Abstract
Adult mice carrying a null mutation of the prostanoid receptor EP3R (EP3R−/− mice) exhibit increased frequency of feeding during the light cycle of the day and develop an obese phenotype under a normal fat diet fed ad libitum. EP3R−/− mice show increased motor activity, which is not sufficient to offset the increased feeding leading to increased body weight. Altered “nocturnal” activity and feeding behavior is present from a very early age and does not seem to require age-dependent factors for the development of obesity. Obesity in EP3R−/− mice is characterized by elevated leptin and insulin levels and >20% higher body weight compared with WT littermates. Abdominal and subcutaneous fat and increased liver weight account for the weight increase in EP3R−/− mice. These observations expand the roles of prostaglandin E2 signaling in metabolic regulation beyond the reported stimulation of leptin release from adipose tissue to involve actions mediated by EP3R in the regulation of sleep architecture and feeding behavior. The findings add to the growing literature on links between inflammatory signaling and obesity.
Keywords: body weight, insulin, leptin, prostanoid receptor, signaling
Prostaglandin E2 (PGE2) is one of the most important inflammatory mediators in the periphery and in the CNS (1–7). PGE2 is synthesized locally in the brain by neurons and glia expressing COX1, COX2, and PGE synthase (8, 9). The activation of specific Toll-like receptors (TLR) by IL-1 or LPS and the subsequent production of PGE2 mediates different CNS responses such as the febrile response (3, 5, 10, 11), activation of the hypothalamic–pituitary–adrenal axis (12–15), and the central activation of brown fat metabolism/uncoupling (16–18). The central and peripheral actions of PGE2 are mediated by four G protein-coupled receptor-type prostanoid receptors (EP1R–EP4R) (for review, see refs. 19 and 20). Transgenic mice carrying a null mutation for each of the four prostanoid receptors have been generated, and different phenotypes have been described for EP1R−/− (4, 21, 22), EP2R−/− (23–29), EP3R−/− (4, 5, 21), and EP4R−/− (30–32) mice. EP1R−/− mice show a decreased aberrant foci formation to azoxymethane (33). EP2R−/− mice have impaired ovulation and fertilization, salt-sensitive hypertension, impaired vasodepressor response to PGE2, and a loss of bronchodilation (29). EP4R−/− mice have an impaired vasodepressor response to i.v. infusion of PGE2 and decreased inflammation and bone resorption (34). EP3R−/− mice have an impaired febrile response (5), impaired duodenal bicarbonate secretion (35), enhanced vasodepressor response to PGE2 (36), and increased bleeding tendency (37). Effects of the loss of EP3R were also observed on tumor-induced angiogenesis (38), colon cancer development (39), allergic inflammation (40), and inflammatory pain (41). PGE2 levels in these mice were not found to be altered in these studies.
Although EP3R−/− mice have been well characterized, there have not been any reports on obesity or altered feeding patterns in these mice. PGE2 has been shown to have an effect on adipocytes by inhibiting lipolysis and stimulating the secretion of leptin, but the specific prostanoid receptor subtype important for mediating these effects has remained unknown (42). The heterozygous COX2+/− mice but not the COX1−/− or COX2−/− transgenic mice have been shown to develop obesity (43), but no mechanistic explanations involving altered PGE2 signaling have been developed.
Effects of prostaglandin D (PGD) and PGE2 on sleep architecture have been studied extensively (cf. ref. 44), and sleep promotion by PGE2 applied in the subarachnoid space was reported by Ram et al. (45), but the repeated night activity described in this report has not been observed.
EP3R is a G protein-coupled receptor (46) with several splice variants (47), but the tissue-specific distribution of these variants is unknown. The EP3R-like immunoreactivity is richly expressed in the rodent brain, with the highest density of EP3R receptors in the different hypothalamic nuclei involved in thermoregulation and sleep regulation (48–50). We have shown that EP3R mediates the effects of PGE2, a potent pyrogen, on the thermosensitivity of anterior hypothalamic neurons (51). EP3R-like immunoreactivity is also abundant in monoaminergic nuclei such as the raphe nuclei and locus ceruleus (48), where this prostanoid receptor may affect appetite and feeding through modulation of the serotonergic and noradrenergic signaling.
We show here that EP3R−/− mice exhibit an obese phenotype with a “night” eating component that is demonstrated by increased feeding during the light cycle of the day. The obese phenotype in EP3R−/− mice is congruent with previous observations that PGE2 stimulated leptin release, affected lipolysis, and had an effect on the overall endocrine state of the organism (43, 52). More broadly, this finding contributes to the increased understanding of the coupling between obesity and inflammatory signaling (53–55).
Results
EP3R−/− Mice Showed Increased Body Weight Caused by Increased Fat Storage.
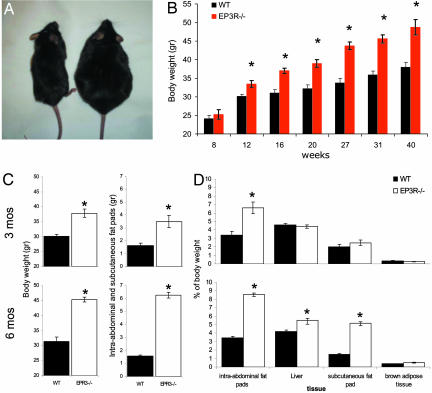
Male EP3R−/− mice fed ad libitum on an 11% fat diet exhibited an increase in body weight that resulted in an obese phenotype (Fig. 1A). EP3R−/− body weights showed a gradual increase, with continuous accumulation up to 20% above that of WT littermate controls by week 20 (t = 4.41; P = 0.001) and continuing thereafter up to a recorded 30% increase by week 40 (t = 4.7; P = 0.006) (Fig. 1B). Body weight gain was primarily attributable to increased adipose tissue deposition (Fig. 1C). Comparison of the body composition of 3- and 6-month-old mice revealed that the early deposition of intraabdominal fat pads (gonadal, retroperitoneal, and mesenteric) in EP3R−/− mice at ≈3 months was followed by significant deposition of fat in adjacent tissues that include the liver and subcutaneous fat pads (inguinal and the groin) in EP3R−/− mice at ≈6 months (Fig. 1D). At 3 and 6 months of age, abdominal fat accounted for 6.62 ± 0.47% and 8.59 ± 0.25% of the total body weight in EP3R−/− mice compared with the 3.37 ± 0.69% and 3.46 ± 0.16% in the WT mice, respectively (Fig. 1C). Subcutaneous fat pads at both ages accounted for 2.49 ± 0.33% and 5.2 ± 0.28% of the total body weight in EP3R−/− mice compared with the 1.99 ± 0.32% and 1.47 ± 0.12% in the WT mice, respectively (Fig. 1D). No significant differences in the brown adipose tissue percentage of body weight were seen between EP3R−/− and WT mice.
Fig. 1.
EP3R−/− mice show an increase in body weight that results in an obese phenotype. (A) A male EP3R−/− mouse (Right) and a WT littermate (Left) at 30 weeks of age. (B) Measurement of body weight at different time points shows that overweight becomes significant on EP3R−/− mice after week 12 (t = 3.17; P = 0.048). Evaluation of body weight at different time points shows a consistent increment of body weight in EP3R−/− mice at weeks 16 (t = 5.29; P = 0.0001), 20 (t = 4.41; P = 0.001), 27 (t = 5.97; P = 0.0001), 31 (t = 6.36; P = 0.0001), and 40 (t = 4.7; P = 0.0006). After week 30, weight differences are ≈30% of the body weight of corresponding WT littermate controls. (C) Early deposition of intraabdominal and subcutaneous fat pads in EP3R−/− mice at 3 months of age becomes an important contributor to body weight gain (t = 3.79; P = 0.0053); at 6 months of age, the contribution becomes higher (t = 18.36; P = 0.0001). (D) At 3 months, intraabdominal fat pads are the main site of fat accumulation in EP3R−/− mice (t = 4.35; P = 0.0024). At 6 months, the intraabdominal fat pads (t = 19.55; P = 0.0001), liver (t = 4.68; P = 0.0016), and subcutaneous fat pads (t = 12.09; P = 0.0001) become important sources of fat deposits in EP3R−/− mice. Asterisks indicate significant differences at each given point. For P values, see above.
EP3R−/− Mice Showed High Serum Levels of Insulin and Leptin at 3 and 6 Months of Age.
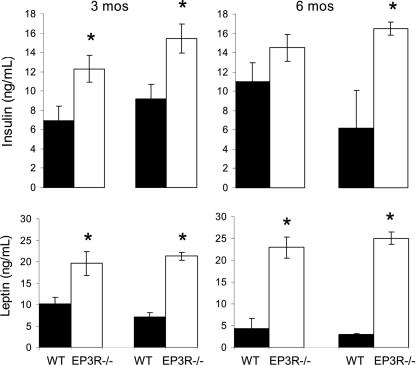
Measurements of circulating levels of insulin and leptin demonstrated that EP3R−/− mice had significantly elevated levels of both hormones. At 3 and 6 months of age, insulin levels accounted for 12.30 ± 1.37 ng/ml and 14.50 ± 1.37 ng/ml in EP3R−/− mice compared with 6.95 ± 1.44 ng/ml and 11.01 ± 1.96 ng/ml in the WT mice during the light cycle (insulin, t = 2.3, P = 0.042, EP3R−/− mice vs. WT mice at 3 months of age; insulin, t = 1.4, P = 0.187, EP3R−/− mice vs. WT mice at 6 months of age); insulin levels accounted for 15.43 ± 1.5 ng/ml and 16.49 ± 0.65 ng/ml in EP3R−/− mice compared with 11.01 ± 1.96 ng/ml and 6.18 ± 3.91 ng/ml in the WT mice during the dark cycle (insulin, t = 2.74, P = 0.033, EP3R−/− mice vs. WT mice at 3 months of age; insulin, t = 4.7, P = 0.003, EP3R−/− mice vs. WT mice at 6 months of age) (Fig. 2Upper).
Fig. 2.
Endocrine changes in EP3R−/− mice vs. WT littermates at 3 and 6 months of age. (Upper) Plasma insulin levels. (Lower) Plasma leptin levels. Asterisks indicate significant differences at each given point. For P values, see text.
Leptin levels were higher in EP3R−/− mice compared with WT mice during both the light and dark cycles. At 3 and 6 months of age, leptin levels were 19.61 ± 2.79 ng/ml and 22.94 ± 2.43 ng/ml in EP3R−/− mice compared with 10.18 ± 1.45 ng/ml and 7.17 ± 1.03 ng/ml in the WT mice during the light cycle (leptin, t = 3.0, P = 0.013, EP3R−/− mice vs. WT mice at 3 months of age; leptin, t = 4.8, P = 0.0019, EP3R−/− mice vs. WT mice at 6 months of age); leptin levels were 21.27 ± 0.95 ng/ml and 25.07 ± 1.47 ng/ml in EP3R−/− mice compared with 7.16 ± 1.03 ng/ml and 2.78 ± 0.11 ng/ml in the WT mice during the dark cycle (leptin, t = 9.1, P = 0.0001, EP3R−/− mice vs. WT mice at 3 months of age; leptin, t = 10.26, P = 0.0001, EP3R−/− mice vs. WT mice at 6 months of age) (Fig. 2 Lower).
EP3R−/− Mice Showed Impaired Glucose Tolerance and Insulin Resistance.
At 3 months of age, the increased adiposity in EP3R−/− mice was accompanied by abnormalities in glucose metabolism. Even though mice were fasted for 24 h before the glucose-tolerance test, basal levels of glucose in EP3R−/− mice were significantly higher compared with WT mice (EP3R−/−, 149.25 ± 15.91 mg/dl; WT, 103.01 ± 10.1 mg/dl; t = 2.45; P = 0.04) (Fig. 3A). After the mice were challenged with a 1.5 mg of glucose per gram of body weight, serum glucose concentrations were similarly elevated at 15 min in EP3R−/− and WT mice, but serum glucose concentrations reached higher levels in EP3R−/− mice at 30 min (445 ± 21.08 mg/dl) than in the WT mice (381 ± 22.54 mg/dl) (t = 2.08; P = 0.04). At 60 min after the glucose challenge, glucose levels were similar in both EP3R−/− and WT mice, but higher levels in EP3R−/− mice were reached again at 120 min (EP3R−/−, 242.25 ± 19.55 mg/dl; WT, 136.25 ± 20.42 mg/dl; t = 3.78; P = 0.0091) and 180 min (EP3R−/−, 157.5 ± 6.38 mg/dl; WT, 119.24 ± 3.72 mg/dl; t = 5.17; P = 0.0021) (Fig. 3A).
Fig. 3.
EP3R−/− mice showed impaired gluclose tolerance and insulin resistance. (A and B) Glucose-tolerance test. Mice were challenged with 1.5 mg of glucose per gram of body weight, and glucose concentrations were measured before glucose challenge and after 15, 30, 60, 120, and 180 min. (C and D) Percentage of changes from baseline on the ability of insulin to acutely stimulate glucose disposal or clearance by performing an acute insulin challenge in EP3R−/− and WT mice at both ages. (C′ and D′) The raw correspondent blood glucose values (in milligrams per deciliter) used for C and D. Asterisks indicate significant differences at each given point. For P values, see text.
At 6 months of age, abnormalities in glucose metabolism were significantly higher in EP3R−/− mice than at 3 months of age (Fig. 3B). Basal levels of glucose in EP3R−/− mice were significantly higher compared with WT mice (EP3R−/−, 196.4 ± 22.84 mg/dl; WT, 105.82 ± 6.17 mg/dl; t = 4.16; P = 0.0024). After a glucose challenge, EP3R−/− mice showed higher glucose levels at 15 min (465.2 ± 22.05 mg/dl) than did WT mice (382.5 ± 22.31 mg/dl) (t = 2.6; P = 0.0283). The same was true at 30 min (EP3R−/−, 487.8 ± 11.7 mg/dl; WT, 404.83 ± 33.35 mg/dl; t = 2.26; P = 0.049), 60 min (EP3R−/−, 420.6 ± 23.54 mg/dl; WT, 282 ± 22.15 mg/dl; t = 4.27; P = 0.0021), 120 min (EP3R−/−, 286.1 ± 25.93 mg/dl; WT, 233.65 ± 16.75 mg/dl; t = 1.7; P = 0.1135), and 180 min (EP3R−/−, 184.6 ± 15.14 mg/dl; WT, 146.5 ± 9.21 mg/dl; t = 2.24; P = 0.048) (Fig. 3B).
We determined the ability of insulin to acutely stimulate glucose disposal or clearance by performing an acute insulin challenge in EP3R−/− and WT mice at both 3 and 6 months of age. At 3 months of age, the ability of insulin to acutely stimulate glucose disposal in EP3R−/− mice was significantly blunted at 15 min, indicating a short-term decrease in insulin sensitivity (EP3R−/−, 158.3 ± 10.59 mg/dl; WT, 125.8 ± 11.79 mg/dl, which corresponded to a 84.36% and 63.63% reduction in baseline values of EP3R−/− and WT, respectively; P = 0.0246) (Fig. 3 C and C′). At 6 months of age, basal levels of glucose were higher in EP3R−/− mice (EP3R−/−, 287.25 ± 48.32 mg/dl; WT, 173.02 ± 17.16 mg/dl), and acute insulin challenge was unable to stimulate glucose disposal in EP3R−/− mice at 15 min (EP3R−/−, 265 ± 53.71 mg/dl; WT, 125.3 ± 16.04 mg/dl, which corresponded to a 90.45% and 73.01% reduction in baseline values of EP3R−/− and WT, respectively; P = 0.046). Even though values at 30 min (EP3R−/−, 280.12 ± 48.5 mg/dl; WT, 148.02 ± 13.21 mg/dl), 60 min (EP3R−/−, 274.07 ± 41.69 mg/dl; WT, 156.23 ± 22.43 mg/dl), or 120 min (EP3R−/−, 290.94 ± 44.14 mg/dl; WT, 185.16 ± 28.89 mg/dl) were significantly higher in EP3R−/− mice, comparisons of the percentage of change from their own baseline were not significant (Fig. 3 D and D′).
EP3R−/− Mice Exhibited Increased Motor Activity and Core Body Temperature.
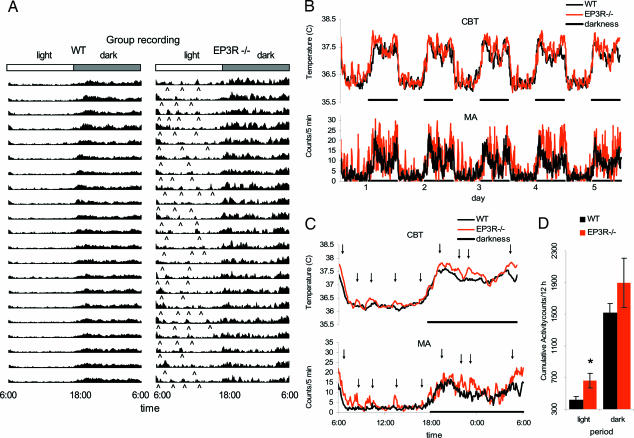
Radiotelemetric evaluation of motor activity indicates that EP3R−/− mice displayed peaks of increased motor activity during the light part of the day, when mice usually spend most of their time sleeping. Increased nocturnal activity was recorded continuously over 21 days in six EP3R−/− mice and six WT littermates. Continuous motor activity profile during 21 days is shown (Fig. 4A), and this profile demonstrates that the peaks of nocturnal activity in EP3−/− mice are not episodic but instead are recurrent daily events occurring with a frequency of between two and four episodes per night (Fig. 4 A and C). The increase in motor activity during the light cycle in EP3R−/− mice compared with WT mice was punctuated and separated by phases in which the motor activity of the EP3R−/− mice was indistinguishable from that of the WT mice (Fig. 4 B and C). However, cumulative analysis demonstrated that these peaks contributed to an overall 60.3% increased motor activity in EP3R−/− mice. Increased motor activity in EP3R−/− mice was also observed during the dark cycle, and this increase accounted for 24.73% of the motor activity (Fig. 4B). Core body temperature of EP3R−/− mice was slightly elevated during the corresponding peaks of increased motor activity (see Fig. 4D).
Fig. 4.
Circadian rhythm profile for WT littermates and EP3R−/− mice. Although diurnal distribution of motor activity follows the light–dark cycle, EP3R−/− mice show bouts of increased activity during the light cycle that are associated with grooming and eating behavior. Those bouts of activity are irregular and better detected during the resting phase (see ⋀). (B) Continuous recording of core body temperature (CBT) and motor activity (MA) during 5 days at normothermic conditions (room temperature was 30°C) confirms that EP3R−/− and WT mice are nocturnal and that they follow the low activity–high resting (light cycle) and high activity–low resting (dark cycle) pattern. (C) Averaged data indicate that EP3R−/− mice have an increase in motor activity characterized by bouts of activity that increases the core body temperature (see arrows). The increase in motor activity is associated with grooming and eating behavior. (D) Cumulative data confirm that EP3R−/− mice are more active during the light period. ∗, P = 0.032.
EP3R−/− Mice Showed Increased Food Consumption.
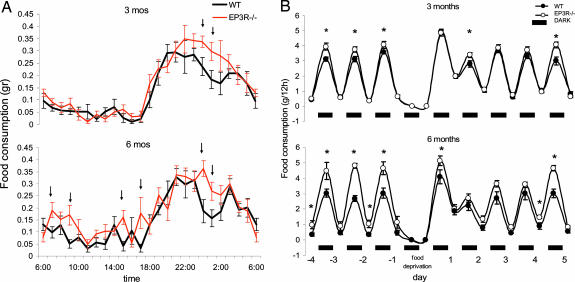
Measurement of food consumption in EP3R−/− and WT mice demonstrated that EP3R−/− ate significantly more food than the WT littermates at both ages. At 3 months of age, hourly food intake was similar between EP3R−/− and WT mice during the light cycle. However, EP3R−/− mice during the dark period showed an increase in food intake characterized by a continuous feeding (monophasic), whereas WT littermate controls showed an increase in feeding followed by a period of temporary decrease and then by a second increase in feeding, making the pattern biphasic (Fig. 5A). As a consequence, statistical differences are only observed in the dark period at 0:00 and 1:00 a.m. (0:00: EP3R−/−, 0.338 ± 0.02 g; WT, 0.236 ± 0.03 g; t = 2.35; P = 0.0466; 1:00: EP3R−/−, 0.298 ± 0.03 g; WT, 0.184 ± 0.03 g; t = 2.33; P = 0.0481). At 6 months of age, the increase in feeding strongly correlated with the peaks of activity and elevated temperature during both the light/inactive and the dark/active part of the day (Fig. 5B). Cumulative food intake was higher in EP3R−/− mice in both periods. During the light phase, significant differences were at 7:00 a.m. (EP3R−/−, 0.19 ± 0.03 g; WT, 0.09 ± 0.02 g; t = 2.25; P = 0.0379), 9:00 a.m. (EP3R−/−, 0.17 ± 0.04 g; WT, 0.05 ± 0.01 g; t = 2.51; P = 0.022), 15:00 p.m. (EP3R−/−, 0.16 ± 0.02 g; WT, 0.04 ± 0.02 g; t = 3.27; P = 0.004), and 17:00 p.m. (EP3R−/−, 0.17 ± 0.06 g; WT, 0.03 ± 0.01 g; t = 2.14; P = 0.047). During the dark phase, significant differences were at 0:00 a.m. (EP3R−/−, 0.36 ± 0.03 g; WT, 0.17 ± 0.04 g; t = 3.08; P = 0.006) and 1:00 a.m. (EP3R−/−, 0.27 ± 0.03 g; WT, 0.15 ± 0.03 g; t = 2.2; P = 0.041).
Fig. 5.
EP3R−/− mice showed increased food consumption. (A and B) Hourly food-intake events as a function of time (A) and effects of 24 h of fasting in EP3R−/− and WT littermates (B). In A, at 3 months of age (Upper), EP3R−/− mice show an increase in food intake characterized by continuous feeding during the dark period (see arrows), whereas WT littermate controls show a similar increase in feeding followed by a period of temporary decrease and then by a second increase in food consumption in the same period. At 6 months of age (Lower), the increase in feeding in the EP3R−/− mice is extended to the light cycle (see arrows; P values are given in the text). In B, food intake was measured every 12 h. At 3 months of age (Upper), baseline measurement for 3 days confirmed that EP3R−/− mice ate more during the dark period (∗, P < 0.05). At 6 months of age (Lower), cumulative data indicate that EP3R−/− mice ate more during both light and dark cycles (∗, P < 0.05). At both ages, food deprivation increases food intake in the next dark and light period in both strains and returns to the baseline pattern after day 4.
Both EP3R−/− and WT mice underwent food deprivation for 24 h, and subsequent food intake was measured every 12 h. Baseline measurement for 3 days confirmed differences in food intake. Food deprivation increased food intake in the next dark and light period in both strains, and full recovery was reached on day 4 (Fig. 5B).
Discussion
The observations reported here that EP3R−/− mice have an obese phenotype include measurements of body weight, as well as endocrine parameters known to increase with obesity, such as insulin and leptin plasma levels. Although it has been reported that PGE2 stimulates leptin release from adipocytes (52), we show that the lack of EP3R signaling in EP3R−/− mice does not prevent the large increase in leptin levels (Fig. 2 Lower), which is commensurate with the increased fat deposits (Fig. 1C). Thus, PGE2 stimulation of leptin release does not exclusively depend on signaling through the EP3 prostanoid receptor subtype.
The levels of PGE2 in obese humans are elevated (56). It will be interesting to test the hypothesis that such elevation could be attributable to disruption of PGE2-negative feedback through EP3R.
EP3R receptors are broadly expressed in the periphery and in the brain. Our study did not determine the site(s) of action that contributes to the obese phenotype of EP3R−/− mice, because both peripheral and central expression of EP3R is absent in the null mouse. The exact localization of the prostanoid receptor(s)-mediated febrile effects by transgenic and lentiviral techniques is currently being studied by Lazarus (57), and similar techniques will be needed to precisely determine the site(s) at which the lack of PGE2 signaling via EP3R contributes to this obese phenotype. Although it is possible to speculate that obesity in EP3R−/− mice may be caused in part by the lack of hypothalamic PGE2 signaling, numerous peripheral actions of PGE2 are also mediated through this prostanoid receptor subtype (34).
The finding of increased feeding coupled with nocturnal motor activity in EP3R−/− mice compared with WT littermates suggests that EP3R−/− mice do not stabilize sleep and may wake up more easily. The role of PGE2 as a somnogenic agent alongside prostaglandin D in sleep has been reported (45). Our data suggest that EP3R may actually mediate a part of this response.
The most likely brain–EP3R-linked response is the febrile response. It has been shown that EP3R−/− mice do not mount fever in response to the pyrogens IL-1β and LPS, suggesting that the PGE2 action in the anterior hypothalamus, raphe pallidus, and other sites involved in the generation of the fever response involves EP3R (5). The basal temperature regulation may also involve EP3R-mediated effects of PGE2, and we observed a slightly elevated core body temperature in freely moving ad libitum-fed EP3R−/− mice. The increased core body temperature and motor activity would be predicted to lead to a decrease in body weight because energy demands are increased when higher body temperature needs to be maintained, but it appears as though the increase in food consumption/energy intake has a more pronounced effect, thus resulting in body weight gain.
The onset of obesity does not occur late in development in EP3R−/− mice and does not seem to require additional age-dependent factors to come into play. It is important to note that the obesity in EP3R−/− mice does not result from the high-fat diet that is now commonly used to achieve an obese state in experimental animals; rather, this obesity in EP3R−/− mice occurs on standard chow and results from increased feeding in the absence of a commensurate increase in energy expenditure. This increase in energy intake relative to energy expenditure is often deemed to be an important factor in the etiology of common forms of human obesity. In addition, it is worth noting that EP3R−/− mice show another common feature of weight-gain scenarios in humans: night eating.
It is important to note that the lack of the full spectrum of inflammatory signaling has been shown recently to lead to obesity in a multitude of transgenic models including IL-1R1−/− (58), IL-1β−/−/IL-6−/− double knockout (59), and IL-18−/− (60) mice. These observations, together with those in the EP3R−/− mice described here, suggest that inflammatory molecules and/or signaling may be required for keeping body weight homeostasis. The exact mechanism leading to obesity in these models is not known, although effects of cytokines on insulin resistance and insulin receptor substrate expression (61) and phosphorylation have been proposed (62). It will be important to determine whether those mechanisms are distinct or similar to those leading to obesity in EP3R−/− mice. Full pharmacological characterization of the contribution of EP3 mediated-signaling in obesity and other phenotypes awaits the introduction of an EP3 prostanoid receptor-specific antagonist, in the same manner that EP1 antagonist-medicated impulsive behavior served to verify the involvement of that receptor subtype in the behavioral effects (63).
In summary, mice that are null for the prostanoid receptor EP3 display increased feeding throughout the day along with additional feeding activity peaks during the night (or light period), resulting in obesity. These mice may represent an important obesity model.
Materials and Methods
Animals.
All procedures were approved by the Institutional Animal Care and Use Committee of the Scripps Research Institute and were carried out on male EP3R−/− mice backcrossed to C57BL/6 background over more than eight generations and on WT littermates.
Food-Intake Measurements.
Mice were fed ad libitum with mouse breeder diet (S-2335 Mouse Breeder, gross energy kcal (1 kcal = 4.18 kJ)/g 4.39, protein % 17.50, fat % 11.72, fiber % 3.36; Harlan Teklad, Madison, WI) and separated in two groups on each age (n = 10 each group). Food intake was monitored every hour during 24 h. For daily consumption, food and body weight were monitored twice per day at the onset of the dark and light period (6:00 a.m. and 6:00 p.m.) for 9 days. Mice were deprived of food for 24 h on day 4. Observation of food consumption was evaluated for 5 additional days after the food deprivation. Body weight was normalized for metabolic demands of body mass according to Kleiber's function (g weight loss/g baseline weight0.75).
Body Weight and Fat Distribution.
At the end of this period, mice were anesthetized, and intraabdominal fat pads (gonadal, retroperitoneal, and mesenteric), liver, subcutaneous fat pad (inguinal and the groin), and brown adipose tissue were dissected and weighed.
Glucose-Tolerance Tests.
A glucose-tolerance test was performed at the onset of the light cycle (6:00 a.m.). Mice were weighed and fasted for 24 h before the glucose-tolerance test. Access to drinking water was allowed during this period. On the day of the test, baseline glucose levels and body weight were determined before challenge with a glucose load of 1.5 mg of glucose per gram of body weight (d-glucose, anhydrous; Sigma–Aldrich, St. Louis, MO) dissolved in sterile distilled water (0.75 g of d-glucose, anhydrous in 10 ml of sterile water). The mouse was restrained by holding the excess skin at the base of the neck between the technician's thumb and forefinger. The mouse's tail was left hanging out and placed on a glass slide, and a segment of ≈1 mm in length was cut off the tip of the tail by using a sharp razor blade. A small drop (≈5 μl) of blood was placed on the glucometer test strip (Home Diagnostics, Fort Lauderdale, FL). After a 5-second developing time, the baseline blood glucose value was recorded (in mg per deciliter), and the mouse was returned to his home cage. After the baseline glucose measurement, the mouse was injected i.p. by using a 1-ml syringe and a 27-gauge needle. The time of the injection was noted, and 15-, 30-, 60-, and 120-min postinjection blood glucose measurements were performed again. It was sometimes necessary to remove a scab that formed at the initial tail-cut site to collect the second blood sample (n = 6 for each group). After the end of the study, mice were returned, and food and water was provided at libitum.
Insulin-Resistance Test.
The effects of insulin injection were assessed in nonfasted male mice. Similar to the glucose-tolerance test, blood was withdrawn from the tail without anesthesia before administration of human insulin (1 unit/kg, i.p.; Sigma–Aldrich). Samples were collected 15, 30, 60, and 120 min after the insulin challenge. Blood glucose levels were determined by a blood glucose meter (Home Diagnostics) (n = 6 for each group).
Plasma Levels of Leptin and Insulin.
Plasma leptin and insulin levels were determined at 6:00 a.m. and 6:00 p.m., at the onset of the light and dark cycle, respectively. Mice were euthanized with isoflorane (5%), decapitated, and bled into EDTA-coated tubes. Blood was centrifuged at 10,000 × g for 10 min at 4°C, and supernatants were taken and stored at −70°C until further analysis. Plasma leptin was measured by using a Mouse Leptin ELISA kit 96-well plate (catalog no. EZML-82K; Millipore, Billerica, MA) used for a nonradioactive quantification of leptin, and values were collected and averaged ± SEM. Mouse insulin was determined by RIA using a Rat Insulin RIA kit (250 tubes; catalog no. RI-13K; Millipore) according to manufacturer's instructions (n = 6 for each group).
Telemetry Device Implant.
EP3R−/− and WT littermate male mice were anesthetized with isoflorane (induction, 3–5%; maintenance, 0.9–1.5%) and implanted with radio telemetry devices (TA10TA-F20; Data Sciences, Inc., St. Paul, MN) into the peritoneal cavity for core body temperature measurement. Mice were allowed to recover for 2 weeks and were then submitted for freely moving recording (n = 10 for each group). Mice were maintained in a temperature-controlled room (25°C) on a 12-h light–dark cycle (light on at 6 a.m.). Core body temperature and motor activity sensors were located in the transmitter implant. The cages were positioned onto the receiver plates. Radio signals from the core body temperature and motor activity of each animal (number of horizontal movements) were continuously monitored with a fully automated data-acquisition system (Dataquest A.R.T.; Data Sciences, Inc.).
Data Analysis.
Data were grouped and analyzed by using the paired t test or ANOVA with repeated measures followed by post hoc Newman–Keuls test. All results are expressed as means ± SE. Metabolic efficiency was calculated as the energy intake divided by the body weight gain over a certain period. Linear relationships were estimated by using Pearson's moment correlation coefficient.
Acknowledgments
We thank Professor Jerold Chun for insightful suggestions. This work was supported by funds from the Skaggs Institute of Chemical Biology at The Scripps Research Institute.
Abbreviation
- PGE2
prostaglandin E2.
Footnotes
The authors declare no conflict of interest.
References
- 1.Alheim K, Bartfai T. Ann N Y Acad Sci. 1998;840:51–58. doi: 10.1111/j.1749-6632.1998.tb09548.x. [DOI] [PubMed] [Google Scholar]
- 2.Ivanov AI, Romanovsky AA. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- 3.Oka T. Front Biosci. 2004;1:3046–3057. doi: 10.2741/1458. [DOI] [PubMed] [Google Scholar]
- 4.Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. J Physiol (London) 2003;551:945–954. doi: 10.1113/jphysiol.2003.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, et al. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 6.Walch L, Clavarino E, Morris PL. Endocrinology. 2003;144:1284–1291. doi: 10.1210/en.2002-220868. [DOI] [PubMed] [Google Scholar]
- 7.Higgs GA. Prog Lipid Res. 1986;25:555–561. doi: 10.1016/0163-7827(86)90113-x. [DOI] [PubMed] [Google Scholar]
- 8.Hoozemans JJ, Veerhuis R, Janssen I, Rozemuller AJ, Eikelenboom P. Exp Gerontol. 2001;36:559–570. doi: 10.1016/s0531-5565(00)00226-6. [DOI] [PubMed] [Google Scholar]
- 9.Pinteaux E, Parker LC, Rothwell NJ, Luheshi GN. J Neurochem. 2002;83:754–763. doi: 10.1046/j.1471-4159.2002.01184.x. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. J Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 11.Saper CB. Ann NY Acad Sci. 1998;856:90–94. doi: 10.1111/j.1749-6632.1998.tb08317.x. [DOI] [PubMed] [Google Scholar]
- 12.Coelho MM, Luheshi G, Hopkins SJ, Pela IR, Rothwell NJ. Am J Physiol. 1995;269:R527–R535. doi: 10.1152/ajpregu.1995.269.3.R527. [DOI] [PubMed] [Google Scholar]
- 13.Derijk R, Van Rooijen N, Tilders FJ, Besedovsky HO, Del Rey A, Berkenbosch F. Endocrinology. 1991;129:330–338. doi: 10.1210/endo-129-1-330. [DOI] [PubMed] [Google Scholar]
- 14.Lenczowski MJ, Bluthe RM, Roth J, Rees GS, Rushforth DA, van Dam AM, Tilders FJ, Dantzer R, Rothwell NJ, Luheshi GN. Am J Physiol. 1999;276:R652–R658. doi: 10.1152/ajpregu.1999.276.3.R652. [DOI] [PubMed] [Google Scholar]
- 15.Murakami N, Watanabe T. Brain Res. 1989;478:171–174. doi: 10.1016/0006-8993(89)91492-3. [DOI] [PubMed] [Google Scholar]
- 16.Arnold J, Little RA, Rothwell NJ. J Appl Physiol. 1989;66:1970–1975. doi: 10.1152/jappl.1989.66.4.1970. [DOI] [PubMed] [Google Scholar]
- 17.Jepson MM, Millward DJ, Rothwell NJ, Stock MJ. Am J Physiol. 1988;255:E617–E620. doi: 10.1152/ajpendo.1988.255.5.E617. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y, Kang X, Ackerman WEt, Belury MA, Koster C, Rovin BH, Landon MB, Kniss DA. Diabetes Obes Metab. 2006;8:83–93. doi: 10.1111/j.1463-1326.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 19.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Narumiya S, Sugimoto Y, Ushikubi F. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka Y, Furuyashiki T, Bito H, Ushikubi F, Tanaka Y, Kobayashi T, Muro S, Satoh N, Kayahara T, Higashi M, et al. Proc Natl Acad Sci USA. 2003;100:4132–4137. doi: 10.1073/pnas.0633341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 23.Audoly LP, Tilley SL, Goulet J, Key M, Nguyen M, Stock JL, McNeish JD, Koller BH, Coffman TM. Am J Physiol. 1999;277:H924–H930. doi: 10.1152/ajpheart.1999.277.3.H924. [DOI] [PubMed] [Google Scholar]
- 24.Stock JL, Shinjo K, Burkhardt J, Roach M, Taniguchi K, Ishikawa T, Kim HS, Flannery PJ, Coffman TM, McNeish JD, et al. J Clin Invest. 2001;107:325–331. doi: 10.1172/JCI6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, Coffman TM, Koller BH. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartney JM, Coggins KG, Tilley SL, Jania LA, Lovgren AK, Audoly LP, Koller BH. Am J Physiol. 2006;290:L105–L113. doi: 10.1152/ajplung.00221.2005. [DOI] [PubMed] [Google Scholar]
- 27.McCoy JM, Wicks JR, Audoly LP. J Clin Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Okada Y, Pilbeam CC, Lorenzo JA, Kennedy CR, Breyer RM, Raisz LG. Endocrinology. 2000;141:2054–2061. doi: 10.1210/endo.141.6.7518. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 30.Mori K, Tanaka I, Kotani M, Miyaoka F, Sando T, Muro S, Sasaki Y, Nakagawa O, Ogawa Y, Usui T, et al. J Mol Med. 1996;74:333–336. doi: 10.1007/BF00207510. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto A, Matsumura J, Mii S, Gotoh Y, Ogawa R. Shock. 2004;22:76–81. doi: 10.1097/01.shk.0000129338.99410.5d. [DOI] [PubMed] [Google Scholar]
- 32.Sakuma Y, Tanaka K, Suda M, Komatsu Y, Yasoda A, Miura M, Ozasa A, Narumiya S, Sugimoto Y, Ichikawa A, et al. Infect Immun. 2000;68:6819–6825. doi: 10.1128/iai.68.12.6819-6825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, Yamamoto H, Maruyama T, Kondo K, Ushikubi F, Narumiya S, et al. Cancer Res. 1999;59:5093–5096. [PubMed] [Google Scholar]
- 34.Narumiya S, FitzGerald GA. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi K, Ukawa H, Kato S, Furukawa O, Araki H, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S. Gastroenterology. 1999;117:1128–1135. doi: 10.1016/s0016-5085(99)70398-7. [DOI] [PubMed] [Google Scholar]
- 36.Audoly LP, Ma L, Feoktistov I, de Foe SK, Breyer MD, Breyer RM. J Pharmacol Exp Ther. 1999;289:140–148. [PubMed] [Google Scholar]
- 37.Ma H, Hara A, Xiao CY, Okada Y, Takahata O, Nakaya K, Sugimoto Y, Ichikawa A, Narumiya S, Ushikubi F. Circulation. 2001;104:1176–1180. doi: 10.1161/hc3601.094003. [DOI] [PubMed] [Google Scholar]
- 38.Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, Kobayashi M, Satoh K, Narita M, Sugimoto Y, et al. J Exp Med. 2003;197:221–232. doi: 10.1084/jem.20021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoji Y, Takahashi M, Kitamura T, Watanabe K, Kawamori T, Maruyama T, Sugimoto Y, Negishi M, Narumiya S, Sugimura T, et al. Gut. 2004;53:1151–1158. doi: 10.1136/gut.2003.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunikata T, Yamane H, Segi E, Matsuoka T, Sugimoto Y, Tanaka S, Tanaka H, Nagai H, Ichikawa A, Narumiya S. Nat Immunol. 2005;6:524–531. doi: 10.1038/ni1188. [DOI] [PubMed] [Google Scholar]
- 41.Minami T, Nakano H, Kobayashi T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Ito S. Br J Pharmacol. 2001;133:438–444. doi: 10.1038/sj.bjp.0704092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fain JN, Leffler CW, Bahouth SW. J Lipid Res. 2000;41:1689–1694. [PubMed] [Google Scholar]
- 43.Fain JN, Ballou LR, Bahouth SW. Prostaglandins Other Lipid Mediat. 2001;65:199–209. doi: 10.1016/s0090-6980(01)00136-8. [DOI] [PubMed] [Google Scholar]
- 44.Hayaishi O, Matsumura H. Adv Neuroimmunol. 1995;5:211–216. doi: 10.1016/0960-5428(95)00010-y. [DOI] [PubMed] [Google Scholar]
- 45.Ram A, Pandey HP, Matsumura H, Kasahara-Orita K, Nakajima T, Takahata R, Satoh S, Terao A, Hayaishi O. Brain Res. 1997;751:81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Xia M, Goetzl EJ, An S. Biochem Biophys Research Commun. 1994;198:999–1006. doi: 10.1006/bbrc.1994.1142. [DOI] [PubMed] [Google Scholar]
- 47.Kotani M, Tanaka I, Ogawa Y, Usui T, Tamura N, Mori K, Narumiya S, Yoshimi T, Nakao K. Genomics. 1997;40:425–434. doi: 10.1006/geno.1996.4585. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. J Comp Neurol. 2000;421:543–569. doi: 10.1002/(sici)1096-9861(20000612)421:4<543::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Ek M, Arias C, Sawchenko P, Ericsson-Dahlstrand A. J Comp Neurol. 2000;428:5–20. doi: 10.1002/1096-9861(20001204)428:1<5::aid-cne2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida K, Nakamura K, Matsumura K, Kanosue K, Konig M, Thiel HJ, Boldogkoi Z, Toth I, Roth J, Gerstberger R, et al. Eur J Neurosci. 2003;18:1848–1860. doi: 10.1046/j.1460-9568.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- 51.Tabarean IV, Behrens MM, Bartfai T, Korn H. Proc Natl Acad Sci USA. 2004;101:2590–2595. doi: 10.1073/pnas.0308718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fain JN, Leffler CW, Bahouth SW, Rice AM, Rivkees SA. Prostaglandins Other Lipid Mediat. 2000;62:343–350. doi: 10.1016/s0090-6980(00)00088-5. [DOI] [PubMed] [Google Scholar]
- 53.Fantuzzi G. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 54.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 55.Wisse BE. J Am Soc Nephrol. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 56.Fain JN, Kanu A, Bahouth SW, Cowan GS, Jr, Hiler ML, Leffler CW. Prostaglandins Leukot Essent Fatty Acids. 2002;67:467–473. doi: 10.1054/plef.2002.0430. [DOI] [PubMed] [Google Scholar]
- 57.Lazarus M. Mol Nutr Food Res. 2006;50:451–455. doi: 10.1002/mnfr.200500207. [DOI] [PubMed] [Google Scholar]
- 58.Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahren B, Enerback S, Ohlsson C, et al. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 59.Chida D, Osaka T, Hashimoto O, Iwakura Y. Diabetes. 2006;55:971–977. doi: 10.2337/diabetes.55.04.06.db05-1250. [DOI] [PubMed] [Google Scholar]
- 60.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, et al. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 61.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Endocrinology. 2007;148:241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JA, Yeh DC, Ver M, Li Y, Carranza A, Conrads TP, Veenstra TD, Harrington MA, Quon MJ. J Biol Chem. 2005;280:23173–23183. doi: 10.1074/jbc.M501439200. [DOI] [PubMed] [Google Scholar]
- 63.Matsuoka Y, Furuyashiki T, Yamada K, Nagai T, Bito H, Tanaka Y, Kitaoka S, Ushikubi F, Nabeshima T, Narumiya S. Proc Natl Acad Sci USA. 2005;102:16066–16071. doi: 10.1073/pnas.0504908102. [DOI] [PMC free article] [PubMed] [Google Scholar]