Abstract
Intracellular granules containing ferric and ferrous iron formed in Shewanella putrefaciens CN32 during dissimilatory reduction of solid-phase ferric iron. It is the first in situ detection at high resolution (150 nm) of a mixed-valence metal particle residing within a prokaryotic cell. The relationship of the internal particles to Fe(III) reduction may indicate a respiratory role.
Iron biominerals that develop within living cells are unusual and far less common than extracellular precipitates, which form copiously during the dissimilatory reduction (DR) of Fe(III) compounds (3, 7, 12). Previously we have observed intracellular iron particles forming during DR by Shewanella putrefaciens CN32, a gram-negative facultative anaerobe (6). One possibility is that the particles serve a respiratory role, perhaps as a reservoir of oxidant as is known for certain Thioplaca spp., freshwater and marine bacteria that use nitrate stored in internal vacuoles to oxidize sulfide (9). The presence of mixed-valence states of Fe in the granules and their disappearance under complete reducing conditions lend further support.
It has not been possible to isolate and purify the intracellular particles because fine-grained extracellular Fe minerals contaminate the growth medium as well as the cell surface. The small size of the internal particles, their intracellular location, and their instability during exposure to a focused electron beam necessitate an in situ, nondestructive spectroscopic approach that can establish the chemical composition, including Fe valence states, at extremely high spatial and chemical resolution. For this, we applied high-energy X-ray microscopy, which combines a spatial resolution of 150 nm with high elemental sensitivity (higher than 1 × 104 atoms within a spot size of 150 nm) (1). X-ray fluorescence (XRF) in concert with X-ray absorption near-edge spectroscopy (XANES) is a recent application of established methods that can achieve the high resolution necessary to articulate chemical information contained in metals associated with bacterial cells (10).
In our study, CN32 was grown to mid- to late exponential growth phase in a defined medium (DM) consisting of mineral salts, phosphate, PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], and lactate (as an electron donor) under aerobic conditions (6). The cells were then washed, gassed with H2-Ar (4:96), and grown in DM to which enough sterile hydrous ferric oxide (HFO) suspension was added as the electron acceptor to produce 4 to 8 mM Fe under strict anaerobic conditions at 22°C (6). Soluble ferric and ferrous iron treatments were included for comparison to treatments with solid ferric iron. Bacteria were inoculated into anaerobic DM containing either 30 mM ferric citrate or 10 mM fumarate and 4 mM FeCl2.
The internal biomineralization products formed during the dissimilatory reduction of Fe(III) by CN32 were investigated in individual cells by XRF microscopy and micro-X-ray absorption spectroscopy (1) (see the supplemental material).
Cells began to exhibit the intracellular granules (Fig. 1) around 3 days after inoculation under anaerobic conditions with HFO as the electron acceptor, as reported previously (6). A combination of electron microscopy and light microscopy allowed discrete regions of selected cells (those containing Fe particles) to be accurately positioned in the X-ray beam. X-ray fluorescence elemental maps were first obtained, followed immediately by XANES spectra at the Fe K-edge at several positions on and within each bacterium. Spectra were compared to Fe mineral standards with Fe valence states from +2 to +3.
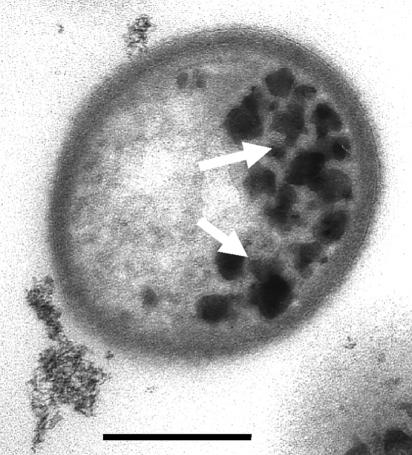
FIG. 1.
Transmission electron microscopy image of S. putrefaciens CN32 cell in cross section, showing intracellular granules of iron oxide (arrows) formed adjacent to the cytoplasmic membrane during anaerobic dissimilatory iron reduction. Bar, 250 nm.
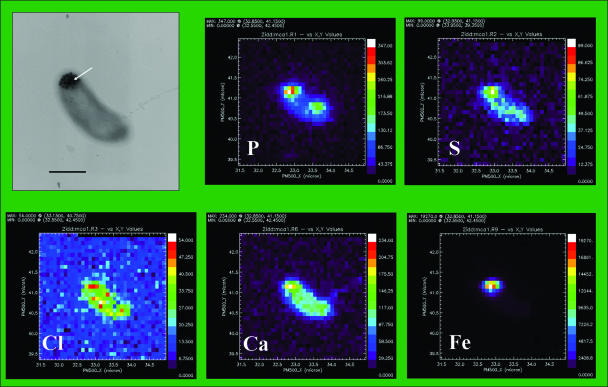
XRF spectra showed that bacteria contained typical cellular elements (Fig. 2). P, Ca, Cl, and S were universal and independent of culture conditions. Differences in distribution between elements could not be correlated with variations exhibited by other elements and were not large enough to exclude possible artifacts due to differences in cell thickness in the region. Potassium was often low or undetectable; however, elevated K was correlated with a high P signal, suggesting potassium phosphate or polyphosphate (not shown). High signals for Fe corresponded to the locations of internal precipitates.
FIG. 2.
X-ray spectromicroscopy analyses of S. putrefaciens CN32. (Top left panel) Transmission electron microscopy image showing a whole mount of a single CN32 cell with cytoplasmic inclusions (arrow) formed during dissimilatory reduction of hydrous ferric oxide. Bar, 500 nm. (Other panels) X-ray fluorescence spectra of bacterium shown in top left panel for P, S, Cl, Ca, and Fe.
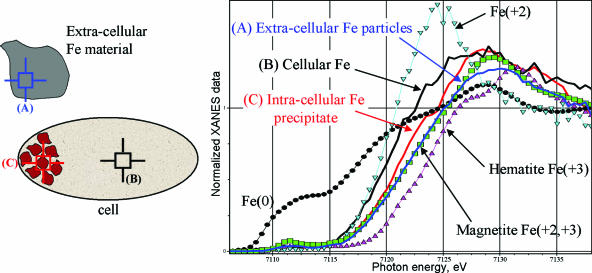
XANES analyses of (i) intracellular granules formed during reduction of HFO, (ii) regions of the bacteria without granules, and (iii) extracellular mineral precipitates provide information on Fe valence state (Fig. 3). This was estimated based on linear calibration obtained by considering the edge position of Fe2+, magnetite, and Fe3+ spectra at 92% of the absorption edge step. Results indicate that the Fe valence states at precipitate-free and precipitate-rich regions of the cell are 2.2 and 2.5, respectively. This places the Fe valence of the granules as more reduced than that of magnetite, or Fe3O4 (average valence of 2.66), and more oxidized than that of green rust, or [Fe(II)(1−x)Fe(III)x(OH)2]x+, where x is the ratio Fe(III)/Fetotal (average valence of 2.25 to 2.33) (4, 14). The average valence state of the extracellular Fe minerals is consistent with that of magnetite and likely results from reduction of the HFO-associated Fe(III) by the bacteria during anaerobic respiration (7, 12).
FIG. 3.
XANES analyses of (A) extracellular Fe-rich material (blue), (B) regions of the cell without granules (black), and (C) intracellular granules formed during ferrihydrite reduction (red) (drawing not to scale). The calibration standard for Fe(III) was hematite (Fe2O3) (magenta triangles), that for Fe(II) and Fe(III) was magnetite (Fe3O4) (green squares), and that for Fe(II) was FeCl2 (light-blue inverted triangles).
Similar analyses of CN32 cultures exposed to Fe(III)-citrate or fumarate-Fe2+ yielded similar valence state results for the intracellular particles; however, due to the lower average concentration of the precipitates (six granules) formed in these treatments, the results are more uncertain. Exposure of dried CN32 cells on electron microscopy grids exposed to air did not appear to change the distribution of Fe valence states either for the granules or for the cell-associated Fe. This is likely due to stabilization by associated anions (e.g., phosphate or organic molecules) (2) and showed the inherent stability of the granules.
Knowing the oxidation state of the granules allows us to propose a mechanism for their formation. The evidence at this point is consistent with uptake of Fe2+ during Fe(III) respiration, followed by segregation and concentration of the Fe within the cytoplasm at the plasma membrane, with which the granules appear to be intimately associated (Fig. 1). Displacement of granules into the cytoplasm accommodates new granule formation on the membrane. This is supported by observations showing that when few granules have developed they are adjacent to the plasma membrane. A higher proportion is located further from the membrane within the cytoplasm when there are more granules. Granules are preferentially located at the ends or poles of the cell under these conditions. The polar location supports specific iron uptake sites at the ends of the cell or rapid segregation to the ends. It is probable that once the poles are exposed to a region of high Fe(III) concentration, DR can result in Fe at sufficient concentrations to trigger uptake; the poles appear to be particularly attractive to Fe oxides (8). Partial oxidation is anticipated to take place after the Fe2+ is moved across the membrane. There is previous evidence for a membrane surrounding each granule (6). Most biological membranes possess proteins having oxidation/reduction power, and it is possible that the membrane controls electron flow to Fe atoms within the granules. This is supported by observations that the granules disappear from the cells once the HFO external electron acceptor is exhausted.
To date, we have observed only the particles within CN32, although many species and strains of Shewanella are capable of DR. We have also tested S. oneidensis, S. algae, S. baltica, and Shewanella sp. strain MR-4, all of which exhibited higher rates of Fe(III) reduction and lower long-term survivability than CN32 when HFO served as the electron acceptor. These strains did not develop the Fe particles. Strain CN32 is a terrestrial bacterium, whereas most isolates are derived from marine systems (15); this may account for a divergence from many of its sister isolates. The presence of unique membrane-bound cytochromes, believed essential for respiration of solid-phase Fe (13), may help to explain the formation of the internal particles during metal respiration. It is unlikely that the cells develop the granules to moderate toxicity effects at high Fe concentrations, which is supported by the observation that fewer granules are actually formed at the higher Fe concentrations of the Fe(III)-citrate treatments.
The fact that the inclusion Fe is intermediate in valency between that of green rust and that of magnetite may be explained by (i) mixed Fe mineral phases, e.g., magnetite and green-rust domains within the particles (5), or (ii) cation-excess magnetite, which has an Fe2+/Fetotal ratio of 0.5 to 0.6 (11). Green rust is a highly reactive reductant in soils and converts to magnetite (4); it is typically unstable under oxic conditions. Vivianite, or Fe3(PO4)2·H2O, cannot be excluded; however, the chemical-state information for the granules as well as their homogeneous size and morphology strongly suggests an Fe(II)/Fe(III) phase.
The regulatory mechanisms for attachment of Shewanella to Fe oxides, dissimilatory Fe reduction, and Fe compartmentalization have been little studied. There is recent evidence that their pathways are interrelated through the ferric uptake regulator modulon fur (16). The scarcity of readily available iron in most natural systems is reflected in the abundance of mechanisms for Fe sequestration by cells (17). Little is known, however, about Fe uptake under conditions of Fe sufficiency, which necessarily must typify the anaerobic environments where dissimilatory Fe reduction occurs. The fact that the granules form best when solid Fe is reduced suggests a unique mechanism closely linked to dissimilatory reduction.
At this point, the most promising routes for future investigations are chemostat culture experiments to optimize conditions for granule formation and molecular genetic studies to articulate mechanisms of Fe uptake and control by Shewanella. We aim to investigate a range of Shewanella spp. under optimized growth conditions to determine the extent to which the intracellular Fe granules may contribute to Fe cycling in subsurface environments.
Supplementary Material
Acknowledgments
We thank S. Vogt and S. Kelly for assistance in using XRF analysis software which they developed, and we thank K. Nealson for his advice.
Partial support was provided to M.B. by the Environmental Molecular Science Institute at the University of Notre Dame. This work is supported by grants to S.G. from the Natural Science and Engineering Research Council of Canada and to T.J.B. and K.K. from the Natural and Accelerated Bioremediation (NABIR) Research Program, Office of Biological and Environmental Research, Office of Science, U.S. Department of Energy (DOE). Additional support was provided to K.K. by the Office of Science, DOE, Early Career Scientist and Engineering Award, and to T.J.B. through the U.S. DOE-NABIR Grand Challenge program administered through the Pacific Northwest National Laboratory in Richland, WA.
Electron microscopy was performed in the Guelph Regional Integrated Imaging Facility (GRIIF), which was partially funded through a Natural Science and Engineering Research Council of Canada Major Facilities Access (NSERC-MFA) grant to T.J.B. Research at the XOR Sector 2 and the Advanced Photon Source (APS) was supported by the Office of Basic Energy Sciences, Office of Science, DOE, under contract W-31-109-Eng-38.
Footnotes
Published ahead of print on 1 December 2006.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Cai, Z., B. Lai, W. Yun, P. P. Ilinski, D. G. Legnini, J. Maser, and W. Rodrigues. 2000. Hard X-ray scanning microprobe for fluorescence imaging and microdiffraction at the advanced photon source, p. 472-477. In W. Meyer-Ilse, T. Warwick, and D. Attwood (ed.), X-ray microscopy. Proceedings of the 6th International Conference. American Institute of Physics, New York, NY.
- 2.Cornell, R. M., and U. Schwertmann. 2003. The iron oxides: structure, properties, reactions, occurrence and uses, 2nd ed. Wiley-VCH, Weinheim, Germany.
- 3.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, H. Dong, T. C. Onstott, N. W. Hinman, and S. Li. 1998. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 62:3239-3257. [Google Scholar]
- 4.Génin, J.-M. R., P. Refait, L. Simon, and S. H. Drissi. 1998. Preparation and Eh-pH diagrams of Fe(II)-Fe(III) green rust compounds; hyperfine interaction characteristics and stoichiometry of hydroxyl-chloride, -sulfate and -carbonate. Hyperfine Interact. 111:313-318. [Google Scholar]
- 5.Génin, J.-M. R., P. Refait, G. Bourrié, M. Abdelmoula, and F. Trolard. 2001. Structure and stability of the Fe(II)-Fe(III) green rust fougerite mineral and its potential for reducing pollutants in soil solutions. Appl. Geochem. 16:559-570. [Google Scholar]
- 6.Glasauer, S., S. Langley, and T. J. Beveridge. 2002. Intracellular iron minerals in a dissimilatory iron-reducing bacterium. Science 295:117-119. [DOI] [PubMed] [Google Scholar]
- 7.Glasauer, S., P. G. Weidler, S. Langley, and T. J. Beveridge. 2003. Controls on Fe reduction and mineral formation by a subsurface bacterium. Geochim. Cosmochim. Acta 67:1277-1288. [Google Scholar]
- 8.Grantham, M. C., P. M. Dove, and T. J. DiChristina. 1997. Microbially catalyzed dissolution of iron and aluminum oxyhydroxide mineral surface coatings. Geochim. Cosmochim. Acta 61:4467-4477. [Google Scholar]
- 9.Jørgensen, B. B., and V. A. Gallardo. 1999. Thioplaca spp.: filamentous sulfur bacteria with nitrate vacuoles. FEMS Microbiol. Ecol. 28:301-313. [Google Scholar]
- 10.Kemner, K. M., S. D. Kelly, B. Lai, J. Maser, E. J. O'Laughlin, D. Sholto-Douglas, Z. H. Cai, M. A. Schneegurt, C. F. Kulpa, and K. H. Nealson. 2004. Elemental and redox analysis of single bacterial cells by X-ray microbeam analysis. Science 306:686-687. [DOI] [PubMed] [Google Scholar]
- 11.Kukkadapu, R. K., J. M. Zachara, J. K. Fredrickson, D. W. Kennedy, A. C. Dohnalkova, and D. E. McCready. 2005. Ferrous hydroxy carbonate is a stable transformation product of biogenic magnetite. Am. Mineral. 90:510-515. [Google Scholar]
- 12.Lovley, D. R., J. F. Stolz, G. L. Nord, Jr., and E. J. P. Philips. 1987. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 330:252-254. [Google Scholar]
- 13.Myers, J. M., and C. R. Myers. 1998. Isolation and sequence of omcA, a gene encoding a decaheme outer membrane cytochrome c of Shewanella putrefaciens MR-1, and detection of omcA homologs in other strains of S. putrefaciens. Biochim. Biophys. Acta 1373:237-251. [DOI] [PubMed] [Google Scholar]
- 14.Sparks, N. H. C., S. Mann, D. A. Bazylinski, D. R. Lovley, H. W. Jannasch, and R. B. Frankel. 1990. Structure and morphology of magnetite anaerobically produced by a marine magnetotactic bacterium and a dissimilatory iron-reducing bacterium. Earth Planet. Sci. Lett. 98:14-22. [Google Scholar]
- 15.Venkateswaran, K., D. P. Moser, M. E. Dolhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackbrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 16.Wan, X.-F., N. C. VerBerkmoes, L. A. McCue, D. Stanek, H. Connelly, L. J. Hauser, L. Y. Wu, X. D. Liu, T. F. Yan, A. Leaphart, R. L. Hettich, J. Z. Zhou, and D. K. Thompson. 2004. Transcriptomic and proteomic characterization of the Fur modulon in the metal-reducing bacterium Shewanella oneidensis. J. Bacteriol. 186:8385-8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wandersman, C., and P. Delepelaire. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611-647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.