Abstract
Polyester-rayon blend wipes were evaluated for efficiency of extraction and recovery of powdered Bacillus atrophaeus spores from stainless steel and painted wallboard surfaces. Method limits of detection were also estimated for both surfaces. The observed mean efficiency of polyester-rayon blend wipe recovery from stainless steel was 0.35 with a standard deviation of ±0.12, and for painted wallboard it was 0.29 with a standard deviation of ±0.15. Evaluation of a sonication extraction method for the polyester-rayon blend wipes produced a mean extraction efficiency of 0.93 with a standard deviation of ±0.09. Wipe recovery quantitative limits of detection were estimated at 90 CFU per unit of stainless steel sample area and 105 CFU per unit of painted wallboard sample area. The method recovery efficiency and limits of detection established in this work provide useful guidance for the planning of incident response environmental sampling following the release of a biological agent such as Bacillus anthracis.
Following a biological agent release such as the Bacillus anthracis incidents of October 2001, environmental samples are collected and analyzed to provide information on initial agent concentration, location, and extent of contamination and ultimately confirmation that clean-up goals are achieved (20). It is critical from a public health perspective that the information obtained is accurate and reproducible. The consequences of an inappropriate public health response founded on information garnered by an ineffective sample collection method or procedure has the potential for undesired social and economic impact. Well-developed and validated procedures for the collection and analysis of biological environmental samples are required to provide the necessary level of confidence in agent characterization information provided.
Researchers and investigators are aware that the Centers for Disease Control and Prevention (CDC)-recommended procedures for the collection of B. anthracis spores by swab, wipe, and vacuum filtration collection methods (6) underestimate the number of spores on surfaces, and attempts are being made to address the knowledge gap (4, 5, 18, 19). Additionally, a number of studies have been conducted to determine the efficiency of the swab sample collection method (1, 2, 3, 7, 8, 10, 17, 18), but limited studies have been conducted to examine the efficiency of the wipe spore collection method (10, 13). A recent study conducted by Sanderson et al. (19) that compared B. anthracis surface sampling results obtained by swab, wipe, and vacuum filtration methods suggests that the wipe collection method outperforms the swab method, but no independent recovery efficiency was established for the wipe method.
The objective of this study was to empirically evaluate the wipe surface sample collection method for recovery efficiency with a polyester-rayon blend wipe material and estimate limits of detection for selected nonporous surfaces seeded with dry deposited Bacillus atrophaeus spores. Additionally, a sonication extraction method was evaluated for effectiveness in removing viable spores from the selected wipe collection material.
MATERIALS AND METHODS
Spore matrix.
The material used as the test agent for this study was a powdered matrix containing B. atrophaeus spores (ATCC 9372; formerly Bacillus subtilis var. niger and subsequently “Bacillus globigii”) (12) and silicon dioxide particles obtained from the U.S. Army Dugway Proving Ground Life Science Division. The spore material was prepared by cultivating B. atrophaeus in tryptic soy broth (Difco, Detroit, MI) containing 3 mg/liter MnSO4 (Fisher Scientific, Pittsburgh, PA). After 80 to 90% sporulation, the spore suspension was centrifuged to obtain a spore suspension containing approximately 20% solids. Dry spore material was then prepared from the unwashed spore suspension with a laboratory spray dryer. The spore material was dry blended with Aerosil R812S fumed silica particles (Degussa, Frankfurt am Main, Germany) at 80% dry spore material to 20% silica and jet milled to a uniform particle size. The final powdered matrix contained approximately 1011 viable spores/g. The B. atrophaeus spore material was expressly designed to enhance aerosol suspension and inhalation characteristics, and the removal, extraction, and recovery characteristics of a different Bacillus species, native spore material, or spore material prepared by a different method may differ.
Reference surface material.
Stainless steel coupons measuring 1.25 by 5 cm (6.25 cm2) were used as reference surfaces. The coupons were cut from 1.2-mm-thick 316-L stainless steel (Neeley Plastic Fabrication Inc., Albuquerque, NM). The stainless steel coupons were washed with Alcojet powdered detergent (Alconox Inc., New York, NY), rinsed in deionized water, air dried, and autoclave sterilized at 121°C and 1,500 kPa for 40 min.
Sample surface material.
Stainless steel and painted wallboard coupons measuring 2.5 by 10 cm (25 cm2) were used for representative sample collection surfaces. The stainless steel coupons were cut from 1.2-mm-thick 316-L stainless steel (Neeley Plastic Fabrication Inc., Albuquerque, NM). The stainless steel coupons were washed with Alcojet powdered detergent (Alconox Inc., New York, NY), rinsed in deionized water, air dried, and autoclave sterilized. The painted wallboard coupons were cut from 6-mm smooth wallboard and painted with white interior latex semigloss paint (catalog no. HM1420; Glidden, Cleveland, OH). To minimize paint surface irregularities, the paint was applied to the wallboard surface with a pressurized paint sprayer. The painted wallboard coupons were air dried and UV-C (254 nm) sterilized at 200 μW/cm2 for 20 min.
Wipe material.
The wipe material evaluated in this study was a sterile polyester-rayon blend gauze wipe (10 by 10 cm, catalog no. 9728; Alliance Medical, Russellville, MO).
Wetting agent.
While it is recognized that addition of a surfactant to the wetting agent potentially enhances particle removal from surfaces (4, 13, 18), the objective of this study was evaluation of the current CDC-recommended wipe collection method. Wetting agents currently recommended by the CDC include sterile, deionized water; sterile saline; and sterile phosphate-buffered saline (6). Sterile, deionized water was selected for use as the wetting agent in this study for this reason and because a recent comparative evaluation of the wipe method by Sanderson et al. (19) also utilized sterile, deionized water as the wetting agent.
Aerosol deposition system.
Components of the aerosol deposition system include a TSI 3400A fluidized bed aerosol generator (TSI Inc., Minneapolis, MN), an aerosol mixing chamber, and an aerosol deposition chamber. The mixing chamber is designed to receive spore material from the aerosol generator and provide a confined volume allowing for concentration equilibration before transfer to the aerosol deposition chamber. The aerosol deposition chamber is designed to receive the aerosolized spore material from the mixing chamber, provide uniform mixing, and allow undisturbed settling of spore material onto reference and sample coupon surfaces.
The aerosol mixing chamber is a cylindrical containment vessel with a diameter of 45 cm, a height of 30 cm, and a volume of 0.048 m3. The chamber is constructed of carbon steel with an enamel-coated surface. Valved feedthrough ports are provided for injecting spores into the chamber from the fluidized bed aerosol generator, transferring spore material to the aerosol deposition chamber, and collecting samples for concentration analysis by a TSI 3110A aerodynamic particle sizer (TSI Inc., Minneapolis, MN).
The aerosol deposition chamber is a cubic containment vessel with dimensions of 90 by 90 by 90 cm providing an interior volume of 0.73 m3. The chamber is constructed of polypropylene sheets welded at the seams to make the chamber watertight. Access doors are located at the front and rear of the chamber, and windows, constructed from 12-mm clear, static-free polyvinyl chloride, are located on the side walls and both doors. A feedthrough port is located in the chamber top to receive aerosol from the mixing chamber. Two muffin fans provide convective airflow and circulation for aerosol dispersion and mixing. A sliding tray is located in the bottom of the chamber for sample access.
Extraction efficiency determination method.
Extraction efficiency, which is the effectiveness of material transfer from the collection medium, such as wipes, to the extraction solution, was determined. Several extraction methods are currently used to extract microorganisms from environmental sample collection media, such as shaking, vortexing, and sonication, but the most widely used are vortexing and sonication (2, 10, 15, 16, 18). A sonication method for extracting spores from the wipe collection medium into a buffer solution containing surfactant was evaluated by the following method.
(i) Wipe inoculation.
A spore suspension was prepared by suspending 2.0 mg of the spore stock (1011 CFU/g) in 200 ml Butterfield buffer solution (3 mmol/liter KH2PO4, pH 7.2) to produce a nominal 106-CFU/ml suspension. Forty wipes were directly inoculated with 1.0 ml (106 CFU) of the suspension. Following inoculation, the wipes were immediately placed into 50-ml Blue Falcon screw-top tubes (Becton Dickinson Labware, Franklin Lakes, NJ) containing 30.0 ml Butterfield buffer with 0.01% Tween 80 (catalog no. BP-338-500; Fisher Scientific, Pittsburgh, PA). Ten additional tubes containing 30.0 ml Butterfield buffer with 0.01% Tween 80 (BBT) was directly inoculated with 1.0 ml (106 CFU) of the same spore suspension as the references.
(ii) Extraction and enumeration.
Spores were extracted from the wipes into the BBT by sonication in a VWR 250T ultrasonic bath (VWR International, Tempe, AZ) for 15 min at sweeping frequencies between 38.5 and 40.5 kHz and an average power of 180 W. The extraction suspension was then heat treated at 65°C for 60 min to kill any bacterial vegetative cells and fungal spores which may be present in the suspension and to activate the Bacillus spores for rapid germination (9, 14). While an estimated 5% of the viable spores were killed by the heat treatment (11), the same relative numbers of viable spores were killed in both the reference and sample suspensions. Following heat treatment, the spore suspension was vortexed for 15 s and five log serial dilutions (10−1 to 10−5) of the extracted spore suspension were prepared in sterile, deionized water. A 1.0-ml aliquot of the suspension and 1.0 ml of each dilution were spread onto Petrifilm aerobic plate count medium (3M Microbiology Products, St. Paul, MN) in triplicate. The Petrifilm plates were then incubated at 37°C for 24 h. Colonies with distinct margins were counted by eye. Only plates with counts between 30 and 300 CFU were included with counts logged into a laboratory notebook. The total number of CFU per sample was determined as a function of the dilution factor and extraction volume. References were subjected to the same procedure.
(iii) Calculations.
Extraction efficiency was calculated as the number of CFU from the wipe extraction suspension relative to the mean number of CFU from the reference suspensions. The mean extraction efficiency was calculated with the equation
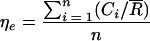 |
where ηe is the mean extraction efficiency, Ci is the average wipe count for three replicates, 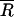 is the mean reference count, i is the sample number, and n is the sample size. Standard deviation was calculated by typical statistical methods for normally distributed data.
is the mean reference count, i is the sample number, and n is the sample size. Standard deviation was calculated by typical statistical methods for normally distributed data.
Recovery efficiency determination method. (i) Experimental design.
For this study, two surface coupons were positioned side by side or colocated in the aerosol deposition chamber and seeded with the dry aerosolized B. atrophaeus spore matrix. One of the surfaces, a stainless steel reference coupon (1.25 by 5 cm), was sized to fit into a sample vial for direct spore removal, while the other surface, a sample surface coupon (2.5 by 10 cm), was sized for a typical wipe application. Deposited spore material was directly removed from the reference coupon surface and cultured for enumeration of CFU, while deposited spore material was collected from the sample coupon by wipe and extracted by sonication for enumeration by culture. Recovery efficiency, which is a measurement of overall effectiveness of transfer from surface to culture, was calculated as the number of CFU from the wipe sample per unit of area relative to the number of CFU from the colocated reference coupon per unit of area.
(ii) Surface material layout.
Reference and sample surface coupons were placed side by side in the sliding tray at the bottom of the aerosol deposition chamber with a separation of 1 cm. The tray was designed to accommodate 20 sets of the colocated coupon pairs. A temporary spray adhesive (catalog no. SF202; J. T. Trading Corporation, Newton, CT) was applied to the bottom of the tray prior to placement of the reference and surface coupons to prevent extraneous spore reaerosolization and redeposition during sample collection.
(iii) Surface seeding.
Reference and surface sample coupons were seeded with the spore mixture by dry aerosol deposition. The spore mixture was aerosolized by the fluidized bed aerosol generator, injected into the mixing chamber, and monitored for volumetric concentration. After the volumetric concentration correlating to the desired surface loading was achieved, the mixing chamber contents were rapidly flushed into the deposition chamber, where they were mixed by circulating fans for 15 min and then allowed to settle onto the coupons for 24 h. Surface loadings in the ranges of 100 to 1,000 and 10,000 to 100,000 CFU/cm2 were evaluated for each surface type. For each surface loading and surface type, 20 sample coupons and 20 colocated reference coupons were seeded. During the surface-seeding and sample collection processes, the temperature was maintained at 25 ± 2°C and the relative humidity was maintained at 30% ±10%.
(iv) Reference collection.
Spores were collected from the reference coupons by gently misting the coupon surface with sterile, deionized water to mitigate spore reaerosolization and carefully placing the reference coupon in a prelabeled, sterile 50-ml Blue Falcon screw-top tube (Becton Dickinson Labware, Franklin Lakes, NJ) containing 30.0 ml of sterile BBT and sealing it with a cap.
(v) Surface sample collection.
By aseptic procedures, spores were collected from the sample coupons by moistening a sterile polyester-rayon blend wipe with 1.0 ml sterile, deionized water, wiping the sample surface with single horizontal strokes to the left and right, folding the exposed side to the interior, and wiping with single upward and downward strokes. After sample collection, the wipe was placed in a prelabeled, sterile 50-ml Blue Falcon screw-top tube (Becton Dickinson Labware, Franklin Lakes, NJ) containing 30.0 ml sterile BBT and sealed with a cap. Samples were collected by two researchers, each collecting half of the samples. Following sample collection, the aerosol deposition chamber was cleaned and sterilized with DF-200, a Sandia National Laboratories-developed spore-sterilizing agent (21, 22).
(vi) Extraction and enumeration.
Spores were removed from the reference coupon and extracted from the sample wipe by the process described in the section on extraction efficiency determination methods.
As recovery efficiency values are calculated relative to the reference coupon count, the effectiveness of the process of spore removal from the reference surface was also measured. The effectiveness of the sonication process for the removal of spores from the reference surface was evaluated for 24 reference coupons by seeding the coupons at approximately 200,000 CFU/cm2, sonication in BBT for 15 min, heat treatment at 65°C for 60 min, removal of the coupon from the buffer, gentle rinsing with deionized water, contact plating to brain heart infusion agar, and incubation at 37°C for 24 h. Colonies forming on the reference surface with distinct margins were counted by eye.
(vii) Calculations.
Recovery efficiency was calculated as the number of CFU from the wipe surface sample per unit of area relative to the number of CFU from the colocated reference coupon per unit of area. The mean recovery efficiency was calculated with the equation
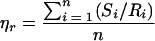 |
where ηr is the mean recovery efficiency, Si is the average sample count for three replicates, Ri is the average reference count for three replicates, i is the colocated sample and reference number, and n is the sample size. Standard deviations, standard errors, and confidence intervals were calculated by typical statistical methods for normally distributed data.
RESULTS
Extraction efficiency.
The efficiency of extraction from polyester-rayon blend wipes by the sonication method ranged from 0.803 to 1.093 with a mean extraction efficiency of 0.932 and a standard deviation (SD) of ±0.087 (n = 40). The efficiency of removal from the reference coupons was 0.999 with an SD of ±0.001 (n = 24).
Recovery efficiency.
Observed efficiencies of recovery from the stainless steel surface ranged from 0.153 to 0.674, with a mean recovery efficiency of 0.346 and an SD of ±0.122 (n = 40). Differences in recovery efficiency between low and high surface loading conditions were noted. The mean efficiency of recovery from stainless steel was 0.312 (n = 20) at low surface loading and 0.392 (n = 20) at high surface loading. However, the differences were not statistically significant at the 0.05 significance level (P = 0.125). Recovery efficiency statistical analysis data for stainless steel are presented in Table 1.
TABLE 1.
Statistics on efficiency of recovery by polyester-rayon blend wipes from stainless steel
| Surface loading (CFU/cm2) | No. of samples | Mean | Median | SD | SE | Range | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| 100-1,000 | 20 | 0.312 | 0.298 | ±0.100 | ±0.021 | 0.153-0.509 | 0.272-0.353 |
| 10,000-100,000 | 20 | 0.392 | 0.394 | ±0.138 | ±0.034 | 0.167-0.674 | 0.326-0.457 |
| Overall | 40 | 0.346 | 0.311 | ±0.122 | ±0.019 | 0.153-0.674 | 0.308-0.384 |
The range of efficiencies of recovery from the painted wallboard surface was wider at 0.081 to 0.574, producing a greater SD of ±0.152 about a mean of 0.285 (n = 40). Differences in recovery efficiency between low and high surface loading conditions were also noted. The mean efficiency of recovery from painted wallboard was 0.325 (n = 20) at low surface loading and 0.252 (n = 20) at high surface loading. However, the differences were not statistically significant at the 0.05 significance level (P = 0.497). Recovery efficiency statistical analysis data for painted wallboard are presented in Table 2.
TABLE 2.
Statistics on efficiency of recovery by polyester-rayon blend wipes from painted wallboard
| Surface loading (CFU/cm2) | No. of samples | Mean | Median | SD | SE | Range | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| 100-1,000 | 20 | 0.325 | 0.331 | ±0.155 | ±0.036 | 0.081-0.574 | 0.254-0.396 |
| 10,000-100,000 | 20 | 0.252 | 0.184 | ±0.148 | ±0.032 | 0.081-0.566 | 0.190-0.315 |
| Overall | 40 | 0.285 | 0.261 | ±0.152 | ±0.024 | 0.081-0.574 | 0.238-0.331 |
DISCUSSION
It must be reiterated that the spore material used in this study was a powdered form expressly designed to enhance aerosol suspension and inhalation characteristics, and the removal and extraction characteristics of a native spore material or spore material prepared by a different method may differ. Also, while the spore preparation contained nonspore material, there was no attempt to evaluate method efficiency in the presence of dust, bacterial vegetative cells, fungal spores, detritus, or other native background material which might interact with removal, extraction, or plating efficiency.
Efficiencies of recovery from different surfaces with similar wipe collection media have been reported in the literature (4, 5). In both studies, a relatively porous surface such as carpet or wood laminate was compared with a nonporous surface such as vinyl tile or metal. Significant differences in recovery efficiency between the surface types were noted. This difference in recovery efficiency was attributed to the porosity differential where particles are more tightly held by porous materials at the surface boundary layer. However, the surfaces evaluated in this study, stainless steel and painted wallboard, are both considered nonporous. Yet a statistically significantly (P = 0.041) lower efficiency of recovery from painted wallboard was observed. While minor differences in porosity between stainless steel and painted wallboard may contribute to the recovery efficiency differential, differences in surface textural and physiochemical adhesive properties are more likely the cause.
Efficiencies of recovery from similar surfaces with different wipe collection media have also been reported in the literature (4, 10). For natural airborne vegetative organism deposition, Kirschner and Puleo (10) reported efficiencies of recovery from stainless steel of 0.904 for polyester-bonded wipes and 0.720 for cellulose cloth wipes. The efficiency of recovery from stainless steel surfaces observed in this study of 0.346 for the polyester-rayon blend wipes, while lower than those observed in previous wipe method evaluations (4, 10), is closer to the efficiencies of recovery from stainless steel of 0.112 and 0.085 for polyester and rayon swabs, respectively, reported by Rose et al. (18). The higher recovery for wipes compared to swabs, also noted by Sanderson et al. (19), may be an artifact of the larger wipe surface area available for spore collection or possibly differences in spore-seeding methods.
Results of this study also reveal a high variability in recovery efficiency values for the wipe sample method with polyester-rayon blend collection material, as evidenced by the relatively high recovery efficiency SD values for both stainless steel (±0.123) and painted wallboard (±0.152). However, this is consistent with previous research, as Kirschner and Puleo (10) reported recovery efficiency SD values of ±0.143 and ±0.343 for polyester-bonded cloth and cellulose cloth, respectively, for deposited natural airborne organisms. Angelotti et al. (1) noted the generally low precision of swab and wipe sampling methods and suggested that these methods are not only subject to errors inherent in the sampling mechanism itself such as wipe material composition, surface composition, and mechanical removal action but are also subject to collection and processing errors. Possible sources of collection and processing error contributing to the low precision cited in the literature include the operator collection technique, such as angle and pressure applied to the surface; variations in the extraction method; and processing errors such as pipetting and counting (1, 18). Additional sources of error, specific to this study, are the potential for nonhomogeneous surface deposition of spore material resulting in unequal surface loading of reference and sample coupons and potentially incomplete removal of spores from the reference coupon.
For quantitative culture analysis, small aliquots of extraction suspension or serial dilutions are plated onto growth medium and incubated and CFU are counted. By this analytical method, the quantitative limit of detection for polyester-rayon blend wipes was calculated from recovery efficiency values.
The following parameters were used to calculate the limit of detection: (i) requirement of at least 1 CFU/ml for culture determination and (ii) 30 ml of extraction suspension. While the surface area evaluated in this study was 25 cm2, the assumption was made that the number of CFU required for detection is independent of the sample surface area and primarily a function of recovery efficiency.
If 1 CFU/ml is required in the extraction suspension for a positive culture, then 30 CFU is required in the total extraction suspension of 30 ml. With the mean recovery efficiency values and the preceding assumptions, the estimated quantitative limit of detection per unit of sample area for wipes is approximately 90 CFU for stainless steel and 105 CFU for painted wallboard surfaces. Thus, for a sample area of 1,000 cm2, a surface loading of approximately 0.1 CFU/cm2 is required to recover 1 CFU from wipe samples. If smaller surface areas are sampled, a surface loading greater than 0.1 CFU/cm2 will be required to recover 1 CFU. Additionally, while the 1-CFU/ml detection requirement proposed by Buttner et al. (5) is theoretically detectable, under actual conditions more than 1 CFU/ml may be required for reliable culture, which would increase the number of CFU required for detection. The 90- to 105-CFU detection limits also represent the method sensitivity for stainless steel and painted wallboard surfaces, respectively; that is, an additional 90 to 105 CFU per unit of sample area is required for each incremental increase in recovered CFU.
The method efficiency and limits of detection established in this work provide useful guidance for the planning of incident response environmental sampling for a spore-forming biological agent such as B. anthracis. The results of this study also provide information necessary for the interpretation of wipe environmental sample collection data; that is, positive wipe samples are indicative of high surface concentrations and may imply a potential for exposure while negative wipe samples do not ensure that organisms are absent from the surfaces sampled and may not ensure the absence of a potential for exposure. This study also emphasizes the need for well-developed and validated procedures for the collection, extraction, and analysis of biological environmental samples to provide the necessary level of confidence in information provided to public health decision makers.
Acknowledgments
Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States Department of Energy's National Nuclear Security Administration under contract DE-AC04-94AL85000. This work was supported by the Department of Homeland Security under WFO-081030926.
We thank H. D. Alan Lindquist, United States Environmental Protection Agency Homeland Security Research Center, and Captain Kenneth F. Martinez, National Institute of Occupational Safety and Health, for review and comment in support of this research effort. We also thank Lloyd Larsen of the U.S. Army Dugway Proving Ground Life Science Division for generously providing the powdered Bacillus spore material.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Angelotti, R., M. J. Foter, K. A. Busch, and K. H. Lewis. 1958. A comparative evaluation of methods for determining the bacterial contamination of surfaces. Food Res. 23:175-185. [Google Scholar]
- 2.Angelotti, R., J. L. Wilson, W. Litsky, and W. G. Walter. 1964. Comparative evaluation of the cotton swab and Rodac methods for the recovery of Bacillus subtilis spore contamination from stainless steel surfaces. Health Lab. Sci. 1:289-296. [PubMed] [Google Scholar]
- 3.Barnes, J. M. 1952. The removal of bacteria from glass surfaces with calcium alginate, gauze, and absorbent cotton wool swabs. Proc. Soc. Appl. Bacteriol. 15:34-40. [Google Scholar]
- 4.Buttner, M. P., P. Cruz, and L. D. Stetzenbach. 2001. Enhanced detection of surface-associated bacteria in indoor environments by quantitative PCR. Appl. Environ. Microbiol. 67:2564-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttner, M. P., P. Cruz, L. D. Stetzenbach, A. K. Klima-Comba, V. L. Stevens, and P. A. Emanuel. 2004. Evaluation of the biological sampling kit (BiSKit) for large-area surface sampling. Appl. Environ. Microbiol. 70:7040-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 26 April 2002, posting date. Procedures for collecting surface environmental samples for culturing B. anthracis. http://www.bt.cdc.gov/Agent/Anthrax/environmental-sampling-apr2002.asp. Accessed 6 February 2006.
- 7.Favero, M. S., J. J. McDade, J. A. Robertson, R. K. Hoffman, and R. W. Edwards. 1968. Microbiological sampling of surfaces. J. Appl. Bacteriol. 31:336-343. [DOI] [PubMed] [Google Scholar]
- 8.Favero, M. S. 1971. Microbiologic assay of space hardware. Environ. Biol. Med. 1:27-36. [PubMed] [Google Scholar]
- 9.Foerster, H. F., and J. W. Foerster. 1966. Response of Bacillus spores to combinations of germinative compounds. J. Bacteriol. 91:1168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirschner, L. E., and J. R. Puleo. 1979. Wipe-rinse techniques for quantitating microbial contamination on large surfaces. Appl. Environ. Microbiol. 38:466-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montville, T. J., R. Dengrove, T. De Siano, M. Bonnet, and D. W. Schaffner. 2005. Thermal resistance of spores from virulent strains of Bacillus anthracis and potential surrogates. J. Food. Prot. 68:2362-2366. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura, L. K. 1989. Taxonomic relationship of black-pigmented Bacillus subtilis strains and a proposal for Bacillus atrophaeus sp. nov. Int. J. Syst. Bacteriol. 39:295-300. [Google Scholar]
- 13.Petersen, N. J., D. E. Collins, and J. H. Marshall. 1973. A microbiological assay technique for hands. Health Lab. Sci. 10:18-22. [PubMed] [Google Scholar]
- 14.Preston, R. A., and H. A. Douthit. 1984. Stimulation of germination of unactivated Bacillus cereus spores by ammonia. J. Gen. Microbiol. 130:1041-1050. [DOI] [PubMed] [Google Scholar]
- 15.Puleo, J. R., M. S. Favero, and N. J. Petersen. 1967. Use of ultrasonic energy in assessing microbial contamination on surfaces. Appl. Microbiol. 15:1345-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puleo, J. R., M. S. Favero, and G. J. Tritz. 1967. Feasibility of using ultrasonics for removing viable microorganisms from surfaces. Contam. Control 6:58-67. [PubMed] [Google Scholar]
- 17.Puleo, J. R., G. S. Oxborrow, N. D. Fields, C. M. Herring, and L. S. Smith. 1973. Microbiological profiles of four Apollo spacecraft. Appl. Microbiol. 26:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose, L., B. Jensen, A. Peterson, S. N. Banerjee, and M. Arduino. 2004. Swab materials and Bacillus anthracis spore recovery from nonporous surfaces. Emerg. Infect. Dis. 10:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanderson, W. T., M. J. Hein, L. Taylor, B. D. Curwin, G. M. Kinnes, T. A. Seitz, T. Popovic, H. T. Holmes, M. E. Kellum, S. K. McAllister, D. N. Whaley, E. A. Tupin, T. Walker, J. A. Freed, D. S. Small, B. Klusaritz, and J. H. Bridges. 2002. Surface sampling methods for Bacillus anthracis endospore contamination. Emerg. Infect. Dis. 8:1145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small, D., B. Klusarwitz, P. Muller. 2001. Evaluation of Bacillus anthracis contamination inside the Brentwood Mail Processing and Distribution Center—District of Columbia, October 2001. Morb. Mortal. Wkly. Rep. 50:1129-1133. [Google Scholar]
- 21.Tucker, M. D., R. G. Betty, and M. E. Tadros. April2004. Concentrated formulations and methods for neutralizing chemical and biological toxants. U.S. patent 6,723,890.
- 22.Tucker, M. D., R. G. Betty, and M. E. Tadros. May2003. Formulations and methods for neutralizing chemical and biological toxants. U.S. patent 6,566,574.


