Abstract
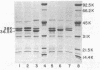
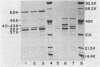
The chromosomal beta-lactamase and outer membrane proteins of Enterobacter cloacae were examined to determine their relative contributions to multiple antibiotic resistance in this organism. Mutants altered in beta-lactamase expression, whether derived in the laboratory or recovered from patients treated with one of the new beta-lactam antibiotics, were found to have no detectable alterations in outer membrane proteins. Derepression of beta-lactamase in these mutants was associated with high-level resistance to multiple beta-lactam antibiotics, while loss of inducible beta-lactamase (i.e., production of basal enzyme levels only) was associated with acquisition of susceptibility to many beta-lactam antibiotics, including cephalothin. In contrast, alteration in outer membrane proteins was associated with only moderate-level resistance to beta-lactam antibiotics. However, this included resistance to such drugs as amdinocillin and Sch 34343, which were unaffected by derepression of beta-lactamase. Resistance to chloramphenicol and tetracycline also accompanied changes in outer membrane proteins. Although the outer membrane proteins of various strains of E. cloacae were similar, there did appear to be some major strain-to-strain variations. Thus, it appears that alterations in both beta-lactamase and outer membrane proteins can affect the susceptibility of E. cloacae to many antibiotics. However, alterations in beta-lactamase alone are sufficient to produce high-level multiple beta-lactam resistance in this organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bavoil P., Nikaido H., von Meyenburg K. Pleiotropic transport mutants of Escherichia coli lack porin, a major outer membrane protein. Mol Gen Genet. 1977 Dec 14;158(1):23–33. doi: 10.1007/BF00455116. [DOI] [PubMed] [Google Scholar]
- Dworzack D. L., Bartelt M. A., Bailey R. T., Jr, Fitzgibbons R. J., Jr, Reich J. W., Picetti G. D., 3rd Emergence of resistance to aztreonam. Clin Pharm. 1984 Sep-Oct;3(5):467–470. [PubMed] [Google Scholar]
- Goering R. V., Pattee P. A. Mutants of Staphylococcus aureus with increased sensitivity to ultraviolet radiation. J Bacteriol. 1971 Apr;106(1):157–161. doi: 10.1128/jb.106.1.157-161.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner M., Glass D. G., Fleming P. C. Spontaneous mutant with loss of beta-lactamase in Aerobacter cloacae. J Bacteriol. 1969 Feb;97(2):961–961. doi: 10.1128/jb.97.2.961-.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C. Characterization of beta-lactamase induction in Enterobacter cloacae. Antimicrob Agents Chemother. 1983 Jan;23(1):91–97. doi: 10.1128/aac.23.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Goering R. V. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. doi: 10.1093/infdis/146.1.34. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Yamaguchi A., Sawai T. Purification and characterization of two kinds of porins from the Enterobacter cloacae outer membrane. J Bacteriol. 1984 Jun;158(3):1179–1181. doi: 10.1128/jb.158.3.1179-1181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M. F., Allan B. J., Minshew B. H., Sherris J. C. Mutational enzymatic resistance of Enterobacter species to beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Apr;21(4):655–660. doi: 10.1128/aac.21.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Sanders C. C. Inducible beta-lactamases and non-hydrolytic resistance mechanisms. J Antimicrob Chemother. 1984 Jan;13(1):1–3. doi: 10.1093/jac/13.1.1. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance to cefamandole: possible role of cefoxitin-inducible beta-lactamases. Antimicrob Agents Chemother. 1979 Jun;15(6):792–797. doi: 10.1128/aac.15.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]