Abstract
Hsp31, the product of the σS- and σD-dependent hchA gene, is a heat-inducible chaperone implicated in the management of protein misfolding at high temperatures. We show here that Hsp31 plays an important role in the acid resistance of starved Escherichia coli but that it has little influence on oxidative-stress survival.
Enteric bacteria have evolved complex protective networks to survive the wide range of adverse environmental conditions that they encounter during their life cycle. One of the most effective triggers of multiple-stress resistance is exposure to nutrient-limited conditions (7), which causes entry into the stationary phase of growth and a large increase in the concentration of the alternative sigma factor σS (11, 12). Stationary-phase Escherichia coli is particularly tolerant to reactive oxygen species (ROS) and low pH, a phenomenon that is partially explained by the fact that some members of the oxyR and soxRS oxidative-stress regulons and of the three E. coli acid resistance systems are regulated by σS (2, 25). Yet, while ROS and acidification cause protein misfolding, only one oxidative-stress-specific cytoplasmic molecular chaperone (Hsp33) and two acid stress-specific periplasmic chaperones (HdeA and HdeB) have been identified to date (5, 10, 13).
Hsp31, the hchA gene product, is a homodimeric protein that is heat inducible and belongs to the ThiI/DJ-1/PfpI superfamily (21, 23). It exhibits molecular chaperone activity and functions as a holdase that stabilizes unfolding intermediates until stress has been abated (18, 23, 24). Although each Hsp31 monomer contains a poorly accessible catalytic triad responsible for a weak, Zn-dependent aminopeptidase activity, Hsp31 is not a protease (16, 17, 21). The hchA gene is transcribed from dual σD- and σS-dependent promoters that are silenced by H-NS (20). While hchA expression in exponential-phase cultures and its thermal induction are primarily regulated by H-NS and σD, EσS-dependent hchA transcription dominates upon entry into stationary phase (20).
Previously, we have shown that deletion of hchA leads to an increase in the temperature sensitivity of dnaK and grpE mutants, causes growth defects as cells transit from exponential to stationary phase at 48°C, and impairs the ability of E. coli to survive severe heat shock and starvation (19, 20). These results, combined with the observation that hchA is a member of the σS general stress regulon, prompted us to study the involvement of Hsp31 in the management of oxidative and acid stresses.
We first examined whether hydrogen peroxide (H2O2) treatment or medium acidification of exponential-phase cultures would lead to hchA activation. For oxidative-stress experiments, triplicate cultures of the hchA::lacZ lysogen MIR510 (Table 1) (20) were grown at 37°C in LB medium supplemented with 50 μg/ml streptomycin (LB-streptomycin medium) to mid-exponential phase (optical density at 600 nm [OD600], ∼0.4). Cultures were split into 25-ml aliquots. One set was left untreated to serve as a control, while the other was challenged with 0.005% (∼1.5 mM) H2O2. For acid induction experiments, cells were grown at 37°C to late exponential phase (OD600, ∼1.0) and culture samples (5 ml) were added to 25 ml of fresh LB-streptomycin medium adjusted to pH 7.0 or pH 4.0 with HCl. In both cases, induction of the hchA promoter and Hsp31 levels were monitored after 1 h of incubation by a β-galactosidase enzymatic activity assay and immunoblotting, as previously described (20). Experiments were repeated with rpoS359, ΔhnS, and ΔhnS rpoS359 mutants (Table 1) to dissect the contribution of each regulatory factor.
TABLE 1.
Bacterial strains and plasmid used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| MC4100 | araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | Laboratory stock |
| MIR401 | MC4100 ΔhchA | 19 |
| RH90 | MC4100 rpoS359::Tn10 | 14 |
| MIR510 | MC4100 λφ(hchA::lacZ) | 20 |
| MIR512 | MC4100 rpoS359::Tn10 λφ(hchA::lacZ) | 20 |
| MIR514 | MC4100 hns::cat λφ(hchA::lacZ) | 20 |
| MIR516 | MC4100 rpoS359::Tn10 hns::cat λφ(hchA::lacZ) | 20 |
| Plasmids | ||
| ptetA3 | pACYC184 derivative containing the tetA followed by MCS, Catr | This work |
| pMM114 | ptetA3 derivative encoding hchA gene under the control of tetracycline-inducible tetA promoter, Catr | This work |
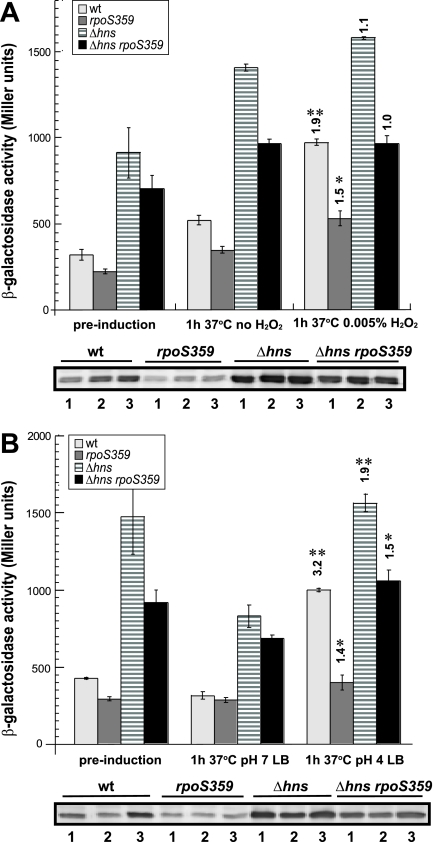
Figure 1 shows that in wild-type cells, H2O2 addition led to an ∼2-fold increase in hchA transcription and Hsp31 protein levels while acidification caused a 3-fold increase. Inactivation of σS significantly reduced, but did not completely abolish, peroxide and acid-mediated induction. In Δhns cells, where hchA transcription is elevated due to lack of promoter silencing (20), acid shock led to a low level of induction (∼50%) while oxidative stress had no significant impact. Similar patterns were observed for the Δhns rpoS359 double mutant. We conclude that although both oxidative and acid shocks lead to Hsp31 upregulation in a process that is at least partially dependent on σS, acid stress is a more effective hchA inducer.
FIG. 1.
hchA is induced by oxidative (A) and acid (B) stresses. The hchA::lacZ lysogens MIR510 (wild type [wt]), MIR512 (rpoS359), MIR514 (Δhns), and MIR516 (Δhns rpoS359) were grown to mid-exponential phase in LB-streptomycin medium at 37°C (preinduction) and treated with either 0.005% hydrogen peroxide or left untreated (A). Acid shock experiments (B) were conducted with late-exponential-phase cells as described in the text. Samples were collected 1 h after challenge, and clarified lysates were assayed for β-galactosidase activities (top panels), while whole-cell fractions were analyzed by immunoblotting with Hsp31 antiserum (bottom panels). Numbers above columns correspond to n-fold induction levels relative to isogenic, unstressed controls. Single asterisks denote a P value of 0.05 or less, while double asterisks denote a P value of 0.005 or less. Labels below blots identify prestress samples (lanes 1) and samples from cultures grown for 1 h without stress (lanes 2) or with imposed stress (lanes 3). Error bars were obtained for triplicate independent experiments.
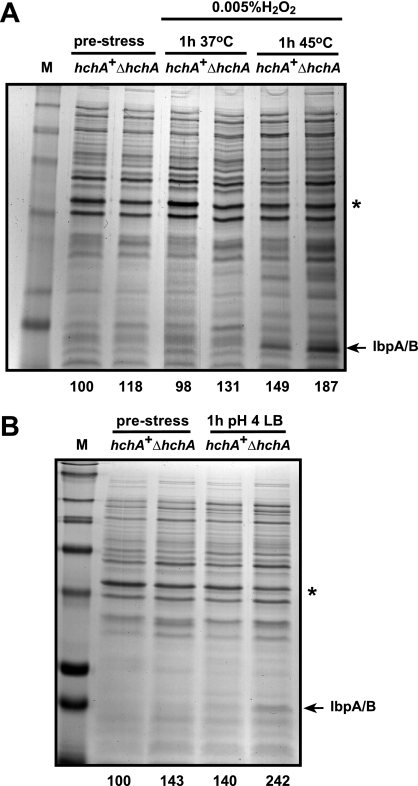
To determine if hchA inactivation would cause increased protein misfolding in cultures exposed to hydrogen peroxide or transferred to low pH, MIR401 cells were grown and challenged as described above and insoluble protein fractions were harvested and processed as described previously (19). Figure 2A shows that treatment of wild-type cells with 0.005% H2O2 at 37°C had essentially no impact on host protein aggregation. Only when oxidative stress was combined with a 1-h heat shock at 45°C (which by itself does not trigger detectable protein misfolding in either hchA+ or hchA null cells) (19) did we observe a small increase in insoluble species relative to unstressed controls. On the other hand, acid shock alone increased host protein aggregation to levels comparable to those achieved upon simultaneous oxidative and thermal shocks. Many sensitive polypeptides had a molecular mass smaller than 36 kDa and included the small heat shock proteins IbpA and IbpB, a marker of aggregated fractions (15). It is worthy to point out here that Hsp31 inactivation does not cause “wholesale” host protein aggregation (as mutations in the DnaK-DnaJ-GrpE system do) and that the increased levels of insoluble proteins in Fig. 2 are similar to those seen when ΔhchA cells are exposed to severe thermal stress (19).
FIG. 2.
Hsp31 contributes to the management of protein misfolding under aggravated oxidative stress and acid stress. Wild-type or ΔhchA cells were subjected to oxidative stress at 37°C or 45°C (A) or to acid shock at 37°C (B) as described in the legend to Fig. 1. Insoluble cell fractions were collected before (prestress lanes) and 1 h after imposition of stress. Numbers at the bottom of the gels correspond to the amount of insoluble proteins in each lane normalized to the intensity of the OmpF band (star) and to the hchA+ prestress lane (arbitrarily set at 100). The migration position of the IbpA and IbpB small heat shock proteins is indicated. Markers (lane M) are 250, 148, 98, 64, 50, 36, 22 and 16 kDa from top to bottom.
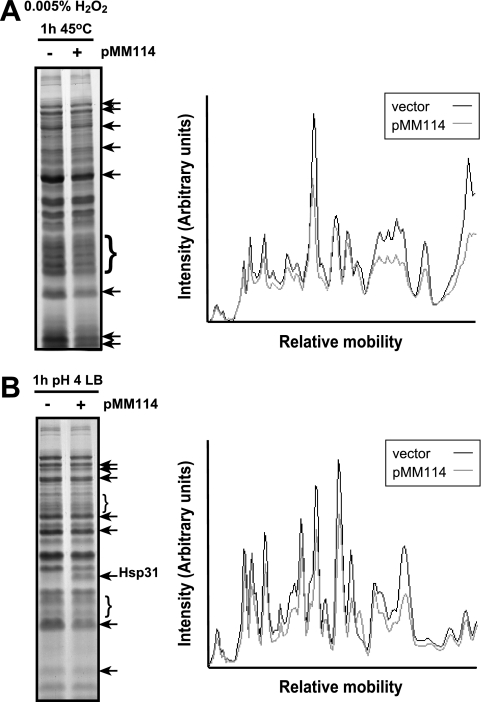
To confirm that the lack of Hsp31 was responsible for the higher levels of protein misfolding in stressed cultures, experiments were repeated using ΔhchA cells transformed with the Hsp31 expression plasmid pMM114 (Table 1). This plasmid was built by amplifying the hchA gene along with its ribosome binding site with primer pair 5′-CCCTAACTAAGCTTCAATAAGGAATAC-3′ and 5′-TTCAAACGTAAGCTTGATTAACCCGCG-3′ and by ligating the HindIII-digested PCR product into the HindIII backbone of ptetA3, a chloramphenicol-resistant derivative of pACYC184 containing a tetA promoter insert from pSKAP/S (6). In the final construct, hchA transcription is under control of the anhydrotetracycline-inducible tetA promoter.
Figure 3 shows that Hsp31 overexpression reduced the aggregation of a number of polypeptides exhibiting enhanced misfolding in the experiments of Fig. 2. Thus, Hsp31 is involved in maintaining the integrity of a subset of cellular proteins that misfold upon aggravated oxidative stress and acid shock.
FIG. 3.
Hsp31 overexpression alleviates protein misfolding in cells subjected to aggravated oxidative stress and acid stress. ΔhchA cells harboring either the cloning vector ptetA3 (− lanes) or the Hsp31 expression vector pMM114 (+ lanes) were grown at 37°C in LB-chloramphenicol medium supplemented with 0.05 μg/ml anhydrotetracycline. Cultures were subjected to oxidative stress at 45°C (A) or to acid shock at 37°C (B). Insoluble cell fractions were collected 1 h after imposition of stress. Arrows and braces show the migration positions of proteins that are present at lower levels in the insoluble fraction of Hsp31-overexpressing cells. The panels on the right show the results of a videodensitometric analysis of the gel lanes. Gray lines correspond to the insoluble fraction of Hsp31-overexpressing cells, while black lines correspond to the insoluble fraction of cultures harboring the ptetA3 vector.
Starved E. coli utilizes three overlapping acid resistance (AR) systems to cope with low pH. AR1 (also known as the oxidative system) is induced upon entry into stationary phase and is important for acid stress survival above pH 3 (1). To date, only two proteins, cyclopropane fatty acid synthase (Cfa) and HdeA, have been implicated in AR1 function. While Cfa increases the outer membrane fatty acid content (thereby reducing permeability by protons) (26), HdeA is an acid-inducible molecular chaperone that relies on low pH-mediated dimer-to-monomer activation to suppress the aggregation of acid-denatured proteins in the periplasm (5, 9). The AR2 (glutamate-dependent) system is induced in stationary-phase cells growing in complex medium supplemented with glucose (which represses AR1) and is very effective in conferring acid resistance at pH values as low as 2. Its major components are the glutamate decarboxylases GadA and GadB and the antiporter GadC (8). GadA and GadB convert glutamate to γ-aminobutyric acid (GABA) in a reaction that consumes an intracellular proton. GadC exports GABA out of the cell while replenishing the cytoplasmic glutamate supply. Exported GABA may also offer acid stress relief by acting as a proton acceptor in the periplasm and the immediate extracellular space (4). The AR3 (arginine-dependent) system works similarly to AR2 except that it relies on AdiA-mediated arginine decarboxylation to consume protons and on the AdiC antiporter to export the resulting agmatine to the extracellular medium (22). AR3 is induced in low-pH complex medium under anaerobic conditions or following growth in brain heart infusion broth supplied with 0.4% glucose (1).
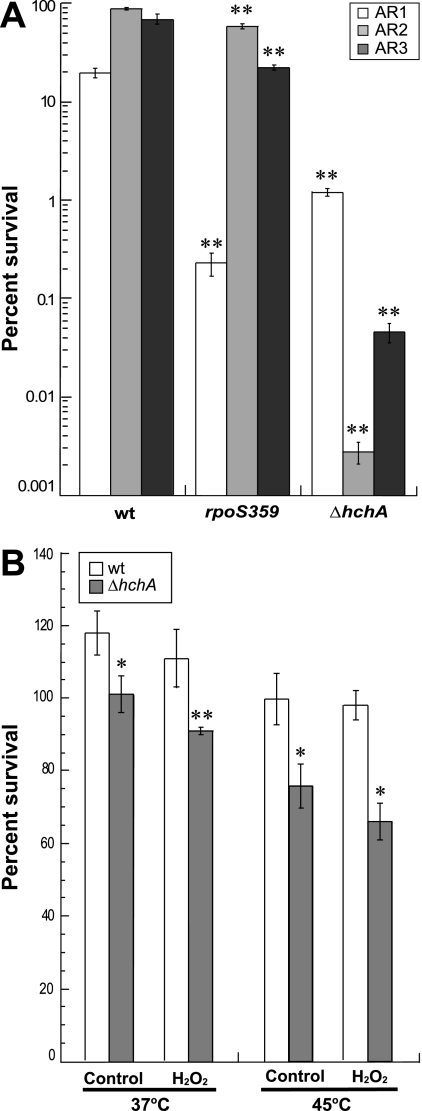
To determine if Hsp31 is implicated in stationary-phase acid resistance, wild-type, ΔhchA, and rpoS359 cells were incubated overnight at 37°C in three different media, each optimal for activating a given acid resistance system. LB medium buffered at a pH 5.5 with 100 mM morpholinethanesulfonic acid was used to induce AR1, while LB supplemented with 20 mM glucose and brain heart infusion broth supplemented with 0.4% glucose were used to activate the AR2 and AR3 systems, respectively (1). All media were supplemented with 50 μg/ml streptomycin. Cultures were then diluted at a 1:40 ratio into 2 ml of minimal E medium containing 0.4% glucose (1) held at pH 2.5 plus no additive (AR1 test), 1.5 mM glutamate (AR2 test), or 0.6 mM arginine (AR3 test). After 1 h of incubation at 37°C, the cells were plated onto LB agar-streptomycin plates and survivors were counted.
In wild-type cells, AR2 proved to be the most efficient defense against acid stress, allowing an 85% survival rate, compared to 70% for AR3 and 20% for AR1 (Fig. 4A). Deletion of rpoS led to a decrease of almost 2 orders of magnitude in AR1-based acid protection but had little impact on the resistance conferred by AR2 or AR3. This result was not surprising in light of the fact that σS is a key regulator of AR1 (1) but does not play a major role in the AR2 and AR3 systems (3). Figure 4A shows that the absence of Hsp31 affected the function of all three acid resistance systems. The biggest impact was on AR2, with a reduction in survival of 4 orders of magnitude, followed by AR3 (with a decrease in viability of 3 orders of magnitude) and AR1 (10-fold decrease in viability). Thus, Hsp31 provides a broad contribution to acid resistance in stationary-phase E. coli.
FIG. 4.
Hsp31 plays an important role in the ability of stationary-phase cells to deal with acid stress. (A) Wild-type (wt), rpoS359, and ΔhchA cells were grown overnight in either LB medium buffered at a pH 5.5 with 100 mM morpholinethanesulfonic acid (to activate AR1), LB medium supplemented with 20 mM glucose (to activate AR2), or brain heart infusion broth supplemented with 0.4% glucose (to activate AR3). Aliquots were subjected to acid shock at pH 2.5 in minimal E medium containing 0.4% glucose that contained no additive (AR1 test), 1.5 mM glutamate (AR2 test), or 0.6 mM arginine (AR3 test). (B) Wild-type or ΔhchA cells were grown at 37°C for 2 days and either left untreated or challenged with 0.005% hydrogen peroxide for 1 h at 37°C or 45°C. Percent survival corresponds to the ratio of the numbers of CFU 1 h after imposition of stress to the numbers of CFU before stress. Error bars were obtained for triplicate independent experiments. Single asterisks denote a P value of 0.05 or less, while double asterisks denote a P value of 0.005 or less relative to the isogenic wild type.
We also assessed the impact of Hsp31 on the ability of stationary-phase cultures to handle oxidative damage. For these experiments, wild-type and ΔhchA cells were grown in LB -streptomycin medium at 37°C for 2 days, with or without exposure to 0.005% hydrogen peroxide for 1 h at 37 or 45°C, and the number of survivors was determined by plating. In agreement with previous results (20), hchA inactivation reduced the viability of stationary-phase cells by 20 to 30% (Fig. 4B). However, H2O2 treatment led to only a small (10%) decrease in the viability of hchA null cells and did not have a significant impact on the viability of the wild type. We conclude that Hsp31 plays only a minor role (if any) in the ability of E. coli to survive stationary-phase oxidative damage.
Based on the observation that its structural gene belongs to the σS general stress regulon (20, 27), we probed the involvement of Hsp31 in oxidative-stress and acid stress resistance. Consistent with the fact that E. coli is already equipped with Hsp33, a specialized chaperone that handles the deleterious effects of ROS (10), we did not find that Hsp31 played a significant role in the management of oxidative damage alone (although it helps cells handle combined oxidative and thermal stresses). On the other hand, our data show that Hsp31 is an important contributor to acid stress resistance. While additional work will be required to determine how it contributes to the process, Hsp31 might act as the cytoplasmic counterpart of the HdeA and HdeB chaperones which alleviate periplasmic aggregation in acid-stressed cells (5, 9, 13). A role for Hsp31 family members in the management of acid stress may have been conserved, since the Hsp31 yeast homolog, YDR533C, is also acid inducible (2).
Acknowledgments
We are grateful to Regine Hengge and Arjen Schots for generous gifts of strains and plasmids.
This work was supported by NSF award BES-0097430 and by the Charles W. H. Matthaei endowment.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Castanie-Cornet, M.-P., T. A. Penfound, D. Smith, J. F. Elliott, and J. W. Foster. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Nobel, H., L. Lawrie, S. Brul, F. Klis, M. Davis, H. Alloush, and P. Coote. 2001. Parallel and comparative analysis of the proteome and transcriptome of sorbic acid-stressed Saccharomyces cerevisiae. Yeast 18:1413-1428. [DOI] [PubMed] [Google Scholar]
- 3.Foster, J. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2:898-907. [DOI] [PubMed] [Google Scholar]
- 4.Foster, J. W. 1993. The acid tolerance response of Salmonella typhimurium involves transient synthesis of key acid shock proteins. J. Bacteriol. 175:1981-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajiwala, K. S., and S. K. Burley. 2000. HDEA, a periplasmic protein that supports acid resistance in pathogenic enteric bacteria. J. Mol. Biol. 295:605-612. [DOI] [PubMed] [Google Scholar]
- 6.Griep, R. A., C. van Twisk, R. J. Kerschbaumer, K. Harper, L. Torrance, G. Himmler, J. M. van der Wolf, and A. Schots. 1999. pSKAP/S: an expression vector for the production of single-chain Fv alkaline phosphatase fusion proteins. Protein Expr. Purif. 16:63-69. [DOI] [PubMed] [Google Scholar]
- 7.Hengge-Aronis, R. 2000. The general stress response in Escherichia coli, p. 161-178. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, DC.
- 8.Hersh, B. M., F. T. Faroo, D. N. Barsatd, D. L. Blankhorn, and J. L. Slonczewski. 1996. A glutamate-dependent acid resistance gene in Escherichia coli. J. Bacteriol. 178:3978-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong, W., W. Jiao, J. Hu, J. Zhang, C. Liu, X. Fu, D. Shen, B. Xia, and Z. Chang. 2005. Periplasmic protein HdeA exhibits chaperone-like activity exclusively within stomach pH range by transforming into disordered conformation. J. Biol. Chem. 280:27029-27034. [DOI] [PubMed] [Google Scholar]
- 10.Jakob, U., W. Muse, M. Eser, and J. C. A. Bardwell. 1999. Chaperone activity with a redox switch. Cell 96:341-352. [DOI] [PubMed] [Google Scholar]
- 11.Jishage, M., and A. Ishihama. 1995. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of sigma 70 and sigma 38. J. Bacteriol. 177:6832-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jishage, M., A. Iwata, S. Ueda, and A. Ishihama. 1996. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J. Bacteriol. 178:5447-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern, R., A. Malki, J. Abdallah, J. Tagourti, and G. Richarme. 3 November 2006. Escherichia coli HdeB is an acid-stress chaperone. J. Bacteriol. doi: 10.1126/JB.01522-06. [DOI] [PMC free article] [PubMed]
- 14.Lange, R., and R. Hengge-Aronis. 1991. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 5:49-59. [DOI] [PubMed] [Google Scholar]
- 15.Laskowska, E., A. Wawrzynow, and A. Taylor. 1996. IbpA and IbpB, the new heat-shock proteins, bind to endogenous Escherichia coli proteins aggregated intracellularly by heat shock. Biochimie 78:117-122. [DOI] [PubMed] [Google Scholar]
- 16.Lee, S. J., S. J. Kim, I. K. Kim, J. Ko, C. S. Jeong, G. H. Kim, C. Park, S. O. Kang, P. G. Suh, H. S. Lee, and S. S. Cha. 2003. Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain. J. Biol. Chem. 278:44552-44559. [DOI] [PubMed] [Google Scholar]
- 17.Malki, A., T. Caldas, J. Abdallah, R. Kern, V. Eckey, S. J. Kim, S. S. Cha, H. Mori, and G. Richarme. 2005. Peptidase activity of the E. coli HSP31 chaperone. J. Biol. Chem. 280:14420-14426. [DOI] [PubMed] [Google Scholar]
- 18.Malki, A., R. Kern, J. Abdallah, and G. Richarme. 2003. Characterization of the Escherichia coli YedU protein as a molecular chaperone. Biochem. Biophys. Res. Commun. 301:430-436. [DOI] [PubMed] [Google Scholar]
- 19.Mujacic, M., M. W. Bader, and F. Baneyx. 2004. Escherichia coli Hsp31 functions as a holding chaperone that cooperates with the DnaK-DnaJ-GrpE system in the management of protein misfolding under severe stress conditions. Mol. Microbiol. 51:849-859. [DOI] [PubMed] [Google Scholar]
- 20.Mujacic, M., and F. Baneyx. 2006. Regulation of Escherichia coli hchA, a stress-inducible gene encoding molecular chaperone Hsp31. Mol. Microbiol. 60:1576-1589. [DOI] [PubMed] [Google Scholar]
- 21.Quigley, P. M., K. Korotkov, F. Baneyx, and W. G. Hol. 2003. The 1.6-Å crystal structure of the class of chaperones represented by Escherichia coli Hsp31 reveals a putative catalytic triad. Proc. Natl. Acad. Sci. USA 100:3137-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard, H. T., and J. W. Foster. 2003. Acid resistance in Escherichia coli. Adv. Appl. Microbiol. 52:167-186. [DOI] [PubMed] [Google Scholar]
- 23.Sastry, M. S. R., K. Korotkov, Y. Brodsky, and F. Baneyx. 2002. Hsp31, the Escherichia coli yedU gene product, is a molecular chaperone whose activity is inhibited by ATP at high temperatures. J. Biol. Chem. 277:46026-46034. [DOI] [PubMed] [Google Scholar]
- 24.Sastry, M. S. R., P. M. Quigley, W. G. Hol, and F. Baneyx. 2004. The linker-loop region of Escherichia coli chaperone Hsp31 functions as a gate that modulates high-affinity substrate binding at elevated temperatures. Proc. Natl. Acad. Sci. USA 101:8587-8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storz, G., and M. Zheng. 2000. Oxidative stress, p. 47-59. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, DC.
- 26.Wang, A.-Y., and J. E. Cronan, Jr. 1994. The growth-phase dependent synthesis of cyclopropane fatty acids in Escherichia coli is the result of an RpoS (KatF)-dependent promoter plus enzyme instability. Mol. Microbiol. 11:1009-1017. [DOI] [PubMed] [Google Scholar]
- 27.Weber, H., T. Polen, J. Heuveling, V. F. Wendisch, and R. Hengge. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187:1591-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]