Abstract
In alkaline conditions, Listeria monocytogenes cells develop higher proportions of branched-chain fatty acids (FAs), including more anteiso forms. In acid conditions, the opposite occurs. Reduced growth of pH-sensitive mutants at adverse pH (5.0/9.0) was alleviated by the addition of 2-methylbutyrate (an anteiso-FA precursor), suggesting that anteiso-FAs are important in adaptation to adverse pH. The balance between anteiso- and iso-FAs may be more important than changes in the amounts and/or degrees of saturation of FAs in pH adaptation.
Listeria monocytogenes can grow under a wide range of pH stress, i.e., 4.1 to 9.0 (38, 46), increasing its abilities to persist during food processing and attempts to decontaminate food-processing environments (15, 42-45). It also has particularly impressive capacities to modulate its membrane lipids to maintain membrane fluidity and transport functions (10, 40, 41) in response to temperature (1), salt (7), and CO2/anaerobiotic (21) stress. Such capacities have been suggested to be related to its atypically high iso and anteiso, odd-numbered, branched-chain fatty acid (BCFA) content (1, 20) and its ability to modulate the overall content and proportions of BCFAs, straight-chain FAs (SCFAs), and unsaturated FAs (22, 23). For example, reductions in environmental temperatures lead to increases in the amount of ai15:0 present in L. monocytogenes cell membranes, while increases in environmental temperatures lead to reductions in the amounts of ai15:0 and other BCFAs present in membranes (1, 14, 32).
Changes in FA profile have been associated with pH adaptation in Streptococcus mutans (16-18), Escherichia coli (5, 48), and Salmonella (24), Pseudomonas (31), and Bacillus species (23). However, little is known about pH stress-associated modulation of FAs in L. monocytogenes (21), the wider role of FA modulation in its responses to nonthermal stresses, or the cross-protection mechanisms which operate in this hardy pathogen (19, 27, 35, 44).
The aims of this study were to investigate the modulation of the FA profile of L. monocytogenes membranes in response to changes in environmental pH, investigate the effects of an exogenous BCFA precursor on the pH stress response of BCFA-deficient mutants (1, 49), and examine possible links between the prevalence of anteiso-BCFAs and the adaptation mechanism(s) of L. monocytogenes under adverse pH conditions.
Modified brain heart infusion broth (MBHIB; Difco Laboratories, Sparks, MD), suitable for adverse-pH studies, was prepared to pH 5.0, 5.5, and 6.0 in 2 M disodium phosphate (Sigma Chemical Company, St. Louis, MO) and 0.1 M citric acid (Fisher Scientific, Fair Lawn, NJ) buffer or to pH 7.0, 8.0, 8.5, and 9.0 in 0.1 M solutions of Trizma-hydrochloride and Trizma-base (Sigma) buffer (6). When required, cultures were supplemented with filter-sterilized 100 μM 2-methylbutyric acid (2MBA).
Washed cells from mid-exponential-phase cultures of L. monocytogenes 10403S, an isogenic sigB null mutant (3), and isogenic non-BCFA-producing cld-1 and cld-2 mutants (1, 49) were inoculated into preheated (30°C) 100-ml volumes of the buffered MBHIB and grown (30°C/200 rpm) to an optical density at 600 nm of 0.5 to 0.6. Growth rates of cultures (doubling times per hour of cultures in exponential growth) were calculated (2). Mid-exponential-phase cells were recovered by centrifugation at 8,000 × g for 10 min at 4°C and washed three times with distilled water.
The FAs in washed-cell pellets were saponified, methylated, and extracted as described previously (1, 49, 50). Methyl ester mixtures were separated using an Agilent 5890 dual-tower gas chromatograph with split/splitless injector, flame ionization detector, 25-m by 0.2-mm Ultra 2 capillary column (Hewlett-Packard), and automatic sampler/integrator and analyzed using an FA identification program (MIDI; Sherlock 4.5 Microbial Identification System). Carrier gas (hydrogen) flow was 80 ml/min. The injector and temperatures were maintained at 250 and 300°C, respectively. Samples (2 ml) were injected into the split mode (ratio, 5:3), and the column temperature was ramped from 170 to 270°C at 5°C/min. Individual FAs comprising less than 1% of the FA content were ignored. FA determination was conducted at Microbial ID Inc. (Newark, DE).
All results represent the average means from three independent experiments. Student's t test was used to make pairwise comparisons between the acid- and alkaline-adapted cultures and the corresponding controls for each condition tested. The confidence interval for a difference in the mean was set at 95% (P ≤ 0.05) for all comparisons.
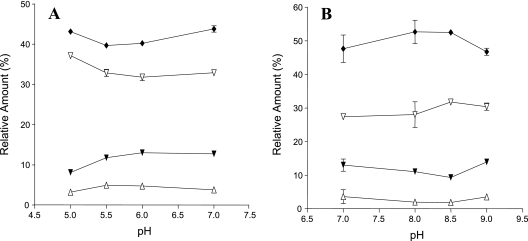
The study established that all samples in all pH conditions contained ai15:0 > ai17:0 > i15:0 > i17:0. Incubation at different pH values induced characteristic and consistent changes in the relative proportions of the above-mentioned major FAs (Table 1); i.e., higher-pH cultures had higher proportions of BCFAs and lower-pH cultures had lower proportions of BCFAs. Figure 1 presents the relative amounts of individual major BCFAs (i15:0, ai15:0, i17:0, and ai17:0) in cells grown at different pH values. Percentages of ai15:0 ranged from 48% (at pH 7.0) to 53% (at pH 8.0 and 8.5) to 47% (at pH 9.0) (Fig. 1A), a pattern also observed for the other three BCFAs examined (Fig. 1B). Cultures grown at pH 8.0 and pH 8.5 (but not at pH 9.0) had higher anteiso/iso ratios than those in control (pH 7.0) samples (Table 1).
TABLE 1.
Effects of growth pH on the total fatty acid composition of L. monocytogenes 10403S
| Growth pH | Buffer | % Total fatty acids (mean ± SD)a
|
ACCLc (mean ± SD) | ||||
|---|---|---|---|---|---|---|---|
| Anteiso | Iso | Anteiso/iso | Straight | Branched | |||
| 7.0 | Tris | 75.16 ± 3.63 | 20.2 ± 4.72 | 3.86 ± 0.95 | 2.85 ± 0.14 | 95.37 ± 1.09 | 15.46 ± 0.16 |
| 8.0 | Tris | 80.77b ± 0.26 | 14.8b ± 0.59 | 5.44b ± 0.23 | 2.04b ± 0.21 | 95.64 ± 0.35 | 15.39 ± 0.08 |
| 8.5 | Tris | 84.30b ± 0.39 | 12.6b ± 0.43 | 6.69b ± 0.22 | 1.55b ± 0.33 | 96.91b ± 0.66 | 15.52 ± 0.01 |
| 9.0 | Tris | 77.17 ± 0.41 | 20.8 ± 0.58 | 3.70 ± 0.12 | 1.38b ± 0.07 | 98.04b ± 0.16 | 15.64 ± 0.03 |
| 7.0 | Phosphate | 76.69 ± 0.39 | 21.0 ± 0.36 | 3.62 ± 0.06 | 2.13 ± 0.61 | 97.86 ± 0.61 | 15.77 ± 0.02 |
| 6.0 | Phosphate | 71.97b ± 1.18 | 25b ± 1.29 | 2.93b ± 0.19 | 3.22b ± 0.48 | 96.56 ± 0.58 | 15.75 ± 0.02 |
| 5.5 | Phosphate | 72.55b ± 0.74 | 24b ± 0.58 | 3.08b ± 0.10 | 3.44b ± 0.16 | 96.06b ± 0.15 | 15.73 ± 0.03 |
| 5.0 | Phosphate | 80.24b ± 0.68 | 16b ± 0.12 | 5.10b ± 0.08 | 3.02 ± 0.37 | 95.97 ± 0.55 | 15.68 ± 0.04 |
Values are from three independent experiments.
Statistically different (P < 0.05) from the control cultures (pH 7.0).
ACCL, average carbon chain length, given by the equation [Σ(FAP × C)]/100, where FAP is the percentage of each fatty acid and C is the number of carbon atoms in the chain.
FIG. 1.
Effect of growth pH on fatty acid composition of L. monocytogenes 10403S cells grown in BHI broth at acid (A) and alkaline (B) pHs. The data represent the means ± standard deviations of three independent experiments. ▾, iso15:0; ⧫, anteiso15:0; ▵, iso17:0; ▿, anteiso17:0.
FAs in cells grown at pH 5.0 contained significantly higher proportions of ai17:0 (5%) and significantly lower proportions of i15:0 than FAs from pH 7.0 (control) cells (Fig. 1A). This pattern was also observed in relation to total anteiso-FAs; i.e., they were in significantly lower concentrations at pH 6.0 and 5.5. At pH 5.0, the total anteiso-FA content was significantly higher than the control values, and the total iso-FA content was significantly lower (P < 0.05) (Table 1). There were no significant differences (P > 0.05) among the average lengths (or degrees of saturation) of test and control samples at all pH values examined. There were no significant differences between the results for L. monocytogenes 10403S and the isogenic sigB null mutant (data not shown).
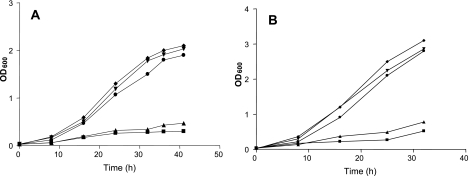
In the absence of 2MBA, BCFA-deficient mutants (cld-1 and cld-2 mutants) grew significantly more slowly (0.28 h−1 and 0.20 h−1 at pH 9.0 and 0.21 h−1 and 0.26 h−1 at pH 5.0, respectively) than the parent strain (0.38 h−1 at pH 9.0 and 0.47 h−1 at pH 5.0) (Fig. 2A and B). In the presence of 2MBA, the growth rates of these BCFA-deficient mutants were almost identical with those of the parent strain.
FIG. 2.
Influence of 2-methylbutyric acid on growth of the cld mutants at pH 5.0 (A) and pH 9.0 (B). Cultures were grown at 30°C in the absence and presence of 2MBA in MBHIB. OD600, optical density at 600 nm; ⧫, 10403S; ▪, cld-1 mutant; •, cld-1 mutant in the presence of 2MBA; ▴, cld-2 mutant; ▾, cld-2 mutant in the presence of 2MBA.
In the absence of 2MBA at pH 5 or 9, the proportions of anteiso-C17:0 and anteiso-C15:0 content were lower (Table 2) (and the proportions of iso-C14:0, C14:0, iso-C16:0, and C16:0 higher [results not shown]) than in the parent strain. In the presence of 2MBA, the proportions of anteiso-C17:0 and anteiso-C15:0 were significantly higher than in the absence of 2MBA. In the presence of 2MBA, the proportions of SCFAs were significantly lower (and the proportions of BCFAs were significantly higher) than in the absence of 2MBA.
TABLE 2.
Fatty acid compositions of parent strain 10403S and cld-1 and cld-2 Bkd mutants in BHIB with or without 2-methylbutyrate at 30°Ca
| Strain and growth conditions | % Total fatty acidsb
|
ACCLd | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| i15:0 | ai15:0 | i17:0 | ai17:0 | Ante | Iso | Ante/iso | Straight- chain | Branched- chain | ||
| 10403S; pH 9.0 | 13.94 | 46.73 | 3.53 | 30.4 | 77.17 | 20.87 | 3.70 | 1.463 | 98.04 | 15.680 |
| cld-1; pH 9.0 | 8.10c | 27.17c | 5.36c | 9.11c | 39.33c | 29.81c | 1.32c | 30.85c | 69.14c | 16.279 |
| clc-1; pH 9.0, 2MBA | 1.26c | 40.84 | NDc | 25.84 | 66.68 | 15.45 | 4.32 | 17.88c | 82.13c | 15.702 |
| cld-2; pH 9.0 | 3.63c | 17.55c | 1.40c | 7.75c | 25.30c | 29.74c | 0.85c | 44.98c | 55.04c | 15.454 |
| cld-2; pH 9.0, 2MBA | 1.22 | 39.54 | NDc | 26.84 | 66.38 | 16.19 | 4.10 | 17.36c | 82.57c | 15.712 |
| 10403S; pH 5.0 | 8.11 | 43.12 | 3.18 | 37.12 | 80.24 | 16.00 | 5.10 | 3.02 | 95.97 | 15.68 |
| cld-2; pH 5.0 | 4.12c | 15.66c | NDc | 5.70c | 21.36c | 35.52c | 0.60c | 43.12c | 56.88c | 15.285 |
| cld-2; pH 5.0, 2MBA | 1.33c | 40.03 | NDc | 33.02 | 73.05 | 10.41c | 7.01 | 16.53c | 83.46 | 15.759 |
| cld-2; pH 5.0 | 3.24c | 15.57c | 0.93c | 5.30c | 20.87c | 35.91c | 0.58c | 43.22c | 56.78c | 15.307 |
| cld-2; pH 5.0, 2MBA | 1.79c | 39.12 | NDc | 35.25 | 74.37 | 13.52c | 5.50 | 12.10c | 87.89 | 15.765 |
Minor fatty acid components are not included in this table.
Values are from three independent experiments. ND, not detected.
Statistically different (P < 0.05) from the control cultures (10403S; pH 9.0 and pH 5.0)
ACCL, average carbon chain length, given by the equation [Σ(FAP × C)]/100, where FAP is the percentage of each fatty acid and C is the number of carbon atoms in the chain.
This study observed that L. monocytogenes 10403S exhibited qualitatively and quantitatively different membrane FA contents at different pH values. Growth at pH 8.0 or 8.5 resulted in higher proportions of BCFAs, especially anteiso forms, changes that have been suggested to increase membrane fluidity (41) and limit alkali and detergent damage (30, 33, 34). Increased BCFA content has been associated with alkali tolerance in alkalophilic species (9, 47). Growth at pH 5.5 or 6.0 resulted in higher proportions of SCFAs. This is different from the patterns of change in other organisms, where such conditions induce increases in monounsaturated long-chain FAs (5, 16-18) and alterations in cyclopropane FA content (11, 39).
pH stress did not induce gross changes in the total amounts of unsaturated FAs. This is interesting because pH stress induces considerable changes in total unsaturated FA content in other bacteria, and L. monocytogenes does make such changes in response to other environmental stresses (4, 13, 25, 26, 29, 32, 49). The absence of significant differences between the responses of L. monocytogenes 10403S and the isogenic sigB null mutant suggests that sigB does not have a major role in pH-induced fatty acid modulation in L. monocytogenes.
The study observed clear and different patterns in L. monocytogenes responses to moderate pH stress (pH 5.5, 6.0, 8.0, or 8.5), although these patterns were not observed at the most extreme pH values studied (i.e., pH 5.0 and pH 9.0). Such discontinuity may reflect a general disruption of membrane fluidity as conditions move beyond the range of compensation of FA modulation-based homeostasis to a state where the imperative is to maintain cytoplasmic pH. Alternatively, more-extreme stress may activate one or more additional “extreme” stress responses, redirecting cellular investment away from moderate stress adaptation mechanisms to more drastic emergency responses.
Shifts between moderate and extreme stress responses have been associated with shifts between metabolic pathways and changes in the concentrations of key enzymes such as β-ketoacyl-acyl carrier protein synthases (32). In L. monocytogenes (49, 50) (and also in Bacillus subtilis [12]), such enzymes and their products have vital roles in membrane adaptation to high- and low-temperature stress. BCFA synthesis involves the transamination of branched-chain amino acids such as isoleucine, valine, and leucine by a branched-chain amino acid transaminase (BcaT) (12, 23) and subsequent oxidative decarboxylation by the branched-chain α-keto acid dehydrogenase (Bkd) (8, 28, 36, 37). Thus, these two enzymes, BcaT and Bkd, are critical for BCFA biosynthesis in L. monocytogenes and represent a possible mechanism for stress regulation and modification of FA profiles in this pathogen (8, 28, 36, 37).
The differences between the growth rates of BCFA-deficient mutants and the BCFA-competent parent strain under pH stress and the resolution of such differences by the provision of exogenous 2MBA, bypassing the branched-chain α-keto acid dehydrogenase step in the biosynthesis of BCFA (49, 50), demonstrated the close correlation between membrane BCFA content and the ability of L. monocytogenes to grow under such adverse environmental conditions.
The results of this study suggest that L. monocytogenes uses subtle manipulation of BCFA content, and of the relative proportions of anteiso and iso FAs, as a very sensitive and effective means of adaptation to mild or moderate pH stress.
Acknowledgments
We thank particularly Kun Zhu and Mudcharee Julotok for their technical advice and practical assistance.
This study took place at the Illinois State University in Normal, IL, during a research visit by Efstathios Giotis, partly funded by the European Society of Clinical Microbiology and Infectious Diseases (ECCMID). The work was partly supported by USDA National Research Initiative competitive grants (2002-35201-12791 and 2006-35201-17386) to B. J. Wilkinson. Efstathios Giotis is supported by a University of Ulster Vice-Chancellor's Ph.D. research scholarship.
Footnotes
Published ahead of print on 17 November 2006.
REFERENCES
- 1.Annous, B. A., L. A. Becker, D. O. Bayles, D. P. Labeda, and B. J. Wilkinson. 1997. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 3.Becker, L. A., S. N. Evans, R. W. Hutkins, and A. K. Benson. 2000. Role of σB in adaptation of Listeria monocytogenes to growth at low temperature. J. Bacteriol. 182:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower, C. K., and M. A. Daeschel. 1999. Resistance responses of microorganisms in food environments. Int. J. Food Microbiol. 50:33-44. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J. L., T. Ross, T. A. McMeekin, and P. D. Nichols. 1997. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 37:163-173. [DOI] [PubMed] [Google Scholar]
- 6.Butler, T., R. W. Frenck, R. B. Johnson, and R. Khakhria. 2001. In vitro effects of azithromycin on Salmonella typhi: early inhibition by concentrations less than the MIC and reduction of MIC by alkaline pH and small inocula. J. Antimicrob. Chemother. 47:455-458. [DOI] [PubMed] [Google Scholar]
- 7.Chihib, N. E., M. Ribeiro da Silva, G. Delattre, M. Laroche, and M. Federighi. 2003. Different cellular fatty acid pattern behaviours of two strains of Listeria monocytogenes Scott A and CNL 895807 under different temperature and salinity conditions. FEMS Microbiol. Lett. 218:155-160. [DOI] [PubMed] [Google Scholar]
- 8.Choi, K. H., R. J. Heath, and C. O. Rock. 2000. Beta-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 182:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clejan, S., T. A. Krulwich, K. R. Mondrus, and D. Setoyoung. 1986. Membrane lipid composition of obligately and facultatively alkalophilic strains of Bacillus spp. J. Bacteriol. 168:334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronan, J. E. 2003. Bacterial membrane lipids: where do we stand? Annu. Rev. Microbiol. 57:203-224. [DOI] [PubMed] [Google Scholar]
- 11.Deinhard, G., J. Saar, W. Krischke, and K. Poralla. 1987. Bacillus cycloheptanicus sp-Nov, a new thermoacidophile containing omega-cycloheptane fatty acids. Syst. Appl. Microbiol. 10:68-73. [Google Scholar]
- 12.De Mendoza, D., G. E. Schujman, and P. S. Aguilar. 2002. Biosynthesis and function of membrane lipids, p. 43-55. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, DC.
- 13.Dubois-Brissonnet, F., C. Malgrange, L. Guerin-Mechin, B. Heyd, and J. Y. Leveau. 2001. Changes in fatty acid composition of Pseudomonas aeruginosa ATCC 15442 induced by growth conditions: consequences of resistance to quaternary ammonium compounds. Microbios 106:97-110. [PubMed] [Google Scholar]
- 14.Edgcomb, M. R., S. Sirimanne, B. J. Wilkinson, P. Drouin, and R. Morse. 2000. Electron paramagnetic resonance studies of the membrane fluidity of the foodborne pathogenic psychrotroph Listeria monocytogenes. Biochim. Biophys. Acta 1463:31-42. [DOI] [PubMed] [Google Scholar]
- 15.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a foodborne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fozo, E. M., and R. G. Quivey, Jr. 2004. Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl. Environ. Microbiol. 70:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fozo, E. M., J. K. Kajfasz, and R. G. Quivey. 2004. Low pH-induced membrane fatty acid alterations in oral bacteria. FEMS Microbiol. Lett. 238:291-295. [DOI] [PubMed] [Google Scholar]
- 18.Fozo, E. M., and R. G. Quivey, Jr. 2004. The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. J. Bacteriol. 186:4152-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gahan, C. G. M., B. O'Driscoll, and C. Hill. 1996. Acid adaptation of Listeria monocytogenes can enhance survival in acidic foods and during milk fermentation. Appl. Environ. Microbiol. 62:3128-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julak, J., M. Ryska, I. Koruna, and E. Mencikova. 1989. Cellular fatty acids and fatty aldehydes of Listeria and Erysipelothrix. Zentbl. Bakteriol. Int. J. Med. Microbiol. 272:171-180. [DOI] [PubMed] [Google Scholar]
- 21.Jydegaard-Axelsen, A.-M., P. E. Høiby, K. Holmstrøm, N. Russell, and S. Knøchel. 2004. CO2- and anaerobiosis-induced changes in physiology and gene expression of different Listeria monocytogenes strains. Appl. Environ. Microbiol. 70:4111-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneda, T. 1977. Fatty acids of genus Bacillus: an example of branched-chain preference. Bacteriol. Rev. 41:391-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneda, T. 1991. Iso-fatty and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55:288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, B. H., S. Kim, H. G. Kim, J. Lee, I. S. Lee, and Y. K. Park. 2005. The formation of cyclopropane fatty acids in Salmonella enterica serovar typhimurium. Microbiology 151:209-218. [DOI] [PubMed] [Google Scholar]
- 25.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, J., M. L. Chikindas, R. D. Ludescher, and T. J. Montville. 2002. Temperature- and surfactant-induced membrane modifications that alter Listeria monocytogenes nisin sensitivity by different mechanisms. Appl. Environ. Microbiol. 68:5904-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lou, Y. Q., and A. E. Yousef. 1997. Adaptation to sublethal environmental stresses protects Listeria monocytogenes against lethal preservation factors. Appl. Environ. Microbiol. 63:1252-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, Y. J., Y. M. Zhang, and C. O. Rock. 2004. Product diversity and regulation of type II fatty acid synthases. Biochem. Cell Biol. 82:145-155. [DOI] [PubMed] [Google Scholar]
- 29.Mastronicolis, S. K., A. Boura, A. Karaliota, P. Magiatis, N. Arvanitis, C. Litos, A. Tsakirakis, P. Paraskevas, H. Moustaka, and G. Heropoulos. 2006. Effect of cold temperature on the composition of different lipid classes of the foodborne pathogen Listeria monocytogenes: focus on neutral lipids. Food Microbiol. 23:184-194. [DOI] [PubMed] [Google Scholar]
- 30.Mendonca, A. F., T. L. Amoroso, and S. J. Knabel. 1994. Destruction of gram-negative food-borne pathogens by high pH involves disruption of the cytoplasmic membrane. Appl. Environ. Microbiol. 60:4009-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann, G., N. Kabelitz, and H. J. Heipieper. 2003. The regulation of the cis-trans isomerase of unsaturated fatty acids in Pseudomonas putida: correlation between cti activity and K+ uptake systems. Eur. J. Lipid Sci. Technol. 105:585-589. [Google Scholar]
- 32.Nichols, D. S., K. A. Presser, J. Olley, T. Ross, and T. A. McMeekin. 2002. Variation of branched-chain fatty acids marks the normal physiological range for growth in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2809-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickerson, K. W., and L. A. Bulla. 1980. Incorporation of specific fatty acid precursors during spore germination and outgrowth in Bacillus thuringiensis. Appl. Environ. Microbiol. 40:166-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen, L. E., D. R. Kadavy, S. Rajagopal, R. Drijber, and K. W. Nickerson. 2005. Survey of extreme solvent tolerance in gram-positive cocci: membrane fatty acid changes in Staphylococcus haemolyticus grown in toluene. Appl. Environ. Microbiol. 71:5171-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Driscoll, B., C. G. M. Gahan, and C. Hill. 1996. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl. Environ. Microbiol. 62:1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oku, H., K. Fujita, T. Nomoto, K. Suzuki, H. Iwasaki, and I. Chinen. 1998. NADH-dependent inhibition of branched-chain fatty acid synthesis in Bacillus subtilis. Biosci. Biotechnol. Biochem. 62:622-627. [DOI] [PubMed] [Google Scholar]
- 37.Oku, H., and T. Kaneda. 1988. Biosynthesis of branched-chain fatty acids in Bacillus subtilis. a decarboxylase is essential for branched-chain fatty acid synthetase. J. Biol. Chem. 263:18386-18396. [PubMed] [Google Scholar]
- 38.Pearson, L. J., and E. H. Marth. 1990. Listeria monocytogenes—threat to a safe food supply: a review. J. Dairy Sci. 73:912-928. [DOI] [PubMed] [Google Scholar]
- 39.Poralla, K., and W. A. Konig. 1983. The occurrence of omega-cycloheptane fatty acids in a thermo-acidophilic Bacillus. FEMS Microbiol. Lett. 16:303-306. [Google Scholar]
- 40.Russell, N. J. 2002. Bacterial membranes: the effects of chill storage and food processing. An overview. Int. J. Food Microbiol. 79:27-34. [DOI] [PubMed] [Google Scholar]
- 41.Russell, N. J., R. I. Evans, P. F. Steeg, J. Hellemons, A. Verheul, and T. Abee. 1995. Membranes as a target for stress adaptation. Int. J. Food Microbiol. 28:255-261. [DOI] [PubMed] [Google Scholar]
- 42.Sharma, M., P. J. Taormina, and L. R. Beuchat. 2003. Habituation of foodborne pathogens exposed to extreme pH conditions: genetic basis and implications in foods and food processing environments. Food Sci. Technol. Res. 9:115-127. [Google Scholar]
- 43.Taormina, P. J., and L. R. Beuchat. 2002. Survival and growth of alkali-stressed Listeria monocytogenes on beef frankfurters and thermotolerance in frankfurter exudates. J. Food Prot. 65:291-298. [DOI] [PubMed] [Google Scholar]
- 44.Taormina, P. J., and L. R. Beuchat. 2001. Survival and heat resistance of Listeria monocytogenes after exposure to alkali and chlorine. Appl. Environ. Microbiol. 67:2555-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taormina, P. J., and L. R. Beuchat. 2002. Survival of Listeria monocytogenes in commercial food-processing equipment cleaning solutions and subsequent sensitivity to sanitizers and heat. J. Appl. Microbiol. 92:71-80. [DOI] [PubMed] [Google Scholar]
- 46.Tienungoon, S., D. A. Ratkowsky, T. A. McMeekin, and T. Ross. 2000. Growth limits of Listeria monocytogenes as a function of temperature, pH, NaCl, and lactic acid. Appl. Environ. Microbiol. 66:4979-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeo, I. H., S. K. Han, J. H. Yu, and D. H. Bai. 1998. Isolation of novel alkalophilic Bacillus alcalophilus subsp. YB380 and the characteristics of its yeast cell wall hydrolase. J. Microbiol. Biotechnol. 8:501-508. [Google Scholar]
- 48.Yuk, H.-G., and D. L. Marshall. 2004. Adaptation of Escherichia coli O157:H7 to pH alters membrane lipid composition, verotoxin secretion, and resistance to simulated gastric fluid acid. Appl. Environ. Microbiol. 70:3500-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, K., D. O. Bayles, A. M. Xiong, R. K. Jayaswal, and B. J. Wilkinson. 2005. Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon-interrupted branched-chain alpha-keto acid dehydrogenase. Microbiology 151:615-623. [DOI] [PubMed] [Google Scholar]
- 50.Zhu, K., X. Ding, M. Julotok, and B. J. Wilkinson. 2005. Exogenous isoleucine and fatty acid shortening ensure the high content of anteiso-C15:0 fatty acid required for low-temperature growth of Listeria monocytogenes. Appl. Environ. Microbiol. 71:8002-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]