Abstract
The myocyte enhancer factor 2 (MEF2) family of transcription factors is not only important for controlling gene expression in normal cellular programs, like muscle differentiation, T-cell apoptosis, neuronal survival, and synaptic differentiation, but has also been linked to cardiac hypertrophy and other pathological conditions. Lysine acetylation has been shown to modulate MEF2 function, but it is not so clear which deacetylase(s) is involved. We report here that treatment of HEK293 cells with trichostatin A or nicotinamide upregulated MEF2D acetylation, suggesting that different deacetylases catalyze the deacetylation. Related to the trichostatin A sensitivity, histone deacetylase 4 (HDAC4) and HDAC5, two known partners of MEF2, exhibited little deacetylase activity towards MEF2D. In contrast, HDAC3 efficiently deacetylated MEF2D in vitro and in vivo. This was specific, since HDAC1, HDAC2, and HDAC8 failed to do so. While HDAC4, HDAC5, HDAC7, and HDAC9 are known to recognize primarily the MEF2-specific domain, we found that HDAC3 interacts directly with the MADS box. In addition, HDAC3 associated with the acetyltransferases p300 and p300/CBP-associated factor (PCAF) to reverse autoacetylation. Furthermore, the nuclear receptor corepressor SMRT (silencing mediator of retinoid acid and thyroid hormone receptor) stimulated the deacetylase activity of HDAC3 towards MEF2 and PCAF. Supporting the physical interaction and deacetylase activity, HDAC3 repressed MEF2-dependent transcription and inhibited myogenesis. These results reveal an unexpected role for HDAC3 and suggest a novel pathway through which MEF2 activity is controlled in vivo.
Protein lysine acetylation refers to transfer of the acetyl moiety from acetyl coenzyme A (acetyl-CoA) to the ɛ-amino group of a lysine residue and is an important posttranslational modification that has recently emerged and rivals phosphorylation (41, 61). Proteins known to be subject to lysine acetylation include histones, over 50 transcription factors, and various other proteins (10, 40, 41, 61, 77). This dynamic modification is controlled by the opposing actions of acetyltransferases and deacetylases in vivo. Histones were the first substrates identified, so these two families of enzymes are known as histone acetyltransferases (HATs) and histone deacetylases (HDACs), although most of them also act on nonhistone proteins. In the past decade, many proteins have been shown to possess HDAC activity (4, 22, 39, 66, 78). On the basis of homology to budding yeast counterparts, human HDACs are grouped into four classes, with HDAC1, -2, -3, and -8, homologs of yeast Rpd3, forming class I. Class II comprises HDAC4, -5, -6, -7, -9, and -10, which possess deacetylase domains highly related to that of yeast Hda1. HDAC4, -5, -7, and -9 have similar domain organization and thus belong to a subgroup known as class IIa. Class III consists of SIRT1 and other Sir2-related proteins. A recent phylogenetic analysis revealed that HDAC11 represents class IV (21). Members of classes I, II, and IV are zinc-dependent enzymes and display some sequence similarity to each other but show no homology to Sir2-related proteins, which require NAD+ for deacetylation.
Human HDACs have both nuclear and cytoplasmic functions. Within the nucleus, these enzymes regulate gene expression and other DNA-templated processes. According to genome-wide analysis (56, 70), orthologs from budding and fission yeast not only display a clear “division of labor” but also act cooperatively. Consistent with this, systematic expression and RNA interference knockdown experiments in Drosophila melanogaster S2 cells have recently revealed distinct roles for different deacetylases (7, 14), raising the possibility that HDACs in Drosophila or higher organisms cooperate with each other or have overlapping roles. Among others, the following lines of evidence suggest that this is the case. First, both HDAC1 and SIRT1 bind to and deacetylate p53, thereby regulating its stability, DNA-binding ability, and transcriptional activity (46, 47, 63). Second, these two deacetylases interact with and deacetylate MyoD and BCL6 (2, 15). Third, HDAC4 has been shown to deacetylate Runx3 (33), whereas Runx1 and Runx2 are known to interact with members of classes I and II (59, 65, 69). Fourth, both HDAC1 and SIRT1 associate with p300/CBP-associated factor (PCAF) (15, 73). Fifth, as integral subunits of HDAC3 complexes (24, 44, 68), the nuclear receptor corepressors SMRT and nuclear receptor corepressor (N-CoR) also interact with class II HDACs (31, 36). Finally, both HDAC6 and SIRT2 efficiently deacetylate α-tubulin acetylated on lysine 40 to regulate microtubule structure (32, 50). Thus, a general notion is that multiple HDACs are able to interact with and/or deacetylate the same target protein in mammalian cells. This notion has led us to investigate whether myocyte enhancer factor 2 (MEF2), a well-known partner of HDAC4 and homologs, is also targeted by other HDACs.
Mammalian MEF2 proteins, MEF2A, -B, -C, and -D, possess a conserved N-terminal domain for specific binding to AT-rich sequences on promoters of target genes that are important for biological processes, such as skeletal myogenesis, cardiac muscle growth and differentiation, T-cell apoptosis, neuronal survival, postsynaptic differentiation, growth factor response, and stress management (13, 49, 60). MEF2 proteins are also of pathological importance by playing a role in cardiac hypertrophy (27, 49), coronary artery disease (18), acute lymphoblastic leukemia (82), virus propagation (45), and perhaps neurodegenerative disorders (13, 60). To repress transcription, MEF2 proteins recruit corepressors such as class IIa HDACs (39, 49, 66). Calcium/calmodulin-dependent kinases (CaMKs) and protein kinases D (PKDs) phosphorylate these deacetylases, promote their nuclear export, and relieve repression (49, 66, 78). Mitogen-activated protein kinases, such as p38 and extracellular signal-regulated kinase 5, directly phosphorylate MEF2 and activate transcription (26, 37, 38, 74, 84). Some other phosphorylation events stimulate sumoylation of MEF2 on a conserved lysine residue and repress transcription (17, 19, 20, 29, 35, 60, 85, 86; reviewed in reference 79). Calcineurin reverses such phosphorylation and derepresses transcription (13, 19, 60). Acetyltransferases like p300 acetylate MEF2 on specific lysine residues to potentiate transcription (48, 60, 85).
Although it is well-known that HDAC4 and other class IIa HDACs repress MEF2-dependent transcription, it remains unclear whether they directly deacetylate MEF2. Here we report that HDAC3, but not HDAC4 and HDAC5, deacetylates MEF2. This finding suggests a novel molecular mechanism by which MEF2 transcriptional activity is regulated in vivo. MEF2 is of pathological importance, and HDAC inhibitors are emerging as promising therapeutic agents, so the HDAC3 link also sheds light on related therapeutic intervention.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney HEK293 cells and mouse C3H10T1/2 fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Sigma), penicillin, and streptomycin (Invitrogen). Mouse C2C12 cells were cultured in the same medium containing 20% FBS.
Plasmid constructs.
Mammalian expression plasmids for Flag- or hemagglutinin (HA)-tagged human MEF2D and MEF2C proteins, as well as baculovirus for Flag-MEF2D, have been described elsewhere (20, 67). A full-length cDNA clone of mouse MEF2B (GenBank accession no. BC045147) was purchased from Open Biosystems, and the coding sequence was subcloned into a pcDNA3.1(−) derivative to express HA-tagged MEF2B (HA-MEF2B). For this, an oligonucleotide duplex consisting of MEF2BL1 (5′-AA TTC ATG GGG AGA AAG AAG ATC CA-3′) and MEF2BL2 (5′-GAT CTG GAT CTT CTT TCT CCC CAT G -3′) was linked to a BglII site overlapping with codons 7 and 8 of the coding sequence. MEF2 mutants were generated by PCR with the Expand thermostable DNA polymerase (Roche) and subcloned into derivatives of pcDNA3.1 (Invitrogen). Mutations were verified by sequencing. Expression vectors for short hairpin RNA (shRNA) specific to human and mouse HDAC3 were derived from pBS/U6 (83) and pSilencer3.0-H1 (Ambion), respectively. Anti-HDAC3 polyclonal rabbit antibody and expression plasmids for HDAC1, -2, -3, and -8 were previously described (43, 75). The HDAC3 mutant H134Q, which contains the mutations H134Q and H135A, was engineered by PCR with primers (5′-CTG GTG GTC TGC AGG CTG CCA AGA AGT TTG-3′ and 5′-CAA ACT TCT TGG CAG CCT GCA GAC CAC CAGC-3′) cloned into a derivative of pcDNA3.1 for expression of a Flag-tagged fusion protein. The mutations were verified by DNA sequencing. Constructs for p300 (myc-tagged), SMRTs (short form), and SMRTe (extended or long form) were provided by E. Chin (81), V. Giguere (6), and J. D. Chen (71), respectively. Baculovirus for Flag-tagged p300 (Flag-p300) was a gift from Y. Nakatani (51), and anti-MEF2D polyclonal rabbit antibody was obtained from R. Prywes (28). Anti-PCAF polyclonal rabbit antibody and baculovirus and the mammalian expression plasmid for Flag-PCAF were described before (80).
Coimmunoprecipitation.
To analyze the interactions of different HDACs with MEF2, expression plasmids for Flag-tagged HDACs were transfected into HEK293 cells along with a construct expressing HA-MEF2. For analysis of interaction between HDAC3 and p300 (or PCAF), an expression plasmid for Flag- or HA-tagged HDAC3 was transfected into HEK293 cells along with a construct expressing Myc-p300 (or Flag-PCAF). About 48 h posttransfection, cells were washed twice with phosphate-buffered saline (PBS) and lysed in buffer K (20 mM sodium phosphate, pH 7.0, 150 mM KCl, 30 mM sodium pyrophosphate, 0.1% NP-40, 5 mM EDTA, 10 mM NaF, 0.1 mM Na3VO4, and protease inhibitors) for extract preparation and affinity purification on M2 agarose (Sigma). Bound proteins were eluted with Flag peptide (Sigma), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and detected by immunoblotting with anti-HA (Covance) and anti-Flag (Sigma) antibodies. PBS with 0.1% Tween 20 was used for membrane blocking and antibody incubation. Blots were developed with Supersignal chemiluminescent substrates (Pierce).
For analysis of interaction between endogenous proteins, subconfluent HEK293 and C2C12 cells were washed twice with PBS and lysed in buffer K. Soluble extracts were prepared for immunoprecipitation with anti-MEF2D or anti-PCAF polyclonal antibody, followed by immunoblotting with anti-HDAC3 polyclonal antibody. To minimize detection interference from the immunoglobulin G (IgG) heavy chain (HDAC3 migrated as a 50- to 55-kDa band on an SDS-acrylamide gel), the light-chain-specific fraction of peroxidase-conjugated monoclonal mouse anti-rabbit IgG (catalog no. 211-032-171; Jackson Immunoresearch Laboratories) was used as the secondary antibody.
Protein-protein interaction in vitro.
Flag-tagged p300, PCAF, and MEF2D were expressed in Sf9 insect cells using recombinant baculoviruses. Extracts from infected cells were used to immobilize these fusion proteins on M2 agarose. After rotation at 4°C for 10 min, agarose beads were incubated with putative interaction partners (i.e., MEF2D, HDAC3, HDAC4, SMRTs, and SMRTe), synthesized in vitro with TNT T7 RNA polymerase-coupled reticulocyte lysate system (Promega) in the presence of [35S]methionine (Amersham Biosciences). After rotation at 4°C for 1 h, agarose beads with bound proteins were washed four times with buffer K. Bound proteins were eluted with Flag peptide for SDS-PAGE and autoradiography. Maltose-binding protein (MBP) pulldown assays were performed as described previously (67).
Protein acetylation and deacetylation in vivo.
Expression plasmids for Flag-tagged proteins were transfected into HEK293 cells as specified in the figure legends. About 40 h posttransfection, cells were treated with 3 μM trichostatin A (TSA) for 1, 1.5, or 6 h. Extracts were prepared in buffer K containing 3 μM TSA for affinity purification on M2 agarose. Bound proteins were eluted with buffer K containing Flag peptide for SDS-PAGE and immunoblotting with anti-Flag antibody (Sigma) and anti-acetyl lysine rabbit polyclonal antibody (Cell Signaling Technology and ImmuneChem Pharmaceuticals Inc.). The latter antibody needed overnight incubation and gentle washing. Specifically, membranes were blocked in PBST (PBS plus 0.1 to 0.15% Tween 20) supplemented with 20% horse serum (Invitrogen) at room temperature for 1 h, with gentle rocking. The membranes were then incubated with anti-acetyl lysine antibody (diluted 1:300 in the blocking buffer) for 16 to 24 h at 4°C, with gentle agitation. After the membranes were washed four to six times with PBST (5 min each time at room temperature), they were incubated with peroxidase-conjugated secondary antibody diluted in the blocking buffer and gently washed with PBST four to six times (5 min each time) prior to visualization with Supersignal chemiluminescent substrates.
Protein acetylation and deacetylation in vitro.
Flag-tagged p300, PCAF, and MEF2D proteins were expressed in Sf9 cells, affinity purified on M2 agarose, and eluted with Flag peptide. Acetylation of the eluted proteins was carried out as described previously (54).
For deacetylation, Flag-HDACs were expressed in and affinity purified from HEK293 cells. Substrates were either acetylated in vitro or in vivo. For the latter, acetylated proteins were expressed in HEK293 cells with TSA treatment as specified in figure legends and immobilized on M2 agarose. Beads were washed once with buffer H (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.1 mM EDTA, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) and mixed with affinity-purified Flag-HDAC proteins in 0.1 ml of buffer H to carry out deacetylation (67). Reaction tubes were rotated in a 37°C incubator for 2 h. The beads were washed once with buffer K, and bound proteins were eluted in buffer K containing Flag peptide. Acetylation levels of the eluted proteins were detected by immunoblotting with the anti-acetyl lysine antibody as described above.
For preparation of acetylated substrates in vitro, extracts from infected Sf9 cells were used to immobilize Flag-tagged proteins on 40 μl of M2 agarose beads. After the agarose beads were washed four times with buffer K and once with buffer A (50 mM Tris-HCl, pH 8.0, 10% glycerol, 1 mM dithiothreitol, 0.1 mM EDTA, 1 mM PMSF, and 10 mM sodium butyrate), they were mixed with buffer A (40 μl) and 2.5 nCi [14C]acetyl-CoA (51 mCi/mmol; Amersham Biosciences) for acetylation. Reaction tubes were rotated in a 30°C incubator for 1 h. Afterwards, the beads were washed once with buffer H and mixed with purified Flag-HDAC proteins in buffer H for deacetylation. Reaction tubes were rotated in a 37°C incubator for 2 h. The beads were washed once with 0.2 ml of buffer K, and bound proteins were eluted in 20 μl buffer K containing Flag peptide prior to SDS-PAGE and autoradiography.
Fluorescence microscopy and reporter gene and myogenic conversion assays.
Fluorescence microscopy and the reporter gene and myogenic conversion assays were performed as previously described (19, 20).
ChIP.
C2C12 cells were grown in 10-cm dishes (five per time point) at 2 × 105 cells per dish and maintained in DMEM containing 20% FBS. Sixteen hours later, the cells were transfected with the Flag-HDAC3 expression vector. Twenty-four hours posttransfection, cells were washed once with PBS and fed with DMEM containing 2% horse serum to induce differentiation. Chromatin immunoprecipitation (ChIP) was carried out as described previously (42). On day 0 or 3, cells were cross-linked in 1% formaldehyde for 10 min at room temperature. Briefly, cells were washed twice with cold PBS and harvested in PBS. The cell pellet was resuspended in 0.2 ml of ChIP lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1, and protease inhibitors) and incubated for 10 min on ice. Cells were sonicated to obtain DNA fragments of about 300 to 2,000 bp (three pulses, 10 seconds each, at a power setting of 10 on a VirTis VirSonic 100 sonicator linked to a microtip). After centrifugation at 4°C, the soluble chromatin was diluted in 1.2 ml of ChIP dilution buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, and 20 mM Tris-HCl, pH 8.1) and then precleared with 45 μl of protein A/G-agarose beads. Ten percent of the precleared chromatin was kept as input, and the rest was divided equally for immunoprecipitation with anti-Flag or anti-HA antibody (2 μl). After rotation overnight at 4°C, 45 μl of protein A/G-agarose beads (preblocked by incubation with salmon sperm DNA) was added. After rotation for 2 h at 4°C, the beads were washed once by incubating in 1 ml of wash buffer I (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, and 150 mM NaCl) for 10 min at 4°C, with rotation. After a brief spin, the beads were similarly washed with 1 ml of buffer II (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, and 500 mM NaCl) and buffer III (0.25 M LiCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, and 10 mM Tris-HCl, pH 8.1). The beads were rinsed once with 1 ml Tris-EDTA and incubated in 0.15 ml of de-cross-linking buffer (1% SDS and 0.1 M NaHCO3) overnight at 65°C. The suspension was mixed with 1 ml of Wizard PCR preps DNA purification resin (Promega). DNA fragments were eluted in 40 μl H2O, and 1 μl was used for 28 cycles of PCR with two primers (5′-TCT AGG CTG CCC ATG TAA GG-3′ and 5′-CAT TCT TGG GAA AAC AAA CC-3′) spanning the MEF2-binding site of the mouse muscle creatine kinase (MCK) promoter. The expected PCR fragment was 0.26 kb.
RESULTS
Multiple deacetylases target MEF2.
To gain further insights into how lysine acetylation may coordinate with other modifications such as phosphorylation and sumoylation in MEF2 regulation, we sought to identify the responsible deacetylase(s). As known partners of MEF2 (39, 49, 66), HDAC4 and homologs were considered the first candidates. Neither HDAC4 nor HDAC5 was found to deacetylate MEF2D (see below and data not shown), a ubiquitously expressed member of the MEF2 family. Treatment of HEK293 cells with the deacetylase inhibitor trichostatin A, however, promoted MEF2D acetylation (Fig. 1A, lanes 1 and 2), suggesting that other zinc-dependent HDACs may be involved. Consistent with the report that SIRT1 deacetylates MEF2 (85), nicotinamide treatment upregulated MEF2 acetylation (lane 3). These findings suggest that different HDACs target MEF2 acetylation in vivo.
FIG. 1.
Interaction of MEF2D with HDAC3. (A) HEK293 cells were transfected with an expression plasmid for Flag-MEF2D. Before harvesting, cells were treated with 3 μM trichostatin A for 6 h or 50 mM nicotinamide for 24 h. Extracts were prepared in buffer K containing the respective inhibitor for affinity purification on M2 agarose, and bound proteins were eluted with Flag peptide for Western blotting (WB) with anti-acetyl lysine (αAcetyl-K) or anti-Flag (αFlag) antibody. (B to D) An expression construct for Flag- or HA-tagged MEF2D was transfected into HEK293 cells along with expression constructs for Flag- or HA-tagged HDACs as indicated. The exception is HDAC1, whose expression vector was for an HA- and Flag-tagged form, leading to a very faint band on lane 5 in panel C (marked with an asterisk). Extracts were prepared in buffer K for coimmunoprecipitation (IP) on M2 agarose, and bound proteins were eluted with Flag peptide for immunoblotting with anti-Flag or anti-HA antibody. The positions of molecular mass markers (in kilodaltons) are shown to the left of the gel. (E) HEK293 extracts were prepared in buffer K for immunoprecipitation with anti-GFP (αGFP) or anti-MEF2D antibody (αMEF2D) and immunoblotting with anti-HDAC3 or anti-MEF2D antibody. For immunoblotting with the former, an IgG light chain (IgG L)-specific secondary antibody was used to avoid detection interference from the IgG heavy chain (IgG H). The asterisk denotes a nonspecific signal in lane 1.
Interaction of MEF2 with HDAC3.
HDACs often directly associate with their substrates, so we next asked whether in addition to HDAC4 and homologs, other HDACs directly bind to MEF2. Related to this, when HA-MEF2B was expressed in HEK293 cells, HA-MEF2B associated with Flag-HDAC3 (data not shown). We thus investigated whether HDAC3 targets MEF2D. For this, coimmunoprecipitation was first performed with epitope-tagged proteins. Expression plasmids for Flag-MEF2D and HA-HDAC3 were cotransfected into HEK293 cells, and extracts were prepared for affinity purification on M2 agarose. Bound proteins were eluted by Flag peptide for immunoblotting with anti-Flag and anti-HA antibodies. As shown in Fig. 1B, HA-HDAC3 specifically coprecipitated with Flag-MEF2D, indicating that they are able to interact with each other in vivo. We next determined whether MEF2D interacts with other members of class I deacetylases. For this, Flag-tagged HDAC1, -2, -3, and -8 were coexpressed individually with HA-MEF2D in HEK293 cells. Extracts were prepared for coimmunoprecipitation and immunoblotting. Among these deacetylases, only HDAC3 coprecipitated with MEF2D (Fig. 1C). Moreover, the efficiency was comparable to that of HDAC4 or HDAC5 (Fig. 1D). To analyze the interaction between endogenous HDAC3 and MEF2D, anti-MEF2D antibody was used for coimmunoprecipitation from extracts of normal HEK293 cells, which are known to express MEF2D (20, 28). The precipitated proteins were analyzed by immunoblotting with anti-MEF2D and anti-HDAC3 antibodies. This assay revealed that anti-MEF2D antibody specifically coprecipitated HDAC3 (Fig. 1E), indicating that endogenous HDAC3 and MEF2D proteins associate with each other in HEK293 cells. Together, these results identify HDAC3 as a class I deacetylase that interacts with MEF2D in vivo.
HDAC3 binds to the MADS box of MEF2.
To determine which region of MEF2D mediates the interaction, we compared the full-length protein with a truncation mutant, 1-86, which contains the N-terminal 86 residues corresponding to the MADS box and the adjacent MEF2-specific domain. These two regions mediate DNA binding and are highly conserved among MEF2 proteins (49). As shown in Fig. 2A, HDAC3 interacted with the mutant, suggesting that the DNA-binding domain mediates HDAC3 binding. Consistent with this finding, HDAC3 also coprecipitated with two MEF2C mutants, 1-178 and 1-116, which contain the N-terminal 178 and 116 residues, respectively (Fig. 2B). Like mutant 1-86, the DNA-binding domain is intact in these two mutants. These results are consistent with the fact that the DNA-binding domain of MEF2D is highly conserved in MEF2C (49). Thus, the DNA-binding domain of MEF2 mediates HDAC3 interaction.
FIG. 2.
Mapping the HDAC3-binding site on MEF2. (A) Flag-tagged MEF2D and deletion mutant 1-86 were expressed with HA-HDAC3 in HEK293 cells as specified. Extracts were prepared in buffer K for purification on M2 agarose, and bound proteins were eluted with Flag peptide for Western blotting (WB) with anti-HA (αHA) or anti-Flag (αFlag) antibody. (B) HA-tagged MEF2C mutants 1-116 (HA-1-116) and 1-178 (HA-1-178) were expressed with Flag-HDAC3 in HEK293 cells as indicated. Extracts were prepared and analyzed as described above for panel A. IP, immunoprecipitation. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the gel. (C) Extracts from bacteria expressing MEF2C fragments fused to MBP were incubated with amylose resin to immobilize the fusion proteins and pull down HA-HDAC3, synthesized in vitro in the presence of [35S]methionine. The bound proteins were resolved by SDS-PAGE for Coomassie blue staining (bottom) and autoradiography (top). Extracts with MBP fused to a small portion of β-galactosidase were used as a negative control (lane 2). About 20% of the total HA-HDAC3 protein used per binding reaction was analyzed on the input lane. (D) Extracts from Sf9 insect cells expressing Flag-HDAC3 were incubated with M2 agarose to immobilize this fusion protein to pull down MEF2D or its deletion mutant 148-522, synthesized in vitro in the presence of [35S]methionine. Plain Sf9 extracts were used as a negative control (lanes 2 and 5). About 20% of each protein used per binding reaction was analyzed on the input lanes. (E) HEK293 cells were transfected with expression constructs for Flag-MEF2D (2 μg) and HA-HDAC3 (6 μg) along with increasing amounts of the expression vector for HA-HDAC4 (0, 1, and 2 μg). Extracts were prepared and analyzed as described above for panel A.
To test whether the interaction is direct, we carried out in vitro pulldown assays. For this, three MEF2C mutants, 1-116, 1-86, and 1-64, were expressed in Escherichia coli as MBP fusion proteins to pull down HDAC3 translated in vitro. Mutant 1-64 contains the N-terminal 64 residues and encompasses only the MADS box. As shown in Fig. 2C, all three mutants were able to interact with HDAC3, indicating that the MADS box is sufficient for the interaction (Fig. 2C). Related to this, the C-terminal part of MEF2D failed to associate with HDAC3 (Fig. 2D). Since HDAC4, as well as HDAC5/7/9, is well-known to interact with the DNA-binding domain of MEF2, we tested whether coexpression of HDAC4 interferes with HDAC3 binding. As shown in Fig. 2E, coexpression of HDAC4 had no detectable effects on association of HDAC3 with MEF2D. This is also consistent with the results presented in Fig. 1D. Therefore, HDAC3 binds directly to the MADS box, a domain that is located away from the MEF2-specific region important for interaction with HDAC4/5/7/9.
Subcellular colocalization of MEF2 with HDAC3.
We next assessed association of HDAC3 with MEF2 by immunofluorescence microscopy. For this, HA-MEF2D and green fluorescent protein-tagged HDAC3 (GFP-HDAC3) were expressed individually or together in HEK293 cells. As expected, MEF2D signals were found exclusively in the nucleus (Fig. 3A), whereas GFP-HDAC3 was enriched in the nucleus (Fig. 3B). When GFP-HDAC3 and MEF2D were coexpressed, GFP-HDAC3 remained predominantly nuclear, but some MEF2D signals were detectable in the cytoplasm (Fig. 3C). In the nucleus, MEF2D and HDAC3 colocalized to distinct dots in ∼50% transfected cells. These dots were observed with the HDAC3 mutant 1-313 but not with 122-428 (Fig. 3D), suggesting that the N-terminal region of HDAC3 is required for targeting MEF2 and HDAC3 to the nuclear dots. Consistent with this, mutant 1-313, but not 122-428, coprecipitated with MEF2D (see Fig. S1 in the supplemental material). In ∼50% of the cells with coexpression of GFP-HDAC3 and HA-MEF2D, some HA signals were also detected in the cytoplasm (Fig. 3C and data not shown). In the cytoplasm, colocalization between MEF2 and HDAC3 could be detected (Fig. 3C). Thus, consistent with protein-binding assays, the results of fluorescence microscopic analysis support the notion that MEF2D can colocalize with HDAC3 in vivo.
FIG. 3.
Subcellular localization of MEF2D and HDAC3. (A to C) HA-MEF2D and GFP-HDAC3 were expressed individually (A and B) or together (C) in HEK293 cells for green fluorescence microscopy to detect GFP signals. Expression of HA-MEF2D was determined by immunostaining with the anti-HA antibody and a Cy3-labeled secondary antibody. Hoechst 33258 was used to stain nuclei. (D) Same as panel C except that GFP-tagged HDAC3 mutants 1-313 and 122-428 were expressed with HA-MEF2D as indicated.
Deacetylation of MEF2 by HDAC3.
Having established that HDAC3 physically associated with MEF2 (Fig. 1 to 3) and that TSA treatment elevated MEF2D acetylation in HEK293 cells (Fig. 1A), we then assessed the ability of HDAC3 to deacetylate MEF2. For this, a previously described shRNA expression vector (83) was employed to deplete endogenous HDAC3 in HEK293 cells. As expected, the shRNA vector was effective in knocking down HDAC3 expression (Fig. 4A). As shown in Fig. 4B, HDAC3 knockdown upregulated MEF2D acetylation, indicating that HDAC3 negatively regulates the acetylation in vivo. Consistent with this, expression of wild-type HDAC3 reduced MEF2D acetylation in HEK293 cells (Fig. 4C, lanes 1 and 2). In contrast, expression of the deacetylase-deficient mutant H134Q slightly increased the acetylation (lane 3), perhaps due to a dominant-negative effect.
FIG. 4.
Deacetylation of MEF2D by HDAC3. (A) The expression construct for hHDAC3i (shRNA specific to human HDAC3) (lane 1) or the corresponding empty vector (lane 2) was transfected into HEK293 cells. Extracts were prepared for immunoblotting with anti-HDAC3 (αHDAC3) and anti-MEF2D (αMEF2D) antibodies. (B) The Flag-MEF2D expression plasmid was transfected into HEK293 cells along with the hHDAC3i expression construct or the corresponding empty vector as indicated. Before harvesting, cells were treated with 3 μM trichostatin A for 1.5 h (+) or not treated with 3 μM TSA (−). Extracts were prepared in buffer K supplemented with the inhibitor for affinity purification on M2 agarose, and bound proteins were eluted for Western blotting (WB) with anti-acetyl lysine (αAc-K) or anti-Flag (αFlag) antibody. (C) The Flag-MEF2D expression plasmid was transfected into HEK293 cells with or without plasmids expressing Flag-tagged HDAC3 and mutant H134Q. Before harvesting, cells were treated with 3 μM TSA for 1 h. Extracts were prepared in buffer K with the inhibitor for immunoprecipitation on M2 agarose, and bound proteins were eluted for immunoblotting with anti-acetyl lysine (αAc-K) or anti-Flag (αFlag) antibody. (D) Flag-HDAC proteins, expressed in and affinity purified from HEK293 cells, were used for in vitro deacetylation of Flag-MEF2D, which was expressed separately in and affinity purified from HEK293 cells. Before harvesting, the cells expressing Flag-MEF2D were treated with 3 μM TSA for 6 h. Flag-MEF2D was then affinity purified on M2 agarose using buffer K containing 3 μM TSA. (E) Same as panel C except that the effects of HDAC4 and CaMKIV-ca (a constitutively active form) were analyzed. (F and G) Flag-tagged HDAC1 and HDAC3 proteins, affinity purified from HEK293 cells, were used for deacetylation of Flag-MEF2D, acetylated in vitro with [14C]acetyl-CoA by Flag-PCAF (F) or Flag-p300 (G). Flag-tagged MEF2D, PCAF, and p300 proteins were affinity purified from Sf9 cells. (H) Expression plasmids for Flag-PCAF and HA-MEF2D were transfected into HEK293 cells individually or in combination. Extracts were prepared for immunoprecipitation (IP) and immunoblotting or Western blotting (WB) with anti-Flag or anti-HA antibody as in Fig. 2A. (I) HEK293 cells were transfected with plasmids expressing the indicated proteins. Before harvesting, cells were treated with 3 μM TSA for 1.5 h. Extracts were prepared in buffer K with the inhibitor for immunoprecipitation on M2 agarose, and bound proteins were eluted for immunoblotting with anti-acetyl lysine or anti-Flag antibody.
We next examined direct MEF2D deacetylation in vitro. Flag-tagged HDAC3 was expressed in HEK293 cells and affinity purified on M2 agarose. For comparison, HDAC1, -2, -4, -5, and -8 were similarly prepared. Eluted proteins were incubated with acetylated Flag-MEF2D, which was separately expressed in and purified from HEK293 cells. As shown in Fig. 4D, HDAC3, but not the other three class I HDACs, deacetylated MEF2D (lanes 1 to 5). Despite their well-characterized interaction of MEF2 (49, 66), neither HDAC4 nor HDAC5 displayed detectable deacetylase activity towards MEF2D (lanes 6 and 7). Consistent with this, expression of CaMKIV-ca, a constitutively active form known to promote nuclear export of HDAC4/5/7/9 (72, 78), had minimal effects on MEF2D acetylation (Fig. 4E).
We subsequently determined the ability of HDAC3 to reverse MEF2 acetylation catalyzed by known acetyltransferases. As reported for MEF2C (48), PCAF and p300 efficiently acetylated recombinant MEF2D protein (Fig. 4F, lanes 1 and 2, and Fig. 4G, lane 1). Consistent with this, both acetyltransferases physically associated with MEF2D (Fig. 4H and data not shown) (58). In agreement with what was observed with acetyl-MEF2D purified from HEK293 cells (Fig. 4D), HDAC3 catalyzed the removal of [14C]acetyl groups from MEF2D acetylated by PCAF and p300 in vitro (Fig. 4F, lane 3, and Fig. 4G, lanes 2 and 3). Similarly, coexpression of HDAC3 inhibited acetylation of MEF2D by PCAF and p300 in HEK293 cells (Fig. 4I). Taken together, the above results indicate that HDAC3 deacetylates MEF2D in vitro and in vivo.
HDAC3 directly targets PCAF and p300.
Reminiscent of autophosphorylation of protein kinases, many HATs are autoacetylated. Moreover, autoacetylation is important for activating p300 (3, 62) and promoting nuclear localization of PCAF (57). During analysis of in vitro deacetylation of MEF2, we noticed that HDAC3 inhibited autoacetylation of p300 and PCAF (Fig. 4F and G), suggesting that HDAC3 not only targets MEF2 but also its acetyltransferases. To substantiate this, we first investigated whether HDAC3 could directly bind to PCAF and p300. Expression plasmids for Flag-PCAF and HA-HDAC3 were cotransfected into HEK293 cells. Proteins were affinity purified on M2 agarose for immunoblotting with anti-Flag and anti-HA antibodies. As shown in Fig. 5A, interaction between HDAC3 and PCAF could be readily detected. Interaction between the endogenous proteins was then analyzed by coimmunoprecipitation with anti-PCAF and anti-HDAC3 polyclonal antibodies. As shown in Fig. 5B, anti-PCAF antibody specifically coprecipitated endogenous HDAC3. Interaction of HDAC3 with p300 could also be detected in HEK293 cells and in vitro (Fig. 5C and D). Functionally, coexpression of HDAC3 promoted cytoplasmic localization of PCAF (Fig. 5E and F), which is consistent with an earlier report that autoacetylation is required for nuclear localization of PCAF (57). Therefore, HDAC3 not only deacetylates MEF2 but also acts on its acetyltransferases.
FIG. 5.
Interaction of HDAC3 with PCAF and p300. (A) Expression plasmids for Flag-PCAF and HA-HDAC3 were cotransfected into HEK293 cells as indicated. Extracts were prepared in buffer K for affinity purification on M2 agarose, and bound proteins were eluted for Western blotting (WB) with anti-HA (αHA) or anti-Flag (αFlag) antibody. (B) HEK293 cells were washed and lysed in buffer K in situ to prepare extracts for immunoprecipitation (IP) with anti-PCAF antibody (αPCAF) and immunoblotting with anti-HDAC3 or anti-PCAF antibody. Immunoblotting with anti-HDAC3 antibody was carried out as described in the legend to Fig. 1E. (C) Flag-HDAC3 and Myc-tagged p300 were expressed and purified as in described above for panel A by immunoblotting anti-Flag and anti-Myc monoclonal antibodies, respectively. (D) Extracts from Sf9 cells expressing Flag-p300 (lane 3) or Flag-PCAF (lane 4) were used to immobilize these fusion proteins on M2 agarose for incubation with HA-HDAC3, synthesized in vitro in the presence of [35S]methionine. Plain Sf9 extracts were used as a negative control (lane 2). About 20% of the total HA-HDAC3 protein used per binding reaction was analyzed on lane 1. (E and F) Flag-PCAF was expressed alone (E) or with GFP-HDAC3 (F) in HEK293 cells. Green fluorescence microscopy was used to detect GFP signals. Expression of Flag-PCAF was visualized by immunostaining with anti-Flag antibody and a Cy3-labeled secondary antibody. Hoechst 33258 was used to stain nuclei.
SMRT promotes deacetylation of MEF2 and PCAF by HDAC3.
As integral subunits of HDAC3 complexes (24, 44, 68), the SMRT and N-CoR corepressors activate HDAC3 to efficiently deacetylate histone substrates (9, 23). However, it remains to be determined whether this is the case with other protein substrates. The ability of HDAC3 to deacetylate MEF2, PCAF, and p300 prompted us to ask whether the SMRT and N-CoR corepressors could activate HDAC3 to deacetylate these nonhistone substrates. To address this, we performed in vitro deacetylation assays in the presence of SMRTs and SMRTe, the short and extended isoforms of the SMRT corepressor, respectively (6, 71). SMRTe, but not SMRTs, contains the SANT domain required for activating the histone-deacetylating activity of HDAC3 (9, 23). Coexpression of SMRTe but not SMRTs stimulated the deacetylase activity of HDAC3 towards MEF2D in vitro (Fig. 6A). To test whether this is the case in vivo, HEK293 cells were transfected with expression plasmids for Flag-MEF2D, HA-HDAC3, SMRTs, and/or SMRTe. Expression of SMRTe, but not SMRTs, potentiated MEF2D deacetylation by HDAC3 (Fig. 6B). HDAC3 knockdown counteracted the effect of SMRTe expression (Fig. 6C), suggesting that SMRTe acts through HDAC3. SMRTe also promoted deacetylation of PCAF by HDAC3 (Fig. 6D). Therefore, as reported for histone substrates (9, 23), SMRTe stimulates the deacetylase activity of HDAC3 towards both MEF2 and PCAF.
FIG. 6.
Effects of SMRT on deacetylation of MEF2D and PCAF by HDAC3. (A) Flag-HDAC3 was coexpressed with SMRTs or SMRTe in HEK293 cells to affinity purify deacetylase complexes for in vitro deacetylation of Flag-MEF2D, which was expressed separately in and affinity purified from HEK293 cells. Before harvesting, cells expressing Flag-MEF2D were treated with 3 μM TSA for 6 h. After deacetylation, Flag-tagged proteins and the acetylation level of Flag-MEF2D were analyzed by Western blotting (WB) with anti-Flag (αFlag) and anti-acetyl lysine (αAcetyl-K) antibodies, respectively. (B) HEK293 cells expressing the indicated proteins were treated with 3 μM TSA for 1.5 h. Extracts were prepared in buffer K in the presence of the inhibitor for immunoprecipitation on M2 agarose and immunoblotting with anti-Flag and anti-acetyl lysine antibodies. (C) HEK293 cells were transfected with plasmids expressing Flag-MEF2D, SMRTe, and hHDAC3i as indicated. Before harvesting, cells were treated with 3 μM TSA for 1 h. Extracts were prepared in buffer K with the inhibitor for immunoprecipitation on M2 agarose, and bound proteins were eluted for immunoblotting with anti-acetyl lysine or anti-Flag antibody. (D) Flag-PCAF autoacetylated with [14C]acetyl-CoA was used for in vitro deacetylation. Flag-PCAF was expressed in and affinity purified from Sf9 cells. Flag-HDAC3 was coexpressed with SMRTe in HEK293 cells for affinity purification of the deacetylase complex. (E) Extracts from Sf9 cells expressing Flag-MEF2D were used to immobilize this fusion protein on M2 agarose. Agarose beads were incubated, as indicated, with HA-HDAC3, SMRT isoforms, and/or HA-HDAC4, synthesized in vitro in the presence of [35S]methionine. Plain Sf9 extracts were used as a negative control (lane 5). For the labeled proteins, 20% of the total amount used per binding reaction was analyzed separately on lanes 1 to 4. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the gel.
SMRTe could promote MEF2 deacetylation through the following mechanisms: (i) directly affecting the structure of HDAC3, (ii) enhancing the interaction between HDAC3 and MEF2, and (iii) forming a trimeric enzyme-substrate complex. To gain further mechanistic insights, we examined the interaction between MEF2D and HDAC3 in the presence and absence of SMRT isoforms. For this, Flag-MEF2D was expressed in Sf9 cells and immobilized on M2 agarose to pull down HDAC3, SMRT, and SMRTe translated in vitro. As shown in Fig. 2E, the presence of HDAC4 had minimal effects on HDAC3 binding to MEF2D (Fig. 6E, lanes 1, 4 to 6, and 10). Neither SMRT isoform affected the interaction between HDAC3 and MEF2D (Fig. 6E, lanes 6 to 9). Interestingly, SMRTe interacted with MEF2D (lane 9). These results indicate that MEF2D, HDAC3, and SMRTe could form a trimeric complex. Within this complex, HDAC3 may be more active for MEF2D deacetylation. The above findings suggest that SMRT is a cofactor required for efficient deacetylation of MEF2 and PCAF by HDAC3, although the precise mechanism through which this occurs awaits further investigation.
Repression of MEF2-dependent transcription by HDAC3.
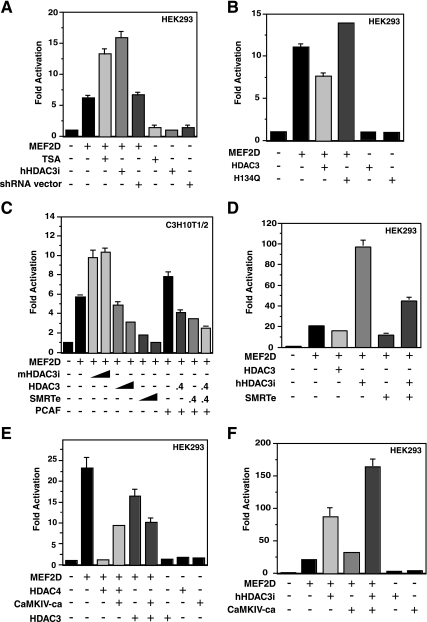
We next performed reporter gene assays to assess whether HDAC3 regulates the transcriptional activity of MEF2D. For this, 3xMEF2-Luc, a reporter with luciferase expression controlled by three copies of a MEF2-binding site (48), was used. As shown in Fig. 7A, TSA treatment of HEK293 cells stimulated the transcriptional activity of MEF2D, suggesting the involvement of zinc-dependent HDACs. To determine whether HDAC3 plays a role, we expressed shRNA to knock down HDAC3 expression in HEK293 cells. As shown in Fig. 7A, expression of shRNA specific to human HDAC3 increased MEF2D-dependent reporter activity. Expression of HDAC3, but not its point mutant H134Q, reduced the reporter activity (Fig. 7B). Similar to what was observed in HEK293 cells, expression of shRNA specific to mouse HDAC3 in murine C3H10T1/2 fibroblasts potentiated MEF2-dependent transcription and expression of HDAC3 reduced the reporter activity (Fig. 7C). Moreover, expression of SMRTe repressed the reporter activity in a dose-dependent manner (Fig. 7C), suggesting that HDAC3 may cooperate with SMRT to repress MEF2-dependent transcription. We also analyzed how HDAC3 affects the ability of PCAF to coactivate MEF2-dependent transcription. As shown in Fig. 7C, PCAF potentiated MEF2D-dependent transcription, but expression of HDAC3 or SMRTe diminished this potentiation. Supporting the synergy between SMRTe and HDAC3, knockdown of this deacetylase reduced the repressive effect of SMRTe (Fig. 7D). To assess the relative contribution of HDAC3 and HDAC4/5/7/9, we expressed CaMKIV-ca. As reported previously (49, 66), this kinase significantly relieved the repression of HDAC4 expression (Fig. 7E). However, it had much smaller effects when no exogenous HDAC4 was present (Fig. 7F), suggesting the existence of HDAC4/5/7/9-independent repression. More interestingly, this kinase slightly stimulated the inhibitory activity of HDAC3 (Fig. 7E) but acted synergistically with HDAC3 knockdown to upregulate MEF2-dependent reporter activity (Fig. 7F). Together, these results indicate that HDAC3 represses MEF2-dependent transcription in a manner that is independent of and different from HDAC4/5/7/9.
FIG. 7.
Repression of MEF2D-dependent transcription by HDAC3. (A) The luciferase reporter 3xMEF2-luc (0.2 μg) and a β-galactosidase expression plasmid (0.05 μg) were cotransfected into HEK293 cells with or without the expression vector for Flag-MEF2D (0.1 μg). Where specified, the expression vector for hHDAC3i (0.1 μg) or the empty shRNA vector (0.1 μg) was cotransfected. About 48 h posttransfection, cells were harvested for determination of luciferase and β-galactosidase activities. Treatment with TSA (0.3 μM) was carried out overnight before harvesting. Normalized luciferase activities from transfection without any effector plasmids were arbitrarily set at 1.0, and the values are shown as average values plus standard deviations (error bars) of three representative experiments. (B) Same as panel A except that expression plasmids for the wild-type and H134Q mutant of HDAC3 were compared. (C) Same as panel A except that C3H10T1/2 cells were used. Expression vectors for shRNA specific to mouse HDAC3 (mHDAC3i) (0.15 or 0.4 μg), HA-HDAC3 (0.15 or 0.4 μg), SMRTe (0.15 or 0.4 μg), and Flag-PCAF (0.05 μg) were cotransfected as indicated. (D) Same as panel A except that the effect of HDAC3 knockdown on SMRTe-mediated repression was determined. (E) Same as panel A except that the effect of CaMKIV-ca expression (0.2 μg) on repression by HDAC3 and HDAC4 was examined. (F) Same as panel A except that the synergy between HDAC3 knockdown and CaMKIV-ca expression was analyzed. In panels D and E, 0.2 μg of the Flag-MEF2D expression plasmid was used.
We also utilized MyoD-dependent myogenic conversion assays to analyze the repressive effect of HDAC3 (55). For this, pluripotent C3H10T1/2 cells were cotransfected with a MyoD expression construct along with the expression plasmid for MEF2D. As expected, wild-type MEF2D stimulated the myogenic potential of MyoD (Fig. 8A and B). Importantly, this activity was reduced in the presence of HDAC3 (Fig. 8A and B). Consistent with results from reporter gene assays (Fig. 7), SMRTe cooperated with HDAC3 to downregulate the myogenic activity of MEF2D (Fig. 8A and B), whereas HDAC3 knockdown increased the myogenic potential in a manner synergistic with CaMKIV-ca expression (Fig. 8C). As shown in Fig. 8D, ChIP assays revealed association of HDAC3 with a muscle-specific promoter with a known MEF2-binding site in vivo. Together, these results suggest that interaction with HDAC3 and SMRTe keeps MEF2 in a repressed state in vivo.
FIG. 8.
Inhibition of myogenic conversion by HDAC3. (A to C) An MyoD expression plasmid (0.4 μg) was transfected into murine C3H10T1/2 fibroblasts along with constructs expressing Flag-MEF2D (0.6 μg), HA-HDAC3 (0.3 μg), SMRTe (0.3 μg), mHDAC3i (0.3 μg), and CaMKIV-ca (0.3 μg). On day 7, myosin heavy chain (MHC) expression was detected by indirect immunofluorescence microscopy with anti-MHC monoclonal antibody and a Cy3-labeled secondary antibody. Average values of three independent experiments are illustrated in panels A and C, with some representative images shown in panel B. (D) C2C12 cells were transfected with the Flag-HDAC3 expression vector. Sixteen hours later, cells were washed once with PBS and fed with DMEM containing 2% horse serum to induce differentiation. Cells were harvested in ChIP lysis buffer on day 0 for chromatin immunoprecipitation with anti-Flag antibody to determine association of Flag-HDAC3 with the MCK promoter. An anti-HA antibody (aHA) was used as a negative control (lane 2). (E) C2C12 cells, maintained in DMEM with 20% FBS, were washed once with PBS and fed with DMEM containing 2% horse serum to induce differentiation. Cells were harvested in buffer K on days 0 and 3 to prepare extracts for immunoprecipitation with anti-MEF2D antibody and immunoblotting with anti-HDAC3 and anti-MEF2D antibodies. An anti-GFP antibody was used as a negative control for immunoprecipitation (IP) (lane 3). Western blotting (WB) with anti-HDAC3 antibody was carried out as described in the legend to Fig. 1E.
Effect of myogenesis on the interaction of HDAC3 with MEF2.
Results shown in Fig. 7 and 8A to D raise an intriguing issue as to whether the repressive role of HDAC3 is regulated in vivo. We thus investigated how the interaction between HDAC3 and MEF2D may be modulated during myogenesis. For this, C2C12 cells were induced to differentiate in 2% horse serum. On days 0 and 3, cells were harvested in buffer K to prepare soluble extracts for immunoprecipitation with anti-MEF2D antibody. Precipitated proteins were analyzed by immunoblotting with anti-MEF2D and anti-HDAC3 antibodies. As shown in Fig. 8E, interaction of HDAC3 with MEF2D was strong on day 0 but diminished on day 3 of differentiation (Fig. 8E), suggesting that the interaction is signal responsive. Therefore, HDAC3 may inhibit MEF2-dependent transcription in a signal-dependent manner (see Discussion below).
DISCUSSION
HDAC3 interacts with and deacetylates MEF2.
Numerous studies have established HDAC4/5/7/9 as signal-responsive binding partners of MEF2 transcription factors (39, 49, 66). Unexpectedly, these deacetylases were unable to deacetylate MEF2 (Fig. 4D and data not shown). Instead, HDAC3 deacetylated MEF2D in vitro and in vivo (Fig. 4). Under similar conditions, other class I HDACs exhibited no obvious deacetylase activity towards MEF2D (Fig. 4D). In agreement with this, HDAC3, but not HDAC1, -2, or -8, physically associated with MEF2D (Fig. 1). Interaction of HDAC3 with MEF2D is also supported by their subcellular colocalization (Fig. 3). Like MEF2D, both MEF2B and MEF2C appeared to bind HDAC3 (Fig. 2 and data not shown). Consistent with this, the HDAC3-binding site was mapped to the DNA-binding domain (Fig. 2), a region that is highly conserved among metazoan MEF2 proteins (49). Functionally, HDAC3 kept MEF2 in a transcriptionally inactive state to prevent myogenesis (Fig. 7 and 8). These results suggest that HDAC3 association may be common to metazoan MEF2 proteins. Therefore, repression by HDAC3 represents an important mechanism for MEF2 regulation in vivo.
HDAC3 may target MEF2 through multiple mechanisms, including direct deacetylation and physical association. Related to the latter, MEF2 may recruit HDAC3 to promoters of target genes for histone deacetylation (Fig. 8D and 9). In addition, HDAC3 is the catalytic subunit of deacetylase complexes containing the nuclear receptor corepressors SMRT and N-CoR (24, 44, 68). The extended form of SMRT formed a trimeric complex with HDAC3 and MEF2D (Fig. 6E). In agreement with this, SMRT recruits MEF2 to nuclear dots (71). SMRT and N-CoR activate HDAC3 to deacetylate histones (9, 23). Similarly, SMRT acted synergistically with HDAC3 to deacetylate MEF2D (Fig. 6A and B). Moreover, SMRT repressed MEF2-dependent transcription (Fig. 7 and 8). Therefore, in cooperation with SMRT and perhaps also N-CoR, HDAC3 associates with and deacetylates MEF2 for transcriptional repression (Fig. 9).
FIG. 9.
Cartoon illustrating distinct pathways by which HDAC3 and HDAC4/5/7/9 repress MEF2-dependent transcription. Association with p300, CBP, and PCAF acetylates MEF2 and proximal nucleosomes (gray ovals) to activate transcription. HDAC3 cooperates with SMRT and perhaps also N-CoR to maintain MEF2 and the proximal nucleosomes in a hypoacetylated state for transcriptional repression. In addition to MEF2 and histones, HDAC3 deacetylates coactivators like PCAF, p300, and CBP. HDAC4/5/7/9 repress MEF2-dependent transcription through multiple repression domains, with the deacetylase domain perhaps deacetylating proximal nucleosomes. While it is well established that CaMKs and PKDs act through HDAC4/5/7/9 to activate MEF2 (pathway a), investigation is needed to identify kinases that may act through HDAC3 to regulate MEF2-dependent transcription (pathway b). HDAC3 is widely expressed, and HDAC4/5/7/9 display tissue-specific expression, so pathway b may be the default in most tissues. HDAC4/5/7/9 directly target MEF2, and there is no evidence indicating direct interaction of these HDACs with the coactivators PCAF and p300/CBP. The broken arrow indicates potential cross talk between the two pathways. IKK, IκB kinase; Ac, acetyl group.
HDAC3 targets autoacetylation of PCAF and p300/CBP.
In addition to MEF2, HDAC3 associated with PCAF and p300 (Fig. 5) and inhibited autoacetylation (Fig. 4F and G). Moreover, the SMRT corepressor promoted PCAF deacetylation by HDAC3 (Fig. 6D). Autoacetylation is known to promote nuclear localization of PCAF (57). Consistent with this, expression of HDAC3 led to translocation of PCAF to the cytoplasm (Fig. 5E and F). Similar to HDAC3, HDAC1 and SIRT1 directly associate with PCAF (15, 73). Thus, multiple HDACs seem to target PCAF. Autoacetylation has been linked to stimulation of acetyltransferase activity and other functions of p300 (3, 62). Its two neighboring acetylatable lysine residues are subject to sumoylation and transcriptional repression (16). Related to this, SIRT1 inhibits acetylation of p300 to upregulate sumoylation (5). By analogy, HDAC3 may act through the acetyltransferase activity and sumoylation of p300 to control its ability to potentiate MEF2-dependent transcription. These findings suggest that deacetylation of acetyltransferases, such as PCAF and p300, is a common mechanism by which different HDACs repress transcription. Consistent with this, a recent report showed that HDAC3 interacts with and deacetylates CBP (8). Therefore, similar to PCAF, different HDACs act on p300 and CBP. These findings also suggest that in addition to promoter-associated histones, HDAC3 targets MEF2 and its acetyltransferase coactivators (Fig. 9).
Cell signaling utilizes distinct pathways for MEF2 regulation.
Mapping the HDAC3-binding site to the MADS box of MEF2 (Fig. 2) raises two intriguing issues. First, this box is conserved in the MADS superfamily of transcription factors, so an interesting question is whether other MADS boxes also associate with HDAC3. Of relevance, the serum response factor SRF interacted with HDAC3 (see Fig. S2 in the supplemental material). Second, dependent on its association with coactivators or corepressors, MEF2 can activate or repress transcription (39, 49, 66). Reporter gene and myogenic conversion assays revealed that HDAC3 is a MEF2 corepressor (Fig. 7 and 8). Similar corepressors include Cabin1 (52) and HDAC4/5/7/9 (39, 49, 66), all of which bind to the DNA-binding domain of MEF2 (25). More interestingly, p300 binds to a similar region (25, 58), suggesting that MEF2 may switch coactivator and corepressor partners by direct competition. In addition, CaMKs phosphorylate HDAC4/5/7/9 (39, 49, 66, 78), as well as Cabin1 (52), to promote nuclear export of these corepressors and derepress MEF2-dependent transcription. Other kinases such as PKDs also negatively regulate nuclear localization of HDAC4 and homologs (11, 53, 64). HDAC3 was found to bind the DNA-binding domain of MEF2 (Fig. 2). By analogy to Cabin1 and class IIa HDACs, the corepressor role of HDAC3 needs to be regulated. In support of this, HDAC3 association with MEF2 diminished after C2C12 myoblasts differentiated (Fig. 8E), and MEF2 acetylation was reported to increase during myogenesis (48), raising an important question as to how cell signaling regulates HDAC3 association with MEF2 (Fig. 9). Potential candidates include IκB kinase α and other kinases known to regulate SMRT and N-CoR (1, 30, 34).
As a MEF2 corepressor, HDAC3 displays several differences from HDAC4/5/7/9. (i) HDAC3 is widely expressed (12, 76), whereas HDAC4/5/7/9 exhibit tissue-specific distribution (66). (ii) SMRT and N-CoR form stable deacetylase complexes with HDAC3 and are required for the deacetylase activity (9, 23, 24, 44, 68), so cell signaling pathways acting on SMRT and N-CoR may also regulate the activity of HDAC3. (iii) While the nuclear localization of HDAC4/5/7/9 is negatively regulated by CaMK- or PKD-mediated phosphorylation and 14-3-3 binding (49, 66), HDAC3 possesses no obvious sites for 14-3-3 binding (12, 76), and at least CaMKIV did not block the repression activity of HDAC3 (Fig. 7E). (iv) HDAC3 interacted with the MADS box of MEF2 (Fig. 2), whereas HDAC4/5/7/9 preferentially bind to the MEF2-specific region (49). Of note, related to the ubiquitous expression of HDAC3 (12, 76), MEF2D is widely expressed in different tissues (49). Thus, the HDAC3 link suggests a novel, and perhaps default, pathway for MEF2 regulation (Fig. 9). It will be interesting to elucidate the upstream regulatory events of this pathway and to determine how it may cross talk with the well-established HDAC4/5/7/9 pathways (Fig. 9) to gain effective spatiotemporal control of MEF2 activity in vivo.
Supplementary Material
Acknowledgments
We thank Ron Prywes, Zhenguo Wu, Minoru Yoshida, Jiahuai Han, Eric Olson, J. Don Chen, and Vincent Giguere for plasmids and antibodies.
This work was supported by NIH grants (to J.W. & E.S.) and funds from Canadian Institutes for Health Research and National Cancer Institute of Canada (to X-.J.Y.).
Footnotes
Published ahead of print on 11 December 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Baek, S. H., K. A. Ohgi, D. W. Rose, E. H. Koo, C. K. Glass, and M. G. Rosenfeld. 2002. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell 110:55-67. [DOI] [PubMed] [Google Scholar]
- 2.Bereshchenko, O. R., W. Gu, and R. Dalla-Favera. 2002. Acetylation inactivates the transcriptional repressor BCL6. Nat. Genet. 32:606-613. [DOI] [PubMed] [Google Scholar]
- 3.Black, J. C., J. E. Choi, S. R. Lombardo, and M. Carey. 2006. A mechanism for coordinating chromatin modification and preinitiation complex assembly. Mol. Cell 23:809-818. [DOI] [PubMed] [Google Scholar]
- 4.Blander, G., and L. Guarente. 2004. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 73:417-435. [DOI] [PubMed] [Google Scholar]
- 5.Bouras, T., M. Fu, A. A. Sauve, F. Wang, A. A. Quong, N. D. Perkins, R. T. Hay, W. Gu, and R. G. Pestell. 2005. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J. Biol. Chem. 280:10264-10276. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J. D., and R. M. Evans. 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454-457. [DOI] [PubMed] [Google Scholar]
- 7.Cho, Y., A. Griswold, C. Campbell, and K. T. Min. 2005. Individual histone deacetylases in Drosophila modulate transcription of distinct genes. Genomics 86:606-617. [DOI] [PubMed] [Google Scholar]
- 8.Chuang, H. C., C. W. Chang, G. D. Chang, T. P. Yao, and H. Chen. 2006. Histone deacetylase 3 binds to and regulates the GCMa transcription factor. Nucleic Acids Res. 34:1459-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Codina, A., J. D. Love, Y. Li, M. A. Lazar, D. Neuhaus, and J. W. Schwabe. 2005. Structural insights into the interaction and activation of histone deacetylase 3 by nuclear receptor corepressors. Proc. Natl. Acad. Sci. USA 102:6009-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, T., and T. P. Yao. 2004. AcK-knowledge reversible acetylation. Sci. STKE 2004:pe42. [DOI] [PubMed] [Google Scholar]
- 11.Dequiedt, F., J. Van Lint, E. Lecomte, V. Van Duppen, T. Seufferlein, J. R. Vandenheede, R. Wattiez, and R. Kettmann. 2005. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J. Exp. Med. 201:793-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emiliani, S., W. Fischle, C. Van Lint, Y. Al-Abed, and E. Verdin. 1998. Characterization of a human RPD3 ortholog, HDAC3. Proc. Natl. Acad. Sci. USA 95:2795-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flavell, S. W., C. W. Cowan, T. K. Kim, P. L. Greer, Y. Lin, S. Paradis, E. C. Griffith, L. S. Hu, C. Chen, and M. E. Greenberg. 2006. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311:1008-1012. [DOI] [PubMed] [Google Scholar]
- 14.Foglietti, C., G. Filocamo, E. Cundari, E. De Rinaldis, A. Lahm, R. Cortese, and C. Steinkuhler. 2006. Dissecting the biological functions of Drosophila histone deacetylases by RNA interference and transcriptional profiling. J. Biol. Chem. 281:17968-17976. [DOI] [PubMed] [Google Scholar]
- 15.Fulco, M., R. L. Schiltz, S. Iezzi, M. T. King, P. Zhao, Y. Kashiwaya, E. Hoffman, R. L. Veech, and V. Sartorelli. 2003. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell 12:51-62. [DOI] [PubMed] [Google Scholar]
- 16.Girdwood, D., D. Bumpass, O. A. Vaughan, A. Thain, L. A. Anderson, A. W. Snowden, E. Garcia-Wilson, N. D. Perkins, and R. T. Hay. 2003. p300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11:1043-1054. [DOI] [PubMed] [Google Scholar]
- 17.Gong, X., X. Tang, M. Wiedmann, X. Wang, J. Peng, D. Zheng, L. A. Blair, J. Marshall, and Z. Mao. 2003. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 38:33-46. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez, P., M. Garcia-Castro, J. R. Reguero, A. Batalla, A. G. Ordonez, R. L. Palop, I. Lozano, M. Montes, V. Alvarez, and E. Coto. 2006. The Pro279Leu variant in the transcription factor MEF2A is associated with myocardial infarction. J. Med. Genet. 43:167-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grégoire, S., A. M. Tremblay, L. Xiao, Q. Yang, K. Ma, J. Nie, Z. Mao, Z. Wu, V. Giguere, and X. J. Yang. 2006. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J. Biol. Chem. 281:4423-4433. [DOI] [PubMed] [Google Scholar]
- 20.Grégoire, S., and X. J. Yang. 2005. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol. Cell. Biol. 25:2273-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregoretti, I. V., Y. M. Lee, and H. V. Goodson. 2004. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J. Mol. Biol. 338:17-31. [DOI] [PubMed] [Google Scholar]
- 22.Grozinger, C. M., and S. L. Schreiber. 2002. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem. Biol. 9:3-16. [DOI] [PubMed] [Google Scholar]
- 23.Guenther, M. G., O. Barak, and M. A. Lazar. 2001. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol. Cell. Biol. 21:6091-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guenther, M. G., W. S. Lane, W. Fischle, E. Verdin, M. A. Lazar, and R. Shiekhattar. 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 14:1048-1057. [PMC free article] [PubMed] [Google Scholar]
- 25.Han, A., J. He, Y. Wu, J. O. Liu, and L. Chen. 2005. Mechanism of recruitment of class II histone deacetylases by myocyte enhancer factor-2. J. Mol. Biol. 345:91-102. [DOI] [PubMed] [Google Scholar]
- 26.Han, J., Y. Jiang, Z. Li, V. V. Kravchenko, and R. J. Ulevitch. 1997. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386:296-299. [DOI] [PubMed] [Google Scholar]
- 27.Han, J., and J. D. Molkentin. 2000. Regulation of MEF2 by p38 MAPK and its implication in cardiomyocyte biology. Trends Cardiovasc. Med. 10:19-22. [DOI] [PubMed] [Google Scholar]
- 28.Han, T.-H., and R. Prywes. 1995. Regulatory role of MEF2D in serum induction of the c-jun promoter. Mol. Cell. Biol. 15:2907-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hietakangas, V., J. Anckar, H. A. Blomster, M. Fujimoto, J. J. Palvimo, A. Nakai, and L. Sistonen. 2006. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc. Natl. Acad. Sci. USA 103:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoberg, J. E., F. Yeung, and M. W. Mayo. 2004. SMRT derepression by the IkappaB kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol. Cell 16:245-255. [DOI] [PubMed] [Google Scholar]
- 31.Huang, E. Y., J. Zhang, E. A. Miska, M. G. Guenther, T. Kouzarides, and M. A. Lazar. 2000. Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev. 14:45-54. [PMC free article] [PubMed] [Google Scholar]
- 32.Hubbert, C., A. Guardiola, R. Shao, Y. Kawaguchi, A. Ito, A. Nixon, M. Yoshida, X. F. Wang, and T. P. Yao. 2002. HDAC6 is a microtubule-associated deacetylase. Nature 417:455-458. [DOI] [PubMed] [Google Scholar]
- 33.Jin, Y. H., E. J. Jeon, Q. L. Li, Y. H. Lee, J. K. Choi, W. J. Kim, K. Y. Lee, and S. C. Bae. 2004. Transforming growth factor-beta stimulates p300-dependent RUNX3 acetylation, which inhibits ubiquitination-mediated degradation. J. Biol. Chem. 279:29409-29417. [DOI] [PubMed] [Google Scholar]
- 34.Jonas, B. A., and M. L. Privalsky. 2004. SMRT and N-CoR corepressors are regulated by distinct kinase signaling pathways. J. Biol. Chem. 279:54676-54686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang, J., C. B. Gocke, and H. Yu. 2006. Phosphorylation-facilitated sumoylation of MEF2C negatively regulates its transcriptional activity. BMC Biochem. 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kao, H. Y., M. Downes, P. Ordentlich, and R. M. Evans. 2000. Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Genes Dev. 14:55-66. [PMC free article] [PubMed] [Google Scholar]
- 37.Kasler, H. G., J. Victoria, O. Duramad, and A. Winoto. 2000. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol. Cell. Biol. 20:8382-8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato, Y., M. Zhao, A. Morikawa, T. Sugiyama, D. Chakravortty, N. Koide, T. Yoshida, R. I. Tapping, Y. Yang, T. Yokochi, et al. 2000. Big mitogen-activated kinase regulates multiple members of the MEF2 protein family. J. Biol. Chem. 275:18534-18540. [DOI] [PubMed] [Google Scholar]
- 39.Khochbin, S., A. Verdel, C. Lemercier, and D. Seigneurin-Berny. 2001. Functional significance of histone deacetylase diversity. Curr. Opin. Genet. Dev. 11:162-166. [DOI] [PubMed] [Google Scholar]
- 40.Kim, S. C., R. Sprung, Y. Chen, Y. Xu, H. Ball, J. Pei, T. Cheng, Y. Kho, H. Xiao, L. Xiao, et al. 2006. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23:607-618. [DOI] [PubMed] [Google Scholar]
- 41.Kouzarides, T. 2000. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 19:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laganiere, J., G. Deblois, C. Lefebvre, A. R. Bataille, F. Robert, and V. Giguere. 2005. Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc. Natl. Acad. Sci. USA 102:11651-11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, H., N. Rezai-Zadeh, and E. Seto. 2004. Negative regulation of histone deacetylase 8 activity by cyclic AMP-dependent protein kinase A. Mol. Cell. Biol. 24:765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, J., J. Wang, Z. Nawaz, J. M. Liu, J. Qin, and J. Wong. 2000. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 19:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, S., P. Liu, A. Borras, T. Chatila, and S. H. Speck. 1997. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J. 16:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo, J., A. Y. Nikolaev, S. Imai, D. Chen, F. Su, A. Shiloh, L. Guarente, and W. Gu. 2001. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107:137-148. [DOI] [PubMed] [Google Scholar]
- 47.Luo, J., F. Su, D. Chen, A. Shiloh, and W. Gu. 2000. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 408:377-381. [DOI] [PubMed] [Google Scholar]
- 48.Ma, K., J. K. Chan, G. Zhu, and Z. Wu. 2005. Myocyte enhancer factor 2 acetylation by p300 enhances its DNA binding activity, transcriptional activity, and myogenic differentiation. Mol. Cell. Biol. 25:3575-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2002. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 27:40-47. [DOI] [PubMed] [Google Scholar]
- 50.North, B. J., B. L. Marshall, M. T. Borra, J. M. Denu, and E. Verdin. 2003. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11:437-444. [DOI] [PubMed] [Google Scholar]
- 51.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 52.Pan, F., A. R. Means, and J. O. Liu. 2005. Calmodulin-dependent protein kinase IV regulates nuclear export of Cabin1 during T-cell activation. EMBO J. 24:2104-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parra, M., H. Kasler, T. A. McKinsey, E. N. Olson, and E. Verdin. 2005. Protein kinase D1 phosphorylates HDAC7 and induces its nuclear export after TCR activation. J. Biol. Chem. 280:13762-13770. [DOI] [PubMed] [Google Scholar]
- 54.Pelletier, N., N. Champagne, H. Lim, and X. J. Yang. 2003. Expression, purification, and analysis of MOZ and MORF histone acetyltransferases. Methods 31:24-32. [DOI] [PubMed] [Google Scholar]
- 55.Puri, P. L., V. Sartorelli, X. J. Yang, Y. Hamamori, L. Kedes, A. Graessmann, Y. Nakatani, and M. Levrero. 1997. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol. Cell 1:35-45. [DOI] [PubMed] [Google Scholar]
- 56.Robyr, D., Y. Suka, I. Xenarios, S. K. Kurdistani, A. Wang, N. Suka, and M. Grunstein. 2002. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109:437-446. [DOI] [PubMed] [Google Scholar]
- 57.Santos-Rosa, H., E. Valls, T. Kouzarides, and M. Martinez-Balbas. 2003. Mechanisms of P/CAF auto-acetylation. Nucleic Acids Res. 31:4285-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sartorelli, V., J. Huang, Y. Hamamori, and L. Kedes. 1997. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol. Cell. Biol. 17:1010-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schroeder, T. M., R. A. Kahler, X. Li, and J. J. Westendorf. 2004. Histone deacetylase 3 interacts with Runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J. Biol. Chem. 279:41998-42007. [DOI] [PubMed] [Google Scholar]
- 60.Shalizi, A., B. Gaudilliere, Z. Yuan, J. Stegmuller, T. Shirogane, Q. Ge, Y. Tan, B. Schulman, J. W. Harper, and A. Bonni. 2006. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 311:1012-1017. [DOI] [PubMed] [Google Scholar]
- 61.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson, P. R., D. Wang, L. Wang, M. Fulco, N. Pediconi, D. Zhang, W. An, Q. Ge, R. G. Roeder, J. Wong, et al. 2004. Regulation of the p300 HAT domain via a novel activation loop. Nat. Struct. Mol. Biol. 11:308-315. [DOI] [PubMed] [Google Scholar]
- 63.Vaziri, H., S. K. Dessain, E. Ng Eaton, S. I. Imai, R. A. Frye, T. K. Pandita, L. Guarente, and R. A. Weinberg. 2001. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107:149-159. [DOI] [PubMed] [Google Scholar]
- 64.Vega, R. B., B. C. Harrison, E. Meadows, C. R. Roberts, P. J. Papst, E. N. Olson, and T. A. McKinsey. 2004. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol. Cell. Biol. 24:8374-8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vega, R. B., K. Matsuda, J. Oh, A. C. Barbosa, X. Yang, E. Meadows, J. McAnally, C. Pomajzl, J. M. Shelton, J. A. Richardson, et al. 2004. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119:555-566. [DOI] [PubMed] [Google Scholar]
- 66.Verdin, E., F. Dequiedt, and H. G. Kasler. 2003. Class II histone deacetylases: versatile regulators. Trends Genet. 19:286-293. [DOI] [PubMed] [Google Scholar]
- 67.Wang, A. H., N. R. Bertos, M. Vezmar, N. Pelletier, M. Crosato, H. H. Heng, J. Th'ng, J. Han, and X. J. Yang. 1999. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol. Cell. Biol. 19:7816-7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen, Y. D., V. Perissi, L. M. Staszewski, W. M. Yang, A. Krones, C. K. Glass, M. G. Rosenfeld, and E. Seto. 2000. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc. Natl. Acad. Sci. USA 97:7202-7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westendorf, J. J., S. K. Zaidi, J. E. Cascino, R. Kahler, A. J. van Wijnen, J. B. Lian, M. Yoshida, G. S. Stein, and X. Li. 2002. Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21CIP1/WAF1 promoter. Mol. Cell. Biol. 22:7982-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiren, M., R. A. Silverstein, I. Sinha, J. Walfridsson, H. M. Lee, P. Laurenson, L. Pillus, D. Robyr, M. Grunstein, and K. Ekwall. 2005. Genomewide analysis of nucleosome density histone acetylation and HDAC function in fission yeast. EMBO J. 24:2906-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu, X., H. Li, E. J. Park, and J. D. Chen. 2001. SMRTe inhibits MEF2C transcriptional activation by targeting HDAC4 and 5 to nuclear domains. J. Biol. Chem. 276:24177-24185. [DOI] [PubMed] [Google Scholar]
- 72.Xu, Q., L. Yu, L. Liu, C. F. Cheung, X. Li, S. K. Yee, X.-J. Yang, and Z. Wu. 2002. The p38 MAPK, CaMK and calcineurin-mediated signaling pathways transcriptionally regulate myogenin expression. Mol. Biol. Cell 13:1940-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamagoe, S., T. Kanno, Y. Kanno, S. Sasaki, R. M. Siegel, M. J. Lenardo, G. Humphrey, Y. Wang, Y. Nakatani, B. H. Howard, et al. 2003. Interaction of histone acetylases and deacetylases in vivo. Mol. Cell. Biol. 23:1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang, C. C., O. I. Ornatsky, J. C. McDermott, T. F. Cruz, and C. A. Prody. 1998. Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res. 26:4771-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang, W. M., S. C. Tsai, Y. D. Wen, G. Fejer, and E. Seto. 2002. Functional domains of histone deacetylase-3. J. Biol. Chem. 277:9447-9454. [DOI] [PubMed] [Google Scholar]
- 76.Yang, W. M., Y. L. Yao, J. M. Sun, J. R. Davie, and E. Seto. 1997. Isolation and characterization of cDNAs corresponding to an additional member of the human histone deacetylase gene family. J. Biol. Chem. 272:28001-28007. [DOI] [PubMed] [Google Scholar]
- 77.Yang, X. J. 2004. Lysine acetylation and the bromodomain: a new partnership for signaling. BioEssays 26:1076-1087. [DOI] [PubMed] [Google Scholar]
- 78.Yang, X. J., and S. Grégoire. 2005. Class II histone deacetylases: from sequence to function, regulation and clinical implication. Mol. Cell. Biol. 25:2873-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang, X. J., and S. Grégoire. 2006. A recurrent phospho-sumoyl switch in transcriptional repression and beyond. Mol. Cell 23:779-786. [DOI] [PubMed] [Google Scholar]
- 80.Yang, X. J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]
- 81.Yuan, Z. L., Y. J. Guan, D. Chatterjee, and Y. E. Chin. 2005. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307:269-273. [DOI] [PubMed] [Google Scholar]
- 82.Yuki, Y., I. Imoto, M. Imaizumi, S. Hibi, Y. Kaneko, T. Amagasa, and J. Inazawa. 2004. Identification of a novel fusion gene in a pre-B acute lymphoblastic leukemia with t(1;19)(q23;p13). Cancer Sci. 95:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang, X., Y. Ozawa, H. Lee, Y. D. Wen, T. H. Tan, B. E. Wadzinski, and E. Seto. 2005. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 19:827-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao, M., L. New, V. V. Kravchenko, Y. Kato, H. Gram, F. D. Padova, E. N. Olson, R. J. Ulevitch, and J. Han. 1999. Regulation of the MEF2 family of transcription factors by p38. Mol. Cell. Biol. 19:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao, X., T. Sternsdorf, T. A. Bolger, R. M. Evans, and T. P. Yao. 2005. Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol. Cell. Biol. 25:8456-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu, B., and T. Gulick. 2004. Phosphorylation and alternative pre-mRNA splicing converge to regulate myocyte enhancer factor 2C activity. Mol. Cell. Biol. 24:8264-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.