Abstract
We have investigated the first events that occur when exponentially grown cells are transferred from a liquid medium (Luria-Bertani [LB]) to a solid medium (LB agar [LBA]). We observed an initial lag phase of 180 min for the wild type MG1655 without any apparent growth. This lack of growth was independent of the bacterial physiological state (either the stationary or the exponential phase), the solid medium composition, or the number of cells on the plate, but it was dependent on the bacterial genotype. Using lacZ-reporter fusions and two-dimensional electrophoresis analysis, we observed that when cells from exponential-phase cultures were plated on LBA, several global regulons, like heat shock regulons (RpoH, RpoE, CpxAR) and oxidative-stress regulons (SoxRS, OxyR, Fur), were immediately induced. Our results indicate that in order to grow on plates, bacteria must not only adapt to new conditions but also perceive a real stress.
Growth and division form the foundation of standard microbiological methods for testing samples for viable bacteria, and viability is equated with culturability. By consequence, plate count remains the method of choice for obtaining a “total viable count,” i.e., the total number of bacteria capable of yielding a population discernible by the observer, usually a visible colony on the surface of a nutrient agar plate. However, it has been proposed that some readily culturable species of bacteria, when subjected to starvation or other stress (5, 6, 14, 22), may enter a long-term survival state in which they are imperceptible by culturability tests but in which they have characteristics compatible with but indirectly related to viability, like conservation of membrane integrity or metabolic activity (24). These cells have been named “viable but nonculturable” (24). It is expected that, for readily culturable species of bacteria, viable but nonculturable cells could be capable of regaining culturability, which has frequently generated a sharp debate (3, 7, 11).
With the plate count method, microbiologists have made one important assumption, that this method has no deleterious effects on bacteria. Since the 1950s, however, it has been reported that apparently dead cells can be reactivated when scavengers of reactive oxygen species, naturally produced during aerobic respiration, are added to agar plates (11, 12, 16-18, 23, 26). These cells have been named “injured cells.” Injury in bacteria is defined as an increased sensitivity to components of growth media that are not normally inhibitory (16, 23). The injured state is transient, resulting from cumulative cellular damage, and can be reversed under appropriate conditions to enable the injured cells to resume growth. For instance, various stresses, like starvation, hypochlorous acid, heat shock, and desiccation, may leave cells in a vulnerable physiological state in which atmospheric oxygen, during the recovery period, increases the toxic effect of the primary stressor, which could in part explain the phenomenon of cells being viable but nonculturable (11, 12, 16-18, 23, 26). Indeed, cells could be detected as viable cells using indirect non-culture-based methods and, when plated on agar, be killed by the switch from liquid to solid growth conditions.
The determination of culturability requires that the bacteria be transferred from one environment to another that is substantially different. This probably necessitates an adaptation by the bacteria. Indeed, several relevant parameters are drastically modified when cells are switched from a liquid to a solid rich medium (such as Luria-Bertani agar [LBA]), like (i) an increase in oxygen pressure, (ii) the presence of hydrogen peroxide on the plate (16), and (iii) increased distances between cells, indicating that bacteria fight stress as individual cells and not as a population. To our knowledge, no research to understand the physiological and genetic bacterial responses to the transfer from liquid to solid media has been performed.
The aim of this study was to understand the first events that occur when cells grown exponentially in liquid medium are transferred to a solid medium. We elaborated the “plating procedure,” allowing us to collect more than 90% of the cells deposited on the plate. We observed a lag time, which was independent of the bacterial physiological state (the stationary or the exponential phase), the solid medium composition, or the number of cells on the plate but was dependent on the bacterial genotype. Moreover, exponential-phase cells, grown and redeposited on a solid medium, still showed a lag time. Using lacZ-reporter fusions and two-dimensional (2D) electrophoresis analysis, we observed that when cells from the exponential-phase culture were plated on LBA, several global regulons, like heat shock regulons (RpoH, RpoE, CpxAR) and oxidative-stress regulons (SoxRS, OxyR), were immediately induced. Our results indicate that for bacteria to grow on plates, they need not only to adapt to new conditions but also to perceive a real stress.
MATERIALS AND METHODS
Bacterial strains and media.
All strains used are Escherichia coli K-12 derivatives (Table 1). Cultures were grown at 37°C under aeration in LB broth at a constant rotation rate of 200 rpm. Growth was monitored by measuring turbidity with a Beckman photometer.
TABLE 1.
E. coli strains used in this study
| Strain | Description | Source |
|---|---|---|
| MG1655 | F− | |
| MC4100 | F−araD139 Δ(lacLPOZA)U169 rpsL thi | |
| SD35 | MC4100 groEL-lacZ | Nyström |
| SD36 | MC4100 λΦRS88 (degP-lacZ) | Nyström |
| SD37 | MC4100 λΦRS88 (cpxP-lacZ) | Nyström |
| SD38 | MC4100 λΦRS45 (rpoH3-lacZ) | Nyström |
| SD51 | MC4100Φ(soxS-lacZ) | Touati |
| SD52 | MC4100Φ(sodA-lacZ)1 | Touati |
| SD53 | MC4100Φ(sodB-lacZ)2 | Touati |
| SD63 | MC4100Φ(sodC-lacZ) | Touati |
General methods.
Culture samples were prepared to produce extracts for resolution on 2D polyacrylamide gels according to the methods of O'Farrell (21), with modifications (30). Samples containing 20 μl of RNase A (0.5 mg/ml), 3 μl of DNase I (1 mg/ml), and cell pellets were lysed with a mini-bead beater and incubated for 10 min at 4°C. A rehydration buffer containing 7 M urea, 2 M thiourea, 4% (wt/vol) CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 100 mM dithiothreitol (DTT), 0.2% (vol/vol) Ampholyte 4-7 (Bio-Rad), and 0.01% (wt/vol) bromophenol blue was then added, and samples were incubated at room temperature for 30 min. Samples were centrifuged (18,000 × g, 10 min, 4°C), supernatant was absorbed onto 17-cm immobilized pH gradient strips (pH 4 to 7, linear) in an IPG reswelling tray, and the strips were then isoelectrically focused on a Protean isoelectric focusing cell (Bio-Rad) according to the manufacturer's instructions. Following isoelectric focusing, the strips were subjected to equilibration for 20 min in 60 mM Tris base containing 2.3% (wt/vol) sodium dodecyl sulfate (SDS), 10% (vol/vol) glycerol, 5% (vol/vol) β-mercaptoethanol, and 0.1% (wt/vol) bromophenol blue. Molecular weight separation was achieved on an 11% acrylamide gel using a Protean II XL multicell slab gel SDS-polyacrylamide gel electrophoresis system (Bio-Rad). The β-galactosidase levels were measured as described by Miller (19), with modifications (2).
Plating procedures.
Bacteria were incubated first at 37°C for 12 h in LB (overnight culture). Following overnight growth, the cells were diluted to 107 cells/ml in the same prewarmed medium (LB, 37°C, 200 rpm) and cultured to an optical density at 600 nm (OD600) of 0.5 (exponential-phase cells) or for 8 h (stationary-phase cells). Then, bacteria were diluted to 108 cells/ml, and 100 μl (107 cells) was plated onto 20 ml LBA, dried for 2 days at room temperature in order to avoid water on the surface, and warmed for 2 h at 37°C before the experiments. After different times of incubation at 37°C, the bacteria were resuspended with 1 ml of phosphate buffer (4°C, 0.05 M, pH 7). The number of bacteria collected was evaluated by plating them on LBA after serial dilutions in cold phosphate buffer. Colonies were counted after 48 h of incubation at 37°C.
RESULTS
Physiological state and initial cell concentration had no influence on lag time during plate growth condition.
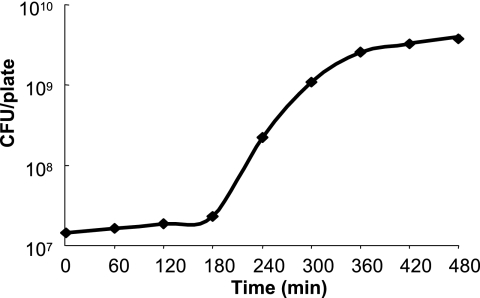
In order to follow bacterial plate growth, we expanded on the “plating procedure” (see Materials and Methods), allowing us to collect more than 90% of the cells deposited on the plates. As shown in Fig. 1, when cells were taken from the exponential growth phase on LB, we observed an initial phase of 180 min without any apparent growth, observed by several groups since 1992 (25). In the liquid growth condition, lag time is dependent on several parameters like bacterial physiological state or concentration. We wanted to know if these parameters also influenced latency on LBA. We first tested the physiological state of the wild type MG1655. As indicated in Table 2, lag time was independent of bacterial physiological state (exponential- or stationary-phase cells) for cells transferred from the liquid to the solid growth conditions. When cells were taken from the exponential and stationary phases and transferred to fresh, prewarmed (37°C) LB, lag times of 0 and 60 min, respectively, were observed. Next we tested the initial bacterial concentration (MG1655). As shown in Table 2, with concentrations ranging from 108 to 103 cells on a plate or per milliliter, the lag time was maintained at 180 min for the transfer from the liquid to the solid medium. However, the lag time for the liquid-to-liquid transfer appeared even in exponential-phase cells at 104 cells/ml (Table 2).
FIG. 1.
Bacterial growth curve on LBA. E. coli MG1655 cells were grown in LB, removed in exponential phase (OD600 = 0.5), and plated on LBA. Growth on the plate was followed as described in Materials and Methods. Experiments were repeated at least four times, and the standard deviations were always below 10%.
TABLE 2.
Influence of the initial physiological state and cell concentration on lag time during growth of E. coli cellsa
| Initial cell count/ml | Lag time (min) for growth of cells transferred from:
|
|||
|---|---|---|---|---|
| Liquid to plate
|
Liquid to liquid
|
|||
| Expo phase | Stat phase | Expo phase | Stat phase | |
| 108 | 180 | 180 | 0 | 60 |
| 107 | 180 | 180 | 0 | 60 |
| 106 | 180 | 180 | 0 | 60 |
| 105 | 180 | 180 | 0 | 60 |
| 104 | 180 | 180 | 20 | NDb |
| 103 | 180 | 180 | 20 | ND |
E. coli MG1655 cells were grown in LB, collected in the exponential (Expo) phase (OD600 = 0.5) or stationary (Stat) phase (8 h), and plated on LBA or grown on LB. Growth on plates was followed as described in Materials and Methods. Experiments were repeated at least four times, and standard deviations were always below 10%.
ND, not determined.
Taken together, these results suggest that bacterial behavior during the transfers from the liquid to the solid medium or from the liquid to the liquid medium were not the same and that bacteria might adapt to or resist environmental modifications involved in these transfers.
Plating of cells appears to be a stress for bacteria.
We wanted to determine whether our observations of bacteria described above were associated with a stress or an adaptation process. In order to answer this question, cells were grown in LBA and collected at the exponential phase (108 cells/plate) or the stationary phase (8 h of culture on a plate; 7 × 109 cells/plate) and transferred first to fresh, prewarmed (37°C) liquid LB at an initial concentration of 107 cells/ml. Results indicate that when the cells from the exponential or stationary phase of growth on an LBA plate were transferred to a prewarmed (37°C) LB at an initial concentration of 107 cells/ml, no lag time or a 60-min lag time, respectively, could be detected. More interestingly, when exponentially grown cells from an LBA plate were transferred to a prewarmed (37°C) LBA plate at an initial concentration of 107 cells per plate, a lag time of 40 min was observed. These results suggest that cells need not only to adapt to new growth conditions (LBA plate) but also to perceive a real stress, which generates a lag time.
Expression of specific stress regulons is induced when plating bacteria.
Next, we determined whether plating exponential-phase bacteria was accompanied by an induction of known stress regulons. We analyzed the evolution of β-galactosidase levels from transcriptional Φ(X-lacZ) fusions (where X represents various genes of stress regulons) to determine whether, during the phase of stress/adaptation, bacteria underwent various stress responses. These fusions are localized in strain MC4100. We first measured the lag time in this new wild-type background when exponential-phase cells were plated on LBA and obtained the lag time of only 60 min. Our results suggest that for an unexpected reason, genotype influences lag time in cell growth.
Induction of SoxRS regulon.
Because oxygen pressure changes between liquid and solid growth conditions, we first wondered whether defenses against superoxide, generated during normal aerobic growth, were induced after plating exponential-phase cells. For this purpose, we tested the induction of the SoxRS regulon involved in the defense against superoxide (29). As shown in Fig. 2A, we found that expression levels of β-galactosidase from the transcriptional Φ(sodA-lacZ) fusion under the control of SoxS (8) and the Φ(soxS-lacZ) fusion were 2.5-fold and 6-fold higher, respectively, after 60 min of plating on agar petri dishes, indicating that the SoxRS regulon is induced during the lag phase on agar plates.
FIG. 2.
Oxidative and heat shock induction after cell plating. Cells (derivatives of MC4100) were grown in LB, sampled in the exponential phase (OD600 = 0.5), and plated on LBA (see Materials and Methods). The expression level of β-galactosidase was measured as described in Materials and Methods and divided by the culturable cell number. (A) Oxidative stress induction; (B) heat shock induction. Experiments were repeated at least three times, and the standard deviations were always below 20%.
Induction of all superoxide dismutases.
As depicted in Fig. 2A, the two other superoxide dismutases, SodB regulated by the iron uptake regulon Fur (9) and SodC regulated by σS (15), were induced in the same manner. In all cases, induction reached a maximum at close to 60 min (the end of the latency time) and then declined to 1, when cells started to grow on the plate.
Induction of heat shock regulons.
Since the oxidative-stress response was induced after plating, we tested whether the heat shock response was also up-expressed. Indeed, oxidative stress will provoke the increased formation of oxidized protein, which will induce the heat shock regulon (13). As depicted in Fig. 2B, we have observed that expression of β-galactosidase from the transcriptional Φ(groEL-lacZ) fusion, under RpoH control (20), was induced fourfold during the 60 min following plating in LBA. On the other hand, levels of expression from Φ(cpxP-lacZ) and F(rpoH3-lacZ) fusions regulated by σE of the extracytoplasmic stress response (1, 10) were induced ninefold and fivefold, respectively, after 60 min following plating on LBA. Induction reached a maximum at close to 60 min (the end of the latency time) and then declined to 1, when cells started to grow, except for expression from the Φ(cpxP-lacZ) fusion, which stayed at a higher value than that of the initial exponential-phase cells collected from LB. Moreover, the expression from the transcriptional Φ(degP-lacZ) fusion, part of the CpxAR regulon, was induced 12-fold after 120 min. The elevated activities of the rpoH3, degP, and cpxP promoters suggest that cells have defects in the management of proteins in the extracytoplasmic compartments (1, 10). In all, these results suggest that all heat shock regulons, RpoH, RpoE, and CpxAR, were induced after cells were plated.
Global response analysis by 2D electrophoresis gels.
Since we observed induction of several regulons, we next analyzed the global bacterial response during the lag time on the plate. We measured the response of an exponential-phase culture of E. coli MG1655 by 2D gel analysis. Because [35S]methionine was used (de novo synthesis), we plated cells in a medium containing 2% glucose (M9). We first tested the lag time when MG1655 exponential-phase cells grown in M9 were plated in a medium which contained both 2% glucose and [35S]methionine (M9A). We obtained a lag time of 180 min, as we did with LBA. Comparative analysis by 2D gel electrophoresis (M9 versus M9A, 10 min of incorporation) revealed a considerable influence of plating in protein synthesis. Indeed, as indicated in Fig. 3, expression of more than 100 proteins changed after 10 min of incubation on the plate. Using a reference gel, we were able to identify several proteins that were up-expressed after plating, like DnaK, GroEL, AhpC, and Fur. DnaK and GroEL up-expression after plating confirmed induction of the RpoH regulon (20). Up-expression of Fur was in good agreement with induction of expression from the Φ(sodB-lacZ) fusion (4, 9). Finally, up-induction of AhpC (27), a member of the OxyR regulon (28) which is involved in hydrogen peroxide detoxification, suggests that the OxyR regulon was induced after plating cells.
FIG. 3.
Global expression monitoring by 2D gel analysis. MC4100 cells were grown in M9, sampled in the exponential phase (OD600 = 0.5), and plated on M9A (medium containing 2% glucose and [35S]methionine on the surface). After 10 min of incorporation in liquid medium (A) or on a plate (B), cells were collected and 2D analysis was performed. Experiments were repeated twice. (A) Circles indicate proteins that were repressed when cells were grown in liquid rather than solid medium; (B) circles indicate proteins that were induced when cells were grown in solid rather than liquid medium.
Taken together, transcriptional fusions and proteomic analyses indicate that several global regulons are immediately induced, like heat shock regulons (RpoH, RpoE, CpxAR) and oxidative-stress regulons (Fur, SoxRS, OxyR), after cells are plated in LBA, suggesting that cells perceive a real stress.
DISCUSSION
Bacteria have developed several complex mechanisms, with a considerable degree of overlap, to allow them to cope with environmental modifications. Probably the most common modifications which occur during the life of bacteria in the laboratory are the switches between liquid and solid growth conditions. However, to our knowledge, bacterial adaptation to the new environment has not yet been studied. In this work, we show that in order to grow on plates, bacteria taken in the exponential phase need to not only adapt to new conditions but also perceive a real stress.
When cells grown in LB were transferred to LBA, an initial phase without any apparent growth was observed. We found that this lag time was not influenced by the initial physiological state of the bacteria (exponential- or stationary-phase cells), by the initial cell concentration on the plate, or by the solid medium composition. In contrast, all these parameters influenced bacterial lag time when cells grown in LB were switched to fresh LB. The only parameter that was determined to modify lag time on the plate was the bacterial genotype. Although they may suggest that lag time was genetically controlled, these results were unexpected, and the reasons were unclear.
The presence of a lag time may suggest that bacteria perceive a stress and/or need to adapt to these new environmental conditions. This adaptation probably involves the modification of the expression of a large number of genes for the bacteria to grow on the plates. As a result, when these modifications occurred, cells adapted to the new conditions. Thus, it was expected that cells taken in the exponential phase from the plate (LBA) and plated on fresh LBA would start to grow directly. However, this was not the case, and 40 min of lag time (for two generations) was observed. These results suggest that bacteria perceive a real stress. Several origins of this stress may be postulated, like (i) the potential presence of a low level of hydrogen peroxide on the LBA plate (16) or (ii) an increase in oxygen pressure when moving from the liquid to the solid medium.
In order to analyze stress perceived by exponentially grown E. coli cells, we tried to identify the bacterial response to plating conditions. Using gene fusions and 2D electrophoresis analysis, we have shown the induction of global stress regulons involved in protection against oxidative stress. Our results suggest the presence of an endogenous oxidative stress generated by aerobic respiration rather than an effect of the potential presence of a low level of hydrogen peroxide. Indeed, although no hydrogen peroxide is present in M9A due to the absence of organic matter, which is able to generate hydrogen peroxide (16), we observed by 2D electrophoresis analysis the induction of OxyR (involved in defense against hydrogen peroxide), Fur (iron uptake), and the heat shock regulon during lag time on the M9A plate. Moreover, the presence of hydrogen peroxide cannot explain the induction of the SoxRS regulon (involved in defense against superoxide anion) observed by several β-galactosidase fusions during the lag time on the LBA plate. One possible explanation for this oxidative stress could be the imbalance between reactive oxygen species (ROS) production due to aerobic respiration and cell defenses against these ROS. A higher ROS production rate will provoke induction of the SoxRS and OxyR regulons and a global heat shock (20, 28, 29). Moreover, fur, which was regulated by OxyR and SoxS, will also be induced as a consequence (31). Finally, induction of RpoE and CpxAR, which deal with unfolded periplasmic or membrane proteins caused by heat shock or environmental stresses (1, 10), also suggested periplasmic and membrane alteration.
In this study, we have shown that cells transferred to LBA from LB are associated with oxidative stress. This stress might have deleterious effects on cells and could explain in part the phenomenon of viable but nonculturable cells.
Acknowledgments
We thank M. Chippaux, D. Brynes, and P. Moreau, Laboratoire de Chimie Bactérienne, Marseille, France, for carefully reading the manuscript. We thank D. Touati and T. Nyström for generous gifts of strains.
This work was supported by ACI Jeunes Chercheurs. C.C. was a recipient of a fellowship from the Ministère de l'Education Nationale.
Footnotes
Published ahead of print on 1 December 2006.
REFERENCES
- 1.Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 52:613-619. [DOI] [PubMed] [Google Scholar]
- 2.Albertson, N. H., and T. Nystrom. 1994. Effects of starvation for exogenous carbon on functional mRNA stability and rate of peptide chain elongation in Escherichia coli. FEMS Microbiol. Lett. 117:181-187. [DOI] [PubMed] [Google Scholar]
- 3.Bogosian, G., and E. V. Bourneuf. 2001. A matter of bacterial life and death. EMBO Rep. 2:770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, V. 2003. Iron uptake by Escherichia coli. Front. Biosci. 8:s1409-s1421. [DOI] [PubMed] [Google Scholar]
- 5.Cappelier, J. M., J. Minet, C. Magras, R. R. Colwell, and M. Federighi. 1999. Recovery in embryonated eggs of viable but nonculturable Campylobacter jejuni cells and maintenance of ability to adhere to HeLa cells after resuscitation. Appl. Environ. Microbiol. 65:5154-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaveerach, P., A. A. H. M. ter Huurne, L. J. A. Lipman, and F. van Knapen. 2003. Survival and resuscitation of ten strains of Campylobacter jejuni and Campylobacter coli under acid conditions. Appl. Environ. Microbiol. 69:711-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colwell, R. R., and D. J. Grimes (ed.). 2000. Nonculturable microorganisms in the environment. American Society for Microbiology, Washington, DC.
- 8.Compan, I., and D. Touati. 1993. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J. Bacteriol. 175:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubrac, S., and D. Touati. 2002. Fur-mediated transcriptional and post-transcriptional regulation of FeSOD expression in Escherichia coli. Microbiology 148:147-156. [DOI] [PubMed] [Google Scholar]
- 10.Duguay, A. R., and T. J. Silhavy. 2004. Quality control in the bacterial periplasm. Biochim. Biophys. Acta 1694:121-134. [DOI] [PubMed] [Google Scholar]
- 11.Dukan, S., S. Belkin, and D. Touati. 1999. Reactive oxygen species are partially involved in the bacteriocidal action of hypochlorous acid. Arch. Biochem. Biophys. 367:311-316. [DOI] [PubMed] [Google Scholar]
- 12.Dukan, S., and T. Nystrom. 1999. Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J. Biol. Chem. 274:26027-26032. [DOI] [PubMed] [Google Scholar]
- 13.Fredriksson, A., M. Ballesteros, S. Dukan, and T. Nystrom. 2006. Induction of the heat shock regulon in response to increased mistranslation requires oxidative modification of the malformed proteins. Mol. Microbiol. 59:350-359. [DOI] [PubMed] [Google Scholar]
- 14.Kell, D. B., A. S. Kaprelyants, D. H. Weichart, C. R. Harwood, and M. R. Barer. 1998. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Leeuwenhoek 73:169-187. [DOI] [PubMed] [Google Scholar]
- 15.Lacour, S., and P. Landini. 2004. σS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of σS-dependent genes and identification of their promoter sequences. J. Bacteriol. 186:7186-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackey, B. M., and D. A. Seymour. 1987. The effect of catalase on recovery of heat-injured DNA-repair mutants of Escherichia coli. J. Gen. Microbiol. 133:1601-1610. [DOI] [PubMed] [Google Scholar]
- 17.Marthi, B., B. T. Shaffer, B. Lighthart, and L. Ganio. 1991. Resuscitation effects of catalase on airborne bacteria. Appl. Environ. Microbiol. 57:2775-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald, L. C., C. R. Hackney, and B. Ray. 1983. Enhanced recovery of injured Escherichia coli by compounds that degrade hydrogen peroxide or block its formation. Appl. Environ. Microbiol. 45:360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 20.Neidhardt, F. C., and R. A. VanBogelen. 1987. Heat shock response, p. 1334-1345. In F. C. Neidhardt, J. I. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, DC.
- 21.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 22.Ohtomo, R., and M. Saito. 2001. Increase in the culturable cell number of Escherichia coli during recovery from saline stress: possible implication for resuscitation from the VBNC state. Microb. Ecol. 42:208-214. [DOI] [PubMed] [Google Scholar]
- 23.Ray, B., J. J. Jezeski, and F. F. Busta. 1971. Effect of rehydration on recovery, repair, and growth of injured freeze-dried Salmonella anatum. Appl. Microbiol. 22:184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roszak, D. B., and R. R. Colwell. 1987. Metabolic activity of bacterial cells enumerated by direct viable count. Appl. Environ. Microbiol. 53:2889-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro, J. A. 1992. Pattern and control in bacterial colony development. Sci. Prog. 76:399-424. [PubMed] [Google Scholar]
- 26.Speck, M. L., B. Ray, and R. B. Read, Jr. 1975. Repair and enumeration of injured coliforms by a plating procedure. Appl. Microbiol. 29:549-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storz, G., F. S. Jacobson, L. A. Tartaglia, R. W. Morgan, L. A. Silveira, and B. N. Ames. 1989. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J. Bacteriol. 171:2049-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tartaglia, L. A., G. Storz, and B. N. Ames. 1989. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J. Mol. Biol. 210:709-719. [DOI] [PubMed] [Google Scholar]
- 29.Touati, D. 2000. Sensing and protecting against superoxide stress in Escherichia coli—how many ways are there to trigger soxRS response? Redox Rep. 5:287-293. [DOI] [PubMed] [Google Scholar]
- 30.VanBogelen, R. A., and F. C. Neidhardt. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:5589-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]