SHP immunoprecipitates from cells infected with either the Ad-empty or Ad-G9a-DN virus (Fig. F). Control IgG immunoprecipitates did not show any HMT activity (Fig. F). These results provide evidence that G9a is present in a hepatic SHP complex and is catalytically active for methylation of H3K9.
G9a increases inhibition of CYP7A1 transcription by SHP.
HNF-4 and its coactivator PGC-1α transactivate the CYP7A1 promoter and are well-known targets of SHP inhibition (4, 10, 24, 29, 42). Therefore, we determined whether exogenously expressed G9a increases the inhibition of HNF-4/PGC-1α activation by SHP using a Gal4 reporter system in transfected Cos-1 cells. Expression of PGC-1α dramatically increased HNF-4 transactivation, and increasing amounts of SHP progressively suppressed the HNF-4/PGC-1α activation (Fig. 3A, lanes 4 to 7). Transfection of increasing amounts G9a expression plasmid with a constant amount of SHP plasmid increased inhibition of HNF-4/PGC-1α activation in a dose-dependent manner (Fig. 3A, lanes 8 to 11, and B, lanes 3 and 4). In contrast, expression of the heterochromatic histone methyltransferase, SUV39H1, did not enhance SHP-mediated inhibition but slightly increased transcription (Fig. 3B, lanes 5 and 6). These results are consistent with our observations that G9a, but not SUV39H1, colocalized with SHP in Cos-1 cells and that G9a is present in SHP-containing complexes (Fig. 1).
FIG. 3.
G9a enhances SHP inhibition of CYP7A1 transcription. (A) Cos-1 cells were cotransfected with 200 ng of 5Gal4-TATA-luc, 300 ng of CMV-β-Gal, and 50 ng of Gal4DBD (G4) or Gal4-HNF-4 (G4HNF-4), 100 ng of pcDNA3-PGC-1α, and increasing amounts of pcDNA3-SHP and pEGFP-hG9a, as indicated. (B) Cos-1 cells were cotransfected with 200 ng of 5Gal4-TATA-luc, 300 ng of CMV-β-Gal, 50 ng of Gal4-HNF-4, 50 ng of pcDNA3-PGC-1, 25 ng of SHP, and increasing amounts of either pcDNA3-G9a or pcDNA3.1-SUV39H1. (C) Cos-1 cells were cotransfected with 200 ng of 5Gal4-TATA-luc, 300 ng of CMV-β-Gal, 10 ng of Gal4-HNF-4, 5 ng of pcDNA3-PGC-1α, 25 ng of pcDNA3-SHP, and increasing amounts of pcDNA3-G9a, as indicated. (D) Cos-1 cells were cotransfected with 200 ng of 5Gal4-TATA-luc, 300 ng of CMV-β-Gal, 200 ng of −1887-hCYP7A1-luc, 50 ng of CMV-HNF-4, 25 ng of pcDNA3-SHP, and increasing amounts of pEGFP-G9a as indicated. In each of the above cases, the values for firefly luciferase activities were normalized by dividing by β-galactosidase activities. Standard error of the mean was calculated from triplicate determinations. Consistent results were obtained from two to four independent triplicate assays. (E) HepG2 cells in which −1887-hCYP7A1-luc reporter plasmid DNA was stably incorporated into the genome were infected with Ad-G9a-DN or Ad-empty virus, and 24 h after infection, cells were treated with 25 or 50 μM CDCA or vehicle for 10 h. Firefly luciferase activities were determined and normalized by dividing by total protein amounts. The standard error of the mean was calculated from triplicate samples, and statistical significance was determined by a Student's t test. *, P < 0.05; NS, statistically not significant. (F) HepG2 cells were infected with Ad-G9a-DN or Ad-empty virus, and 24 h after infection, cells were treated with 25 μM CDCA or vehicle for 10 h. Total RNA was isolated and subjected to real-time RT-PCR. CYP7A1 mRNA levels were normalized to β-actin mRNA levels, and the standard error of the mean was calculated from triplicate samples. Differences between Ad-empty- and Ad-G9a-DN-infected samples in CDCA-treated samples were analyzed by a Student t test. *, P < 0.05.
To test if G9a inhibition of HNF-4/PGC-1α activation is dependent on SHP, G9a was expressed in transfected Cos-1 cells with or without expression of SHP. In the absence of exogenous SHP, G9a had little effect on the HNF-4/PGC-1α transactivation (Fig. 3C, lanes 6 to 8) while increasing inhibition was observed with SHP (lanes 3 to 5), suggesting that SHP is required for the inhibitory effect of G9a. We also tested if G9a enhances SHP inhibition of HNF-4-mediated transactivation of the natural CYP7A1 promoter rather than the Gal4 reporter used above. Exogenous expression of SHP inhibited HNF-4 transactivation of CYP7A1 promoter activity and cotransfection of increasing amounts of G9a vector potentiated the SHP inhibition in a dose-dependent manner in Cos-1 cells while expression of G9a in the absence of SHP had much less of an effect (Fig. 3D). Transfection of increasing amounts of G9a also potentiated the inhibited of CYP7A1 promoter activity by exogenously expressed SHP in HepG2 cells (not shown). These functional reporter studies suggest that G9a cooperatively enhances inhibition of CYP7A1 transcription with SHP.
Overexpression of G9a-DN reverses CYP7A1 inhibition in bile acid-treated HepG2 cells.
It has been shown that bile acid treatment results in the induction of SHP, which inhibits expression of CYP7A1 in HepG2 cells and in animals in vivo (8, 9, 15, 24, 31). To evaluate the role of G9a in the suppression of the CYP7A1 promoter activity mediated by bile acid-induced SHP, HepG2 cells, in which CYP7A1 promoter-luciferase reporter plasmid DNA is stably incorporated into the genome, were infected with either Ad-empty or Ad-G9a-DN virus, and 2 days later cells were treated with 25 or 50 μM CDCA, a primary bile acid, and reporter activity was measured. Luciferase activity was inhibited about 30% and 40% by 25 μM and 50 μM CDCA treatment, respectively (Fig. 3E, lanes 1, 2, and 5), and infection with the increasing amounts of Ad-G9a-DN completely reversed the inhibition (lanes, 3, 4, 6, and 7).
We further tested if expression of G9a-DN reversed the SHP-mediated inhibition of endogenous CYP7A1 gene expression after bile acid treatment. HepG2 cells were infected with Ad-empty or Ad-G9a-DN virus, and 2 days after infection, cells were treated with 25 μM CDCA. Levels of the endogenous CYP7A1 mRNA in HepG2 cells were measured by quantitative real-time RT-PCR. After CDCA treatment, CYP7A1 mRNA levels were decreased about 50%, and infection of HepG2 cells with Ad-G9a-DN completely reversed the decrease (Fig. 3F). These results indicate that G9a is involved in SHP-mediated suppression of endogenous CYP7A1 expression, most likely by catalyzing H3K9 methylation at the native promoter in bile acid-treated HepG2 cells.
G9a is recruited to the endogenous CYP7A1 and CYP8B1 promoters and H3K9 is methylated in mice fed cholic acid and in bile acid-treated HepG2 cells.
If the interaction of G9a with SHP is physiologically relevant for bile acid metabolism, then G9a should be recruited to the transcriptionally suppressed promoters of the SHP target genes, Cyp7a1 and Cyp8b1, in mice fed cholic acid. In mice fed chow containing 0.5% cholic acid for 24 h, SHP mRNA levels were increased, whereas Cyp7a1 and Cyp8b1 mRNA levels were substantially decreased as determined by real-time RT-PCR (Fig. 4A).
FIG. 4.
G9a is recruited to the endogenous CYP7A1 and CYP8B1 promoters and H3K9 is methylated in mice fed cholic acids and in CDCA-treated HepG2 cells. Mice were fed normal or 0.5% cholic acid (C.A.)-supplemented chow for 24 h, and livers were collected for real-time RT-PCR or ChIP assays. (A) Total RNA was isolated from mouse liver and subjected to RT-PCR using primers specific to mouse Cyp7a1, Cyp8b1, and SHP and 36B4 as a control, as described in Materials and Methods. (B) At the top, schematic diagrams of mouse Cyp7a1, Cyp8b1, and GAPDH genes with the positions of the primers used for the PCRs are shown. Soluble chromatin was isolated and precleared as described in Materials and Methods and immunoprecipitated with antibodies against SHP, G9a, diacetylated H3 K9/K14, or dimethylated H3K9, or with normal serum (NS). Precipitated chromatin was extensively washed. Genomic DNA was isolated from the input chromatin before precipitation (total) or from the precipitated chromatin and was analyzed by semiquantitative PCR using primer sets specific for Cyp7a1, Cyp8b1, and GAPDH. (C) The intensities of bands were determined using the Image J program. The amount of Cyp7a1 promoter sequence precipitated by each antibody was normalized by dividing by the amount of the promoter sequence in the input DNA sample, and the value in the control group for each factor was assigned as a value of 100%. (D to F) HepG2 cells treated with 50 μM CDCA or vehicle dimethyl sulfoxide in serum-free medium for 18 h were collected for real-time RT-PCR and ChIP assays. (D) Total RNA was isolated and subjected to real-time RT-PCR using primers specific to human CYP7A1, SHP, and 36B4 as a control. (E) At the top, a schematic diagram of the human CYP7A1 gene and the position of the primer set specific for CYP7A1 used for semiquantitative PCR are shown. ChIP assays were performed using antibodies against SHP, G9a, acetylated histone H3, diacetylated H3 K9/K14, or dimethylated H3K9, or using normal serum (NS). (F) Results were quantified and analyzed from three experiments as described for panel C. For panels A, C, D, and F, the standard error of the mean is calculated from triplicates, and statistical significance was determined by a Student's t test.  , P < 0.05;
, P < 0.05; 
 , P < 0.01. di-AcH3 K9/K14, diacetylated H3 K9/K14; di-Met H3K9, dimethylated H3K9; hCYP7A1, human CYP7A1; mCyp7a1, mouse Cyp7a1; mCyp8b1, mouse Cyp8b1; mSHP, mouse SHP; mGAPDH, mouse GAPDH.
, P < 0.01. di-AcH3 K9/K14, diacetylated H3 K9/K14; di-Met H3K9, dimethylated H3K9; hCYP7A1, human CYP7A1; mCyp7a1, mouse Cyp7a1; mCyp8b1, mouse Cyp8b1; mSHP, mouse SHP; mGAPDH, mouse GAPDH.
In mice fed cholic acid, there was an increased association of SHP with the promoters of the hepatic Cyp7a1 and Cyp8b1 genes compared to mice fed normal chow, as detected by ChIP assays (Fig. 4B and C). In addition, increased association of G9a was observed in these mice (Fig. 4B and C). These results indicate that both SHP and G9a are recruited to these promoters in response to increased bile acid levels, which is consistent with a functional interaction between SHP and G9a.
We previously reported that bile acid treatment resulted in a decrease in the acetylation of H3 and H4 at the human CYP7A1 promoter in HepG2 cells but did not determine whether H3K9 was the modified residue (24). Further, since the recruitment of G9a to the Cyp7a1 and Cyp8b1 promoters was increased after bile acid treatment and methylation of H3K9 has been inversely correlated with acetylation at H3K9 and H3K14 (27, 41, 43), it seemed likely that methylation of H3K9 should also increase. Therefore, these histone modifications were examined by ChIP assay using antisera specific for either dimethyl H3K9 or diacetyl H3K9/K14. In mice fed cholic acid, Cyp7a1 and Cyp8b1 promoter sequences precipitated by antisera against diacetyl H3 K9/K14 were substantially decreased, while the promoter sequences precipitated by antisera against dimethyl H3K9 were markedly increased (Fig. 4B and C). The control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) coding region was not precipitated by any of the antisera. These results indicate that H3K9 at the Cyp7a1 and Cyp8b1 promoters is deacetylated and then methylated in livers of mice fed cholic acid.
We also examined the effects of treatment of HepG2 cells with CDCA. Reporters containing FXR-responsive elements were induced in the HepG2 cells by CDCA treatment, indicating that FXR signaling pathway was functional in HepG2 cells (data not shown). CDCA treatment markedly increased SHP mRNA levels and decreased CYP7A1 mRNA levels in HepG2 cells (Fig. 4D). ChIP assays showed that CDCA treatment increased recruitment of SHP and G9a, decreased H3K9 acetylation, and increased H3K9 methylation at the promoters of CYP7A1 and CYP8B1 (Fig. 4E and F), which is consistent with the studies above on mice fed cholic acid (Fig. 4B and C). These studies in vivo in mice fed cholic acid and in bile acid-treated HepG2 cells establish that endogenous SHP and G9a are recruited to the transcriptionally repressed native CYP7A1 and CYP8B1 promoters. Consistent with this recruitment and that of HDACs observed previously (24), H3K9 at the native CYP7A1 and CYP8B1 promoters is deacetylated and methylated.
G9a recruitment and subsequent H3K9 methylation after bile acid treatment are dependent on SHP expression.
To determine if SHP is directly involved in the recruitment of G9a and subsequent H3K9 methylation at the native CYP7A1 promoter after bile acid treatment, HepG2 cells were either transiently transfected with pSuper-siSHP (24) or infected with adenoviral vector encoding human SHP siRNA (Ad-siSHP). Treatment of HepG2 cells with CDCA increased and decreased mRNA levels of SHP and CYP7A1, respectively (Fig. 5A). Transient transfection of SHP siRNA vectors significantly reversed the changes in mRNA for SHP and CYP7A1 after CDCA treatment, which is consistent with previous studies (24).
FIG. 5.
G9a recruitment to the CYP7A1 promoter and subsequent H3K9 methylation after bile acid treatment is dependent on SHP expression. (A and B) HepG2 cells were transiently transfected with either pSUPER or pSUPER-siSHP and treated with 50 μM CDCA for 18 h. (A) Levels of mRNA were determined by real-time RT-PCR relative to 36B4 mRNA levels. The standard error of the mean was calculated from triplicate samples, and statistical significance was determined by a Student's t test. 
 , P < 0.01. (B) ChIP assays were performed using antibodies against SHP and G9a. Precipitated chromatin was extensively washed and analyzed as described in the legend of Fig. 4E. (C) HepG2 cells were infected with Ad-empty or Ad-siSHP virus and treated with 50 μM CDCA or vehicle for 18 h. Cells were then collected for ChIP assays using antibodies against SHP, G9a, diacetylated H3K9/K14, or dimethylated H3K9, or using normal serum (NS). Precipitated chromatin was extensively washed and analyzed as described in the legend of Fig. 4E. di-AcH3 K9/K14, diacetylated H3 K9/K14; di-Met H3K9, dimethylated H3K9; hCYP7A1, human CYP7A1; hGAPDH, human GAPDH.
, P < 0.01. (B) ChIP assays were performed using antibodies against SHP and G9a. Precipitated chromatin was extensively washed and analyzed as described in the legend of Fig. 4E. (C) HepG2 cells were infected with Ad-empty or Ad-siSHP virus and treated with 50 μM CDCA or vehicle for 18 h. Cells were then collected for ChIP assays using antibodies against SHP, G9a, diacetylated H3K9/K14, or dimethylated H3K9, or using normal serum (NS). Precipitated chromatin was extensively washed and analyzed as described in the legend of Fig. 4E. di-AcH3 K9/K14, diacetylated H3 K9/K14; di-Met H3K9, dimethylated H3K9; hCYP7A1, human CYP7A1; hGAPDH, human GAPDH.
As determined by ChIP assay, SHP was recruited to the native CYP7A1 promoter by CDCA treatment, but the recruitment was decreased in cells transiently transfected with pSuper-siSHP or infected with Ad-siSHP, and association of G9a with the promoter was also reduced in these cells (Fig. 5B and C). The state of histone methylation and acetylation was examined in the adenovirus-infected cells. The decrease in histone acetylation and increase in methylation at H3K9 observed after bile acid treatment were markedly reversed in the cells infected with Ad-siSHP (Fig. 5C). These results suggest that recruitment of G9a to the native CYP7A1 promoter and subsequent H3K9 methylation are contingent on expression of bile acid-induced SHP. These results, together with in vitro protein interaction studies (Fig. 1), suggest that G9a is recruited by SHP to CYP7A1 promoter after bile acid treatment, probably by direct interaction with SHP.
Blocking the H3K9 methylation by G9a-DN partially inhibits histone deacetylation at the CYP7A1 promoter chromatin.
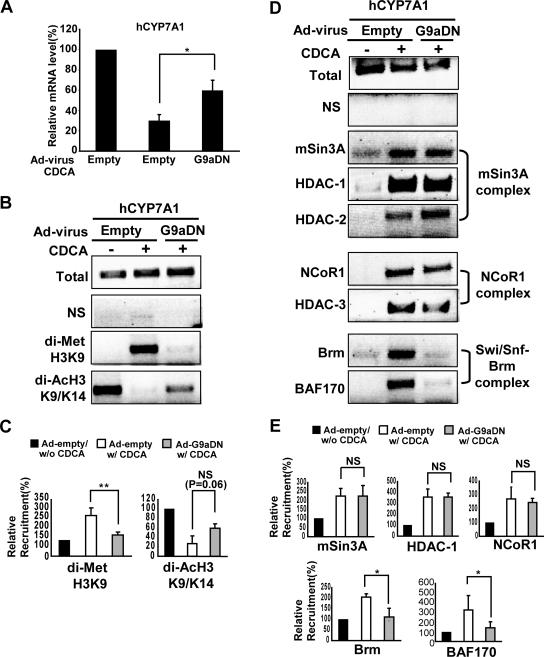
Methylated H3K9 by G9a could serve as a binding platform to recruit SHP-associated chromatin-modifying complexes for transcriptional silencing. For example, it has been suggested that H3K9 methylation affects deacetylation by enhancing recruitment and/or activity of HDACs and by inhibiting the association and/or the activity of histone acetyltransferases (HATs) (41, 43, 44). Therefore, to test if inhibition of H3K9 methylation at the CYP7A1 promoter affects deacetylation at H3K9, HepG2 cells were infected with either Ad-G9a-DN or Ad-empty virus, treated with CDCA, and analyzed by ChIP assays using antibodies against either dimethylated H3K9 or diacetylated H3K9/K14. In addition, the infected cells were subjected to real-time RT-PCR to analyze the effects of G9a-DN on CYP7A1 expression. After CDCA treatment, CYP7A1 mRNA levels were decreased about 70%, and infection of HepG2 cells with Ad-G9a-DN partially but significantly reversed the decrease (Fig. 6A).
FIG. 6.
Expression of the G9a-DN mutant inhibits H3K9 deacetylation as well as methylation and blocks recruitment of the Swi/Snf-Brm complex to the CYP7A1 promoter in CDCA-treated HepG2 cells. HepG2 cells were infected at a multiplicity of infection of 10 with either Ad-G9a-DN or Ad-empty virus, and 24 h after infection, cells were treated with 50 μM CDCA or vehicle for 18 h. (A) The mRNA levels of CYP7A1 were measured by real-time PCR. (B and D) The infected cells were immunoprecipitated using antibodies against dimethylated H3K9 or diacetylated H3K9/K14, antibodies against Brm, BAF170, mSin3A, HDAC-1, HDAC-2, NcoR1, or HDAC-3 and normal serum (NS) as described in the legend to Fig. 4E. Precipitated chromatin was extensively washed, and isolated DNA was analyzed by semiquantitative PCR using primer sets specific for human CYP7A1 as described in the legend to Fig. 4E. (C and E) The intensities of bands in three independent experiments were determined using the Image J program and analyzed as described in the legend of Fig. 4C. For panels A, C, and E, the standard error of the mean for triplicate determinations is shown, and the statistical significance was determined by a Student's t test.  , P < 0.05;
, P < 0.05; 
 , P < 0.01; NS, statistically not significant. di-AcH3 K9/K14, diacetylated H3 K9/K14; di-Met H3K9, dimethylated H3K9; hCYP7A1, human CYP7A1.
, P < 0.01; NS, statistically not significant. di-AcH3 K9/K14, diacetylated H3 K9/K14; di-Met H3K9, dimethylated H3K9; hCYP7A1, human CYP7A1.
CYP7A1 promoter chromatin precipitated with the dimethylated H3K9 antibody was increased after CDCA treatment in cells infected with Ad-empty virus (Fig. 6B and C), as expected from ChIP studies with uninfected cells (Fig. 4 and 5). However, in CDCA-treated HepG2 cells infected with Ad-G9a-DN, methylation was reduced about 50% (Fig. 6B and C). The control GAPDH coding region was not precipitated by any of the antisera (data not shown). These results indicate that increased dimethylation of H3K9 at the native CYP7A1 promoter after CDCA treatment is dependent on G9a activity.
CYP7A1 promoter sequence precipitated with diacetylated H3K9/K14 antibody was reduced about 80% after CDCA treatment in cells infected with Ad-empty virus (Fig. 6B and C) as expected from studies with uninfected cells (Fig. 4 and 5). However, the amount of precipitated promoter DNA was markedly increased about twofold in CDCA-treated cells infected with the Ad-G9a-DN virus compared to cells infected with Ad-empty although this increase was just short of statistical significance (Fig. 6B and C). These results indicate that inhibition of the endogenous G9a by G9a-DN inhibited H3K9 methylation as expected and also partially blocked diacetylation of H3K9. These results are consistent with the idea that acetylation of H3K9 is decreased after CDCA treatment even if methylation is blocked by recruitment of HDACs and possibly dissociation of HATs. Methylation by G9a may block reacetylation of H3K9, resulting in nearly complete deacetylation.
In bile acid-treated HepG2 cells, Ad-G9a-DN blocks recruitment of the Swi/Snf-Brm complex, but not of HDAC-containing corepressor complexes, to the CYP7A1 promoter.
Since blocking methylation partially reversed deacetylation, it is possible that methylation is required for recruitment of chromatin-modifying complexes, such as HDAC corepressor and the Swi/Snf-Brm chromatin remodeling complexes to the native CYP7A1 promoter. To examine this question, HepG2 cells were infected with either Ad-G9a-DN or Ad-empty virus, treated with CDCA, and subjected to ChIP assays using antibodies against mSin3A, HDAC-1, HDAC-2, NcoR1, HDAC-3, BAF170, or Brm. CYP7A1 promoter sequences precipitated with antibodies against all these cofactors were increased in HepG2 cells infected with Ad-empty after CDCA treatment, indicating that corepressor mSin3A/HDAC-1/2, corepressor NcoR1/HDAC3, and the Swi/Snf-Brm remodeling complexes are recruited after CDCA treatment (Fig. 6D and E) as previously shown (24). CYP7A1 promoter sequences precipitated with antibodies against mSin3A, HDAC-1, HDAC-2, NcoR1, or HDAC-3 were not reduced in HepG2 cells infected with Ad-G9a-DN, suggesting that recruitment of these corepressor complexes is not dependent on dimethylation at H3K9 even though deacetylation is partially blocked. The amounts of the CYP7A1 promoter sequence precipitated with antibodies against Brm or BAF170 were reduced about 50% in cells infected with Ad-G9a-DN (Fig. 6D and E). These results suggest that G9a is critical for the inhibitory SHP action by catalyzing dimethylation of H3K9, which prevents reversible acetylation, thereby “locking” H3K9 in a deacetylated state, and is a prerequisite for recruitment of the Brm complex, which in turn catalyzes chromatin remodeling, ultimately resulting in transcriptional silencing of the CYP7A1 gene.
Inhibition of HDAC activity by TSA blocks H3K9 methylation but not recruitment of G9a to the CYP7A1 promoter.
Histone deacetylation and methylation are coordinated events and are functionally associated with gene silencing (19, 27). H3K9 deacetylation is required in order for methylation to occur at H3K9, but it is not clear whether deacetylation is required for the recruitment of the G9a methyltransferase. To examine this question, the effect of TSA, an inhibitor of HDAC activity, on bile acid-induced chromatin modification and G9a recruitment in HepG2 cells was examined. First, to examine the functional correlation between histone deacetylation and methylation, Cos-1 cells were cotransfected with a Gal4-TATA-luc reporter and expression plasmids for Gal4DBD-HNF-4, PGC-1α, SHP, and G9a, and the transfected cells were treated with increasing amounts of TSA. Expression of SHP reduced transactivation mediated by HNF-4/PGC-1α, and coexpression of G9a further enhanced the SHP inhibitory activity (Fig. 7A, lanes 1 to 3). Increasing amounts of TSA completely reversed the inhibitory effects of SHP and G9a on the reporter activity in a dose-dependent manner (lanes 4 to 7).
FIG. 7.
Functional interplay between SHP-recruited HDACs and G9a in altering CYP7A1 promoter chromatin structure. (A) TSA treatment completely reversed enhancement of SHP inhibitory activity by G9a. Cos-1 cells were cotransfected with 200 ng Gal4-TATA-luc, 300 ng CMV-β-Gal, 100 ng of Gal4-HNF-4, 50 ng of pcDNA3-PGC-1, 25 ng of pcDNA3-SHP, and 500 ng of pCMV-G9a, and 6 h after transfection, cells were treated with increasing amounts of TSA for 16 h as indicated. Luciferase and β-galactosidase activities were determined in cell extracts, and firefly luciferase activities were normalized by dividing by β-galactosidase activities. (B and C). Deacetylation at H3K9 is a prerequisite for G9a-mediated methylation. HepG2 cells were pretreated with 100 nM TSA, and 1 h later, cells were additionally treated with 25 μM CDCA or vehicle for 7 h. (B) CYP7A1 mRNA levels were measured by real-time RT-PCR. (C) Cells were collected for ChIP assays using antibodies against diacetylated H3K9/K14, dimethylated H3K9, or G9a, or using normal serum (NS). (D) The intensities of bands were determined by the Image J program and analyzed as described in the legend of Fig. 4C. For panels B and D, the standard error of the means from triplicate determinations are shown, and statistical significance was determined by a Student's t test.  , P < 0.05;
, P < 0.05; 
 , P < 0.01; NS, statistically not significant; di-AcH3 K9/K14, diacetylated H3 K9/K14; di-Met H3K9, dimethylated H3K9; hCYP7A1, human CYP7A1.
, P < 0.01; NS, statistically not significant; di-AcH3 K9/K14, diacetylated H3 K9/K14; di-Met H3K9, dimethylated H3K9; hCYP7A1, human CYP7A1.
To assess the effects of TSA on bile acid regulation of endogenous CYP7A1 expression, cells were pretreated with TSA and then additionally treated with CDCA. Treatment with CDCA suppressed CYP7A1 mRNA levels by about 50% (Fig. 7B). Treatment with TSA resulted in a complete reversal of the decreased in CYP7A1 mRNA levels, indicating that histone deacetylation is important for the suppression of CYP7A1 expression by bile acids.
In ChIP assays under the same experimental conditions described in Fig. 7B, addition of TSA reversed the decrease in diacetylation and the increase in dimethylation at H3K9 after bile acid treatment (Fig. 7C and D). Interestingly, TSA treatment did not reduce the amount of CYP7A1 promoter sequence precipitated by G9a antibody in the bile acid-treated cells, indicating that G9a recruitment to the promoter was not dependent on deacetylation (Fig. C and D). In contrast, the control GAPDH coding region was not precipitated by any of the antisera (not shown). This result is consistent with the studies above indicating that both HDACs and G9a are recruited to the CYP7A1 promoter by bile acid-induced SHP.
Results from ChIP assays and these functional reporter assays suggest that histone deacetylation by HDACs at H3K9 is required for dimethylation by G9a and, conversely, that dimethylation by G9a is important in HDAC-mediated deacetylation and transcription suppression. These results further suggest that recruitment and activity of chromatin-modifying cofactors including G9a and HDACs are coordinated and interdependent events in the transcriptional silencing of CYP7A1 mediated by bile acid-induced SHP.
Acute liver-specific inhibition of G9a disrupts bile acid homeostasis and partially reverses suppression of Cyp7a1 and Cyp8b1 genes in mice in vivo.
To determine the in vivo significance of G9a in the SHP-mediated negative feedback inhibition of hepatic bile acid metabolism, the activity of G9a in mouse liver was inhibited by the expression of G9a-DN. Mice were injected with Ad-G9a-DN or Ad-empty virus via the tail vein. Three days later, mice were fed either normal or 0.5% cholic acid-supplemented chow for 5 h or 24 h, and then the liver, gall bladder, and small intestine were collected for further analyses. Expression of G9a-DN in the liver was confirmed by Western blotting and similar infection efficiencies of over 80% between experimental groups were observed based on GFP expression (not shown).
First, to ensure that G9a-DN expressed in mouse liver blocked endogenous G9a activity, liver extracts were prepared from mice infected with Ad-G9a-DN or Ad-empty virus and immunoprecipitated with SHP antibody or control IgG. Then, the immunoprecipitates were incubated with purified GST-H3(1-84) or GST-H3R9 mutant as histone substrates in an in vitro HMT assay. The control IgG immunoprecipitates did not show any HMT activity (Fig. 8A). In contrast, hepatic SHP complex immunoprecipitated from mice infected with Ad-empty virus methylated GST-H3(1-84), whereas the complex from mice infected with Ad-G9aDN virus did not methylate GST-H3(1-84) (Fig. 8A), consistent with the results obtained with HepG2 cells (Fig. 2F). These results indicate that overexpression of G9a-DN inhibited H3K9 methylation by blocking endogenous G9a activity present in the hepatic SHP complex.
FIG. 8.
Acute liver-specific inhibition of G9a activity by G9a-DN disrupted bile acid homeostasis and derepresses Cyp7a1 and Cyp8b1 in mice fed cholic acid. About 1 × 109 active viral particles (Ad-G9a-DN or Ad-empty) were injected into a mouse tail vein, and 3 days after infection, mice were fed normal or 0.5% cholic acid-containing chow for 5 h or 24 h. Liver, gall bladder, and small intestine were collected for further analyses. (A) Liver nuclear extracts were prepared from 3 mice for each group infected with Ad-empty or Ad-G9a-DN virus and immunoprecipitated with SHP antibody (Ab) or control IgG. The immunoprecipitates were used as the source of enzyme in in vitro HMT assays. GST-H3(1-84) (WT) or GST-H3 mutant (H3R9) was used as histone substrates. The HMT reaction samples were analyzed as described in the legend of Fig. 2A. (B) Total bile acid pool size was measured from bile acid levels in gall bladder, liver, and the entire small intestine in mice fed normal or 0.5% cholic acid-supplemented chow for 5 h or 24 h. Total bile acid pool size was determined as described in Materials and Methods. The standard errors of the mean from samples from 3 mice are shown.  , P < 0.05; NS, statistically not significant. (C and D) Mice were infected with either Ad-G9a-DN or Ad-empty virus, and 3 days later, mice were fed normal or 0.5% cholic acid-containing chow for 5 h or 24 h. As a control, normal uninfected mice were also analyzed in parallel. Total RNA was isolated from liver and subjected to quantitative real-time RT-PCR using primer sets specific for Cyp7a1, Cyp8b1, and SHP, and the amounts of PCR product were divided by the amounts of 36B4 PCR product. The standard error of the mean is shown for samples from 6 mice for 5 h feeding and from 3 mice for 24 h feeding groups. Differences between Ad-empty- and Ad-G9a-DN-infected samples were analyzed by a Student t test.
, P < 0.05; NS, statistically not significant. (C and D) Mice were infected with either Ad-G9a-DN or Ad-empty virus, and 3 days later, mice were fed normal or 0.5% cholic acid-containing chow for 5 h or 24 h. As a control, normal uninfected mice were also analyzed in parallel. Total RNA was isolated from liver and subjected to quantitative real-time RT-PCR using primer sets specific for Cyp7a1, Cyp8b1, and SHP, and the amounts of PCR product were divided by the amounts of 36B4 PCR product. The standard error of the mean is shown for samples from 6 mice for 5 h feeding and from 3 mice for 24 h feeding groups. Differences between Ad-empty- and Ad-G9a-DN-infected samples were analyzed by a Student t test.  , P < 0.05; NS, statistically not significant.
, P < 0.05; NS, statistically not significant.
To determine if blocking hepatic G9a activity by G9a-DN affects bile acid homeostasis, we measured the total bile acid pool size in mice infected with either Ad-empty or Ad-G9a-DN virus. Bile acid pools in these two groups were similar when mice were fed with normal chow (Fig. 8B). In contrast, the total bile acid pools were slightly elevated in mice fed cholic acid for 5 h and significantly increased by twofold after 24 h in mice infected with Ad-G9a-DN compared to those infected with Ad-empty (Fig. 8B). Consistent with these observations, the size of the gall bladder was markedly increased in mice infected with G9a-DN and fed cholic acid for either 5 h or 24 h (data not shown).
To better understand the molecular basis of elevated bile acid pools in mice expressing G9a-DN, we examined the effects of G9a-DN on the mRNA levels of the SHP target genes in bile acid biosynthesis, Cyp7a1 and Cyp8b1. As expected, in uninfected mice fed 0.5% cholic acid-supplemented chow, the levels of mRNA for Cyp7a1 and Cyp8b1 were decreased about 50% after 5 h of feeding (Fig. 8C) and about 80% after 24 h of feeding (Fig. 8D). In mice infected with Ad-empty virus, similar effects were observed although the inhibition at 5 h was greater than in the uninfected controls (Fig. 8C and D). Interestingly, at 5 h suppression of Cyp7a1 in the Ad-G9a-DN-infected mice fed cholic acid was significantly reversed compared to the group infected with Ad-empty virus, while at 24 h of cholic acid feeding, a modest nonsignificant reversal was observed (Fig. 8C and D). Compared to regulation of Cyp7a1, suppression of Cyp8b1 was modestly, but significantly, reversed in the Ad-G9a-DN-infected mice after 5 h of feeding, although the difference was not significant at 24 h (Fig. 8C and D). SHP mRNA levels, as expected, were increased after bile acid feeding in all groups at 5 h and more dramatically at 24 h (Fig. 8C and D). Compared to the Ad-empty-infected mice, SHP mRNA levels in the Ad-G9a-DN-infected mice after cholic acid feeding were slightly reduced, but the changes were not statistically significant. These results indicate that inhibition of endogenous G9a activity by G9a-DN elevated the total bile acid pool size, which in part may be caused by transient reversal of suppression of Cyp7a1 and Cyp8b1 expression by bile acid feeding.
These in vivo animal studies, along with the studies in HepG2 cells and in vitro mechanistic studies, establish a physiological role of G9a as a key downstream cofactor of the bile acid-activated SHP pathway, resulting in acute transcriptional suppression of Cyp7a1 and Cyp8b1 genes.
DISCUSSION
In this paper we present evidence that G9a plays a critical role in the SHP-mediated suppression of hepatic bile acid biosynthesis by catalyzing H3K9 methylation at the CYP7A1 and CYP8B1 promoters, which is required for recruiting the Swi/Snf-Brm complex and transcriptional silencing. Our major findings to support this conclusion are as follows: endogenous G9a was associated with SHP in mouse liver and HepG2 cells, and G9a in hepatic SHP complexes methylated H3K9 in vitro. SHP and G9a were recruited to the native CYP7A1 and CYP8B1 promoters after bile acid treatment, and recruitment of G9a and subsequent H3K9 methylation were impaired if SHP expression was reduced by siRNA, indicating that SHP is required for G9a recruitment. G9a expression enhanced the inhibition of CYP7A1 transcription by SHP, which was correlated with the methylation of H3K9 by G9a. Inhibition of G9a activity by expression of G9a-DN in HepG2 cells inhibited H3K9 methylation after bile acid treatment and partially reversed bile acid-mediated suppression of the endogenous CYP7A1 gene expression. Interestingly, expression of G9a-DN also partially reversed deacetylation of H3K9 and blocked recruitment of the Brm complex to the promoter, indicating that deacetylation and methylation were required for recruitment of the Brm complex and ultimately for transcriptional silencing. Acute liver-specific inhibition of G9a by G9a-DN elevated the total bile acid pool size in mice fed cholic acid and partially derepressed Cyp7a1 and Cyp8b1, indicating that bile acid homeostasis was disrupted. These results establish a critical role for G9a in the SHP-mediated suppression of Cyp7a1 and Cyp8b1 in cholesterol and bile acid metabolism.
From chromatin immunoprecipitation studies in mouse liver and in HepG2 cells, we observed that SHP was recruited to the native CYP7A1 promoter in mice fed cholic acid and CDCA-treated HepG2 cells (Fig. 4). Acetylation of histones H3 and H4 and specific acetylation of H3K9 were markedly decreased and H3K9 methylation was substantially increased after feeding mice cholic acid or treating HepG2 cells with bile acids, consistent with recruitment of HDACs and G9a. Consistent with our observations, a recent study showed that SHP was detected at the Cyp7a1 and Cyp8b1 promoters in transgenic mice expressing SHP in the liver at levels equivalent to those observed after mice were fed cholic acid (5). Interestingly, chromatin changes at these two promoters differed in that study. In contrast to our observations, HDACs and changes in histone acetylation and methylation were not detected at the Cyp7a1 promoter in the transgenic mice, while in parallel to our studies, histone acetylation was decreased and H3K9 methylation was increased at the Cyp8b1 promoter. This discrepancy for Cyp7a1 may result from the length of time that SHP was elevated in the two systems. Our studies were done in a transient acute setting to assess direct effect of bile acids on the association of regulatory proteins with the native promoters, whereas in the transgenic animal model, high levels of SHP were sustained continuously in the liver, which may lead to secondary effects that affect regulation of Cyp7a1 expression, but not that of Cyp8b1.
Differential responses to different lengths of cholic acid feeding were also observed in our in vivo study. Suppression of Cyp7a1 was substantially reversed in the Ad-G9a-DN-infected mice after 5 h of cholic acid feeding compared to the control Ad-empty-infected mice, while the reversal was very modest at 24 h. Suppression of Cyp8b1 was only modestly reversed at both time points suggesting that regulation of these two genes is not identical, which has also been observed with long cholic acid feeding times in SHP-null mice (47). These results imply that at longer times of cholic acid feeding, the influence of the FXR/SHP pathway on Cyp7a1 regulation may decrease and SHP-independent pathways may become predominant. This interpretation is consistent with the trends observed in previous SHP-null mice studies in which Cyp7a1 was still mostly repressed after 1 week of cholic acid feeding, whereas it was almost completely reversed 1 day after cholic acid feeding. These studies were interpreted to suggest that the FXR/SHP pathway was more important acutely after cholic acid feeding (25, 47-49). In our studies, however, at 24 h after cholic acid feeding, the reversal of Cyp7a1 expression was already largely lost. One interpretation of this result is that the role of G9a is even more transient than that of SHP/FXR in the suppression of Cyp7a1. A second is that inactivation of G9a will have effects on the activity of multiple transcription factors, in addition to SHP, which might have indirect effects on Cyp7a1 expression not observed in the SHP-null mice. Nevertheless, these data are consistent with an important role for G9a in the acute regulation of Cyp7a1 in vivo. This is consistent with an increase in bile acid pools although other factors probably also contribute to the increased pool size.
H3K9 methyltransferase enzymes can be recruited to the target promoters by direct interaction with gene-specific DNA binding proteins or with non-DNA binding corepressors. For instance, G9a has been shown to interact with PRD-BF1, CtBP, CDP/cut, Gfi1, and SHP and is mainly responsible for silencing of target genes in the euchromatin region (6, 12, 16, 36, 43). SHP was also shown to be localized exclusively with nuclease-sensitive euchromatin and not with the heterochromatic DNA binding protein HP1 (6). Consistent with these findings, we observed that SHP and the euchromatic methyltransferase G9a, but not the heterochromatic methyltransferase SUV39H1, were colocalized in the nucleus (Fig. 1). Further, inhibition of CYP7A1 transcription by SHP was increased by expression of G9a but not by expression of SUV39H1 (Fig. 3). Interestingly, SHP, like other inhibitory factors, interacts with G9a and corepressor HDACs through distinct domains, its N-terminal and C-terminal domains, respectively (6, 24). These results suggest that SHP could recruit both HDAC-containing corepressor complexes and G9a to the native CYP7A1 promoter simultaneously.
Although the role of H3K9 methylation by G9a has been established in epigenetic gene silencing, little is known about how this enzyme is recruited and coordinately works with other chromatin-modifying cofactors at the native target promoters. Inhibition of SHP expression by siRNA in HepG2 cells demonstrated that G9a recruitment to the CYP7A1 promoter and subsequent H3K9 methylation are contingent on SHP expression (Fig. 5). Since we along with others (6) have shown that SHP can interact with G9a, the simplest conclusion from these SHP siRNA studies is that G9a recruitment is mediated by a direct interaction with SHP. The siRNA data, however, do not eliminate the possibility that G9a is recruited indirectly by another SHP-dependent protein. Inhibition of H3K9 deacetylation by a specific HDAC inhibitor, TSA, inhibited the H3K9 methylation at the promoter chromatin but, interestingly, did not inhibit recruitment of G9a (Fig. 7). The recruitment of G9a alone, therefore, was not sufficient for methylation at H3K9 but required previous deacetylation at H3K9. Functional reporter assays showed that G9a increased SHP inhibition of transactivation mediated by HNF-4/PGC-1, but these effects were blocked by TSA. These results suggest a central role of histone deacetylation in mediating transcriptional repression either directly or indirectly by permitting G9a-mediated methylation. Coordinated sequential modification of deacetylation and methylation of H3K9 have been shown in other systems as well, for example, the CtBP corepressor complex in oncogenesis (41), Gfi1 in the regulation of development and oncogenesis (12), PRDI-BF1 in beta interferon gene transcription (16), and HP1 recruitment for gene silencing (34, 43).
Conversely, H3K9 deacetylation at the CYP7A1 promoter after bile acid treatment was markedly affected by inhibition of H3K9 methylation by G9a-DN, whereas the recruitment of HDAC-containing corepressor complexes was not affected (Fig. 7). Methylation appears to be required to “lock” the H3K9 in a deacetylated state and prevent reversible acetylation rather than for recruitment of HDACs. Interestingly, deacetylation and methylation at H3K9 are required for recruitment of the Brm remodeling complex during transcriptional silencing of CYP7A1 (Fig. 6). Therefore, these results collectively suggest that recruitment and activity of chromatin-modifying cofactors are temporally coordinated sequential events in SHP-mediated repression of CYP7A1.
Based on the in vitro, in vivo, and cell culture results, we propose a model for SHP-mediated active suppression of CYP7A1 transcription (Fig. 9). As proposed earlier (20, 21, 29), bile acid-induced SHP may compete with coactivator HATs, including p300, CBP, and SRC-1, for binding at the target promoters initially. SHP then directly interacts with HDAC-containing mSin3A and NcoR1 corepressor complexes and G9a methyltransferase through its C terminus and N terminus, respectively, recruiting these factors to the promoter, which results in deacetylation and subsequent dimethylation. Deacetylation of H3K9 is required for methylation, and, conversely, methylation of H3K9 is also required to prevent reversal of the deacetylation. H3K9 methylation and deacetylation create a binding surface for the Swi/Snf-Brm complex which allows the SHP-dependent recruitment of this remodeling complex to the CYP7A1 promoter. The Swi/Snf complex then catalyzes the ATP-dependent remodeling of the promoter chromatin, as demonstrated previously (24), which establishes a transcriptionally suppressed chromatin structure. These coordinated biochemical and enzymatic events at the native CYP7A1 promoter help convert the chromatin from an active to a repressed state, which underlies the feedback inhibition of bile acid synthesis by SHP.
FIG. 9.
Coordinated sequential recruitment of HDACs, G9a, and Swi/Snf-Brm in the SHP-mediated suppression of CYP7A1 in the hepatic bile acid metabolism. Bile acid-induced SHP may compete with coactivator HATs, including p300, CBP, and SRC-1, at the CYP7A1 promoter, which contributes to a decrease in histone acetylation initially as proposed before. SHP directly interacts with HDACs and G9a through its C and N terminus, respectively, recruiting these cofactors to the promoter, which results in deacetylation and subsequent dimethylation at the H3K9. H3K9 methylation and deacetylation create a binding surface for the recruitment of the Swi/Snf-Brm complex, which results in ATP-dependent chromatin remodeling and subsequent gene silencing of CYP7A1.
In the present and previous studies, we demonstrated that chromatin-modifying enzymes, such as histone deacetylases, G9a histone methyltransferase, and Swi/Snf-Brm, are critical downstream cofactors in mediating SHP activity in the regulation of hepatic cholesterol and bile acid metabolism (24). Abnormal SHP function has been implicated in metabolic disorders such as hypercholesterolemia, cholestasis, obesity, and diabetes (2). Interestingly, heterozygous mutations of the SHP gene in humans are associated with a predisposition for moderate obesity and diabetes (35). Therefore, it will be interesting to test whether these naturally occurring SHP mutants show an impaired ability to recruit these chromatin-modifying enzymes to target genes. Aberrant regulation of histone methylation and deacetylation is now emerging as a major contributor to carcinogenesis, and molecules that affect epigenetic regulation, such as HDAC inhibitors, are now actively being tested as therapeutic agents (17). In this regard, modulation of SHP function by targeting these chromatin-modifying enzymes may help lead to new pharmacological agents for metabolic disorders.
Acknowledgments
We are grateful to Y. Nakatani and B. Vogelstein for generously providing the G9a antibody and Ad-Easy system, respectively. We thank K. Wright, C. Mizzen, M. Horwitz, M. Walsh, J. Chiang, Yoon-Kwang Lee, M. Evans, Y. Shinkai, J. Auwerx, T. Kuzarides, and I. Talianidis for kindly providing plasmids and reagents for this study. We also thank B. Kemper for helpful comments on the manuscript.
This study was supported by Swedish Research Council K2004-32X-14963-01A to E.T. and by NIH DK062777 and AHA 557772 to J.K.K.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Bae, Y., J. K. Kemper, and B. Kemper. 2004. Repression of CAR-mediated transactivation of CYP2B genes by the orphan nuclear receptor, short heterodimer partner (SHP). DNA Cell Biol. 23:81-91. [DOI] [PubMed] [Google Scholar]
- 2.Bavner, A., S. Sanyal, J. A. Gustafsson, and E. Treuter. 2005. Transcriptional corepression by SHP: molecular mechanisms and physiological consequences. Trends Endocrinol. Metab. 16:478-488. [DOI] [PubMed] [Google Scholar]
- 3.Bhalla, S., C. Ozalp, S. Fang, L. Xiang, and J. K. Kemper. 2004. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1α. Functional implications in hepatic cholesterol and glucose metabolism. J. Biol. Chem. 279:45139-45147. [DOI] [PubMed] [Google Scholar]
- 4.Borgius, L. J., K. R. Steffensen, J. A. Gustafsson, and E. Treuter. 2002. Glucocorticoid signaling is perturbed by the atypical orphan receptor and corepressor SHP. J. Biol. Chem. 277:49761-49766. [DOI] [PubMed] [Google Scholar]
- 5.Boulias, K., N. Katrakili, K. Bamberg, P. Underhill, A. Greenfield, and I. Talianidis. 2005. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. EMBO J. 24:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulias, K., and I. Talianidis. 2004. Functional role of G9a-induced histone methylation in small heterodimer partner-mediated transcriptional repression. Nucleic Acids Res. 32:6096-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brendel, C., K. Schoonjans, O. A. Botrugno, E. Treuter, and J. Auwerx. 2002. The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol. Endocrinol. 16:2065-2076. [DOI] [PubMed] [Google Scholar]
- 8.Chiang, J. Y. L., R. Kimmel, C. Weinberger, and D. Stroup. 2000. Farnesoid X receptor responds to bile acids and represses cholesterol 7a-hydroxylase gene (CYP7A1) transcription. J. Biol. Chem. 275:10918-10924. [DOI] [PubMed] [Google Scholar]
- 9.De Fabiani, E., N. Mitro, F. Gilardi, D. Caruso, G. Galli, and M. Crestani. 2003. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J. Biol. Chem. 278:39124-39132. [DOI] [PubMed] [Google Scholar]
- 10.del Castillo-Olivares, A., J. A. Campos, W. M. Pandak, and G. Gil. 2004. The role of α1-fetoprotein transcription factor/LRH-1 in bile acid biosynthesis: a known nuclear receptor activator that can act as a suppressor of bile acid biosynthesis. J. Biol. Chem. 279:16813-16821. [DOI] [PubMed] [Google Scholar]
- 11.Denson, L. A., E. Sturm, W. Echevarria, T. L. Zimmerman, M. Makishima, D. J. Mangelsdorf, and S. J. Karpen. 2001. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 121:140-147. [DOI] [PubMed] [Google Scholar]
- 12.Duan, Z., A. Zarebski, D. Montoya-Durango, H. L. Grimes, and M. Horwitz. 2005. Gfi1 coordinates epigenetic repression of p21Cip/WAF1 by recruitment of histone lysine methyltransferase G9a and histone deacetylase 1. Mol. Cell. Biol. 25:10338-10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eloranta, J. J., and G. A. Kullak-Ublick. 2005. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch. Biochem. Biophys. 433:397-412. [DOI] [PubMed] [Google Scholar]
- 14.Gobinet, J., S. Carascossa, V. Cavailles, F. Vignon, J. C. Nicolas, and S. Jalaguier. 2005. SHP represses transcriptional activity via recruitment of histone deacetylases. Biochemistry 44:6312-6320. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin, B., S. A. Jones, R. R. Price, M. A. Watson, D. D. McKee, L. B. Moore, C. Galardi, J. G. Wilson, M. C. Lewis, M. E. Roth, P. R. Maloney, T. M. Wilson, and S. A. Kliewer. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6:517-526. [DOI] [PubMed] [Google Scholar]
- 16.Gyory, I., J. Wu, G. Fejer, E. Seto, and K. L. Wright. 2004. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat. Immunol. 5:299-308. [DOI] [PubMed] [Google Scholar]
- 17.Hake, S. B., A. Xiao, and C. D. Allis. 2004. Linking the epigenetic “language” of covalent histone modifications to cancer. Br. J. Cancer 90:761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA. 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 20.Johansson, L., A. Bavner, J. S. Thomsen, M. Farnegardh, J. A. Gustafsson, and E. Treuter. 2000. The orphan nuclear receptor SHP utilizes conserved LXXLL-related motifs for interactions with ligand-activated estrogen receptors. Mol. Cell. Biol. 20:1124-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson, L., J. S. Thomsen, A. E. Damdimopoulos, G. Spyrou, J.-A. Gustafsson, and E. Treuter. 1999. The orphan nuclear receptor SHP inhibits agonist-dependent transcriptional activity of estrogen receptors ERα and ERβ. J. Biol. Chem. 274:345-353. [DOI] [PubMed] [Google Scholar]
- 22.Kadam, S., and B. M. Emerson. 2003. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell 11:377-389. [DOI] [PubMed] [Google Scholar]
- 23.Kadonaga, J. 1998. Eukaryotic transcription: An interlaced network of transcription factors and chromatin-modifying machines. Cell 92:307-315. [DOI] [PubMed] [Google Scholar]
- 24.Kemper, J., H. Kim, J. Miao, S. Bhalla, and Y. Bae. 2004. Role of an mSin3A-Swi/Snf chromatin remodeling complex in the feedback repression of bile acid biosynthesis by SHP. Mol. Cell. Biol. 24:7707-7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr, T. A., S. Saeki, M. Schneider, K. Schaefer, S. Berdy, T. Redder, B. Shan, D. W. Russell, and M. Schwarz. 2002. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev. Cell 2:713-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornberg, R. D., and Y. Lorch. 1999. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98:285-294. [DOI] [PubMed] [Google Scholar]
- 27.Kouzarides, T. 2002. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12:198-209. [DOI] [PubMed] [Google Scholar]
- 28.Ktistaki, E., and I. Talianidis. 1997. Modulation of hepatic gene expression by hepatocyte nuclear factor 1. Science 277:109-112. [DOI] [PubMed] [Google Scholar]
- 29.Lee, Y., H. Dell, D. H. Dowhan, M. Hadzopoulou-Cladaras, and D. D. Moore. 2000. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol. Cell. Biol. 20:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, Y., and D. D. Moore. 2002. Dual mechanism for repression of the monomeric orphan receptor liver receptor homologous protein-1 (LRH-1) by the orphan small heterodimer partner (SHP). J. Biol. Chem. 277:2463-2467. [DOI] [PubMed] [Google Scholar]
- 31.Lu, T. T., M. Makishima, J. J. Repa, K. Schoonjans, T. A. Kerr, J. Auwerx, and D. J. Mangelsdorf. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 6:507-515. [DOI] [PubMed] [Google Scholar]
- 32.Miao, J., S. Fang, Y. Bae, and J. K. Kemper. 2006. Functional inhibitory cross-talk between car and HNF-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J. Biol. Chem. 281:14537-14546. [DOI] [PubMed] [Google Scholar]
- 33.Min, G., J. K. Kemper, and B. Kemper. 2002. Glucocorticoid receptor interacting protein-1 (GRIP-1) and constitutive androstane receptor (CAR) mediate ligand-independent activation of the CYP2B1 gene in vivo. J. Biol. Chem. 277:26356-26363. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen, S. J., R. Schneider, U. M. Bauer, A. J. Bannister, A. Morrison, D. O'Carroll, R. Firestein, M. Cleary, T. Jenuwein, R. E. Herrera, and T. Kouzarides. 2001. Rb targets histone H3 methylation and HP1 to promoters. Nature 412:561-565. [DOI] [PubMed] [Google Scholar]
- 35.Nishigori, H., H. Tomura, N. Tonooka, M. Kanamori, S. Yamada, K. Sho, I. Inoue, N. Kikuchi, K. Onigata, I. Kojima, T. Kohama, K. Yamagata, Q. Yang, Y. Matsuzawa, T. Miki, S. Seino, M. Y. Kim, H. S. Choi, Y. K. Lee, D. D. Moore, and J. Takeda. 2001. Mutations in the small heterodimer partner gene are associated with mild obesity in Japanese subjects. Proc. Natl. Acad. Sci. USA. 98:575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishio, H., and M. J. Walsh. 2004. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. Proc. Natl. Acad. Sci. USA. 101:11257-11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa, H., K. Ishiguro, S. Gaubatz, D. M. Livingston, and Y. Nakatani. 2002. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296:1132-1136. [DOI] [PubMed] [Google Scholar]
- 38.Rice, J. C., and C. D. Allis. 2001. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr. Opin. Cell Biol. 13:263-273. [DOI] [PubMed] [Google Scholar]
- 39.Sanyal, S., J. Y. Kim, H. J. Kim, J. Takeda, Y. K. Lee, D. D. Moore, and H. S. Choi. 2002. Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J. Biol. Chem. 277:1739-1748. [DOI] [PubMed] [Google Scholar]
- 40.Seol, W., H. Choi, and D. D. Moore. 1996. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272:1336-1339. [DOI] [PubMed] [Google Scholar]
- 41.Shi, Y., J. Sawada, G. Sui, B. Affar el, J. R. Whetstine, F. Lan, H. Ogawa, M. P. Luke, and Y. Nakatani. 2003. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 422:735-738. [DOI] [PubMed] [Google Scholar]
- 42.Shin, D. J., J. A. Campos, G. Gil, and T. F. Osborne. 2003. PGC-1alpha activates CYP7A1 and bile acid biosynthesis. J. Biol. Chem. 278:50047-50052. [DOI] [PubMed] [Google Scholar]
- 43.Stewart, M. D., J. Li, and J. Wong. 2005. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol. Cell. Biol. 25:2525-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 45.Tachibana, M., K. Sugimoto, T. Fukushima, and Y. Shinkai. 2001. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 276:25309-25317. [DOI] [PubMed] [Google Scholar]
- 46.Tachibana, M., K. Sugimoto, M. Nozaki, J. Ueda, T. Ohta, M. Ohki, M. Fukuda, N. Takeda, H. Niida, H. Kato, and Y. Shinkai. 2002. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 16:1779-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, L., Y. Han, C. S. Kim, Y. K. Lee, and D. D. Moore. 2003. Resistance of SHP-null mice to bile acid-induced liver damage. J. Biol. Chem. 278:44475-44481. [DOI] [PubMed] [Google Scholar]
- 48.Wang, L., Y. Lee, D. Bundman, Y. Han, S. Thevananther, C. Kim, S. Chua, P. Wei, R. Heyman, M. Karin, and D. Moore. 2002. Redundant pathways for negative feedback regulation of bile acid production. Dev. Cell 2:721-731. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe, M., S. M. Houten, L. Wang, A. Moschetta, D. J. Mangelsdorf, R. A. Heyman, D. D. Moore, and J. Auwerx. 2004. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 113:1408-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Workman, J. L., and R. E. Kingston. 1998. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67:545-579. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, H. S., M. Gavin, A. Dahiya, A. A. Postigo, D. Ma, R. X. Luo, J. W. Harbour, and D. C. Dean. 2000. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 101:79-89. [DOI] [PubMed] [Google Scholar]