Abstract
Lactobacillus reuteri inhibits Staphylococcus aureus growth on Baird-Parker agar. This activity required the presence of tellurite and was not shared with other lactic acid bacteria or an L. reuteri mutant defective in cystine metabolism. Secreted products generated from L. reuteri cystine metabolism and thiols were shown to augment tellurite toxicity.
Tellurite (TeO32−)-containing Baird-Parker agar (BPA) is used as a selective and differential medium for the isolation and enumeration of Staphylococcus aureus from foods (2). Tellurite is also used in media for the selection of pathogens, including Corynebacterium diphtheriae, Vibrio cholerae, Shigella spp., and Escherichia coli O157 (14). In previous work, Lactobacillus reuteri BR11 (formerly Lactobacillus fermentum BR11) was engineered to express the S. aureus-killing bacteriocin lysostaphin (18). L. reuteri and S. aureus coculture experiments were performed to investigate the killing effect of secreted lysostaphin (18). In the course of that study, a result was obtained that was not fully understood. It was found, using the methods described in reference 18, that when an undiluted aliquot of an overnight coculture in MRS (buffered with potassium phosphate, pH 7) containing a non-lysostaphin-expressing L. reuteri BR11 (9 × 108 CFU/ml) and S. aureus (9 × 108 CFU/ml) was plated onto BPA, no S. aureus grew. Here we report the investigation of the basis of this phenomenon.
L. reuteri (BR11 and ATCC 55730) but not other lactic acid bacteria inhibit the growth of S. aureus and Listeria monocytogenes on tellurite-containing media.
The first questions addressed in this study were whether the L. reuteri-mediated S. aureus growth inhibition or killing is occurring in the coculture or on BPA and the extent of dependence of the phenomenon on the number and species of Lactobacillus cells. All strains used are shown in Table 1, and all growth media and growth additives were obtained from Oxoid (Basingstoke, United Kingdom). Culture conditions were as described previously (18) except for L. reuteri BR11 (Mlp-His6-CFTR) and L. reuteri PNG201, which were grown in MRS containing 10 μg/ml erythromycin at 40°C overnight and then diluted and grown to log phase at 37°C. Dilution of the L. reuteri-S. aureus coculture by 100-fold or greater resulted in S. aureus growth on BPA, indicating that S. aureus is viable in the coculture (data not shown). Growth inhibition was found to take place on BPA, since adding aliquots of separately grown and washed L. reuteri BR11 and S. aureus ATCC 49476 resulted in a lack of S. aureus growth (Fig. 1). Inhibition of S. aureus growth on BPA was less effective, with reduced numbers of L. reuteri BR11 cells, and did not occur when L. reuteri BR11 was replaced with Lactobacillus plantarum, Lactococcus lactis, Lactobacillus delbrueckii, or Lactobacillus rhamnosus (Fig. 1). Another L. reuteri strain (ATCC 55730) was also found to significantly inhibit S. aureus growth (Fig. 1). Growth inhibition caused by L. reuteri BR11 was found to be entirely dependent upon the presence of tellurite, since omission of this compound from BPA resulted in a confluent lawn of S. aureus growth (L. reuteri BR11 is unable to grow on BPA with or without tellurite). L. reuteri BR11 was able to significantly inhibit S. aureus growth on other growth media supplemented with tellurite, including Luria-Bertani agar (containing 0.2 mM tellurite) and brain heart infusion (BHI) agar (containing 0.4 mM tellurite). L. reuteri BR11 was also found to inhibit other S. aureus strains (ATCC 35556 and SS21c) and L. monocytogenes on tellurite-containing medium (Fig. 2).
TABLE 1.
Strains used in this study
| Strain | Details | Source or reference |
|---|---|---|
| L. reuteri strains | ||
| BR11 | Guinea pig vaginal isolate (formerly L. fermentum BR11) | 13 |
| PNG201 | L. reuteri BR11 mutant defective in CyuC expression and cystine uptake (formerly L. fermentum PNG201) | 19 |
| BR11 (Mlp-His6-CFTR) | L. reuteri BR11 containing pJRS233 (Mlp-His6-CFTR) plasmid integrated in the mlp locus (not affected in cystine metabolism) | 16 |
| ATCC 55730 | Probiotic strain isolated from commercial tablet (Forbiotic) | Blackmores Australia Ltd. |
| L. lactis MG1363 | Plasmid-free L. lactis subsp. cremoris | 6 |
| L. rhamnosus GG | Commercial probiotic strain | ATCC 53103 |
| L. plantarum ATCC 14917 | Natural cabbage fermentation isolate | |
| L. delbrueckii ATCC 4797 | L. delbrueckii subsp. lactis DSM 20076 | DSMZ |
| S. aureus strains | ||
| ATCC 49476 | Methicillin-resistant S. aureus | Graeme Nimmoa |
| SS21c | Methicillin-sensitive S. aureus clinical isolate | Graeme Nimmoa |
| ATCC 35556 | Methicillin-sensitive S. aureus | |
| L. monocytogenes ATCC 19112 | Serovar 1/2c | ATCC |
Queensland Health Pathology Services, Microbiology Department, Princess Alexandra Hospital, Brisbane, Australia.
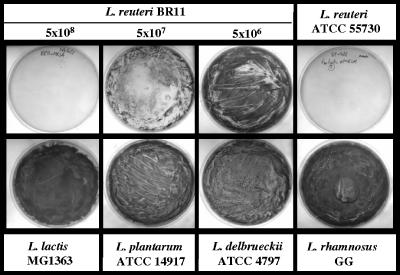
FIG. 1.
Inhibition of growth of S. aureus on BPA in the presence of L. reuteri. Exponential-phase S. aureus ATCC 49476 (6 × 106 CFU) was plated onto BPA (containing 0.4 mM tellurite) along with washed exponential-phase L. reuteri BR11 (CFU indicated), L. reuteri ATCC 55730 (4 × 108 CFU), L. lactis MG1363 (5 × 109 CFU), L. plantarum ATCC 14917 (2 × 109 CFU), L. delbrueckii ATCC 4797 (2 × 108 CFU), or L. rhamnosus GG (1 × 109 CFU). Following 24 h of incubation, growth of S. aureus is seen as a black confluent lawn. The lactic acid bacteria were unable to grow on this medium (data not shown).
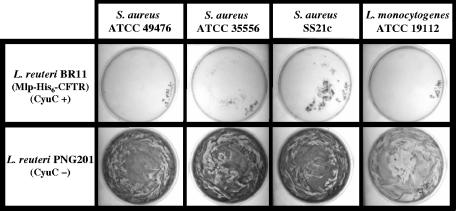
FIG. 2.
The cyuC mutant of L. reuteri is unable to significantly inhibit S. aureus growth on BPA or L. monocytogenes growth on BHI containing tellurite. Exponential-phase S. aureus ATCC 49476 (7 × 106 CFU), S. aureus ATCC 35556 (3 × 106 CFU), and S. aureus SS21c (1 × 107 CFU) were plated onto BPA (containing 0.4 mM tellurite), while L. monocytogenes (5 × 106 CFU) was plated onto BHI (containing 0.2 mM tellurite). Also plated were washed exponential-phase L. reuteri BR11 (Mlp-His6-CFTR; 3 × 108 CFU) or L. reuteri PNG201 (2 × 108 CFU) cells. Following 24 h of incubation, growth of S. aureus strains and L. monocytogenes is seen as black confluent lawns.
Lactic acid bacteria can produce antimicrobial substances, including bacteriocins, lactic acid, and hydrogen peroxide. It is likely that the inhibitor is not lactic acid, since inhibition of S. aureus growth by L. reuteri BR11 also occurred on BHI agar containing tellurite that was buffered with 0.2 M potassium phosphate (pH 7). L. reuteri BR11 produces hydrogen peroxide in the presence of oxygen but not under anaerobic conditions (8). However, hydrogen peroxide is not likely to be the inhibitor, since S. aureus growth was inhibited by L. reuteri BR11 in both the presence and absence of oxygen and on medium containing catalase (500 U/ml; from bovine liver [Sigma-Aldrich]). Therefore, our model at this point indicated that L. reuteri secretes a substance that potentiates tellurite toxicity.
An L. reuteri cystine uptake-deficient mutant does not inhibit S. aureus growth on tellurite-containing media.
In the case of S. aureus, it is known that cysteine metabolism is important for tellurite resistance (12). It has also been shown that tellurium compounds interact with thiols (1), and tellurite decreases the level of reduced thiols in E. coli and Pseudomonas spp. (15, 20). It was hypothesized that L. reuteri BR11 inhibits the growth of S. aureus on tellurite-containing media by modifying the extracellular thiol or disulfide levels. L. reuteri and close relatives possess a unique operon whose products are devoted to the uptake and conversion of the disulfide amino acid cystine (9). One of the products of this operon is a very abundant high-affinity cystine binding protein, CyuC, which is essential for cystine uptake (9, 17, 19). A mutant (L. reuteri PNG201) defective in CyuC expression is unable to import cystine or generate extracellular thiol from cystine (19).
It was found that L. reuteri PNG201 is unable to inhibit S. aureus or L. monocytogenes growth on tellurite-containing medium (Fig. 2). This suggests that the tellurite-mediated S. aureus growth-inhibiting activity of L. reuteri BR11 is either due to the depletion of cystine in the growth medium or the production of a compound derived from cystine. To test this, we investigated whether cystine potentiates or lessens the S. aureus-inhibitory activity of L. reuteri. L. reuteri cells were washed and resuspended in potassium phosphate magnesium (KPM) buffer (19), and 1.5 ml of a suspension with an optical density (OD) of ∼1.5 was incubated with 0.4 mM cystine and 21 mM glucose at 37°C for 75 min. Five microliters of this suspension was spotted onto filter paper disks on BPA inoculated with S. aureus (Fig. 3). L. reuteri BR11 cells, but not L. reuteri PNG201 (CyuC mutant) cells, incubated with cystine plus glucose inhibited growth of S. aureus on tellurite-containing medium (Fig. 3). Also, L. reuteri ATCC 55730, L. plantarum, and L. rhamnosus cells that were incubated with cystine and glucose were able to inhibit growth of S. aureus (Fig. 3). L. lactis and L. delbrueckii cells incubated with cystine and glucose had no significant S. aureus-inhibiting activity (Fig. 3). These experiments suggest that products of Lactobacillus cystine metabolism heighten the toxicity of tellurite to S. aureus.
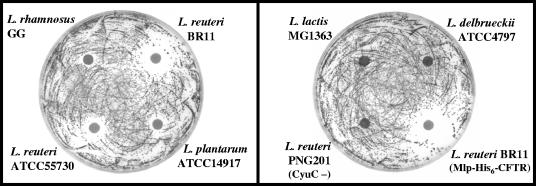
FIG. 3.
Inhibition of S. aureus growth on BPA by lactic acid bacterial cells incubated with cystine and glucose. Following incubation of lactic acid bacteria with cystine and glucose, 5 μl of this cell suspension was spotted onto filter paper disks (6-mm diameter) on top of BPA plates containing 0.4 mM tellurite that had been inoculated with S. aureus ATCC 49476 (9 × 104 CFU). Plates were incubated for 48 h. Significant S. aureus growth-inhibitory activity is seen for disks containing L. reuteri BR11, L. reuteri ATCC 55730, L. reuteri BR11 (Mlp-His6-CFTR), L. plantarum, and L. rhamnosus, while no or little inhibition is seen for disks containing L. lactis, L. delbrueckii, and L. reuteri PNG201. No inhibition of growth of S. aureus was seen on BPA plates that did not contain tellurite.
Thiols inhibit the growth of S. aureus and L. monocytogenes in the presence of tellurite.
We hypothesized that cystine-derived thiol(s) heightens the toxicity of tellurite. To test this, the products secreted by L. reuteri BR11 (Mlp-His6-CFTR) and L. reuteri PNG201 (CyuC mutant) during incubation with cystine and glucose (as described above) were obtained following removal of the cells by centrifugation. Under these conditions, the only difference between the strains should be the amount of thiols secreted. One hundred microliters of the supernatant (either filtered or not filtered) was spotted onto disks on BPA inoculated with S. aureus (Fig. 4). The products secreted by L. reuteri BR11 (Mlp-His6-CFTR) but not L. reuteri PNG201 (CyuC mutant) were found to inhibit S. aureus growth in the presence of tellurite (Fig. 4). Cystine is a likely substrate for the L. reuteri BR11 cystathionine γ-lyase (which is encoded in the same operon as cyuC [17]), and the predicted products of such a reaction would be thiocysteine, pyruvate, and ammonia (4). Thiocysteine is unstable and can spontaneously degrade to form cysteine and elemental sulfur (7) and can react nonenzymatically with thiols to generate H2S. Previously, H2S was found to be produced by L. reuteri BR11 cells when incubated with cystine and glucose (8). Here, a significant quantity of cysteine was found to be secreted by L. reuteri BR11, but not L. reuteri PNG201 (CyuC mutant), during incubation with cystine and glucose (data not shown) as determined using the cysteine-specific acid ninhydrin assay (5). Therefore, at least two different thiols (H2S and cysteine) are secreted by L. reuteri BR11 following incubation with cystine and glucose, and it was concluded that either or both of these increase the toxicity of tellurite.
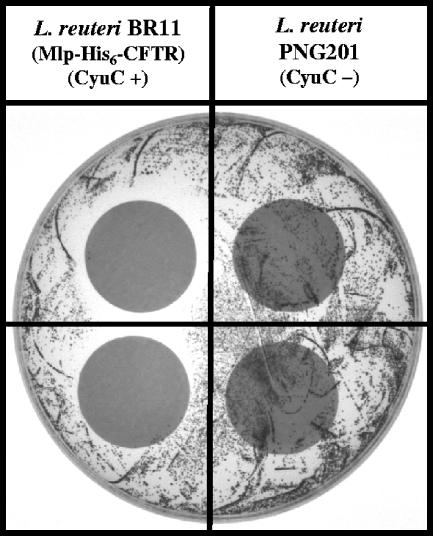
FIG. 4.
The cystine-derived products secreted by L. reuteri BR11 (Mlp-His6-CFTR), but not L. reuteri PNG201, inhibit S. aureus growth on BPA. L. reuteri strains were incubated with cystine and glucose, and the products from this reaction were obtained following centrifugation of the cells. Portions of the supernatant (100 μl) were spotted before (top quadrants) or after (bottom quadrants) filtering through a 0.2-μm syringe filter onto filter paper disks (24-mm diameter) on a BPA plate (containing 0.4 mM tellurite) inoculated with S. aureus ATCC 49476 (9 × 104 CFU). The plate was incubated at 37°C for 24 h. S. aureus growth-inhibitory activity is seen from the secreted products from L. reuteri BR11 (Mlp-His6-CFTR) by the lack of colonies underneath and near the disks on the left side of the plate. No inhibition of growth of S. aureus was seen on BPA plates that did not contain tellurite.
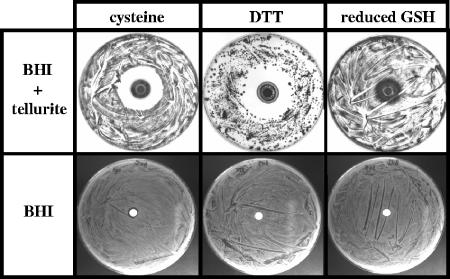
Next, we determined if specific thiols can inhibit the growth of S. aureus on tellurite-containing medium. Cysteine and dithiothreitol, but not reduced glutathione, were found to inhibit the growth of S. aureus on BHI medium containing 0.4 mM tellurite but did not inhibit growth on BHI alone (Fig. 5). To quantify thiol concentrations needed for this inhibitory effect, growth of S. aureus and L. monocytogenes in liquid BHI containing 0.4 mM tellurite and various concentrations of cysteine was investigated (Table 2). Ten microliters of exponential-phase-grown S. aureus cells (OD at 600 nm [OD600], ∼0.01) or L. monocytogenes (OD600, ∼0.8) was inoculated into 5 ml liquid medium, incubated overnight at 37°C without shaking, and then examined for growth. Concentrations of cysteine between 1 mM and 0.25 mM prevented growth of both S. aureus and L. monocytogenes in the presence of tellurite. Addition of higher concentrations of cysteine (5 mM and 10 mM) to BHI containing tellurite caused a rapid reduction of tellurite to black elemental tellurium. S. aureus and L. monocytogenes growth occurred under these high concentrations of cysteine (Table 2).
FIG. 5.
Effects of various thiol compounds on growth of S. aureus in the presence of tellurite. Ten microliters of 0.45 M cysteine, dithiothreitol (DTT) or reduced glutathione (GSH) was spotted onto filter paper disks (6-mm diameter) placed on BHI agar containing 0.4 mM tellurite or BHI agar that had been inoculated with S. aureus ATCC 49476 (∼1 × 106 CFU). Plates were incubated for 48 h at 37°C. The dark ring around the filter paper disks is likely due to tellurium formation, and within these black zones are some S. aureus colonies. Significant S. aureus growth inhibition is seen with cysteine and DTT, but not with reduced GSH on BHI containing tellurite. Little or no S. aureus growth inhibition is seen with the thiols on BHI without tellurite. DTT reduced the S. aureus lawn into discrete colonies on the BHI plate containing tellurite, even though the same number of S. aureus CFU was plated.
TABLE 2.
Growth of S. aureus ATCC 49476 and L. monocytogenes in BHI containing 0.4 mM potassium tellurite and various concentrations of supplemented cysteine
| Species | Growtha with supplemented cysteine at (mM):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.031 | 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 5 | 10 | |
| S. aureus | + | + | + | + | − | − | − | + | + |
| L. monocytogenes | + | + | + | + | − | − | − | + | + |
+, growth; −, no growth.
The results presented here clearly demonstrate that thiols, including those produced by cystine metabolism of L. reuteri, can augment the toxicity of tellurite. This was unexpected, since it was predicted that thiol-mediated reduction of tellurite would lead to detoxification. This was the case with higher thiol levels, which caused the abiotic production of nontoxic black elemental tellurium. However, in the presence of lower levels of thiols, little or no abiotic production of tellurium occurred, and tellurite toxicity was increased. It is possible that the increase is due to S. aureus having heightened thiol levels, which result in an increase in reactive oxygen species generated during intracellular tellurite reduction. It has recently been shown that intracellular tellurite reduction causes production of reactive oxygen species and that this is linked to its mechanism of toxicity (15). Selenite toxicity appears to have a similar basis (10). Glutathione- and thioredoxin-deficient mutants of E. coli or Salmonella are hyper-resistant to selenite, which suggests that the intracellular interaction of selenite with thiol results in toxic oxidative by-products (3, 11). Also, selenite mutagenicity is the result of an intracellular but not an extracellular reaction with thiols, possibly due to proximity with DNA (11).
The results of the current study have clear implications in the development and application of tellurite-containing selective media for the isolation of pathogens and provide insight into the role of thiols in tellurite detoxification and toxicity.
Acknowledgments
We thank Graeme Nimmo and Flavia Huygens for Staphylococcus strains. We also thank Terry Walsh for his helpful comments during this work.
This research was supported by grants from ARC (grant no. DP0665546) and NHMRC (grant no. 290526).
Footnotes
Published ahead of print on 1 December 2006.
REFERENCES
- 1.Albeck, A., H. Weitman, B. Sredni, and M. Albeck. 1998. Tellurium compounds: selective inhibition of cysteine proteases and model reaction with thiols. Inorg. Chem. 37:1704-1712. [Google Scholar]
- 2.Baird-Parker, A. 1962. An improved diagnostic and selective medium for isolating coagulase positive staphylococci. J. Appl. Bacteriol. 25:12-19. [Google Scholar]
- 3.Bébien, M., G. Lagniel, J. Garin, D. Touati, A. Verméglio, and J. Labarre. 2002. Involvement of superoxide dismutases in the response of Escherichia coli to selenium oxides. J. Bacteriol. 184:1556-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Angelis, M., A. C. Curtin, P. L. McSweeney, M. Faccia, and M. Gobbetti. 2002. Lactobacillus reuteri DSM 20016: purification and characterization of a cystathionine gamma-lyase and use as adjunct starter in cheesemaking. J. Dairy Res. 69:255-267. [DOI] [PubMed] [Google Scholar]
- 5.Gaitonde, M. K. 1967. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 104:627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentry-Weeks, C. R., J. M. Keith, and J. Thompson. 1993. Toxicity of Bordetella avium β-cystathionase toward MC3T3-E1 osteogenic cells. J. Biol. Chem. 268:7298-7314. [PubMed] [Google Scholar]
- 8.Hung, J., D. Cooper, M. S. Turner, T. Walsh, and P. M. Giffard. 2003. Cystine uptake prevents production of hydrogen peroxide by Lactobacillus fermentum BR11. FEMS Microbiol. Lett. 227:93-99. [DOI] [PubMed] [Google Scholar]
- 9.Hung, J., M. S. Turner, T. Walsh, and P. M. Giffard. 2005. BspA (CyuC) in Lactobacillus fermentum BR11 is a highly expressed high-affinity L-cystine-binding protein. Curr. Microbiol. 50:33-37. [DOI] [PubMed] [Google Scholar]
- 10.Kessi, J., and K. W. Hanselmann. 2004. Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J. Biol. Chem. 279:50662-50669. [DOI] [PubMed] [Google Scholar]
- 11.Kramer, G. F., and B. N. Ames. 1988. Mechanisms of mutagenicity and toxicity of sodium selenite (Na2SeO3) in Salmonella typhimurium. Mutat. Res. 201:169-180. [DOI] [PubMed] [Google Scholar]
- 12.Lithgow, J. K., E. J. Hayhurst, G. Cohen, Y. Aharonowitz, and S. J. Foster. 2004. Role of a cysteine synthase in Staphylococcus aureus. J. Bacteriol. 186:1579-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rush, C. M., L. M. Hafner, and P. Timms. 1994. Genetic modification of a vaginal strain of Lactobacillus fermentum and its maintenance within the reproductive tract after intravaginal administration. J. Med. Microbiol. 41:272-278. [DOI] [PubMed] [Google Scholar]
- 14.Taylor, D. E. 1999. Bacterial tellurite resistance. Trends Microbiol. 7:111-115. [DOI] [PubMed] [Google Scholar]
- 15.Tremaroli, V., F. Stefano, and D. Zannoni. 2006. Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress of cells of Pseudomonas pseudoalcaligenes KF707. Arch. Microbiol. [Epub ahead of print.] [DOI] [PubMed]
- 16.Turner, M. S., L. M. Hafner, T. Walsh, and P. M. Giffard. 2003. Peptide surface display and secretion using two LPXTG-containing surface proteins from Lactobacillus fermentum BR11. Appl. Environ. Microbiol. 69:5855-5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner, M. S., P. Timms, L. M. Hafner, and P. M. Giffard. 1997. Identification and characterization of a basic cell-surface-located protein from Lactobacillus fermentum BR11. J. Bacteriol. 179:3310-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner, M. S., F. Waldherr, M. J. Loessner, and P. M. Giffard. 2007. Antimicrobial activity of lysostaphin and a Listeria monocytogenes bacteriophage endolysin produced and secreted by lactic acid bacteria. Syst. Appl. Microbiol. 30:58-67. [DOI] [PubMed] [Google Scholar]
- 19.Turner, M. S., T. Woodberry, L. M. Hafner, and P. M. Giffard. 1999. The bspA locus of Lactobacillus fermentum BR11 encodes an l-cystine uptake system. J. Bacteriol. 181:2192-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner, R., J. Weiner, and D. Taylor. 1999. Tellurite-mediated thiol oxidation in Escherichia coli. Microbiology 145:2549-2557. [DOI] [PubMed] [Google Scholar]